Abstract
Water contamination with organochlorine pesticides (OCPs) is strongly linked to agricultural practices, and it still represents an environmental issue, despite the OCPs bans in many countries and despite the reported sustainable remediation technologies for their removal. Considering the environmental persistence of OCPs, the imbalances produced in the ecosystem, and the bioaccumulation tendency in living organisms through the food chain, the monitoring of OCPs and of their metabolites has crucial importance. The accuracy of the results obtained is strongly connected to the selection of reliable and accurate analytical procedures, especially considering the multitude of challenges related to OCP quantification. The purpose of this paper is to present an overview of the analytical techniques and protocols reported for OCP assessment in water, and to offer scientists a presentation of the current state of the literature on this subject. Nevertheless, it must be considered that each method has advantages and disadvantages, and, in most cases, the protocols reported in the literature must be adapted and improved. In addition, the levels of OCPs identified in surface water, groundwater, and rainwater have been reviewed. This review paper is directly connected to sustainability practices, since environmental sustainability is related to the responsibility to conserve natural resources and to prevent pollution, and for scientists, these objectives are fulfilled by conducting chemical analyses to track and quantify pollutants, as part of environmental studies.
Keywords:
analytical techniques; DDT; OCPs; organochlorine pesticide; metabolite; POPs; sustainability; water 1. Introduction
Organochlorine pesticides (OCPs) represent a class of persistent organic pollutants (POPs) that are highly toxic and resistant to degradation, which have a tendency to bioaccumulate in the environment. During World War II, these compounds were used to control disease-carrying insects. At the end of the war, OCPs were adapted for the control of pests in agriculture and public health, and were regarded as “miracle” pesticides due to their high efficacy and low cost [1]. Nevertheless, the discovery of pesticidal residues in various sections of the environment has prompted serious concerns regarding their continued use, which outweigh the overall benefits derived from their use. Even though several have been banned in many countries since the 1970s due to their slow degradation rate, they are still being detected in environmental samples worldwide. More than half of all insecticides used globally come from Asia. India occupies the third place in pesticide usage in Asia [2] after China and Turkey.
