Optimizing Nitrogen Fertilization for Enhanced Rice Straw Degradation and Oilseed Rape Yield in Challenging Winter Conditions: Insights from Southwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Location
2.2. Soil Sampling and Measurements
2.3. Nitrogen Using Efficiency
2.4. Determination of Physiochemical Properties
2.5. Soil Enzyme Activity
2.6. Soil Organic Matter and Lignocellulose Content
2.7. Bacterial Diversity and Composition Analysis
2.8. Statistical Analysis
3. Results
3.1. Yield and Yield Parameters
3.2. Plant Dry Weight
3.3. Nitrogen Use Efficiency
3.4. Soil Physiochemical Properties
3.5. Soil Nutrients
3.6. Soil Enzyme Activities
3.7. Soil Organic Matter and Lignocellulose Content
3.8. Pearsons Correlation Coefficient between Nutrients and Physiochemical Properties
3.9. Bacterial Diversity and Composition
3.9.1. Impact of Rice Straw on Bacterial Alpha Diversity and Total Composition
3.9.2. Bacterial Diversity and Composition
3.9.3. Impact of Nitrogen Fertilization on Bacterial Community Composition
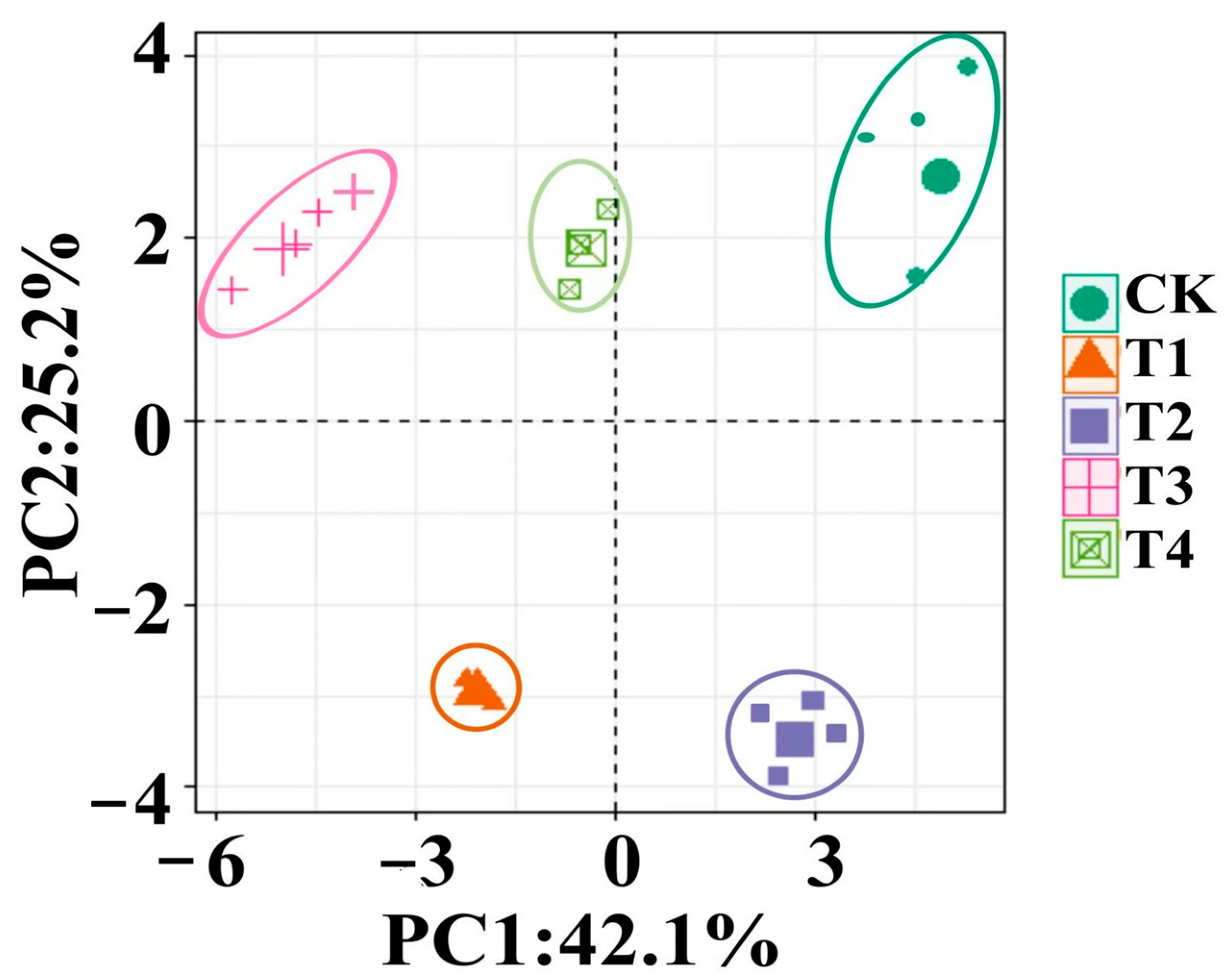
3.9.4. Principal Component Analysis of Bacterial Composition (Order Level)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef]
- Khan, N.; Ray, R.L.; Sargani, G.R.; Ihtisham, M.; Khayyam, M.; Ismail, S. Current progress and future prospects of agriculture technology: Gateway to sustainable agriculture. Sustainability 2021, 13, 4883. [Google Scholar] [CrossRef]
- Chen, L.; Sun, S.; Yao, B.; Peng, Y.; Gao, C.; Qin, T.; Zhou, Y.; Sun, C.; Quan, W. Effects of straw return and straw biochar on soil properties and crop growth: A review. Front. Plant Sci. 2022, 13, 986763. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiong, Y.; Qu, Z.; Xu, X.; Huang, Q.; Huang, G. Impact of biochar addition on soil properties and water-fertilizer productivity of tomato in semi-arid region of Inner Mongolia, China. Geoderma 2018, 331, 100–108. [Google Scholar] [CrossRef]
- Wang, Y.J.; Bi, Y.Y.; Gao, C.Y. The assessment and utilization of straw resources in China. Agric. Sci. China 2010, 9, 1807–1815. [Google Scholar] [CrossRef]
- Jin, Z.; Shah, T.; Zhang, L.; Liu, H.; Peng, S.; Nie, L. Effect of straw returning on soil organic carbon in rice–wheat rotation system: A review. Food Energy Secur. 2020, 9, e200. [Google Scholar] [CrossRef]
- Zhang, R.; Li, X.; Fadel, J. Oyster mushroom cultivation with rice and wheat straw. Bioresour. Technol. 2002, 82, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Binod, P.; Sindhu, R.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Bioethanol production from rice straw: An overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Mothe, S.; Polisetty, V.R. Review on anaerobic digestion of rice straw for biogas production. Environ. Sci. Pollut. Res. 2021, 28, 24455–24469. [Google Scholar] [CrossRef]
- Roca-Pérez, L.; Martínez, C.; Marcilla, P.; Boluda, R. Composting rice straw with sewage sludge and compost effects on the soil–plant system. Chemosphere 2009, 75, 781–787. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, W.; Li, T.; Cheng, X.; Liu, Q. Balancing straw returning and chemical fertilizers in China: Role of straw nutrient resources. Renew. Sustain. Energy Rev. 2018, 81, 2695–2702. [Google Scholar] [CrossRef]
- Ma, E.; Zhang, G.; Ma, J.; Xu, H.; Cai, Z.; Yagi, K. Effects of rice straw returning methods on N2O emission during wheat-growing season. Nutr. Cycl. Agroecosyst. 2010, 88, 463–469. [Google Scholar] [CrossRef]
- Su, W.; Lu, J.; Wang, W.; Li, X.; Ren, T.; Cong, R. Influence of rice straw mulching on seed yield and nitrogen use efficiency of winter oilseed rape (Brassica napus L.) in intensive rice–oilseed rape cropping system. Field Crops Res. 2014, 159, 53–61. [Google Scholar] [CrossRef]
