Molecular Mechanisms of the Cyanobacterial Response to Different Phosphorus Sources
Abstract
1. Introduction
2. P Forms in Water
3. Use of DOP by Cyanobacteria
3.1. Hydrolysis of DOP
3.1.1. Hydrolysis Pathways of C-O-P-Bonded DOP
3.1.2. Hydrolysis Pathway of C-P Bonded DOP
| System | Gene | Function |
|---|---|---|
| C-O-P hydrolysate | phoX | Alkaline phosphatase |
| phoA | Alkaline phosphatase | |
| phoD | Alkaline phosphatase | |
| phoV | Alkaline phosphatase | |
| nucH | Extracellular nuclease | |
| C-P lyase | phnF | Repressor proteins |
| PhnG | C-P lyase complex protein | |
| PhnK | C-P lyase complex protein | |
| PhnL | C-P lyase complex protein | |
| phnM | C-P lyase complex protein | |
| phnO | C-P lyase complex protein | |
| phnJ | C-P lyase complex protein | |
| phnI | C-P lyase complex protein | |
| phnH | C-P lyase complex protein | |
| phnF | C-P lyase complex protein |
3.2. P Transport System
4. Use of DIP by Cyanobacteria
4.1. Intracellular Poly-P Accumulation
4.2. Use of Intracellular Poly-Ps
4.3. Intracellular Phosphate Metabolism Pathway
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, T.; Falkowski, P.G. Genome evolution in cyanobacteria: The stable core and the variable shell. Proc. Natl. Acad. Sci. USA 2008, 105, 2510–2515. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.R.; Lv, H.; Isabwe, A.; Liu, L.; Yu, X.; Chen, H.; Yang, J. Disturbance-induced phytoplankton regime shifts and recovery of cyanobacteria dominance in two subtropical reservoirs. Water Res. 2017, 120, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Song, L.R.; Jia, Y.L.; Qin, B.Q.; Li, R.H.; Carmichael, W.W.; Gan, N.Q.; Xu, H.; Shan, K.; Sukenik, A. Harmful cyanobacterial blooms: Biological traits, mechanisms, risks, and control strategies. Annu. Rev. Environ. Resour. 2023, 48, 123–147. [Google Scholar] [CrossRef]
- Burford, M.A.; Carey, C.C.; Hamilton, D.P.; Huisman, J.; Paerl, H.W.; Wood, S.A.; Wulff, A. Perspective: Advancing the research agenda for improving understanding of cyanobacteria in a future of global change. Harmful Algae 2020, 91, 101601. [Google Scholar] [CrossRef]
- Tanaka, T.; Rassoulzadegan, F.; Thingstad, T.F. Orthophosphate uptake by heterotrophic bacteria, cyanobacteria, and autotrophic nanoflagellates in Villefranche Bay, northwestern Mediterranean: Vertical, seasonal, and short-term variations of the competitive relationship for phosphorus. Limnol. Oceanogr. 2004, 49, 1063–1072. [Google Scholar] [CrossRef]
- Vershinina, O.A.; Znamenskaya, L.V. The Pho regulons of bacteria. Microbiology 2002, 71, 497–511. [Google Scholar] [CrossRef]
- Blank, L.M. The cell and P: From cellular function to biotechnological application. Curr. Opin. Biotech. 2012, 23, 846–851. [Google Scholar] [CrossRef]
