Two-Step Macromolecule Separation Process with Acid Pretreatment and High-Shear-Assisted Extraction for Microalgae-Based Biorefinery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biochemical Composition Analysis
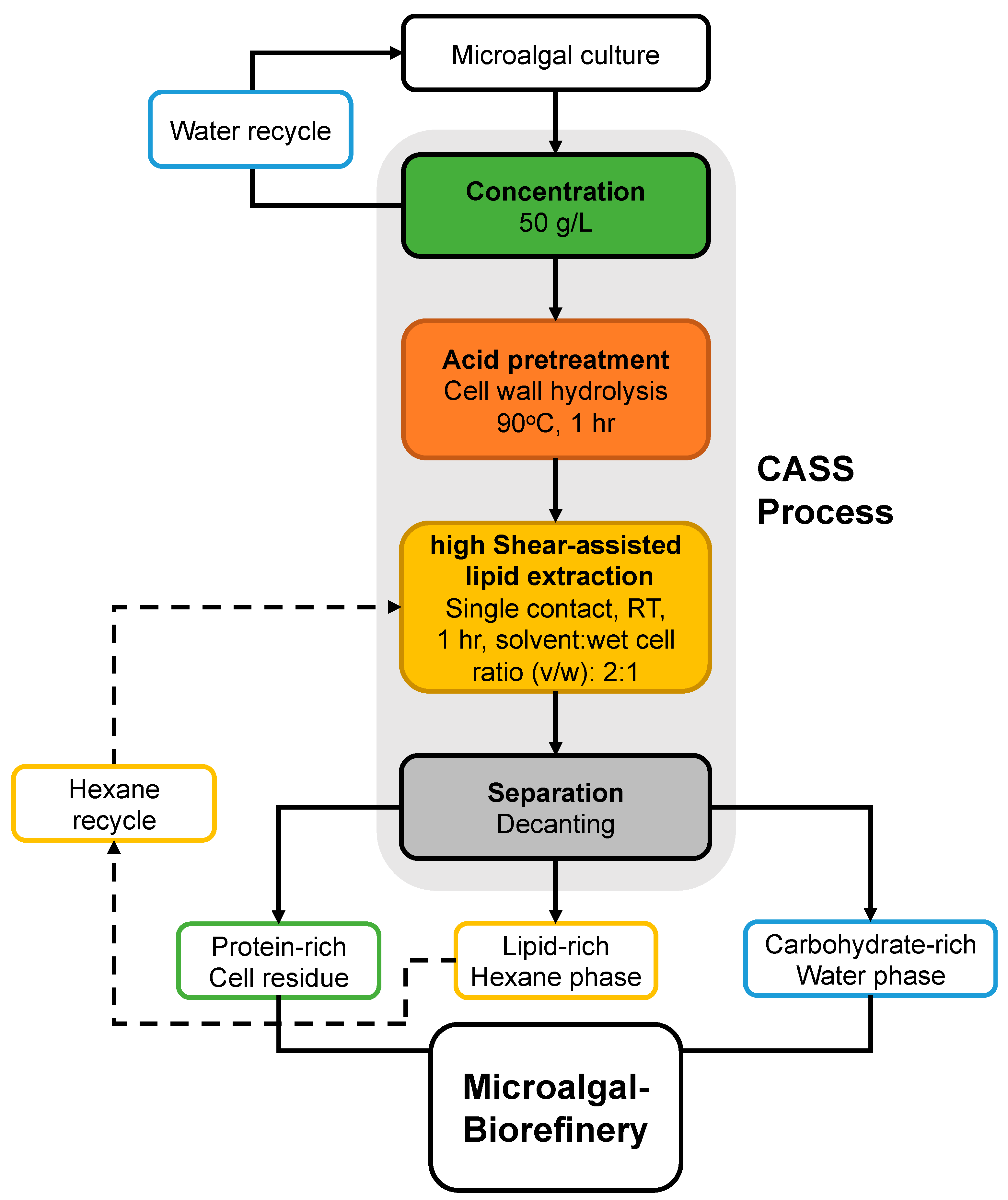
2.3. Acid Pretreatment of Wet Biomass
2.4. High Shear-Assisted Lipid Extraction
2.5. Characterization of Oil, Water, and Solid Phase Products
3. Results and Discussion
3.1. Biochemical Composition of Chlorella sp. ABC-001
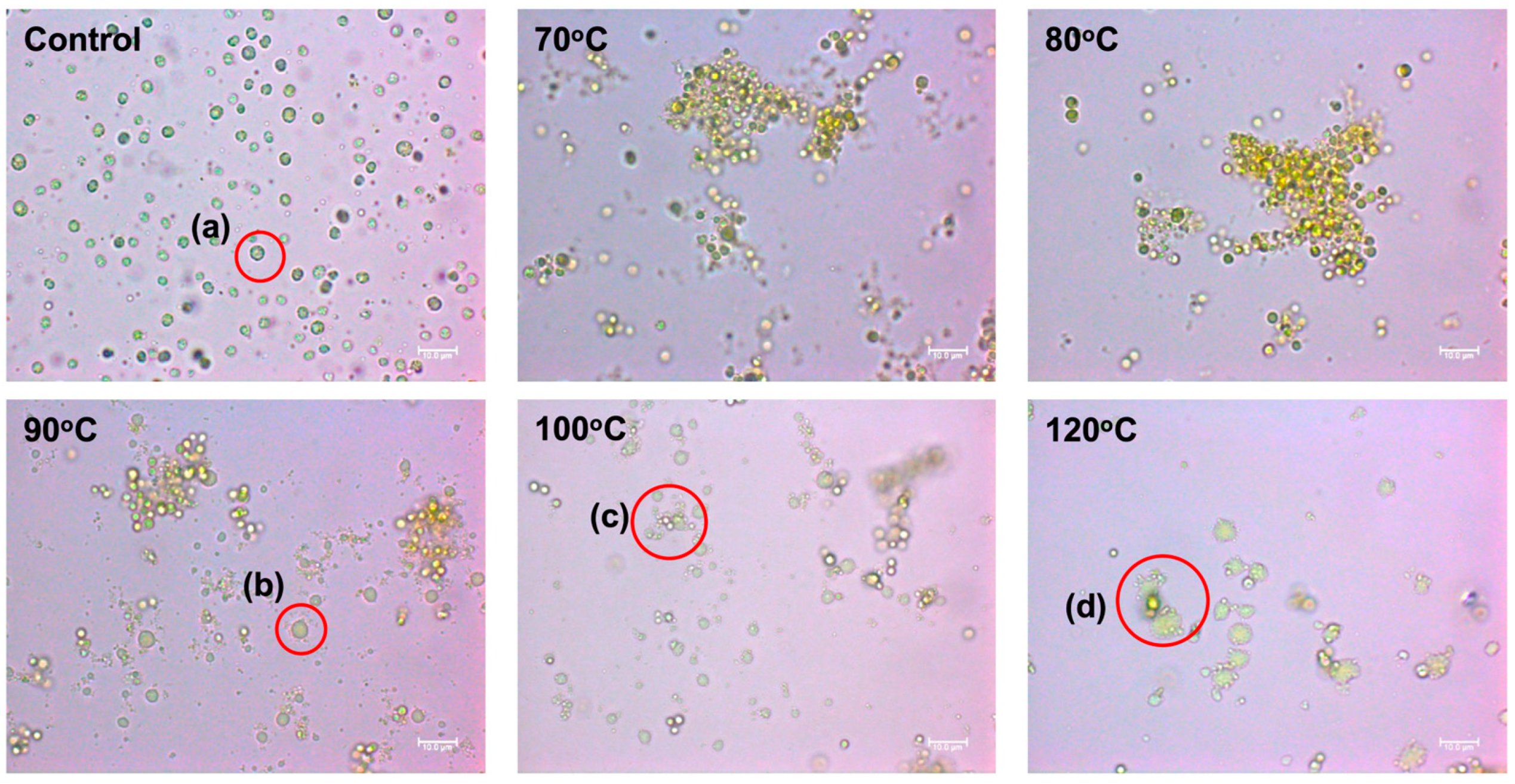
3.2. Acid Pretreatment of Concentrated Wet Biomass
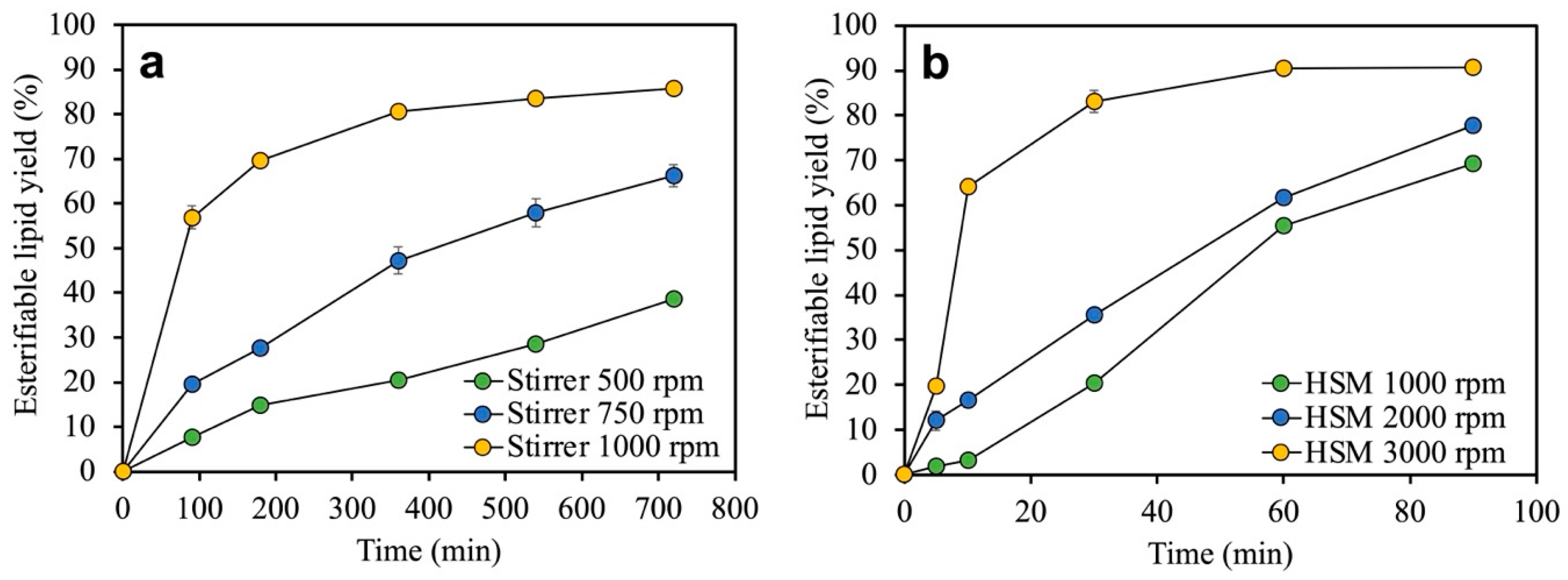
3.3. Effects of Mixing Efficiency on the Lipid Recovery Process
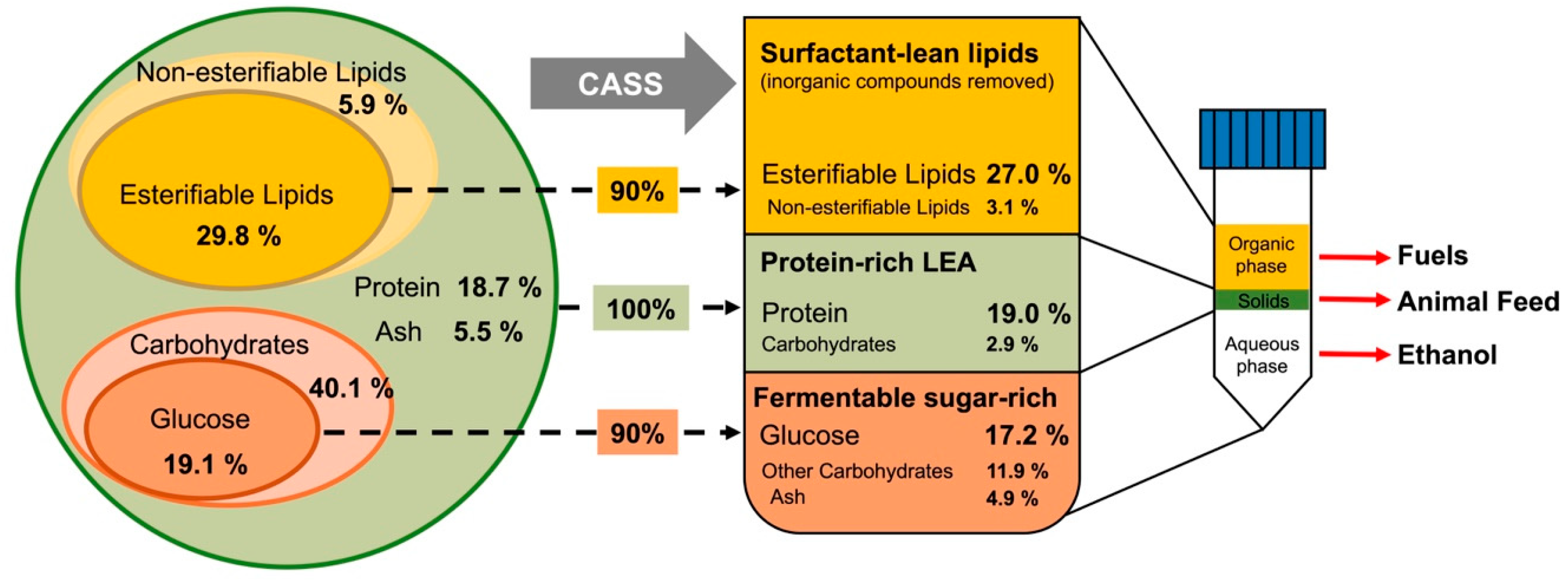
3.4. Separation of Macromolecules in Post-Extracted Mixture: Biorefinery Concept
3.4.1. Lipid Phase: Esterifiable Lipids Recovery
3.4.2. Water Phase: Fermentable Glucose Recovery
3.4.3. Solid Phase: Maintaining Protein Integrity
3.4.4. Impurities Removal Effects on Extracted Lipids
3.5. Comparison with Other Biorefinery Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spandagos, C. Achieving decarbonization goals through biofuels: Policy challenges and opportunities in the European Union and the United States. In Advances in Biofuels Production, Optimization and Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 269–283. [Google Scholar]
- Bracmort, K. The Renewable Fuel Standard (RFS): An Overview; Congressional Research Service: Washington, DC, USA, 2023.
- Bhatt, A.H.; Zhang, Y.; Milbrandt, A.; Newes, E.; Moriarty, K.; Klein, B.; Tao, L. Evaluation of performance variables to accelerate the deployment of sustainable aviation fuels at a regional scale. Energy Convers. Manag. 2023, 275, 116441. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, R.H.; Barbosa, M.J. An Outlook on Microalgal Biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef]
- Klein, B.C.; Chagas, M.F.; Davis, R.E.; Watanabe, M.D.; Wiatrowski, M.R.; Morais, E.R.; Laurens, L.M. A systematic multicriteria-based approach to support product portfolio selection in microalgae biorefineries. Chem. Eng. J. 2024, 481, 148462. [Google Scholar] [CrossRef]