- Jansson, J.K.; Taş, N. The microbial ecology of permafrost. Nat. Rev. Microbiol. 2014, 12, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, M.U.F. The temperature dependence of organic-matter decomposition—Still a topic of debate. Soil Biol. Biochem. 2006, 38, 2510–2518. [Google Scholar] [CrossRef]
- Pullens, J.W.M.; Sharif, B.; Trnka, M.; Balek, J.; Semenov, M.A.; Olesen, J.E. Risk factors for European winter oilseed rape production under climate change. Agric. For. Meteorol. 2019, 272, 30–39. [Google Scholar] [CrossRef]
- Moradtalab, N.; Ahmed, A.; Geistlinger, J.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Synergisms of microbial consortia, N forms, and micronutrients alleviate oxidative damage and stimulate hormonal cold stress adaptations in maize. Front. Plant Sci. 2020, 11, 396. [Google Scholar] [CrossRef]
- Gous, P.W.; Warren, F.; Mo, O.W.; Gilbert, R.G.; Fox, G.P. The effects of variable nitrogen application on barley starch structure under drought stress. J. Inst. Brew. 2015, 121, 502–509. [Google Scholar] [CrossRef]
- Andrianary, B.H.; Tsujimoto, Y.; Rakotonindrina, H.; Oo, A.Z.; Rabenarivo, M.; Ramifehiarivo, N.; Razakamanarivo, H. Phosphorus application affects lowland rice yields by changing phenological development and cold stress degrees in the central highlands of Madagascar. Field Crops Res. 2021, 271, 108256. [Google Scholar] [CrossRef]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef]
- Han, S.-W.; Yoshikuni, Y. Microbiome engineering for sustainable agriculture: Using synthetic biology to enhance nitrogen metabolism in plant-associated microbes. Curr. Opin. Microbiol. 2022, 68, 102172. [Google Scholar] [CrossRef] [PubMed]
- Løvdal, T.; Lillo, C. Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal. Biochem. 2009, 387, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Chang, L.; Khalid, M.; Zhang, J.; Huang, D. Alleviation of drought stress by nitrogen application in Brassica campestris ssp. Chinensis L. Agronomy 2018, 8, 66. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Zhou, J.; Wang, Y.; Ai, Y.; Li, X.; Zhang, P.; Zhou, S. Responses of the root morphology and photosynthetic pigments of ryegrass to fertilizer application under combined petroleum–heavy metal stress. Environ. Sci. Pollut. Res. 2022, 29, 87874–87883. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.; Khan, M.; Mohammad, F.; Khan, M. Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop Sci. 2008, 194, 214–224. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, M.; Yang, L.; Gu, X.; Jin, J.; Fu, M. Regulation of straw decomposition and its effect on soil function by the amount of returned straw in a cool zone rice crop system. Sci. Rep. 2023, 13, 15673. [Google Scholar] [CrossRef]
- Zhu, S.; Vivanco, J.M.; Manter, D.K. Nitrogen fertilizer rate affects root exudation, the rhizosphere microbiome and nitrogen-use-efficiency of maize. Appl. Soil Ecol. 2016, 107, 324–333. [Google Scholar] [CrossRef]
- Yang, G.; Nabi, F.; Sajid, S.; Kaleri, A.R.; Jakhar, A.M.; Cheng, L.; Raspor, M.; Muhammad, N.; Ma, J.; Hu, Y. Response of root development and nutrient uptake of two chinese cultivars of hybrid rice to nitrogen and phosphorus fertilization in Sichuan Province, China. Mol. Biol. Rep. 2021, 48, 8009–8021. [Google Scholar] [CrossRef]
- Nabi, F.; Chen, H.; Sajid, S.; Yang, G.; Kyung, Y.; Shah, S.M.M.; Wang, X.; Hu, Y. Degradation of agricultural waste is dependent on chemical fertilizers in long-term paddy-dry rotation field. J. Environ. Manag. 2024, 355, 120460. [Google Scholar] [CrossRef]
- Vereecken, H.; Maes, J.; Feyen, J.; Darius, P. Estimating the soil moisture retention characteristic from texture, bulk density, and carbon content. Soil Sci. 1989, 148, 389–403. [Google Scholar] [CrossRef]