In addition to environmental-related issues, concerns about their chronic effects have also emerged from both animal and human studies, which have associated many OCPs with different types of cancer [3,4,5].
Organochlorine pesticides can contaminate freshwater resources, including lakes and rivers, through several routes. These include agricultural runoff, the discharge of wastewater from industrial plants, leakage from storage sites, rainfall, and atmospheric deposition. Aquatic ecosystems frequently serve as the ultimate repository for these compounds, representing the terminal link in their accumulation chain [6].
Nowadays, human contact with pesticides is more frequent than that with any other pollutants, and considering their known high toxicity, monitoring and quantification in different samples is becoming increasingly important to avoid environmental pollution and health impacts. Considering the complexity of sample matrices, the protocols for the sampling, conditioning, extraction, enrichment, and quantification of target compounds are continuously being improved and developed. Moreover, the analyte in environmental samples is rarely found at trace levels; therefore, sample preparation is the most time-consuming and challenging step. Furthermore, lowering the detection limit when using conventional techniques is possible by adopting pre-concentration methods or certain sample preparation protocols.
Even if OCPs have been restricted for a very long time and the use of those that are allowed under certain conditions is strictly monitored, they are still being found in the environment and represent a current problem.
This review paper structures the scientific information related to analytical techniques and protocols used for the extraction and quantification of OCPs from different water sources. This information was gathered from a multitude of studies on the concentrations of OCPs and their metabolites in surface water, groundwater, and rainwater.
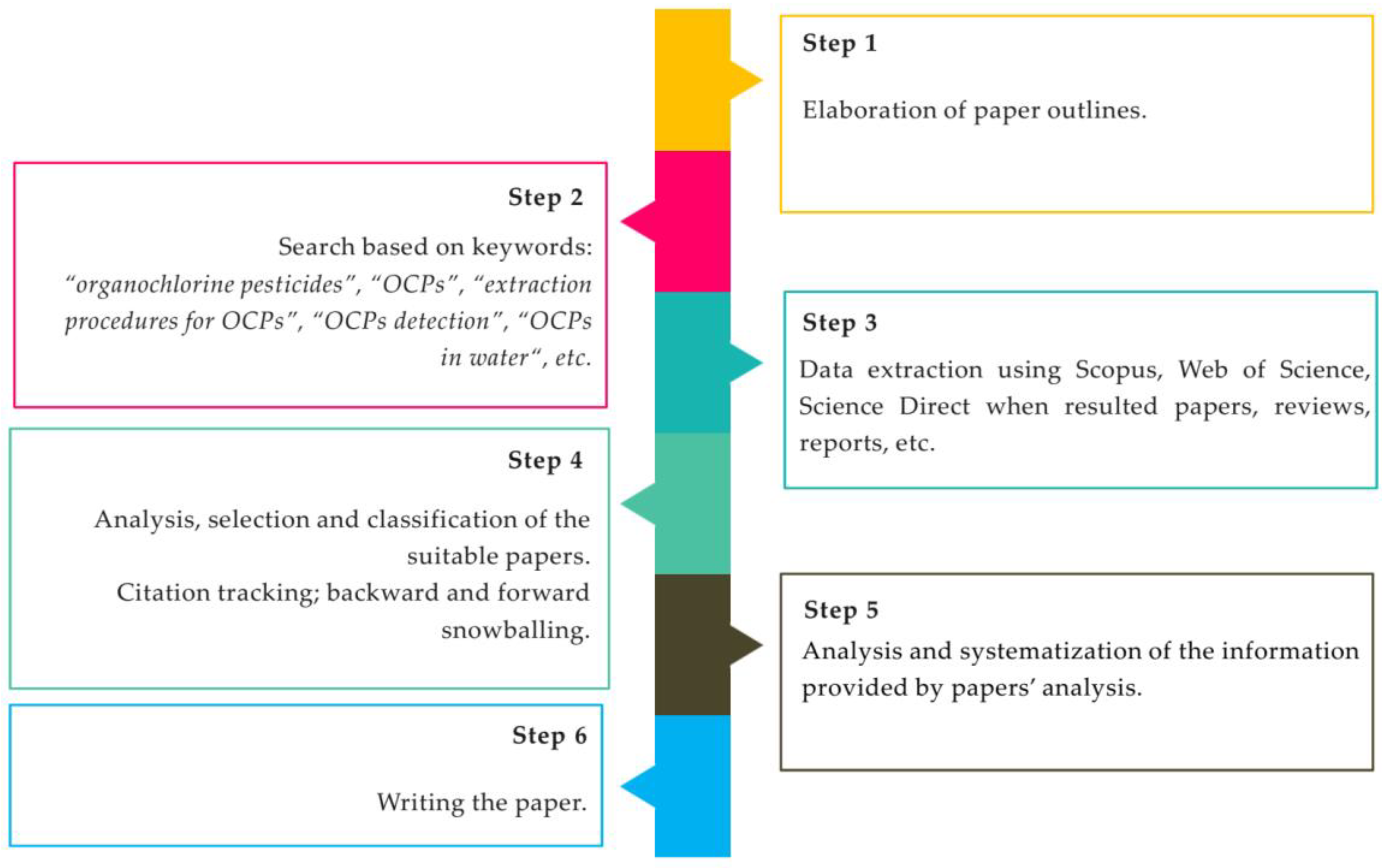
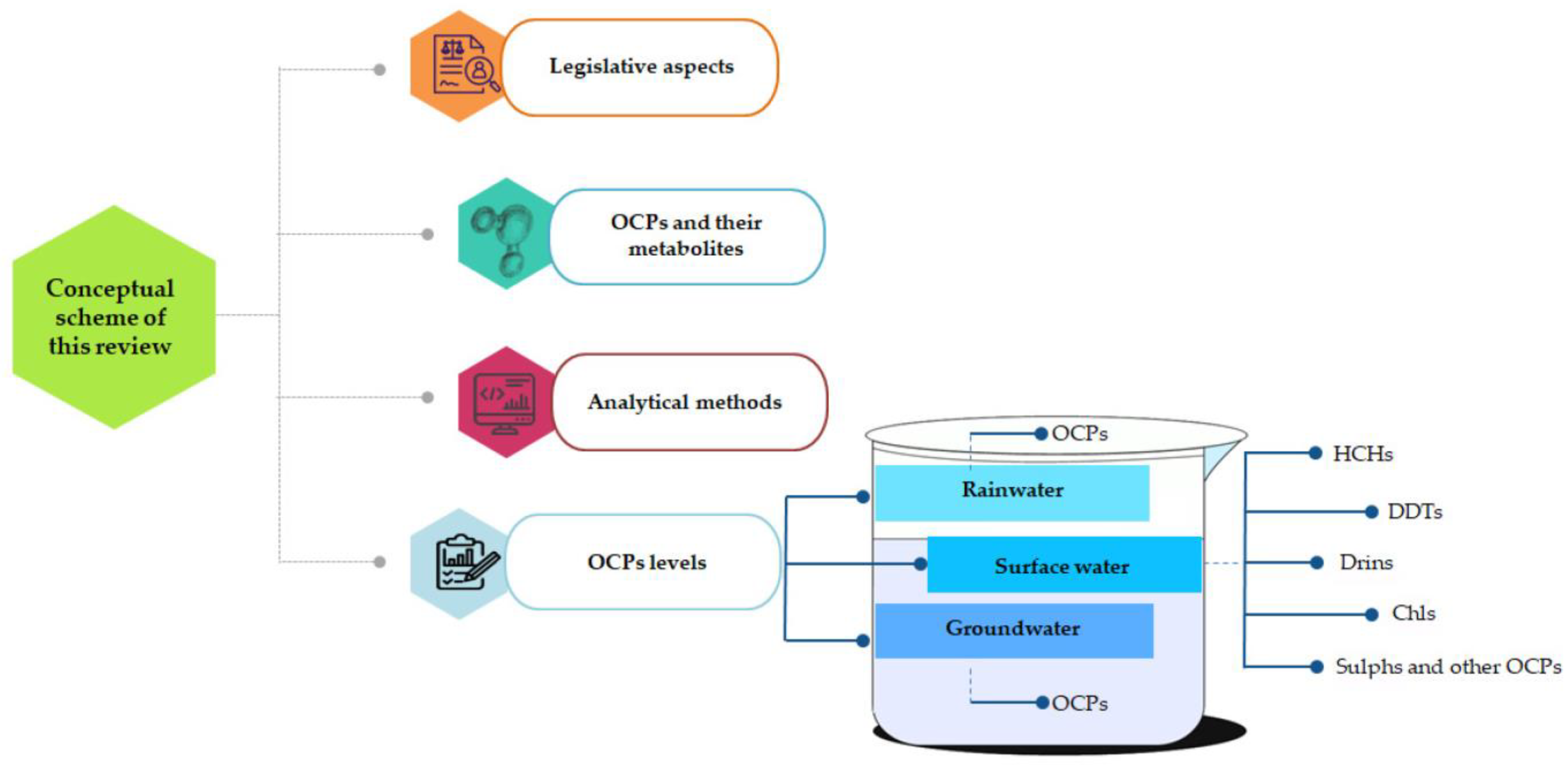
The data collection for this review paper and research steps are highlighted in Figure 1; meanwhile, the conceptual scheme of this paper is depicted in Figure 2.
Figure 1.
Literature research procedures.