- Kruger, J.S.; Wiatrowski, M.; Davis, R.E.; Dong, T.; Knoshaug, E.P.; Nagle, N.J.; Laurens, L.M.; Pienkos, P.T. Enabling production of algal biofuels by techno-economic optimization of co-product suites. Front. Chem. Eng. 2022, 3, 803513. [Google Scholar] [CrossRef]
- Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-based biorefineries: Challenges and future trends to produce carbohydrate enriched biomass, high-added value products and bioactive compounds. Biology 2022, 11, 1146. [Google Scholar] [CrossRef]
- Andreeva, A.; Budenkova, E.; Babich, O.; Sukhikh, S.; Dolganyuk, V.; Michaud, P.; Ivanova, S. Influence of carbohydrate additives on the growth rate of microalgae biomass with an increased carbohydrate content. Mar. Drugs 2021, 19, 381. [Google Scholar] [CrossRef]
- Sed, G.; Cicci, A.; Jessop, P.G.; Bravi, M. A novel switchable-hydrophilicity, natural deep eutectic solvent (NaDES)-based system for bio-safe biorefinery. RSC Adv. 2018, 8, 37092–37097. [Google Scholar] [CrossRef]
- Kwak, M.; Roh, S.; Yang, A.; Lee, H.; Chang, Y.K. High shear-assisted solvent extraction of lipid from wet biomass of Aurantiochytrium sp. KRS101. Sep. Purif. Technol. 2019, 227, 115666. [Google Scholar] [CrossRef]
- Ramasubramania, I. The issue of reducing or removing phospholipids from total lipids of a microalgae and an oleaginous fungus for preparing biodiesel. Biofuels 2016, 7, 55–72. [Google Scholar]
- Arora, P. Deactivation of Catalysts and Reaction Kinetics for Upgrading of Renewable Oils. Ph.D. Thesis, Chalmers Tekniska Hogskola, Gothenburg, Sweden, 2019. [Google Scholar]
- Nitsos, C.; Filali, R.; Taidi, B.; Lemaire, J. Current and novel approaches to downstream processing of microalgae: A review. Biotechnol. Adv. 2020, 45, 107650. [Google Scholar] [CrossRef]
- Aveldano, M.I.; Horrocks, L.A. Quantitative release of fatty acids from lipids by a simple hydrolysis procedure. J. Lipid Res. 1983, 24, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Schüth, F. Acid hydrolysis of cellulose as the entry point into biorefinery schemes. ChemSusChem Chem. Sustain. Energy Mater. 2009, 2, 1096–1107. [Google Scholar] [CrossRef]