- Mahe, G.; Paturel, J.-E.; Servat, E.; Conway, D.; Dezetter, A. The impact of land use change on soil water holding capacity and river flow modelling in the Nakambe River, Burkina-Faso. J. Hydrol. 2005, 300, 33–43. [Google Scholar] [CrossRef]
- Bradstreet, R.B. Kjeldahl method for organic nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- Norman, R.; Stucki, J. The determination of nitrate and nitrite in soil extracts by ultraviolet spectrophotometry. Soil Sci. Soc. Am. J. 1981, 45, 347–353. [Google Scholar] [CrossRef]
- Robertson, G.P.; Wedin, D.; Groffman, P.; Blair, J.; Holland, E.; Nedelhoffer, K.; Harris, D. Soil carbon and nitrogen availability. Nitrogen mineralization, nitrification and soil respiration potentials. Stand. Soil Methods Long-Term Ecol. Res. 1999, 258–271. [Google Scholar]
- Dong, J.; Xing, Y.H.; Gong, X.M. Study on Novel Structures Phosphorus Sorption Capacity of Surficial Sediment in Dongping Lake and Risk Assessment of Potential Phosphorus Release. Adv. Mater. Res. 2012, 518, 1089–1092. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Kumar, A.; Singh, D.; Sharma, K.K.; Arora, S.; Singh, A.K.; Gill, S.S.; Singhal, B. Gel-based purification and biochemical study of laccase isozymes from Ganoderma sp. and its role in enhanced cotton callogenesis. Front. Microbiol. 2017, 8, 674. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Li, C.; Ma, Y. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. MicrobiologyOpen 2019, 8, e00813. [Google Scholar] [CrossRef]
- Sajid, S.; Zveushe, O.K.; de Dios, V.R.; Nabi, F.; Lee, Y.K.; Kaleri, A.R.; Ma, L.; Zhou, L.; Zhang, W.; Dong, F. Pretreatment of rice straw by newly isolated fungal consortium enhanced lignocellulose degradation and humification during composting. Bioresour. Technol. 2022, 354, 127150. [Google Scholar] [CrossRef]
- Eiland, F.; Leth, M.; Klamer, M.; Lind, A.M.; Jensen, H.; Iversen, J. C and N turnover and lignocellulose degradation during composting of Miscanthus straw and liquid pig manure. Compos. Sci. Util. 2001, 9, 186–196. [Google Scholar] [CrossRef]
- Ibáñez, A.B.; Bauer, S. Downscaled method using glass microfiber filters for the determination of Klason lignin and structural carbohydrates. Biomass Bioenergy 2014, 68, 75–81. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lönnerdal, B. Cloning and characterization of the human lactoferrin receptor gene promoter. Biometals 2018, 31, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Shao, S.; Fu, Z.; Li, Y.; Ni, H.; Chen, S.; Zhou, Y.; Jin, B.; Shi, Z. Identifying heavy metal pollution hot spots in soil-rice systems: A case study in South of Yangtze River Delta, China. Sci. Total Environ. 2019, 658, 614–625. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, B.Y.; Liu, S.L.; Qi, J.Y.; Wang, X.; Pu, C.; Li, S.S.; Zhang, X.Z.; Yang, X.G.; Lal, R. Sustaining crop production in China’s cropland by crop residue retention: A meta-analysis. Land Degrad. Dev. 2020, 31, 694–709. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xu, M.; Feng, G.; Zhang, W.; Lu, C. Crop yield and soil organic matter after long-term straw return to soil in China. Nutr. Cycl. Agroecosyst. 2015, 102, 371–381. [Google Scholar] [CrossRef]
- Xu, P.; Sun, C.-X.; Ye, X.-Z.; Xiao, W.-D.; Zhang, Q.; Wang, Q. The effect of biochar and crop straws on heavy metal bioavailability and plant accumulation in a Cd and Pb polluted soil. Ecotoxicol. Environ. Saf. 2016, 132, 94–100. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Timmer, V.R. Fertilizer-induced changes in rhizosphere electrical conductivity: Relation to forest tree seedling root system growth and function. New For. 2005, 30, 147–166. [Google Scholar] [CrossRef]