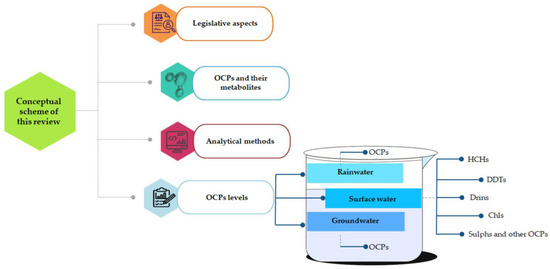
Figure 2.
Conceptual scheme of this review paper (Surface water: HCHs (Table 3), DDTs (Table 4), Drins (Table 5), Chls (Table 6), Sulphs and other OCPs (Table 7). Groundwater: OCPs (Table 8). Rainwater: OCPs (Table 9)).
2. Legislative Aspects–Brief Presentation
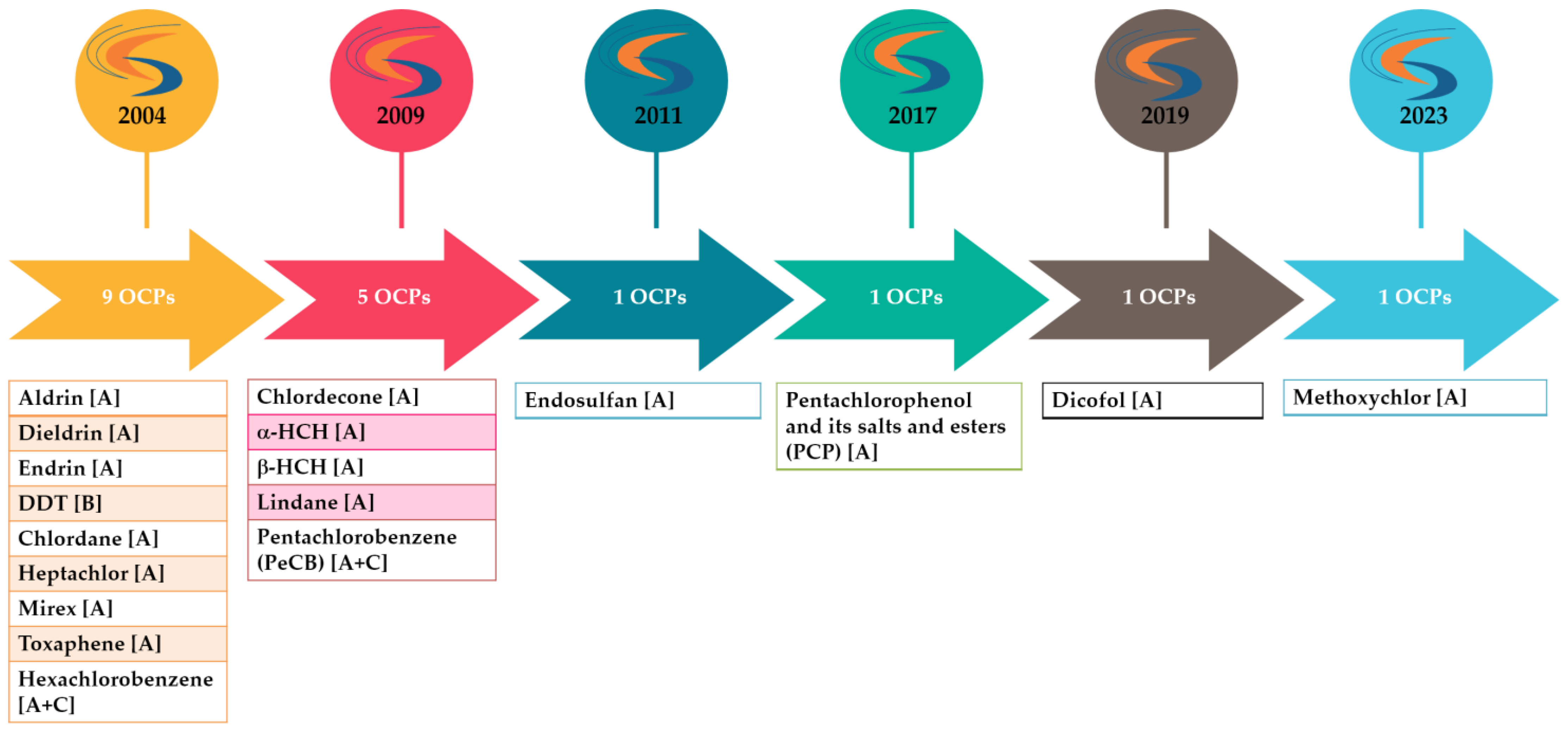
POPs, and thus OCPs, are subject to a multitude of stringent regulations (Figure 3). This arises from the fact that they exert long-lasting impacts on both the environment and human health. The global regulation of POPs is governed by several international agreements, including the Stockholm Convention (“POPs Convention”) and the Aarhus Protocol (“POPs Protocol”). Within the European Union, these two initiatives have been translated into the European Regulation (EU) 2019/1021 (“POPs Regulation”) [7]. The objective of this regulatory framework is to minimize and to potentially eliminate the release of these substances, and to manage waste containing or contaminated by them.
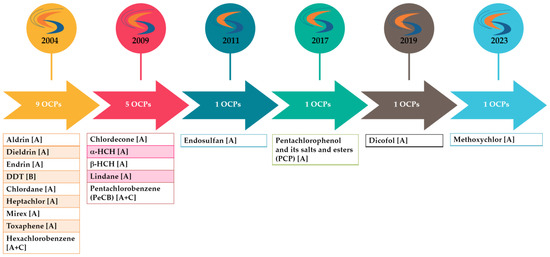
Figure 3.