- Wijaya, Y.P.; Putra, R.D.D.; Widyaya, V.T.; Ha, J.-M.; Suh, D.J.; Kim, C.S. Comparative study on two-step concentrated acid hydrolysis for the extraction of sugars from lignocellulosic biomass. Bioresour. Technol. 2014, 164, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Sukias, J.; Craggs, R. Enhanced methane yields from microalgal digestion with various pre-treatments. In Proceedings of the 7th IWA Specialist Group Conference on Waste Stabilization Ponds, Bangkok, Thailand, 25–27 September 2006; pp. 25–27. [Google Scholar]
- Sposob, M.; Kim, D.-H.; Yun, G.-S.; Yun, Y.-M. Assessment of the relationship between solubilization and biogas production on anaerobic digestion of pretreated lipid-extracted microalgae waste. Biomass Bioenergy 2020, 141, 105702. [Google Scholar] [CrossRef]
- Santos, N.O.; Oliveira, S.M.; Alves, L.C.; Cammarota, M.C. Methane production from marine microalgae Isochrysis galbana. Bioresour. Technol. 2014, 157, 60–67. [Google Scholar] [CrossRef]
- Samson, R.; Leduy, A. Influence of mechanical and thermochemical pretreatments on anaerobic digestion of Spirulina maxima algal biomass. Biotechnol. Lett. 1983, 5, 671–676. [Google Scholar] [CrossRef]
- Rincón-Pérez, J.; Razo-Flores, E.; Morales, M.; Alatriste-Mondragón, F.; Celis, L.B. Improving the biodegradability of Scenedesmus obtusiusculus by thermochemical pretreatment to produce hydrogen and methane. BioEnergy Res. 2020, 13, 477–486. [Google Scholar] [CrossRef]
- Marques, A.d.L.; Pinto, F.P.; Araújo, O.Q.d.F.; Cammarota, M.C. Assessment of methods to pretreat microalgal biomass for enhanced biogas production. J. Sustain. Dev. Energy Water Environ. Syst. 2018, 6, 394–404. [Google Scholar] [CrossRef]
- Juárez, J.M.; Pastor, E.R.; Sevilla, J.M.F.; Torre, R.M.; García-Encina, P.A.; Rodríguez, S.B. Effect of pretreatments on biogas production from microalgae biomass grown in pig manure treatment plants. Bioresour. Technol. 2018, 257, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Deng, F.; Li, H.; Qin, Z.; Wang, M.; Li, J. Nutrients removal from the secondary effluents of municipal domestic wastewater by Oscillatoria tenuis and subsequent co-digestion with pig manure. Environ. Technol. 