- Miller, J.; Beasley, B.; Larney, F.; Olson, B. Soil salinity and sodicity after application of fresh and composted manure with straw or wood-chips. Can. J. Soil Sci. 2005, 85, 427–438. [Google Scholar] [CrossRef]
- Piedallu, C.; Gégout, J.-C.; Bruand, A.; Seynave, I. Mapping soil water holding capacity over large areas to predict potential production of forest stands. Geoderma 2011, 160, 355–366. [Google Scholar] [CrossRef]
- Bravo, E.L.; Tijskens, E.; Suárez, M.H.; Cueto, O.G.; Ramon, H. Prediction model for non-inversion soil tillage implemented on discrete element method. Comput. Electron. Agric. 2014, 106, 120–127. [Google Scholar] [CrossRef]
- Fan, T.; Stewart, B.; Yong, W.; Luo, J.; Zhou, G. Long-term fertilization effects on grain yield, water-use efficiency and soil fertility in the dryland of Loess Plateau in China. Agric. Ecosyst. Environ. 2005, 106, 313–329. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Liu, S.; Li, J.; Geng, J.; Wang, L. Key soil properties influencing infiltration capacity after long-term straw incorporation in a wheat (Triticum aestivum L.)–maize (Zea mays L.) rotation system. Agric. Ecosyst. Environ. 2023, 344, 108301. [Google Scholar] [CrossRef]
- Ostmeyer, T.; Parker, N.; Jaenisch, B.; Alkotami, L.; Bustamante, C.; Jagadish, S.K. Impacts of heat, drought, and their interaction with nutrients on physiology, grain yield, and quality in field crops. Plant Physiol. Rep. 2020, 25, 549–568. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Shanahan, J.F.; Schepers, J.S.; Caldwell, B. Agronomic responses of corn hybrids from different eras to deficit and adequate levels of water and nitrogen. Agron. J. 2004, 96, 1660–1667. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Chen, X.; Yang, S.-H.; Jiang, Z.-W.; Ding, J.; Sun, X. Biochar as a tool to reduce environmental impacts of nitrogen loss in water-saving irrigation paddy field. J. Clean. Prod. 2021, 290, 125811. [Google Scholar] [CrossRef]
- Yang, S.; Li, F.; Malhi, S.S.; Wang, P.; Suo, D.; Wang, J. Long-term fertilization effects on crop yield and nitrate nitrogen accumulation in soil in northwestern China. Agron. J. 2004, 96, 1039–1049. [Google Scholar] [CrossRef]
- Zhou, M.; Butterbach-Bahl, K. Assessment of nitrate leaching loss on a yield-scaled basis from maize and wheat cropping systems. Plant Soil 2014, 374, 977–991. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, D.; Luo, W.; Hafeez, M.B.; Li, J.; Wen, P.; Wang, X. Combined nitrogen and phosphorus management based on nitrate nitrogen threshold for balancing crop yield and soil nitrogen supply capacity. Agric. Ecosyst. Environ. 2022, 337, 108071. [Google Scholar] [CrossRef]
- Baligar, V.; Fageria, N.; He, Z. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Nishigaki, T.; Tsujimoto, Y.; Rinasoa, S.; Rakotoson, T.; Andriamananjara, A.; Razafimbelo, T. Phosphorus uptake of rice plants is affected by phosphorus forms and physicochemical properties of tropical weathered soils. Plant Soil 2019, 435, 27–38. [Google Scholar] [CrossRef]
- Lopez-Bucio, J.; De la Vega, O.M.; Guevara-Garcia, A.; Herrera-Estrella, L. Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nat. Biotechnol. 2000, 18, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Jianlin, S.; Minghong, S.; Yajun, H.; Jiang, W.; Juan, W.; Yong, L.; Jinshui, W. Soil phosphorus availability and rice phosphorus uptake in paddy fields under various agronomic practices. Pedosphere 2021, 31, 103–115. [Google Scholar] [CrossRef]