- Feng, S.; Qin, B.Q.; Gao, G. The relationships between phosphorus-transmuting bacteria and phosphorus forms in Lake Taihu. J. Lake Sci. 2008, 20, 428–443. (In Chinese) [Google Scholar]
- Halemejko, G.Z.; Chrost, R.J. The role of phosphatases in phosphorus mineralization during decomposition of lake phytoplankton blooms. Arch. Fur Hydrobiol. 1984, 101, 489–502. [Google Scholar]
- Bai, X.L.; Zhou, Y.K.; Sun, J.H.; Ma, J.H.; Zhao, H.Y.; Liu, X.F. Classes of dissolved and particulate phosphorus compounds and their spatial distributions in the water of a eutrophic lake: A 31P NMR study. Biogeochemistry 2015, 126, 227–240. [Google Scholar] [CrossRef]
- Dyhrman, S.T.; Ammerman, J.W.; Van Mooy, B.A. Microbes and the marine phosphorus cycle. Oceanography 2007, 20, 110–116. [Google Scholar] [CrossRef]
- Su, Z.; Olman, V.; Xu, Y. Computational prediction of Pho regulons in cyanobacteria. BMC Genom. 2007, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, Y.; Fu, Y.; Bhaya, D. The role of three-tandem Pho Boxes in the control of the C-P lyase operon in a thermophilic cyanobacterium. Environ. Microbiol. 2021, 23, 6433–6449. [Google Scholar] [CrossRef] [PubMed]
- Santos-Beneit, F. The Pho regulon: A huge regulatory network in bacteria. Front. Microbiol. 2015, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Luque, E.; Bhaya, D.; Grossman, A.R. Polyphosphate: A multifunctional metabolite in cyanobacteria and algae. Front. Plant Sci. 2020, 11, 938. [Google Scholar] [CrossRef] [PubMed]
- Kulaev, I.S.; Vagabov, V.; Kulakovskaya, T. The Biochemistry of Inorganic Polyphosphates; John Wiley & Sons: Hoboken, NJ, USA, 2004; p. 1. [Google Scholar]
- Kulaev, I.S.; Vagabov, V.M. Polyphosphate metabolism in micro-organisms. Adv. Microb. Physiol. 1983, 24, 83–171. [Google Scholar] [PubMed]
- Kornberg, A.; Rao, N.N.; Ault-Riche, D. Inorganic polyphosphate: A molecule of many functions. Annu. Rev. Biochem. 1999, 68, 89–125. [Google Scholar] [CrossRef]
- Lin, S.; Litaker, R.W.; Sunda, W.G. Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. J. Phycol. 2016, 52, 10–36. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, S.S.; Pandey, V.D.; Mishra, A.K. Factors modulating alkaline phosphatase activity in the diazotrophic rice-field cyanobacterium, Anabaena oryzae. World J. Microbiol. Biot. 2006, 22, 927–935. [Google Scholar] [CrossRef]
- Harke, M.J.; Berry, D.L.; Ammerman, J.W.; Gobler, C.J. Molecular response of the bloom-forming cyanobacterium, Microcystis aeruginosa, to phosphorus limitation. Microb. Ecol. 2012, 63, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.; Shilova, I.N.; Zehr, J.P. Use of the high-affinity phosphate transporter gene, pstS, as an indicator for phosphorus stress in the marine diazotroph Crocosphaera watsonii (Chroococcales, Cyanobacteria). J. Phycol. 2019, 55, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Y.C.; Wang, M.; Zhang, J.Y.; Chen, Q.W.; Liu, D.S. Molecular responses to inorganic and organic phosphorus sources in the growth and toxin formation of Microcystis aeruginosa. Water Res. 2021, 196, 117048. [Google Scholar] [CrossRef]