2018, 39, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Phwan, C.K.; Chew, K.W.; Sebayang, A.H.; Ong, H.C.; Ling, T.C.; Malek, M.A.; Ho, Y.-C.; Show, P.L. Effects of acids pre-treatment on the microbial fermentation process for bioethanol production from microalgae. Biotechnol. Biofuels 2019, 12, 191. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, S.; Li, W. High shear mixers: A review of typical applications and studies on power draw, flow pattern, energy dissipation and transfer properties. Chem. Eng. Process. Process Intensif. 2012, 57–58, 25–41. [Google Scholar] [CrossRef]
- Cho, J.M.; Oh, Y.K.; Park, W.K.; Chang, Y.K. Effects of Nitrogen Supplementation Status on CO(2) Biofixation and Biofuel Production of the Promising Microalga Chlorella sp. ABC-001. J. Microbiol. Biotechnol. 2020, 30, 1235–1243. [Google Scholar] [CrossRef]
- Bilad, M.; Arafat, H.A.; Vankelecom, I.F. Membrane technology in microalgae cultivation and harvesting: A review. Biotechnol. Adv. 2014, 32, 1283–1300. [Google Scholar] [CrossRef]
- Kim, D.; Kwak, M.; Kim, K.; Chang, Y.K. Turbulent jet-assisted microfiltration for energy efficient harvesting of microalgae. J. Membr. Sci. 2019, 575, 170–178. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kwak, M.; Kang, S.G.; Hong, W.-K.; Han, J.-I.; Chang, Y.K. Simultaneous cell disruption and lipid extraction of wet Aurantiochytrium sp. KRS101 using a high shear mixer. Bioprocess Biosyst. Eng. 2018, 41, 671–678. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Seon, G.; Joo, H.W.; Kim, Y.J.; Park, J.; Chang, Y.K. Hydrolysis of lipid-extracted Chlorella vulgaris by simultaneous use of solid and liquid acids. Biotechnol. Prog. 2019, 35, e2729. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Dempster, T.A.; Jones, H.D.T.; Wolfrum, E.J.; Van Wychen, S.; McAllister, J.S.P.; Rencenberger, M.; Parcher, K.J.; Gloe, L.M. Algal Biomass Constituent Analysis: Method Uncertainties and Investigation of the Underlying Measuring Chemistries. Anal. Chem. 2012, 84, 1879–1887. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.-J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, X.; Yang, X. Overcoming the cell wall recalcitrance of heterotrophic Chlorella to promote the efficiency of lipid extraction. J. Clean. Prod. 2018, 198, 1224–1231. [Google Scholar] [CrossRef]