- Borase, D.; Nath, C.; Hazra, K.; Senthilkumar, M.; Singh, S.; Praharaj, C.; Singh, U.; Kumar, N. Long-term impact of diversified crop rotations and nutrient management practices on soil microbial functions and soil enzymes activity. Ecol. Indic. 2020, 114, 106322. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Yang, L.; Ahmad, S.; Zhou, X. Stover return and nitrogen application affect soil organic carbon and nitrogen in a double-season maize field. Plant Biol. 2022, 24, 387–395. [Google Scholar] [CrossRef]
- Gianfreda, L.; Rao, M.A.; Piotrowska, A.; Palumbo, G.; Colombo, C. Soil enzyme activities as affected by anthropogenic alterations: Intensive agricultural practices and organic pollution. Sci. Total Environ. 2005, 341, 265–279. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Carter, M.R. Soil quality for sustainable land management: Organic matter and aggregation interactions that maintain soil functions. Agron. J. 2002, 94, 38–47. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Haynes, R.; Beare, M. Influence of six crop species on aggregate stability and some labile organic matter fractions. Soil Biol. Biochem. 1997, 29, 1647–1653. [Google Scholar] [CrossRef]
- Saroa, G.; Lal, R. Soil restorative effects of mulching on aggregation and carbon sequestration in a Miamian soil in central Ohio. Land Degrad. Dev. 2003, 14, 481–493. [Google Scholar] [CrossRef]
- Tan, D.; Jin, J.; Huang, S.; Li, S.; He, P. Effect of long-term application of K fertilizer and wheat straw to soil on crop yield and soil K under different planting systems. Agric. Sci. China 2007, 6, 200–207. [Google Scholar]
- Cui, X.; Guo, L.; Li, C.; Liu, M.; Wu, G.; Jiang, G. The total biomass nitrogen reservoir and its potential of replacing chemical fertilizers in China. Renew. Sustain. Energy Rev. 2021, 135, 110215. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Geng, P.; Yang, Q.; Chen, K.; Liu, N.; Fan, Y.; Zhan, X.; Han, X. Effects of different returning method combined with decomposer on decomposition of organic components of straw and soil fertility. Sci. Rep. 2021, 11, 15495. [Google Scholar] [CrossRef]
- Jin, S.; Huang, Y.; Dong, C.; Bai, Y.; Pan, H.; Hu, Z. Effects of different straw returning amounts and fertilizer conditions on bacteria of rice’s different part in rare earth mining area. Sci. Rep. 2023, 13, 412. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Y.; Wu, J.; Peng, F. Research progress on the effects of returning straw to fields on soil quality and crop growth. Chin. J. Soil Sci. 2014, 45, 1527–1532. [Google Scholar]
- Qiu, J.; Chen, Y.; Feng, Y.; Li, X.; Xu, J.; Jiang, J. Adaptation of Rhizosphere Microbial Communities to Continuous Exposure to Multiple Residual Antibiotics in Vegetable Farms. Int. J. Environ. Res. Public Health 2023, 20, 3137. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, L.; Liu, Y.; Zhang, Q.; Ruan, R.; Luo, X. Effect of acclimatized paddy soil microorganisms using swine wastewater on degradation of rice straw. Bioresour. Technol. 2021, 332, 125039. [Google Scholar] [CrossRef] [PubMed]
- Faoro, H.; Alves, A.; Souza, E.; Rigo, L.; Cruz, L.; Al-Janabi, S.; Monteiro, R.; Baura, V.; Pedrosa, F. Influence of soil characteristics on the diversity of bacteria in the Southern Brazilian Atlantic Forest. Appl. Environ. Microbiol. 2010, 76, 4744–4749. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Jones, K.L.; Peterson, D.E.; Garrett, K.A.; Hulbert, S.H.; Paulitz, T.C. Members of soil bacterial communities sensitive to tillage and crop rotation. Soil Biol. Biochem. 2010, 42, 2111–2118. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; Han, X.; Wang, X.; Guo, Z.; Lu, X.; Zou, W.; Yan, J. Microbial Responses of Soil Fertility to Depth of Tillage and Incorporation of Straw in a Haplic Chernozem in Northeast China. Chin. Geogr. Sci. 2023, 33, 693–707. [Google Scholar] [CrossRef]
- Limon-Ortega, A.; Sayre, K.D.; Drijber, R.A.; Francis, C.A. Soil attributes in a furrow-irrigated bed planting system in northwest Mexico. Soil Tillage Res. 2002, 63, 123–132. [Google Scholar] [CrossRef]