OCPs listed under the Stockholm Convention. The letter(s) in brackets indicate in which annex(es) the POP is listed (Annex A (elimination) = Parties must take measures to eliminate the production and use of the chemicals listed under Annex A. Annex B (restriction) = Parties must take measures to restrict the production and use of the chemicals listed under Annex B in light of any applicable acceptable purposes and/or specific exemptions listed in the Annex. Annex C (unintentional production) = Parties must take measures to reduce the unintentional release of chemicals listed under Annex C with the goal of continuing minimization and, where feasible, ultimate elimination.).
In force since 2004, the Stockholm Convention on Persistent Organic Pollutants (POPs) calls for the reduction or elimination of the release of these chemicals, both at the national and global levels. Parties to the Convention commit to the non-production and non-use of the chemicals listed in its annexes; these chemicals are subject to regular updates to reflect the latest scientific developments. As of today, 185 countries have ratified the Convention and 34 POPs have been listed under it, with 17 pesticides, 15 industrial chemicals, and 7 unintentional by-products included [8]. Furthermore, EU POP Regulation 2019/1021 is intended to protect the environment and human health and therefore bans or restricts the production and/or use of POPs in the European Union [7].
Nine OCPs were initially listed amongst the twelve initial POPs [9], colloquially known as the “dirty dozen”, namely aldrin, dieldrin, endrin, dichlorodiphenyltrichloroethane (DDT), chlordane, hexachlorobenzene (HCB), mirex, toxaphene, and heptachlor. In the subsequent period, the list of prohibited substances was expanded to chlordecone and hexachlorocyclohexanes (HCH), including lindane (γ-HCH), pentachlorobenzene, endosulfan, pentachlorophenol (PCP), dicofol, and methoxychlor [10] (Figure 3).
Despite the ban on their production in the European Union, some of the remaining stocks were still utilized following the adoption of the Stockholm Convention, contributing to a historical contamination of agricultural soils, groundwater, and surface waters by these substances. Persistence in the environment is a characteristic of these substances, as reflected in the acronym POPs. Moreover, it has been shown that OCPs are resistant to environmental degradation processes [11].
Concerning the OCP and metabolite levels in water, there are guideline values set by the WHO to protect human health and maximum acceptable levels/standards set by different countries. In the European Union, environmental quality standards for inland waters, as well as other surface waters, to protect the aquatic environment against pollution are regulated by Directive 2008/105/EC [12] and those for drinking water intended for human consumption are regulated by Directive (EU) 2020/2184 [13].
3. Main Characteristics of OCPs and Their Metabolites
Organochlorine pesticides (OCPs) consist of a diverse array of chemical compounds, including herbicides, insecticides, and fungicides. Each of these compounds possesses unique physicochemical properties that contribute to their efficacy in controlling various pests [14]. OCPs are characterized by three key properties: low polarity, low aqueous solubility, and high lipophilicity [15]. Compared with other OCPs, HCHs (especially lindane) are more soluble (Table 1), which represents a serious inconvenience for the environment, especially since it is still used in developing countries. Their persistence in the environment varies from moderate to high, as can be observed in Table 1. Data from Table 1 were collected from information provided by the following resources: [6,16,17,18,19,20,21].

Table 1.
Main characteristics of OCPs and metabolites.
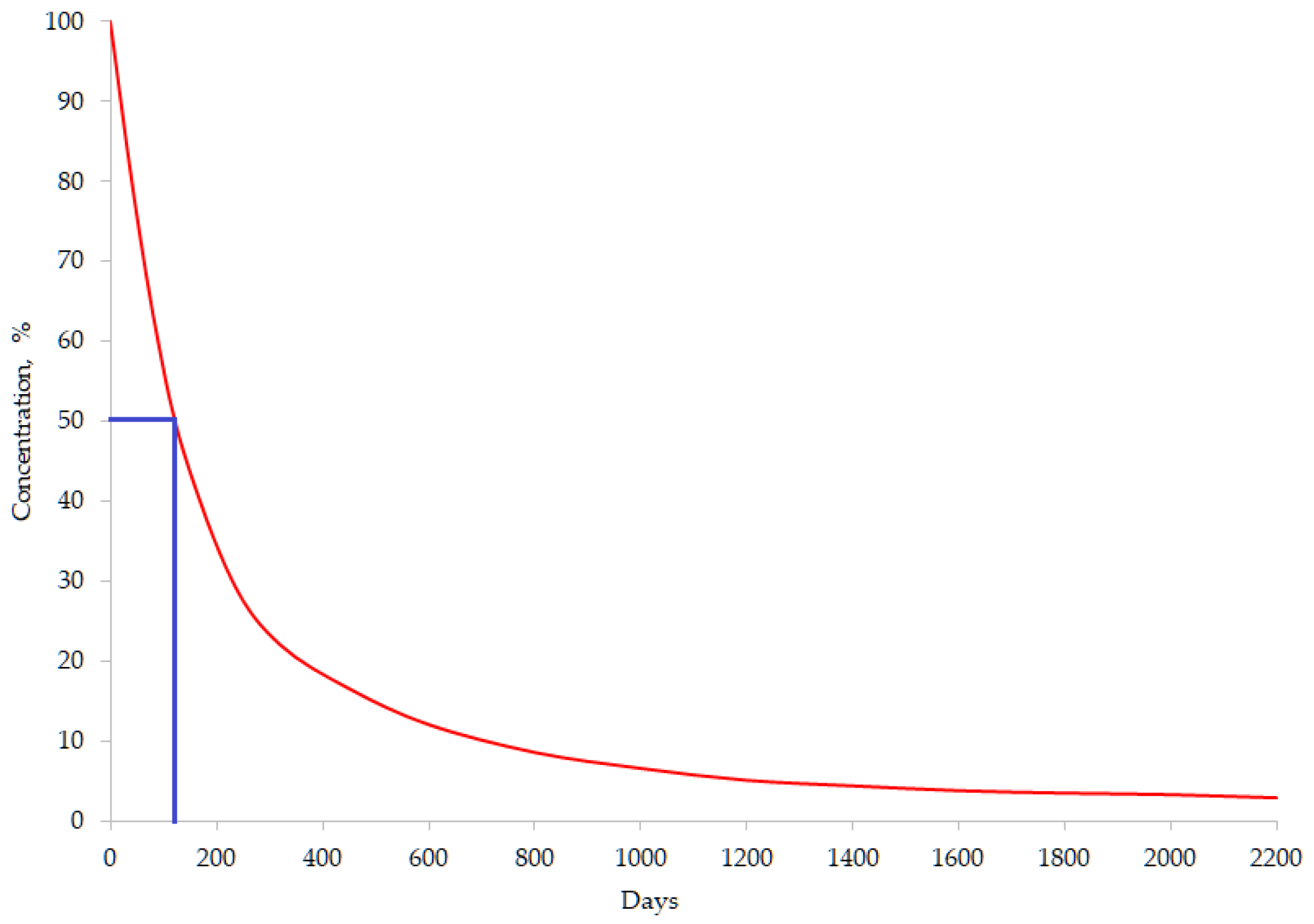
The persistence of OCPs in the environment is evaluated based on their half-life degradation time (DT50), which represents the time needed for initial concentration in a specific medium to be reduced to half (Figure 4) [22]. HCHs and DDTs are characterized by high persistence in the environment, where they may persist for decades (Table 1).