- Martins, L.B.; Soares, J.; da Silveira, W.B.; Sousa, R.d.C.S.; Martins, M.A. Dilute sulfuric acid hydrolysis of Chlorella vulgaris biomass improves the multistage liquid-liquid extraction of lipids. Biomass Convers. Biorefinery 2021, 11, 2485–2497. [Google Scholar] [CrossRef]
- Ranjan, A.; Patil, C.; Moholkar, V.S. Mechanistic assessment of microalgal lipid extraction. Ind. Eng. Chem. Res. 2010, 49, 2979–2985. [Google Scholar] [CrossRef]
- Kwak, M.; Kim, D.; Kim, S.; Lee, H.; Chang, Y.K. Solvent screening and process optimization for high shear-assisted lipid extraction from wet cake of Nannochloropsis sp. Renew. Energy 2020, 149, 1395–1405. [Google Scholar] [CrossRef]
- Gong, M.; Hu, Y.; Yedahalli, S.; Bassi, A. Oil extraction processes in microalgae. Recent Adv. Renew. Energy 2017, 1, 377–411. [Google Scholar]
- Huber, G.W.; Corma, A. Synergies between bio-and oil refineries for the production of fuels from biomass. Angew. Chem. Int. Ed. 2007, 46, 7184–7201. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Oh, Y.-K.; Chang, Y.K.; Choi, M. Optimum utilization of biochemical components in Chlorella sp. KR1 via subcritical hydrothermal liquefaction. ACS Sustain. Chem. Eng. 2017, 5, 7240–7248. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, D.; Lin, Y.; Wang, X.; Kong, H.; Tanaka, S. Substrate and Product Inhibition on Yeast Performance in Ethanol Fermentation. Energy Fuel 2015, 29, 1019–1027. [Google Scholar] [CrossRef]
- Yun, J.-H.; Nam, J.-W.; Yang, J.H.; Lee, Y.J.; Cho, D.-H.; Choi, H.I.; Hong, J.S.; Ahn, K.H.; Kim, H.-S. Toward a zero-waste microalgal biorefinery: Complete utilization of defatted Chlorella biomass as a sole heterotrophic substrate for Chlorella sp. HS2 and an improved composite filler. Chem. Eng. J. 2024, 480, 147998. [Google Scholar] [CrossRef]
- Gehrke, C.W.; Wall Sr, L.L.; Absheer, J.S.; Kaiser, F.E.; Zumwalt, R.W. Sample preparation for chromatography of amino acids: Acid hydrolysis of proteins. J. Assoc. Off. Anal. Chem. 1985, 68, 811–821. [Google Scholar] [CrossRef]