- Ranjit, P.; Varkey, D.; Shah, B.S.; Paulsen, I.T. Substrate specificity and ecological significance of PstS homologs in phosphorus uptake in marine Synechococcus sp. WH8102. Microbiol. Spectr. 2024, 12, e02786-23. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, R. Limnology: Lake and River Ecosystems, 3rd ed.; Gulf Professional Publishing: San Diego, CA, USA, 2001. [Google Scholar]
- Peng, S.H.; Huang, S.M.; Zeng, Y.; Zhao, L. Advances on cyanobacteria phosphonate metabolism and its ecological significance. J. Lake Sci. 2023, 35, 43–56. (In Chinese) [Google Scholar]
- McGrath, J.W.; Chin, J.P.; Quinn, J.P. Organophosphonates revealed: New insights into the microbial metabolism of ancient molecules. Nat. Rev. Microbiol. 2013, 11, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.L.; Ingall, E.D.; Benner, R. Marine phosphorus is selectively remineralized. Nature 1998, 393, 426. [Google Scholar] [CrossRef]
- Kolowith, L.C.; Ingall, E.D.; Benner, R. Composition and cycling of marine organic phosphorus. Limnol. Oceanogr. 2001, 46, 309–320. [Google Scholar] [CrossRef]
- Ishii, Y.; Harigae, S.; Tanimoto, S.; Yabe, T.; Yoshida, T.; Taki, K.; Tatsumoto, H. Spatial variation of phosphorus fractions in bottom sediments and the potential contributions to eutrophication in shallow lakes. Limnology 2010, 11, 5–16. [Google Scholar] [CrossRef]
- Reitzel, K.; Ahlgren, J.; DeBrabandere, H.; Waldebäck, M.; Gogoll, A.; Tranvik, L.; Rydin, E. Degradation rates of organic phosphorus in lake sediment. Biogeochemistry 2007, 82, 15–28. [Google Scholar] [CrossRef]
- Ahlgren, J.; Reitzel, K.; Danielsson, R.; Gogoll, A.; Rydin, E. Biogenic phosphorus in oligotrophic mountain lake sediments: Differences in composition measured with NMR spectroscopy. Water Res. 2006, 40, 3705–3712. [Google Scholar] [CrossRef]
- Villarreal-Chiu, J.F.; Quinn, J.P.; McGrath, J.W. The genes and enzymes of phosphonate metabolism by bacteria, and their distribution in the marine environment. Front. Microbiol. 2012, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Mahieu, N.; Condron, L.M. Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH–EDTA extracts. Soil Sci. Soc. Am. J. 2003, 67, 497–510. [Google Scholar] [CrossRef]