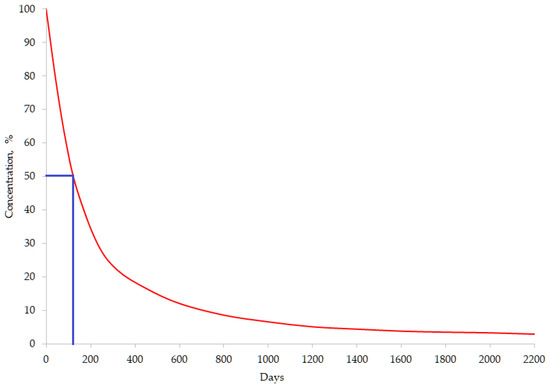
Figure 4.
Aspect of life-time curve for a certain OCP (DT50 = 120 days).
The presence and persistence of OCPs in the environment are related to their structures and physical properties, and also to conditions associated with the location where they are found. For example, aerobic biodegradation may occur faster than the anaerobic process. Also, OCP decomposition is strongly influenced by ultraviolet light, which is valued in photocatalytic degradation strategies [23].
Although OCPs are very stable, some molecular alterations are possible in certain circumstances, and the resultant metabolites are either as toxic and persistent as the parent compound, or, fortunately, less so.
Albeit the degradation of lindane, a gamma enantiomer of hexachlorocyclohexane, may occur either under aerobic or anaerobic conditions, it occurs frequently in aerobic conditions mediated by bacterial strains [24,25]. A metabolite of lindane obtained under aerobic degradation is γ-pentachlorocyclohexene [24]. Lindane’s microbial degradation is intensively investigated because it could be employed in remediation strategies.
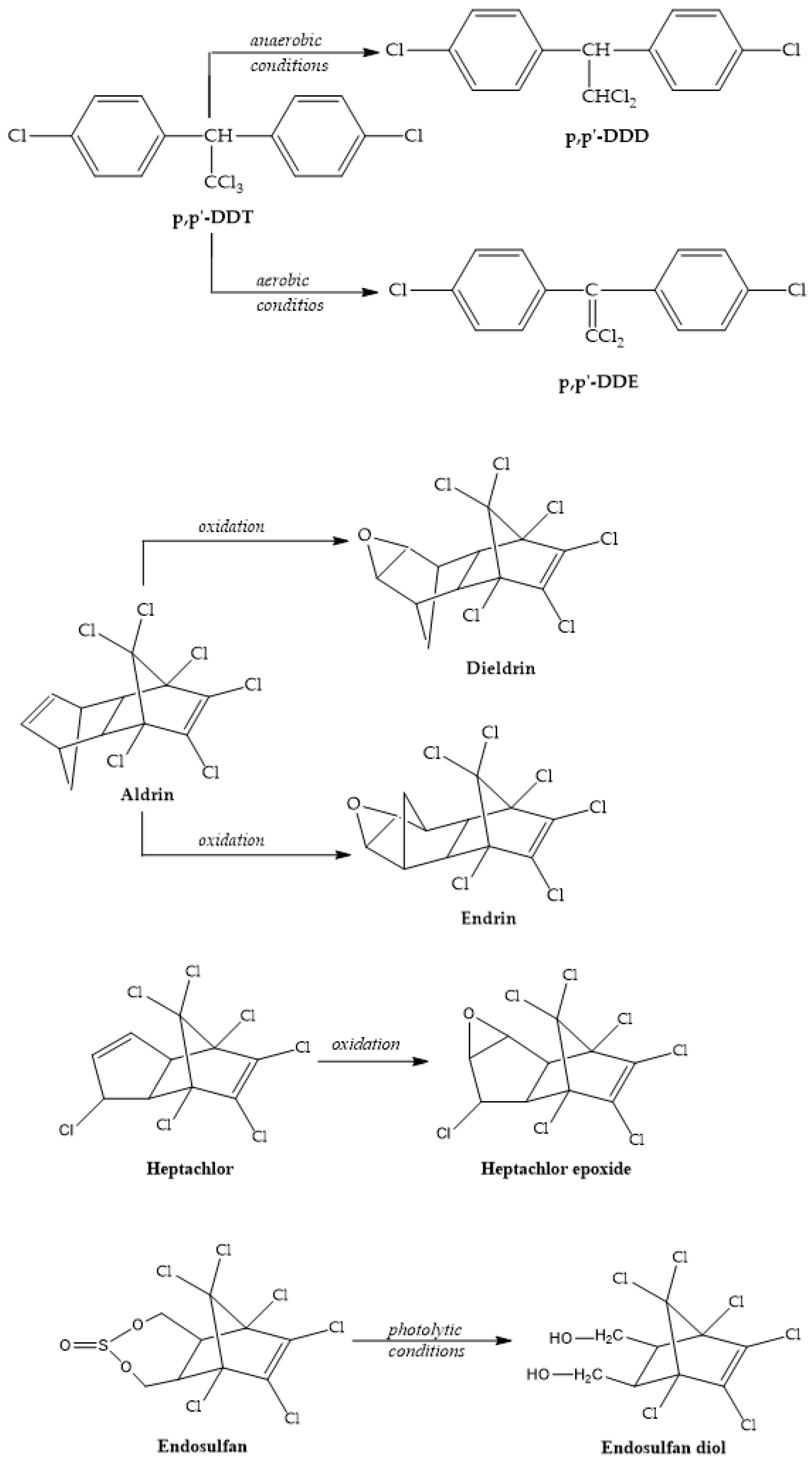