- Singh, A.K.; Prajapati, K.S.; Shuaib, M.; Kushwaha, P.P.; Kumar, S. Microbial proteins: A potential source of protein. In Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Springer: Cham, Switzerland, 2020; pp. 139–147. [Google Scholar]
- Abdus Salam, M.; Creaser, D.; Arora, P.; Tamm, S.; Lind Grennfelt, E.; Olsson, L. Influence of bio-oil phospholipid on the hydrodeoxygenation activity of NiMoS/Al2O3 catalyst. Catalysts 2018, 8, 418. [Google Scholar] [CrossRef]
- van der Bij, H.E.; Weckhuysen, B.M. Phosphorus promotion and poisoning in zeolite-based materials: Synthesis, characterisation and catalysis. Chem. Soc. Rev. 2015, 44, 7406–7428. [Google Scholar] [CrossRef]
- Paisan, S.; Chetpattananondh, P.; Chongkhong, S. Assessment of water degumming and acid degumming of mixed algal oil. J. Environ. Chem. Eng. 2017, 5, 5115–5123. [Google Scholar] [CrossRef]
- Law, S.Q.; Chen, B.; Scales, P.J.; Martin, G.J. Centrifugal recovery of solvent after biphasic wet extraction of lipids from a concentrated slurry of Nannochloropsis sp. biomass. Algal Res. 2017, 24, 299–308. [Google Scholar] [CrossRef]
- Law, S.Q.; Mettu, S.; Ashokkumar, M.; Scales, P.J.; Martin, G.J. Emulsifying properties of ruptured microalgae cells: Barriers to lipid extraction or promising biosurfactants? Colloids Surf. B Biointerfaces 2018, 170, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Webley, P.A.; Martin, G.J. The CIDES process: Fractionation of concentrated microalgal paste for co-production of biofuel, nutraceuticals, and high-grade protein feed. Algal Res. 2016, 19, 299–306. [Google Scholar] [CrossRef]
- Wang, H.; Ji, C.; Bi, S.; Zhou, P.; Chen, L.; Liu, T. Joint production of biodiesel and bioethanol from filamentous oleaginous microalgae Tribonema sp. Bioresour. Technol. 2014, 172, 169–173. [Google Scholar] [CrossRef]
- Laurens, L.; Nagle, N.; Davis, R.; Sweeney, N.; Van Wychen, S.; Lowell, A.; Pienkos, P. Acid-catalyzed algal biomass pretreatment for integrated lipid and carbohydrate-based biofuels production. Green Chem. 2015, 17, 1145–1158. [Google Scholar] [CrossRef]