- Read, E.K.; Ivancic, M.; Hanson, P.; Cade-Menun, B.J.; McMahon, K.D. Phosphorus speciation in a eutrophic lake by 31P NMR spectroscopy. Water Res. 2014, 62, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Garcia, M.R.; Davison, M.; Blain-Hartnung, M.; Grossman, A.R.; Bhaya, D. Alternative pathways for phosphonate metabolism in thermophilic cyanobacteria from microbial mats. ISME J. 2011, 5, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Xie, E.; Su, Y.; Deng, S.; Kontopyrgou, M.; Zhang, D. Significant influence of phosphorus resources on the growth and alkaline phosphatase activities of Microcystis aeruginosa. Environ. Pollut. 2021, 268, 115807. [Google Scholar] [CrossRef]
- Kononova, S.V.; Nesmeyanova, M.A. Phosphonates and their degradation by microorganisms. Biochem. Moscow. 2002, 67, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lin, L.Z.; Chen, M.Y.; Teng, W.K.; Zheng, L.L.; Peng, L.; Shu, W.S. The widespread capability of methylphosphonate utilization in filamentous cyanobacteria and its ecological significance. Water Res. 2022, 217, 118385. [Google Scholar] [CrossRef]
- Teikari, J.E.; Fewer, D.P.; Shrestha, R.; Hou, S.; Leikoski, N.; Mäkelä, M.; Sivonen, K. Strains of the toxic and bloom-forming Nodularia spumigena (cyanobacteria) can degrade methylphosphonate and release methane. ISME J. 2018, 12, 1619–1630. [Google Scholar] [CrossRef]
- Zaheer, R.; Morton, R.; Proudfoot, M.; Yakunin, A.; Finan, T.M. Genetic and biochemical properties of an alkaline phosphatase PhoX family protein found in many bacteria. Environ. Microbiol. 2009, 11, 1572–1587. [Google Scholar] [CrossRef]
- Tiwari, B.; Singh, S.; Kaushik, M.S.; Mishra, A.K. Regulation of organophosphate metabolism in cyanobacteria. A review. Microbiology 2015, 84, 291–302. [Google Scholar] [CrossRef]
- Luo, H.; Benner, R.; Long, R.A.; Hu, J. Subcellular localization of marine bacterial alkaline phosphatases. Proc. Natl. Acad. Sci. USA 2009, 106, 21219–21223. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.D.; Saito, M.A. Proteomic responses of oceanic Synechococcus WH8102 to phosphate and zinc scarcity and cadmium additions. Front. Microbiol. 2013, 4, 387. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, S.; Diaz, J.M.; Adams, J.C.; Djaoudi, K.; Steck, V.; Waggoner, E.M. Phosphorus as an integral component of global marine biogeochemistry. Nat. Geosci. 2021, 14, 359–368. [Google Scholar] [CrossRef]
- Dai, J.Y.; Gao, G.; Wu, S.; Wu, X.; Zhou, J.; Xue, W.; Chen, D. Bacterial alkaline phosphatases and affiliated encoding genes in natural waters: A review. J. Lake Sci. 2016, 28, 1153–1166. (In Chinese) [Google Scholar]
- Ragot, S.A.; Kertesz, M.A.; Bünemann, E.K. phoD alkaline phosphatase gene diversity in soil. Appl. Environ. Microbiol. 2015, 81, 7281–7289. [Google Scholar] [CrossRef]
- Wagner, K.U.; Masepohl, B.; Pistorius, E.K. The cyanobacterium Synechococcus sp. strain PCC 7942 contains a second alkaline phosphatase encoded by phoV. Microbiology 1995, 141, 3049–3058. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Su, Z.; Xu, Y. The evolution of microbial phosphonate degradative pathways. J. Mol. Evol. 2005, 61, 682–690. [Google Scholar] [CrossRef]