- Dai, W.; Wang, J.; Fang, K.; Cao, L.; Sha, Z.; Cao, L. Wheat straw incorporation affecting soil carbon and nitrogen fractions in Chinese paddy soil. Agriculture 2021, 11, 803. [Google Scholar] [CrossRef]
- Björnsson, L.; Prade, T. Sustainable cereal straw management: Use as feedstock for emerging biobased industries or cropland soil incorporation? Waste Biomass Valorization 2021, 12, 5649–5663. [Google Scholar] [CrossRef]
- García-Gutiérrez, S.; García-Marco, S.; Jiménez-Horcajada, R.; Montoya, M.; Vallejo, A.; Guardia, G. Maize residue input rather than cover cropping influenced N2O emissions and soil–crop N dynamics during the intercrop and cash crop periods. Agric. Ecosyst. Environ. 2024, 363, 108873. [Google Scholar] [CrossRef]
- Chen, H.; Yang, L.; Mickan, B.S.; Li, Z.; Zhang, F. Long–term (25 years) continuous cotton cropping combined with residue incorporation affects the fungal communities in reclaimed saline soil. Pedobiologia 2024, 102, 150928. [Google Scholar] [CrossRef]
- Ahmad, A.; Arif, M.S.; Shahzad, S.M.; Yasmeen, T.; Shakoor, A.; Iqbal, S.; Riaz, A.; Zahid, A.; Chapman, S.J. Long-term raw crop residue but not burned residue incorporation improved soil multifunctionality in semi-arid agroecosystems. Soil Tillage Res. 2024, 240, 106073. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, M.; Zeng, F.; Xu, P.; Ma, S.; Zhang, B.; Li, Z.; Wang, Y.; Zhu, B. How do soil organic carbon pool, stock and their stability respond to crop residue incorporation in subtropical calcareous agricultural soils? Agric. Ecosyst. Environ. 2022, 332, 107927. [Google Scholar] [CrossRef]
- Choudhary, V.; Gurjar, D.; Meena, R.S. Crop residue and weed biomass incorporation with microbial inoculation improve the crop and soil productivity in the rice (Oryza sativa L.)-toria (Brassica rapa L.) cropping system. Environ. Sustain. Indic. 2020, 7, 100048. [Google Scholar] [CrossRef]
- Canalli, L.B.d.S.; Santos, J.B.d.; Silva, A.A.; Muchinski, A.; Feliciano, E.; Penteado, D. Residues decomposition in crop rotations under no-till system. Braz. Arch. Biol. Technol. 2020, 63, e20190609. [Google Scholar] [CrossRef]
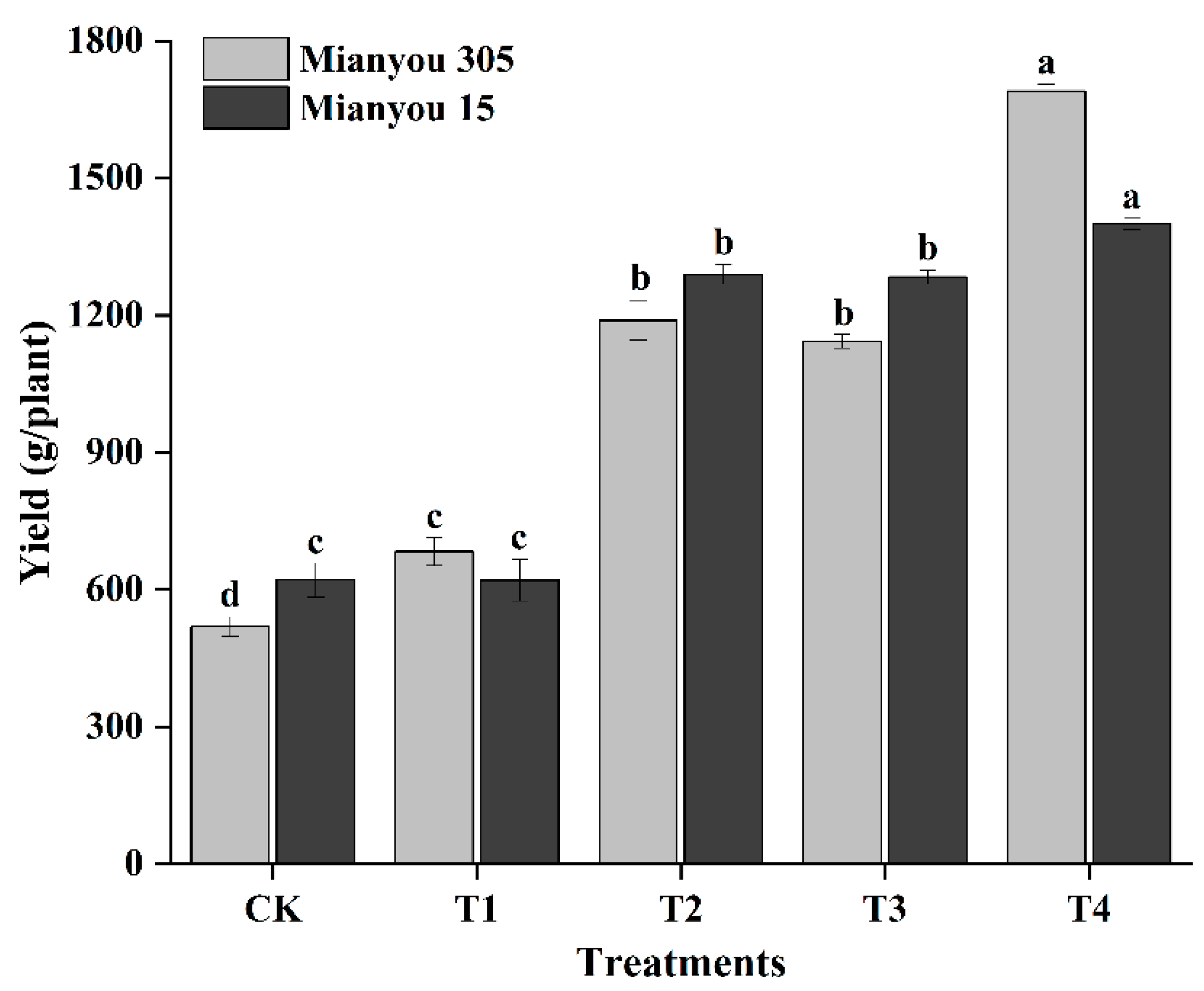
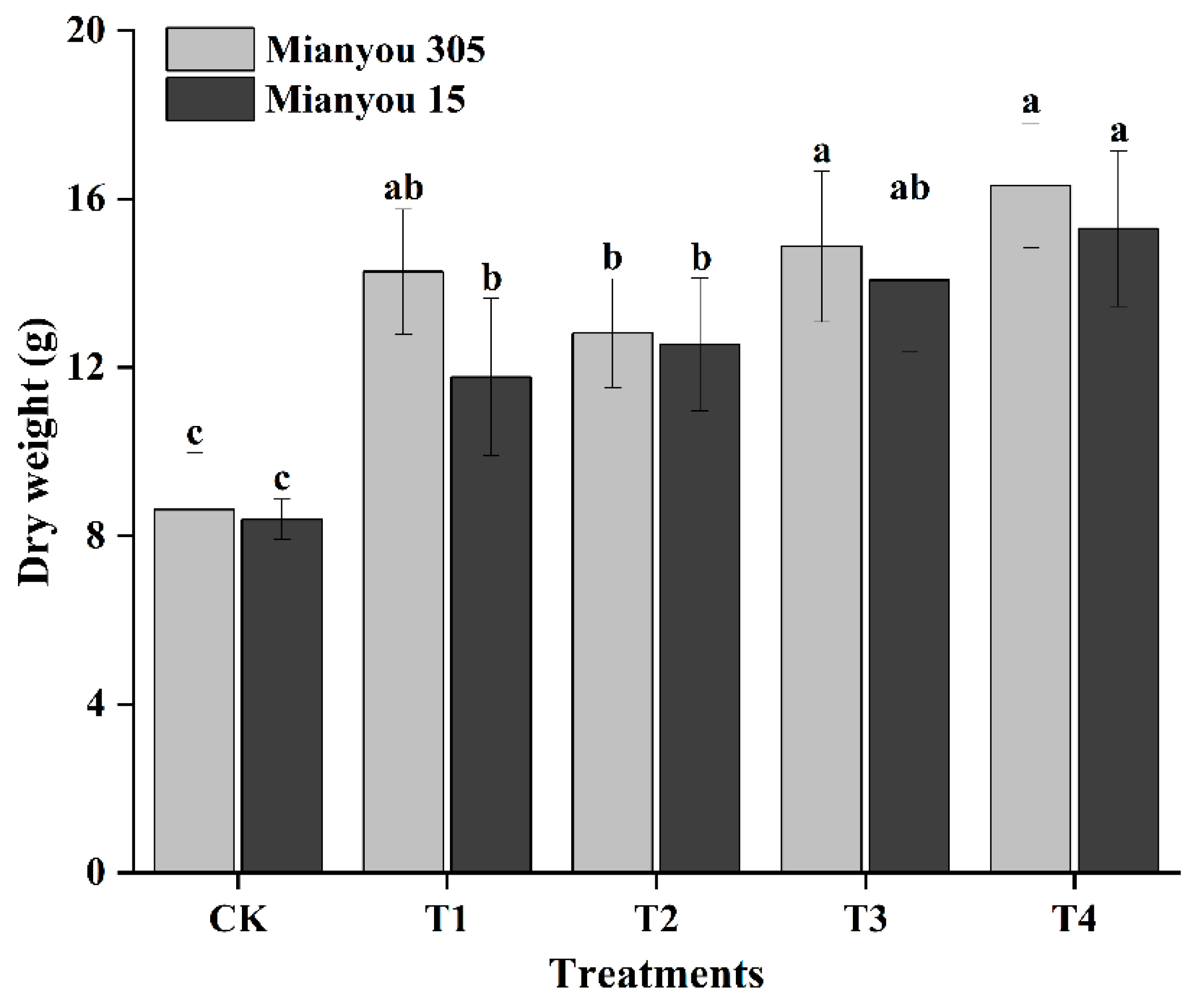
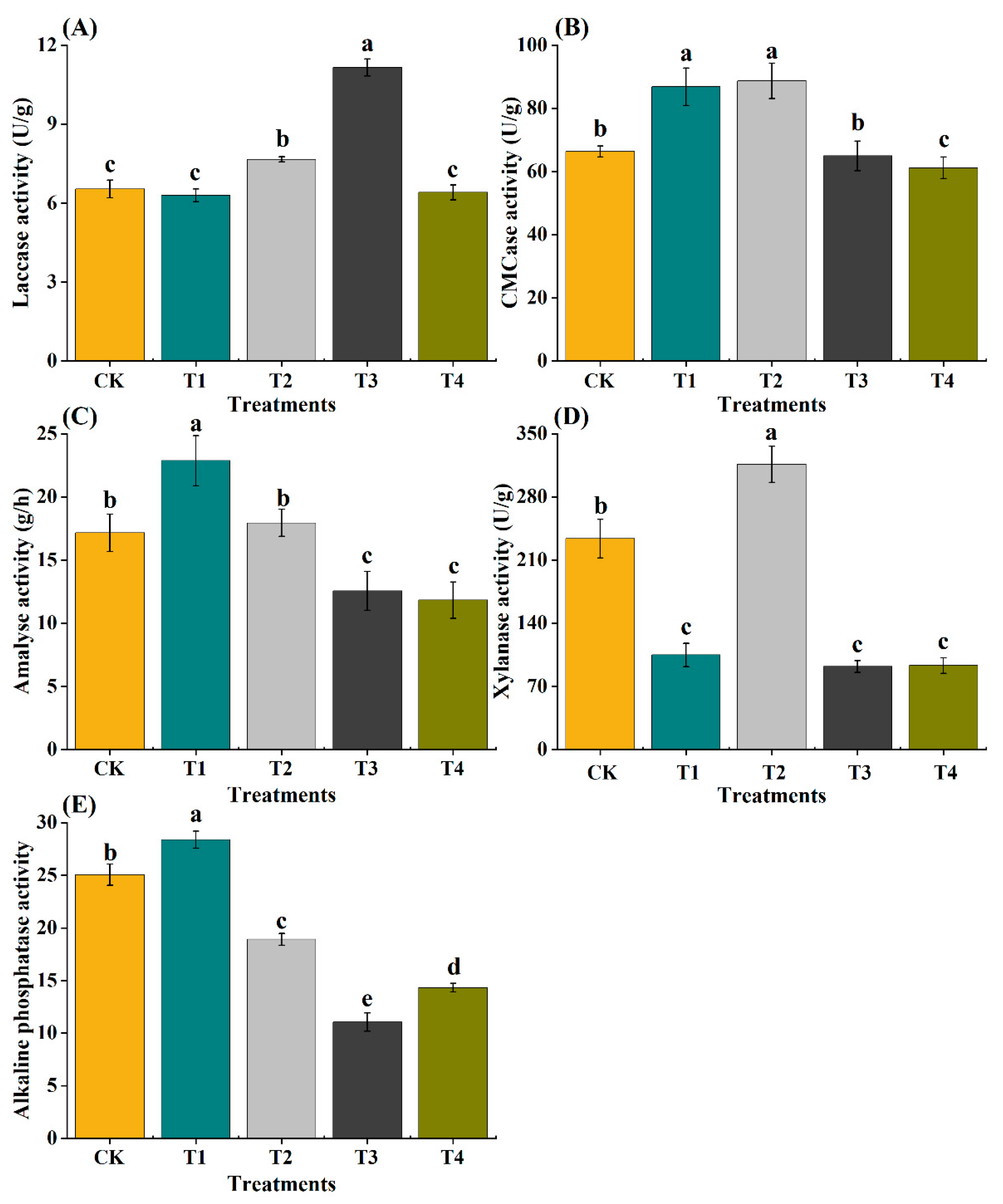

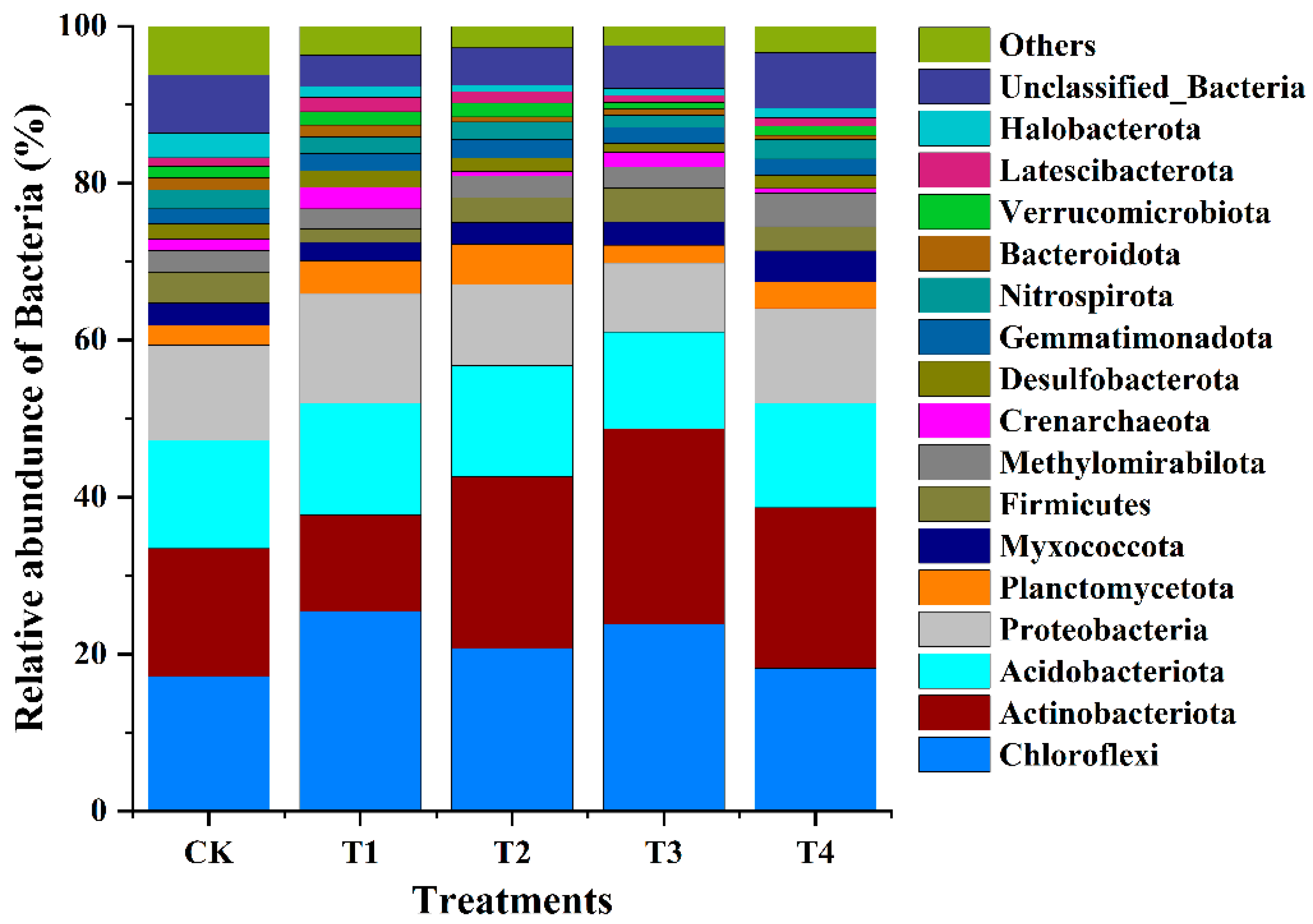
| Cultivars | Treatments | Main Branch | Primary and Secondary Branches | |||||
|---|---|---|---|---|---|---|---|---|
| Number of Pods | Number of Grains | 1000-Grains Weight | Number of Branches | Number of Pods | Number of Grains | 1000-Grains Weight | ||
| Mianyou 305 | CK | 64 ± 7.54 c | 1567 ± 110 d | 3.05 ± 0.20 a | 5 ± 0.58 c | 81 ± 11.2 e | 2299 ± 122 d | 3.85 ± 1.68 c |
| T1 | 68 ± 5.03 c | 1647 ± 302 c | 3.42 ± 0.53 a | 5 ± 0.52 c | 109 ± 9.54 d | 2349 ± 223 d | 4.28 ± 1.55 b | |
| T2 | 78 ± 15.50 b | 1714 ± 102 b | 3.49 ± 0.09 a | 7 ± 0.64 b | 168 ± 8.71 c | 3133 ± 168 c | 4.42 ± 2.06 b | |
| T3 | 78 ± 8.96 b | 1776 ± 336 b | 3.56 ± 0.13 a | 8 ± 0.78 b | 185 ± 13.6 b | 3776 ± 731 b | 5.46 ± 1.89 ab | |
| T4 | 91 ± 11.51 a | 2502 ± 874 a | 3.62 ± 0.12 a | 14 ± 1.16 a | 384 ± 10.5 a | 7047 ± 445 a | 6.56 ± 0.79 a | |
| Mianyou 15 | CK | 62 ± 9.64 d | 1431 ± 300 d | 3.53 ± 0.13 b | 3 ± 1.0 d | 67 ± 3.46 e | 1119 ± 156 d | 3.59 ± 0.12 c |
| T1 | 80 ± 6.19 c | 1515 ± 336 c | 3.68 ± 0.81 b | 4 ± 1.5 d | 120 ± 27.7 d | 2368 ± 276 c | 3.71 ± 0.36 c | |
| T2 | 87 ± 3.60 b | 1714 ± 749 b | 3.74 ± 0.43 b | 5 ± 1.0 c | 159 ± 27.1 c | 3133 ± 168 b | 4.13 ± 1.34 b | |
| T3 | 86 ± 2.64 bc | 1780 ± 222 b | 4.07 ± 0.19 a | 6 ± 0.3 b | 203 ± 36.9 b | 3318 ± 482 b | 4.21 ± 1.47 b | |