- Seon, G.; Kim, M.; Lee, Y.W.; Cho, J.M.; Kim, H.; Park, W.-K.; Chang, Y.K. Development of an integrated biomass refinery process for whole cell biomass utilization of Chlorella sp. ABC-001. Chem. Eng. J. 2023, 451, 138543. [Google Scholar] [CrossRef]

| Composition | Biomass (wt.%) | Crude Lipids 2 (wt.%) | CASS Process 3 | |||
|---|---|---|---|---|---|---|
| Oil Phase (wt.%) | Water Phase (wt.%) | Solid Phase 4 (wt.%) | ||||
| Macromolecule composition 1 | Lipid | 35.7 ± 0.6 | 35.7 ± 0.6 | 30.1 ± 0.2 | N/A | 2.2 ± 0.01 |
| Esterifiable Lipid (FAME) | 29.8 ± 0.2 | 29.8 ± 0.2 | 27.0 ± 0.5 | 0.5 ± 0.2 | ||
| Carbohydrate | 40.1 ± 1.3 | N/A | N/A | 29.1 ± 0.8 | 2.9 ± 0.1 | |
| Glucose | 19.1 ± 0.1 | 17.2 ± 0.3 | 0.2 ± 0.1 | |||
| Protein | 18.7 ± 2.3 | N/A | 19.0 ± 0.4 | |||
| Ash | 5.5 ± 0.1 | 0.1 ± 0.1 | <0.1 | 4.9 ± 0.0 | 0.5 ± 0.0 | |
| Elemental composition | C | 51.8 ± 0.1 | 74.1 ± 0.6 | 75.4 ± 0.4 | N/A | 44.5 ± 0.1 |
| H | 8.2 ± 0.1 | 11.8 ± 0.1 | 12.2 ± 0.1 | 6.5 ± 0.1 | ||
| O | 29.7 ± 0.1 | 11.8 ± 0.1 | 11.4 ± 0.1 | 27.4 ± 0.0 | ||
| N | 2.6 ± 0.0 | 0.4 ± 0.1 | 0.2 ± 0.0 | 8.2 ± 0.1 | ||
| S | 0.2 ± 0.1 | N/D | N/D | 2.5 ± 0.1 | ||
| Content | Biomass (wt. ppm) | Crude Lipid 1 (wt. ppm) | CASS Process | ||
|---|---|---|---|---|---|
| Oil Phase (wt. ppm) | Water Phase (wt. ppm) | Solid Phase 2 (wt. ppm) | |||
| P | 27,021 ± 49 | 1130 ± 56 | 206.5 ± 1.9 | 24,318 ± 155 | 3346 ± 155 |
| Ca | 3168 ± 69 | 74.5 ± 5.1 | N/D | 2738 ± 21 | 461.2 ± 5.3 |
| Na | 623 ± 5 | N/D | N/D | 792.8 ± 6.7 | 137.4 ± 3.3 |
| K | 19,844 ± 509 | 65.3 ± 1.8 | N/D | 17,344 ± 242 | 312.9 ± 2.0 |
| Cu | 889 ± 37 | 271.8 ± 8.2 | 24.9 ± 1.3 | 700.7 ± 0.4 | 352.1 ± 5.1 |
| Zn | 232 ± 53 | 17.4 ± 1.8 | N/D | 298.1 ± 0.7 | N/D |
| Mg | 3325 ± 44 | N/D | N/D | 2891 ± 5 | 574.0 ± 10.2 |
| Process | Strains | Biomass Conc. (g/L) | Operating Conditions | Macromolecule Yield (%) | References |
|---|---|---|---|---|---|
| High-pressure homogenizer | Nannochloropsis sp. | 110–230 | (1) Cell weakening (35 °C, 7–24 h) (2) High-pressure homogenizer (800–1000 bar, 1 pass) (3) Lipid recovery (2 h, biomass (paste):solvent 5:2 w/w) | Lipid: 25 Carbohydrate in water: 41 Protein in biomass: 51 | [54] |