- Seweryn, P.; Van, L.B.; Kjeldgaard, M.; Russo, C.J.; Passmore, L.A.; Hove-Jensen, B.; Brodersen, D.E. Structural insights into the bacterial carbon–phosphorus lyase machinery. Nature 2015, 525, 68–72. [Google Scholar] [CrossRef]
- Dyhrman, S.T.; Chappell, P.D.; Haley, S.T.; Moffett, J.W.; Orchard, E.D.; Waterbury, J.B.; Webb, E.A. Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 2006, 439, 68–71. [Google Scholar] [CrossRef]
- Horsman, G.P.; Zechel, D.L. Phosphonate biochemistry. Chem. Rev. 2017, 117, 5704–5783. [Google Scholar] [CrossRef]
- Manav, M.C.; Sofos, N.; Hove-Jensen, B.; Brodersen, D.E. The Abc of phosphonate breakdown: A mechanism for bacterial survival. BioEssays 2018, 40, 1800091. [Google Scholar] [CrossRef] [PubMed]
- White, A.K.; Metcalf, W.W. Microbial metabolism of reduced phosphorus compounds. Annu. Rev. Microbiol. 2007, 61, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Orchard, E.D.; Webb, E.A.; Dyhrman, S.T. Molecular analysis of the phosphorus starvation response in Trichodesmium spp. Environ. Microbiol. 2009, 11, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Tyson, G.W.; DeLong, E.F. Widespread known and novel phosphonate utilization pathways in marine bacteria revealed by functional screening and metagenomic analyses. Environ. Microbiol. 2010, 12, 222–238. [Google Scholar] [CrossRef]
- Chen, Z.J.; Ruan, Z.X.; Cheng, N.; Xiao, L.J.; Peng, L.; Han, B.P.; Lei, L.M. Whole-genome sequencing and phosphorus uptake and transport pathway comparative analysis of Cylindrospermopsis raciborskii N8. Acta Hydrobiol. Sin. 2022, 46, 1130–1141. [Google Scholar]
- Money, V.A.; Moreland, G.M.C.; Lott, J.S.; Edward, N. Crystal Structure of PhnF, a GntR-Family. J. Bacteriol. 2014, 196, 3472. [Google Scholar]
- Rosenberg, H.; Gerdes, R.; Chegwidden, K. Two systems for the uptake of phosphate in Escherichia coli. J. Bacteriol. 1977, 131, 505–511. [Google Scholar] [CrossRef]
- Willsky, G.R.; Malamy, M.H. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J. Bacteriol. 1980, 144, 356–365. [Google Scholar] [CrossRef]
- Lebens, M.; Lundquist, P.; Söderlund, L.; Todorovic, M.; Carlin, N.I. The nptA gene of Vibrio cholerae encodes a functional sodium-dependent phosphate cotransporter homologous to the type II cotransporters of eukaryotes. J. Bacteriol. 2002, 184, 4466–4474. [Google Scholar] [CrossRef]
- Kamennaya, N.A.; Geraki, K.; Scanlan, D.J.; Zubkov, M.V. Accumulation of ambient phosphate into the periplasm of marine bacteria is proton motive force dependent. Nat. Commun. 2020, 11, 2642. [Google Scholar] [CrossRef] [PubMed]