| T4 | 101 ± 19.5 a | 2026 ± 265 a | 4.89 ± 0.54 a | 8 ± 2.0 a | 267 ± 91.5 a | 4827 ± 582 a | 6.87 ± 2.56 a | |
| Treatments | Mianyou 305 | Mianyou 15 | ||||
|---|---|---|---|---|---|---|
| NAE (kg Nkg−1) | NUE (%) | NPE (kg Nkg−1) | NAE (kg Nkg−1) | NUE (%) | NPE (kg Nkg−1) | |
| CK | - | - | - | - | - | - |
| T1 | - | - | - | - | - | - |
| T2 | 8.43 a | 25.50 c | 33.04 b | 11.16 a | 19.83 c | 56.23 a |
| T3 | 8.39 a | 36.42 b | 46.07 a | 6.49 b | 32.42 b | 40.05 b |
| T4 | 2.56 b | 42.24 a | 18.14 c | 3.69 c | 38.24 a | 28.91 c |
| Treatments | pH | EC (dS/m) | WHC (%) | BD (g/cm3) | SP (%) |
|---|---|---|---|---|---|
| CK | 7.79 ± 0.05 a | 0.07 ± 0.01 e | 21.36 ± 2.12 d | 1.32 ± 0.02 a | 41.88 ± 1.90 c |
| T1 | 7.30 ± 0.04 e | 0.14 ± 0.01 b | 32.65 ± 1.98 b | 1.08 ± 0.02 b | 62.32 ± 2.12 a |
| T2 | 7.45 ± 0.05 c | 0.13 ± 0.01 b | 32.99 ± 2.57 b | 1.06 ± 0.11 b | 63.92 ± 2.19 a |
| T3 | 7.38 ± 0.03 d | 0.16 ± 0.01 b | 30.19 ± 2.23 c | 1.14 ± 0.21 b | 58.63 ± 2.82 b |
| T4 | 7.59 ± 0.06 b | 0.18 ± 0.01 a | 34.85 ± 2.32 a | 1.04 ± 0.11 b | 65.39 ± 2.56 a |
| Treatments | TN (mg/kg) | NN (mg/kg) | AN (mg/kg) | TP (mg/kg) | AP (mg/kg) |
|---|---|---|---|---|---|
| CK | 480.6 ± 6.59 d | 14.1 ± 1.58 c | 12.4 ± 1.89 d | 446.6 ± 27 d | 12.2 ± 1.67 c |
| T1 | 682.6 ± 9.46 c | 14.2 ± 2.16 c | 17.4 ± 2.06 c | 675.8 ± 19 c | 30.5 ± 1.76 b |
| T2 | 818.6 ± 12.2 b | 15.3 ± 2.28 c | 22.4 ± 2.35 b | 1049 ± 29 b | 31.6 ± 2.49 b |
| T3 | 839.5 ± 7.76 b | 23.1 ± 2.33 b | 26.3 ± 1.66 a | 1168 ± 32 a | 31.3 ± 2.22 b |
| T4 | 926.3 ± 12.2 a | 25.3 ± 2.30 a | 27.7 ± 2.53 a | 1195 ± 29 a | 34.3 ± 2.32 a |
| Treatments | Valid Sequences | OTUs | Chao1 | Simpson |
|---|---|---|---|---|
| CK | 122,112 ± 365 a | 114 ± 18 b | 3212 ± 112 b | 0.05 ± 0.01 b |
| T1 | 101,985 ± 556 b | 117 ± 25 b | 3217 ± 195 b | 0.02 ± 0.01 c |
| T2 | 98,634 ± 532 c | 101 ± 12 c | 3012 ± 145 d | 0.01 ± 0.01 c |
| T3 | 102,125 ± 523 b | 128 ± 12 a | 3841 ± 125 a | 0.21 ± 0.03 a |
| T4 | 100,365 ± 668 b | 105 ± 13 c | 3115 ± 142 c | 0.06 ± 0.01 b |
| Treatments | Phylum | Class | Order | Family | Genus |
|---|---|---|---|---|---|
| CK | 52 ± 1.74 a | 151 ± 06.23 a | 312 ± 11.23 a | 472 ± 22.18 a | 719 ± 42.12 a |
| T1 | 48 ± 2.14 b | 142 ± 11.12 c | 299 ± 10.96 b | 427 ± 29.65 c | 624 ± 34.12 c |
| T2 | 49 ± 2.98 b | 141 ± 09.23 c | 291 ± 09.66 c | 421 ± 14.52 d | 602 ± 22.45 d |
| T3 | 50 ± 4.12 ab | 148 ± 09.65 b | 310 ± 09.24 a | 445 ± 31.55 b | 642 ± 54.26 b |
| T4 | 50 ± 3.12 ab | 140 ± 08.51 c | 290 ± 08.65 c | 402 ± 18.22 e | 603 ± 19.45 d |
| Study | Design | Environmental Conditions | Results on Straw Degradation | Results on Crop Yield | Methodological Approaches | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| Current Study | Short-term field experiment | Cold winter, Southwest China | Significant enhancement with optimized N | Increased yield of oilseed rape | Standardized N and P levels, multiple N treatments | Regional focus, practical application insights, detailed enzyme activity analysis | Short-term duration, specific to Southwest China |
| Chen et al. [86] | Short-term field experiment | Cold climate, Northeast China | Moderate improvement with N addition | Consistent yield increase over years | Variation in conventional tillage and straw incorporation | Short-term insights, broader environmental applicability | Limited enzyme activity analysis, high variability in conditions |
| Limon-Ortega et al. [87] | Long-term field experiment | Arid conditions, Northern Mexico | Limited impact with N fertilization | Variable yield results | Standardized N and P levels | Insights for arid conditions, practical recommendations | Long-term duration, specific to arid regions |