| Acid hydrolysis | Tribonema sp. | 50 | (1) Acid hydrolysis (3 wt%, sulfuric acid, 121 °C, 45 min) (2) Lipid recovery (ethanol-hexane (1:3, v/v) on shaker incubation, 2.5 h, 50 °C, biomass (paste):solvent = 1:6 w/v) | Biodiesel: 98.5 Monosaccharides: 81.5 | [55] |
| Acid hydrolysis | Chlorella sp. | 250 | (1) Acid hydrolysis (2 wt%, sulfuric acid, 155 °C, 10 min) (2) Lipid recovery (hexane, 2 h, biomass (paste):solvent 1:1 v/v) | Lipid: 22.2 Glucose: 81.2 | [56] |
| Scenedesmus sp. | Lipid: 92.5 Glucose: 73.1 | ||||
| Acid hydrolysis | Chlorella sp. ABC-001 | 50 | (1) Acid hydrolysis (0.1 N, sulfuric acid, 170 °C, 4 min) (2) Phase separation using centrifugation (1000 rpm, 5 min) (3) Lipid recovery (hexane with vortexing, 20 min) | Lipid: 100 Monosaccharides: 89 | [57] |
| Hydrothermal liquefaction | Chlorella sp. KR1 | 50 | (1) Hydrothermal liquefaction (180 °C, 1 h) (2) Lipid recovery (hexane with sonication, 2 h) | Lipid: 87 Glucose: 70 | [49] |
| CASS | Chlorella sp. ABC-001 | 50 | (1) Acid hydrolysis (5 wt.% sulfuric acid, 100 °C, 1 h) (2) Lipid recovery (hexane with high-shear mixing, 3000 rpm, 30 min, biomass (wet):solvent = 1:1 v/v) | Lipid: 90.6 Glucose: 90.0 Protein: 100 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kang, S.-G.; Chang, Y.K.; Kwak, M. Two-Step Macromolecule Separation Process with Acid Pretreatment and High-Shear-Assisted Extraction for Microalgae-Based Biorefinery. Sustainability 2024, 16, 7589. https://doi.org/10.3390/su16177589
Kim D, Kang S-G, Chang YK, Kwak M. Two-Step Macromolecule Separation Process with Acid Pretreatment and High-Shear-Assisted Extraction for Microalgae-Based Biorefinery. Sustainability. 2024; 16(17):7589. https://doi.org/10.3390/su16177589
Chicago/Turabian StyleKim, Donghyun, Seul-Gi Kang, Yong Keun Chang, and Minsoo Kwak. 2024. "Two-Step Macromolecule Separation Process with Acid Pretreatment and High-Shear-Assisted Extraction for Microalgae-Based Biorefinery" Sustainability 16, no. 17: 7589. https://doi.org/10.3390/su16177589
APA StyleKim, D., Kang, S.-G., Chang, Y. K., & Kwak, M. (2024). Two-Step Macromolecule Separation Process with Acid Pretreatment and High-Shear-Assisted Extraction for Microalgae-Based Biorefinery. Sustainability, 16(17), 7589. https://doi.org/10.3390/su16177589