- Dyhrman, S.T.; Haley, S.T. Phosphorus scavenging in the unicellular marine diazotroph Crocosphaera watsonii. Appl. Environ. Microb. 2006, 72, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Hirani, T.A.; Suzuki, I.; Murata, N.; Hayashi, H.; Eaton-Rye, J.J. Characterization of a two-component signal transduction system involved in the induction of alkaline phosphatase under phosphate-limiting conditions in Synechocystis sp. PCC 6803. Plant Mol. Biol. 2001, 45, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Hudek, L.; Premachandra, D.; Webster, W.A.J.; Bräu, L. Role of phosphate transport system component PstB1 in phosphate internalization by Nostoc punctiforme. Appl. Environ. Microb. 2016, 82, 6344–6356. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.Y.; Yu, R.C.; Du, K.; Zhou, Z.C.; Li, X. Genome sequence analysis of phosphorus metabolism pathways of Microcystis aeruginosa Chao 1910 isolated from Chaohu Lake. Microbiol. China 2023, 50, 1491–1510. (In Chinese) [Google Scholar]
- Taylor, B.L.; Zhulin, I.B. PAS domains: Internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. R 1999, 63, 479–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Ye, Q.Z.; Zhu, Z.M.; Wanner, B.L.; Walsh, C.T. Molecular biology of carbon-phosphorus bond cleavage. Cloning and sequencing of the phn (psiD) genes involved in alkylphosphonate uptake and CP lyase activity in Escherichia coli B. J. Biol. Chem. 1990, 265, 4461–4471. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.S.; Ford, B.A.; Varkey, D.; Mikolajek, H.; Orr, C.; Mykhaylyk, V.; Paulsen, I.T. Marine picocyanobacterial PhnD1 shows specificity for various phosphorus sources but likely represents a constitutive inorganic phosphate transporter. ISME J. 2023, 17, 1040–1051. [Google Scholar] [CrossRef]
- Li, J.; Dittrich, M. Dynamic polyphosphate metabolism in cyanobacteria responding to phosphorus availability. Environ. Microbiol. 2019, 21, 572–583. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Ismagulova, T.T.; Lukyanov, A.A.; Vasilieva, S.G.; Konyukhov, I.V.; Pogosyan, S.I.; Gorelova, O.A. Luxury phosphorus uptake in microalgae. J. Appl. Phycol. 2019, 31, 2755–2770. [Google Scholar] [CrossRef]
- Falkner, G.; Falkner, R. The complex regulation of the phosphate uptake system of cyanobacteria. In Bioenergetic Processes of Cyanobacteria: From Evolutionary Singularity to Ecological Diversity; Springer: Berlin/Heidelberg, Germany, 2011; pp. 109–130. [Google Scholar]
- Martin, P.; Dyhrman, S.T.; Lomas, M.W.; Poulton, N.J.; Van Mooy, B.A. Accumulation and enhanced cycling of polyphosphate by Sargasso Sea plankton in response to low phosphorus. Proc. Natl. Acad. Sci. USA 2014, 111, 8089–8094. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.P.; Amrhein, N.; Freimoser, F.M. Novel method for the quantification of inorganic polyphosphate (iPoP) in Saccharomyces cerevisiae shows dependence of iPoP content on the growth phase. Arch. Microbiol. 2005, 184, 129–136. [Google Scholar] [CrossRef]