| Dai et al. [88] | Short-term crop rotation study | Subtropical climate, Southern China | Improved soil organic carbon and microbial biomass | Enhanced yield over multiple crops | Crop rotation, single-year assessment | Short-term crop insights, nutrient cycling understanding | Complex interaction effects, regional focus |
| Björnsson and Prade [89] | Multi-site field experiment | Cold climate climates, Sweden | Variable results based on site | Yield improvement at most sites | Standardized protocols across sites | Broad applicability, multi-site validation | High variability, site-specific recommendations |
| García-Gutiérrez et al. [90] | Field study | Mediterranean climate, Spain | Maize residue was incorporated into soil | Yield improved in N-fertilized treatments | Crop rotation single-year assessment | Short-term crop insights, GHG emission analysis | Specific regional focus, without assessing the degradation rate of maize residues |
| Chen et al. [91] | Long-term field study | Cold climate, North | Cotton residue was incorporated into soil | Increased yield over several years | Long-term, detailed environmental monitoring | Long-term insights to reclaim saline soil | Regional focus, limited to cold climate regions |
| Ahmad et al. [92] | Field study with different residues | Semi-arid climate, Pakistan | Enhanced degradation | Yield improvement with residue management | Diverse residue types, practical agricultural implications | Long-term insights to improve soil multifunctionality | Soil metagenomics and enzymes were not analyzed |
| Lin et al. [93] | Field study | Subtropical climate, Southwest China | Crop residues increased SOC up to 25% | Organic improvement noted | Diverse residue types, wheat-maize rotation | Long-term field study focusing on soil organic carbon | Moderate field applicability, Crop residue degradation rate was not determined |
| Choudhary et al. [94] | Field study over multiple seasons | Rain-fed climate, India | Mixed residue incorporation increased available N | Yield increases with crop residue return | Multi-season data | Long-term field study focusing on crop yield and biomass | Limited long-term insights, soil physiochemical properties were not explained |
| Canalli et al. [95] | Crop residue management study | Humid climate, Brazil | Improved degradation with mixed management | Yield improvement with residue techniques | Practical recommendations, humid climate focus | Applicability to humid regions, practical insights | Short-term duration, specific to humid conditions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Nabi, F.; Sajid, S.; Kama, R.; Shah, S.M.M.; Wang, X. Optimizing Nitrogen Fertilization for Enhanced Rice Straw Degradation and Oilseed Rape Yield in Challenging Winter Conditions: Insights from Southwest China. Sustainability 2024, 16, 5580. https://doi.org/10.3390/su16135580
Wang H, Nabi F, Sajid S, Kama R, Shah SMM, Wang X. Optimizing Nitrogen Fertilization for Enhanced Rice Straw Degradation and Oilseed Rape Yield in Challenging Winter Conditions: Insights from Southwest China. Sustainability. 2024; 16(13):5580. https://doi.org/10.3390/su16135580
Chicago/Turabian StyleWang, Hongni, Farhan Nabi, Sumbal Sajid, Rakhwe Kama, Syed Muhammad Mustajab Shah, and Xuechun Wang. 2024. "Optimizing Nitrogen Fertilization for Enhanced Rice Straw Degradation and Oilseed Rape Yield in Challenging Winter Conditions: Insights from Southwest China" Sustainability 16, no. 13: 5580. https://doi.org/10.3390/su16135580
APA StyleWang, H., Nabi, F., Sajid, S., Kama, R., Shah, S. M. M., & Wang, X. (2024). Optimizing Nitrogen Fertilization for Enhanced Rice Straw Degradation and Oilseed Rape Yield in Challenging Winter Conditions: Insights from Southwest China. Sustainability, 16(13), 5580. https://doi.org/10.3390/su16135580