DDT’s metabolites are DDD and DDE (Figure 5), but it has been found that DDT is metabolized mainly into DDE and that is a possible explanation for the higher DDE levels compared with DDT, reported in some cases [26]. According to Wei and his team [27], DDT is converted to DDD under anaerobic conditions; meanwhile, aerobic conditions enable the transformation of DDT into DDE. This behavior could be utilized to gather information on the pollution source using DDD/DDE or (DDE + DDD)/ΣDDTs ratios.
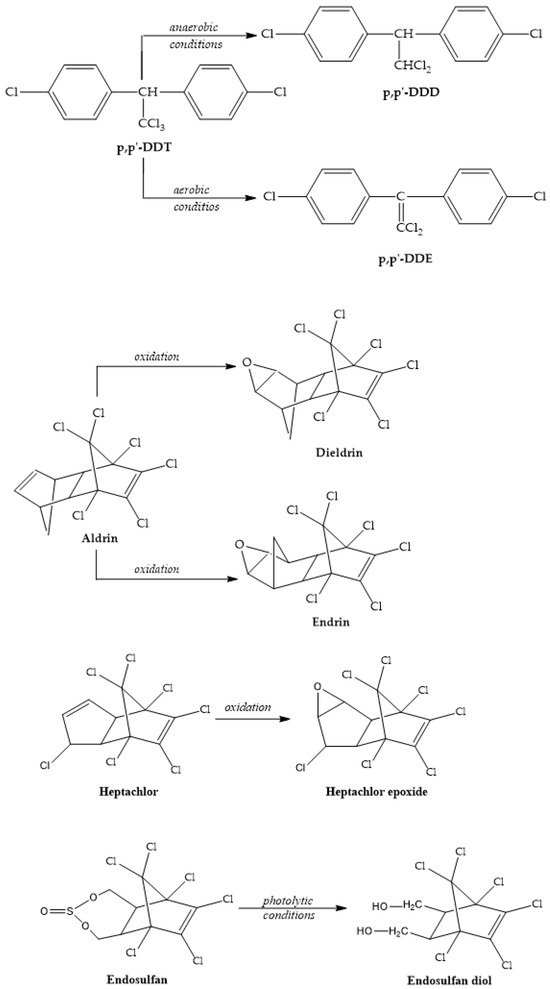
Figure 5.
OCPs and their metabolites.
It has been shown that DDT degradation is influenced by pH, while hydrolysis is increased in alkaline waters [28].
In environmental studies, there are also frequently monitored levels of OCPs and metabolites. For instance, Sibali and co-workers [29] assessed the levels of DDT and its metabolites in surface water from the Jukskei River in South Africa, and the low levels of DDT metabolites indicated recent contamination with DDT. If high levels of DDD and DDE had been found, this would have clearly indicated past DDT application practices and the detected metabolites would have resulted from DDT.
4. Analytical Methods and Protocols Used for OCP Assessment
OCP analysis is very challenging because there are many aspects that may interfere with the analysis: extraction and cleanup issues, sample type, the level of pesticide in the sample, etc. In addition, the analysis is complicated when there are chemical changes in the pesticide caused by hydrolysis, under UV light or sunlight, or in the case of complex matrices.