- De Mazancourt, C.; Schwartz, M.W. Starve a competitor: Evolution of luxury consumption as a competitive strategy. Theor. Ecol. 2012, 5, 37–49. [Google Scholar] [CrossRef][Green Version]
- Nierzwicki-Bauer, S.A.; Balkwill, D.L.; Stevens, S.E., Jr. Three-dimensional ultrastructure of a unicellular cyanobacterium. J. Cell Biol. 1983, 97, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Liberton, M.; Austin, J.R.; Berg, R.H.; Pakrasi, H.B. Unique thylakoid membrane architecture of a unicellular N2-fixing cyanobacterium revealed by electron tomography. Plant Physiol. 2011, 155, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.H.; Fan, D.D.; Wang, M.M.; Zhao, Q.S.; Yang, L.Y. Structure and function of granular polyphosphate organelle in biological cells. Acta Microbiol. Sin. 2022, 62, 4713–4730. [Google Scholar]
- Schwarz, R.; Forchhammer, K. Acclimation of unicellular cyanobacteria to macronutrient deficiency: Emergence of a complex network of cellular responses. Microbiology 2005, 151, 2503–2514. [Google Scholar] [CrossRef]
- Van Mooy, B.A.; Fredricks, H.F.; Pedler, B.E.; Dyhrman, S.T.; Karl, D.M.; Koblížek, M.; Webb, E.A. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 2009, 458, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Siuda, W.; Chróst, R.J. Utilization of selected dissolved organic phosphorus compounds by bacteria in lake water under non-limiting orthophosphate conditions. Pol. J. Environ. Stud. 2001, 10, 475–484. [Google Scholar]
- Jentzsch, L.; Grossart, H.P.; Plewe, S.; Schulze-Makuch, D.; Goldhammer, T. Response of cyanobacterial mats to ambient phosphate fluctuations: Phosphorus cycling, polyphosphate accumulation and stoichiometric flexibility. ISME Commun. 2023, 3, 6. [Google Scholar] [CrossRef]
- Rychter, A.M.; Rao, I.M. Role of phosphorus in photosynthetic carbon metabolism. Handb. Photosynth. 2005, 2, 123–148. [Google Scholar]
- Liu, Y.N. The Regulation Mechanism of Glycerophosphatide in Ganoderic Acid Biosynthesis under Heat Stress in Ganoderma lucidum; Nanjing Agricultural University: Nanjing, China, 2018. (In Chinese) [Google Scholar]
- Karl, D.M. Microbially mediated transformations of phosphorus in the sea: New views of an old cycle. Annu. Rev. Mar. Sci. 2014, 6, 279–337. [Google Scholar] [CrossRef]
- Haeder, D.P. Photosynthesis in Plants and Algae. Anticancer Res. 2022, 42, 5035–5041. [Google Scholar] [CrossRef] [PubMed]
- Mullineaux, C.W. Electron transport and light-harvesting switches in cyanobacteria. Front. Plant Sci. 2014, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y.; Altman-Gueta, H.; Wolff, Y.; Gurevitz, M. Rubisco mutagenesis provides new insight into limitations on photosynthesis and growth in Synechocystis PCC6803. J. Exp. Bot. 2011, 62, 4173–4182. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, A.S. Activation of spinach ribulose 1, 5-bisphosphate carboxylase by inorganic phosphate. Plant Sci. Lett. 1981, 23, 197–206. [Google Scholar] [CrossRef]
- Lu, K.J.; Chang, C.W.; Wang, C.H.; Chen, F.Y.; Huang, I.Y.; Huang, P.H.; Liao, J.C. An ATP-sensitive phosphoketolase regulates carbon fixation in cyanobacteria. Nat. Metab. 2023, 5, 1111–1126. [Google Scholar] [CrossRef]
- Shinde, S.; Zhang, X.; Singapuri, S.P.; Kalra, I.; Liu, X.; Morgan-Kiss, R.M.; Wang, X. Glycogen metabolism supports photosynthesis start through the oxidative pentose phosphate pathway in cyanobacteria. Plant Physiol. 2020, 182, 507–517. [Google Scholar] [CrossRef]
- Tiwari, B. Phosphate metabolism in cyanobacteria: Fundamental prospective and applications. In Cyanobacteria; Academic Press: Cambridge, MA, USA, 2024; pp. 159–175. [Google Scholar]
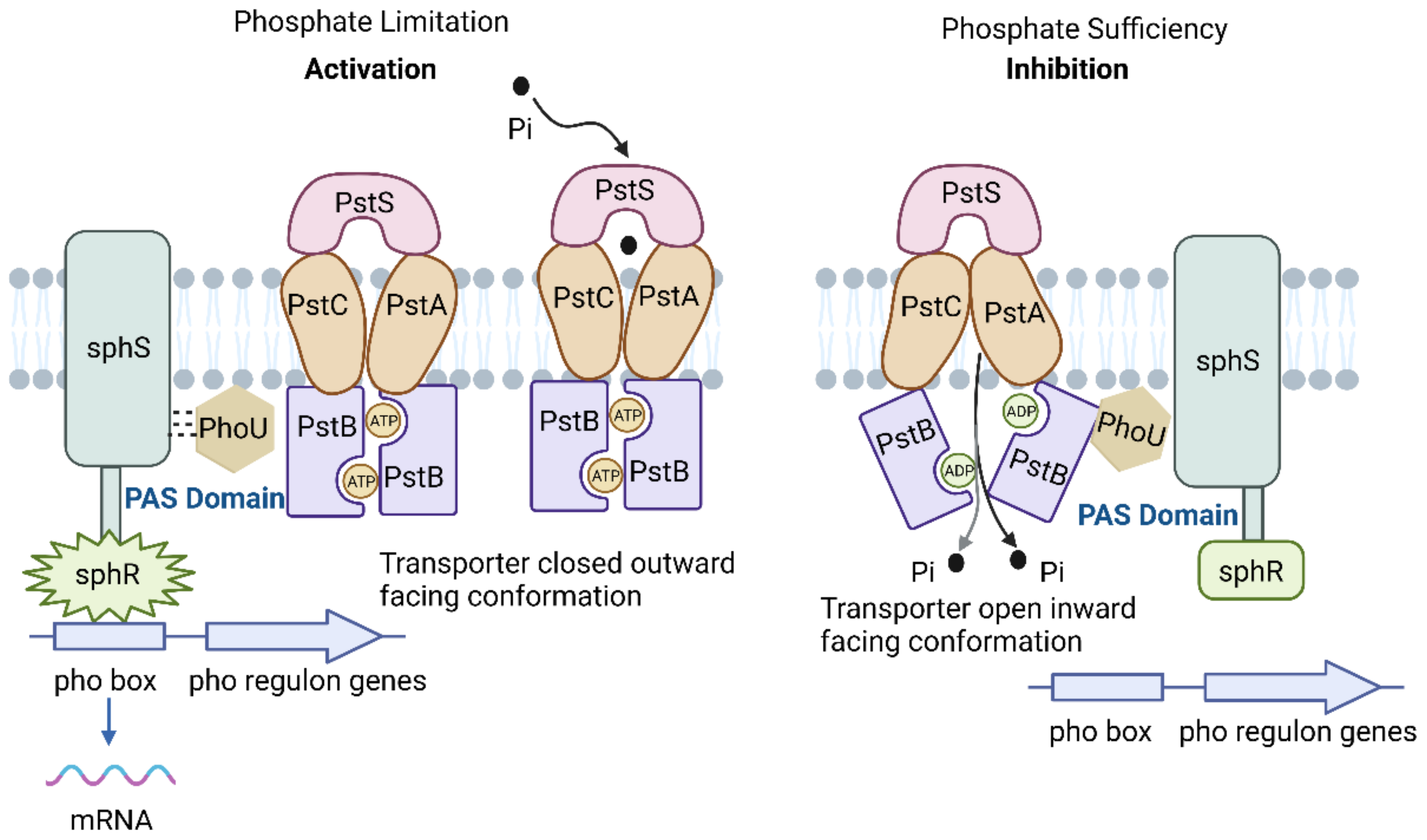
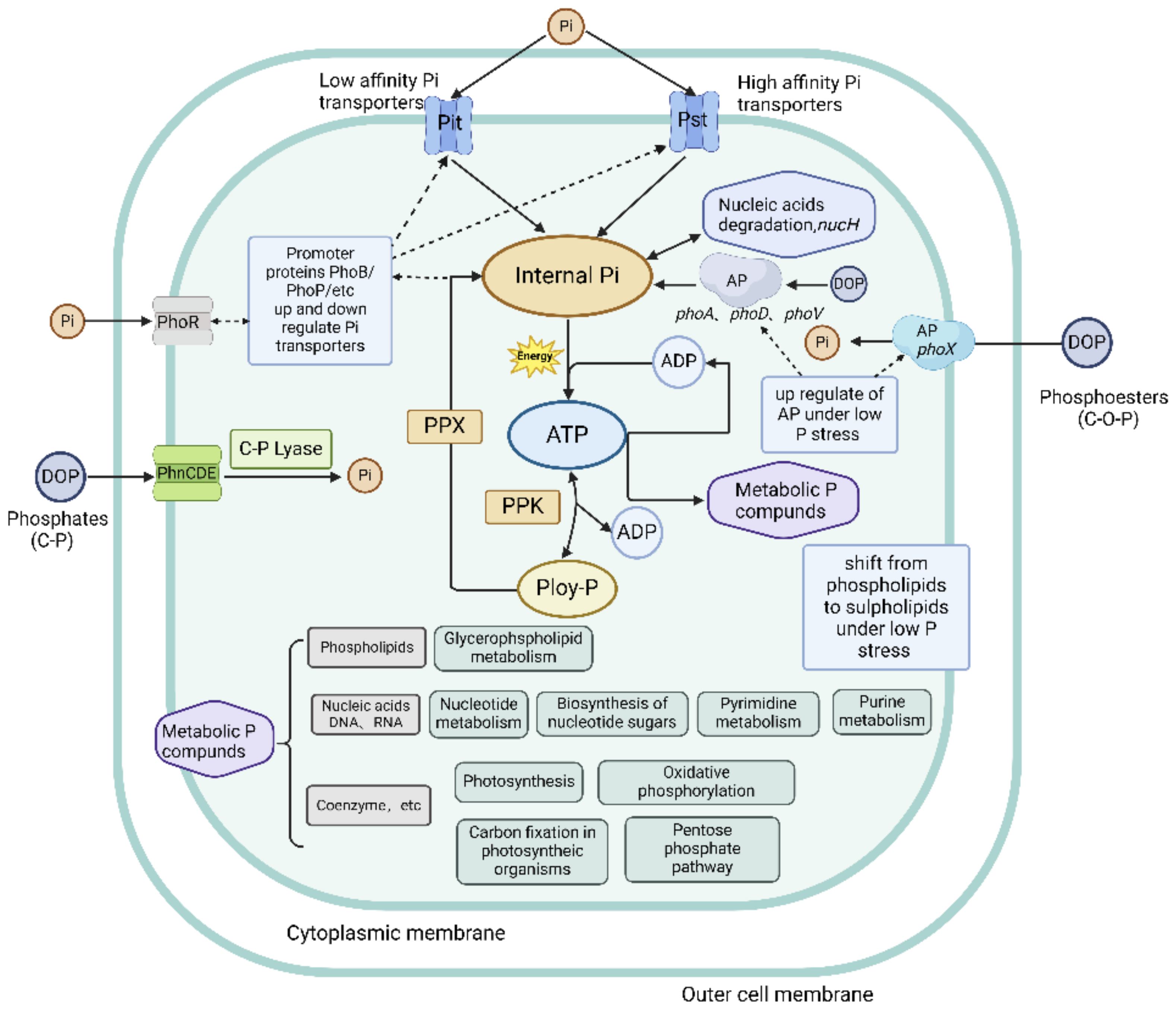
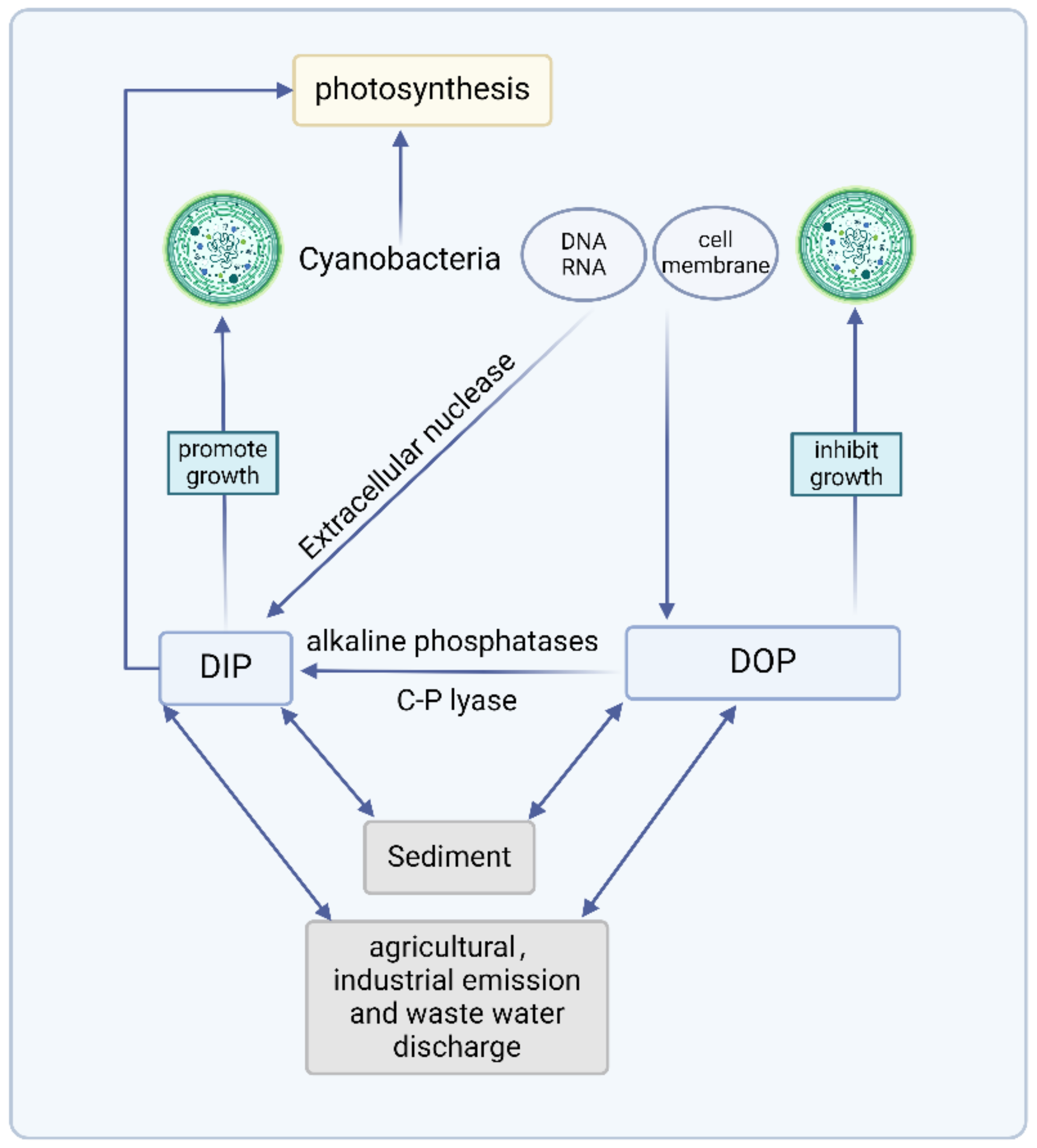
| System | Gene | Function |
|---|---|---|
| Two-component regulatory system | sphR | Response regulator |
| sphS | Sensor kinase | |
| Low-affinity inorganic phosphorus transport system | pit | Inorganic phosphorus transporter protein |
| High-affinity inorganic phosphorus transport system | sphX | ABC-type phosphate transporter phosphate-binding protein |
| pstS | ABC-type phosphate transporter phosphate-binding protein | |
| pstC | ABC-type phosphate transporter permease protein | |
| pstA | ABC-type phosphate transporter permease protein | |
| pstB | ABC-type phosphate transporter ATP-binding protein | |
| Organophosphonate transport complex | phnC | Organophosphonates transport ATP-binding protein |
| phnD | Organophosphonates transport substrate-binding protein | |
| phnE | Organophosphonates transport permease protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Jia, L.; Chen, Y.; Yan, H.; Chen, Q.; Zhang, J.; Sun, H. Molecular Mechanisms of the Cyanobacterial Response to Different Phosphorus Sources. Sustainability 2024, 16, 5642. https://doi.org/10.3390/su16135642
Zhang Q, Jia L, Chen Y, Yan H, Chen Q, Zhang J, Sun H. Molecular Mechanisms of the Cyanobacterial Response to Different Phosphorus Sources. Sustainability. 2024; 16(13):5642. https://doi.org/10.3390/su16135642
Chicago/Turabian StyleZhang, Qi, Lu Jia, Yuchen Chen, Hanlu Yan, Qiuwen Chen, Jianmin Zhang, and Hao Sun. 2024. "Molecular Mechanisms of the Cyanobacterial Response to Different Phosphorus Sources" Sustainability 16, no. 13: 5642. https://doi.org/10.3390/su16135642
APA StyleZhang, Q., Jia, L., Chen, Y., Yan, H., Chen, Q., Zhang, J., & Sun, H. (2024). Molecular Mechanisms of the Cyanobacterial Response to Different Phosphorus Sources. Sustainability, 16(13), 5642. https://doi.org/10.3390/su16135642