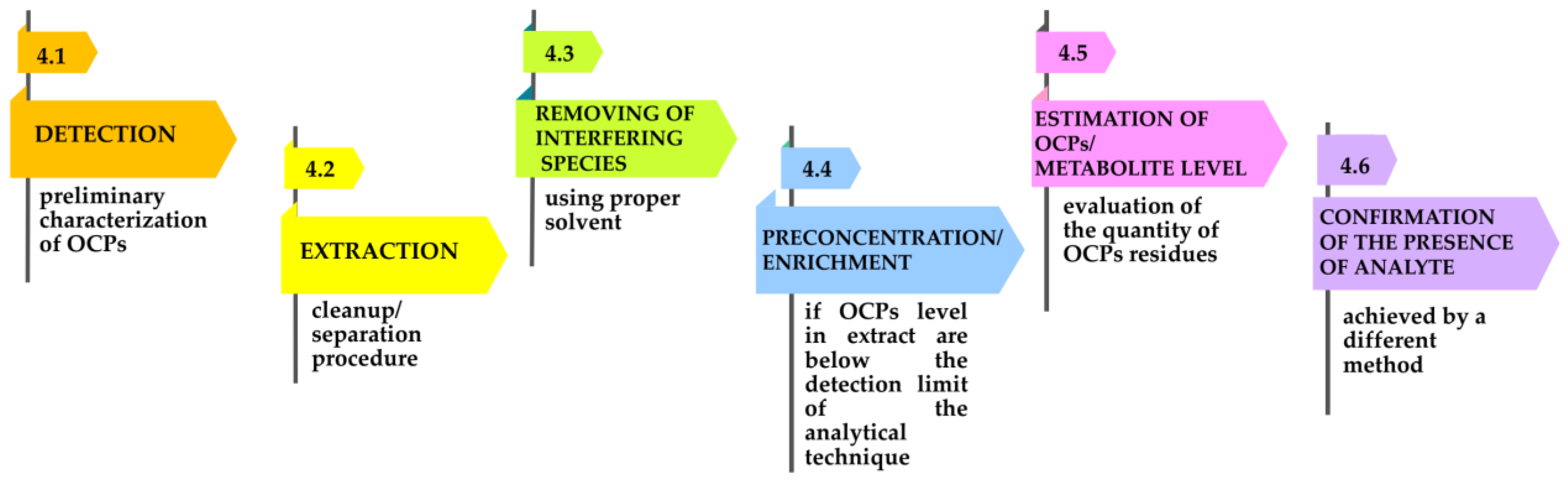
Nevertheless, the analysis of OCPs from different samples follows several steps, as suggested in Figure 6 [31].
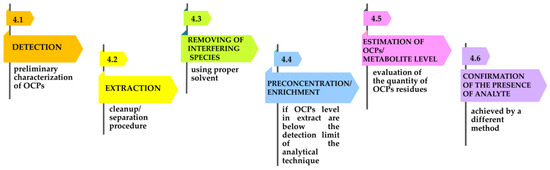
Figure 6.
Diagram of the steps of OCP analysis.
4.1. Detection
As OCP analysis is both expensive and time-consuming, it is highly recommended to perform a preliminary evaluation with a cheaper test before the quantitative analysis. Nowadays, there are rapid pesticide tests for different OCPs from various matrices. Also, there are reported screening or color tests for common groups of pesticides, including OCPs [32].
4.2. Extraction
The most important and laborious step in OCP analysis is represented by extraction, because the association between the proper extraction technique and suitable solvents assures the efficient extraction of the OCPs and the correctness of the obtained results. In addition, the more complex the sample matrix, the more complicated and laborious the extraction protocols are.
The extraction procedure is performed because it enriches the analyte level and lowers the interferences, and it is selected based on the type of pesticide and the nature of the sample to be analyzed [33]. Furthermore, the effectiveness of extraction is influenced by solvent polarity, dispersion coefficient, hydrogen bonding, and the solubility of OCPs in certain solvents [34]. According to the literature [35], the extraction procedure should be characterized by recoveries of at least 80% (lower values are synonymous to erroneous results) to require a small amount of organic solvent and minimum cleanup before quantification.
The most frequently used methods reported for OCP extraction are presented in the following paragraphs.
- Liquid–liquid extraction (LLE) is widely used for OCP extraction from aqueous matrices. It is considered as a simple method and it involves the dissolution of analytes in two different immiscible liquids (mainly water and organic solvents, such as ethyl acetate, dichloromethane, and petroleum ether) (Table 2). LLE is time-consuming and it also has other disadvantages; one that it is worth mentioning is the large volume of solvents that are associated with environmental issues and, worse, with carcinogenic effects on humans [35], and low enrichment of the analytes [33].
LLE with ethyl acetate on water samples collected from the Kabul River near an abandoned pesticide factory provided extracts that were quantified by GC with an electron capture detector and evidenced an alarming presence of p,p’-DDT [36]. Furthermore, LLE extraction with petroleum ether of OCPs from surface and groundwater samples of the Shaying River (China), followed by analysis by GC with a micro-cell electron capture detector (μECD), reported total OCP levels of 21.0 to 61.4 ng∙L−1 for groundwater and 12.3 to 77.5 ng∙L−1 for surface water [37]. Another team [38] used dichloromethane for the LLE of DDT and its metabolites from ditches and ponds near a closed-down factory in Bangladesh, followed by GC-ECD analysis. The values ranged from 590 to 3010 ng∙L−1. In addition, Fatoki and Awofolu [39] used light petroleum, hexane, and dichloromethane for LLE extraction, but from all the extracting solvents, dichloromethane gave the best results. For instance, the mean percentage recoveries of OCPs with dichloromethane ranged between 90.09 ± 8.03 and 102.95 ± 2.84%, the recoveries using light petroleum were between 68.18 ± 13.80 and 96.02 ± 4.90, whilst the recoveries using hexane were between 64.49 ± 3.04 and 98.92 ± 3.55.
A modification of LLE is solvent microextraction (SME), which involves the partitioning of the analyte between the aqueous phase and a very small volume of organic solvent. This extraction procedure combined with a GC technique, was used by de Jager and Andrews [40] to detect organochlorine from water samples. The proposed protocol was completed in less than 9 min and allowed the extraction and preconcentration of pesticides into a 2 μL drop of hexane. Via this procedure, OCP concentrations down to 0.25 ng∙mL−1 were detectable.
Other modifications of LLE, namely liquid–liquid extraction with low-temperature partition (LLE-LTP), were presented by Mesquita and his team [41] for the assessment of OCPs in water samples. In the LLE-LTP procedure, a water sample and acetonitrile are mixed and placed in a freezer at −20 °C for 1 h for phase separation. When the temperature is lowered, partitioning occurs between water and acetonitrile and results in the extraction of OCPs in the organic phase.
- Dispersive liquid–liquid microextraction (DLLME) requires injecting an appropriate mixture of extraction solvent and disperser solvent rapidly into an aqueous sample, resulting in a cloudy solution. DLLME is a low-cost, rapid, and easy-to-operate procedure with high recovery and it is also environmentally friendly, if we consider that very low volumes of solvents are used. This method is used to extract hydrophobic compounds. In addition to OCP extraction, this method has proven its utility for organophosphorus pesticides, herbicides, and polycyclic aromatic hydrocarbons [42]. For instance, the extraction of 14 OCPs (α-HCH, β-HCH, δ-HCH, γ-HCH, aldrin, dieldrin, endrin, heptachlor, heptachlor oxide, α-chlordane, β-chlordane, p,p’-DDT, p,p’-DDD, and p,p’-DDE) from the water of the Jajrood River in Iran was performed by DLLME using 0.5 mL of ether (disperser solvent) and 13.5 μL of carbon disulfide (extraction solvent), which were injected into the sample solution.
The DLLME procedure was also adopted by Jorfi and his team [43] to extract OCPs (chlordane, dieldrin, DDT, heptachlor, lindane, and endrin) from the water of a water treatment plant in Ahvaz City, Iran. They used 10 μL of tetrachloroethylene (extraction solvent) and 1000 μL of acetone (disperser solvent).
Eighteen OCPs were extracted from water samples with an optimized DLLME procedure using 10 μL of tetrachloroethylene (extraction solvent) and 1 mL of acetone (disperser solvent), and afterward, 2 μL of the extractant was subjected to GC-MS analysis. The detection limits of the method proposed were in the range of 1–25 ng∙L−1; the method was suitable for assessing ultra trace levels of OCPs in dirty and clean water samples [44].
Furthermore, another team [45] used DLLME coupled with GC-ECD for the extraction and quantification of 14 OCPs. The extraction protocol, which lasted for less than 5 min, consisted of using a mixture composed of 13.5 μL of carbon disulfide (extraction solvent) and 0.50 mL of acetone (dispenser solvent), which was injected into the sample. It was found that the enrichment factors were between 647 and 923 at room temperature and the OCP recoveries at two spiking levels (2.00 μg∙L−1 and 10 μg∙L−1) were 88.0–111.0% and 95.8–104.1%, respectively.
A modification of DLLME was reported by Tsai and Huang [46]. In this case, a smaller volume of disperser solvent than that used for DLLME was used; hence, the name of the method is dispersive liquid–liquid microextraction with little solvent consumption (DLLME-LSC). DLLME-LSC presents good repeatability and high sensitivity, and it could be used for the investigation of OCPs from different types of water. The authors [46] used 13 μL of solvent mixture (tetrachloroethylene:tert-butyl methyl ether = 4:6, v/v) to extract five OCPs from clean water.
- Vortex-assisted liquid–liquid microextraction (VALLME) was developed by Ozcan [47] for OCP quantification from aqueous samples. This optimized method used bromoform (50 μL) for extraction, followed by vortex extraction for 2 min at 3000 rpm with no NaCl addition for ionic strength adjustment, centrifugation for 5 min at 4000 rpm, and a 5 mL water sample. The mean recoveries for the OCPs assessed were between 71% and 104%. The performance of this extraction procedure was compared with that of LLE, and the conclusion was that recovery values were comparable (75–105%). This extraction method is suitable for the qualitative and quantitative assessment of OCPs from aqueous matrices, and it is easy to use and rapid.
- Solid-phase extraction (SPE) involves the use of disks or columns able to retain analytes, which are then released with small volumes of solvents. This is a great advantage over LLE. The conditioning of cartridges containing octadecyl groups chemically bonded to silica is performed using different solvents, such as methanol [48], hexane [49], acetonitrile [50], ethyl acetate [14], or solvent mixtures [51].
A common disadvantage of both LLE and SPE is that volatile analytes may be lost during the evaporation process [35].
Table 2 summarizes the SPE protocols with experimental details, instrumentation, and all other details.
The extraction of OCPs from shallow groundwater samples from the Taihu Lake region in China was performed by an SPE procedure using C18 cartridges that were washed with 5 mL of ethyl acetate, conditioned with 5 mL of methanol, and then washed with distilled water. Water samples were passed through the cartridges and the elution of OCPs was performed with 6 mL of ethyl acetate. After performing drying and concentrating the extract, the OCP residue levels were determined by GC-μECD [52].
Chen and his team [53] adopted a similar SPE protocol to extract OCPs from the surface waters of Shanghai, China. Unlike the above-mentioned study, 5 mL of methanol and 5 mL of dichloromethane were used for elution, and the OCPs were quantified via the GC-MS technique in the EI mode.
The extraction of OCPs from surface water in Greece [54] was also performed by SPE, but the elution of pesticides was performed with 10 mL of hexane and afterward, the detection was performed by GC equipped with 63Ni-ECD. The recoveries for OCPs for three concentration levels (0.04 μg∙L−1; 0.2 μg∙L−1; and 0.4 μg∙L−1) were between 28.65% and 201.34%, 38.12% and 177.32%, and 49.67% and 144.59%, respectively. The OCP concentrations obtained were higher than the EU target levels.
For OCP determination from water samples, a modification of the SPE protocol was proposed [55], the so-called magnetic-SPE, which involves the use of Fe3O4 magnetic nanoparticles coated with oleic acid and then quantification by GC-MS. The recoveries of the proposed method were in the range 44% to108% for three fortification levels.
Another extraction procedure for OCPs from water [29] is activated carbon extraction (ACE), which could be employed in monitoring DDT and its metabolites in water. The activation of carbon is performed using methanol and double-distilled water. Among the advantages of ACE, the low cost of obtaining activated carbon (coconut shell, coal, and wood) and good recoveries ranging from 75% to 84% (compared with SPE, 56% to 70%) are worth mentioning.
- Solid-phase microextraction (SPME) was developed during the 1990s and presents superiority over the above-mentioned extraction procedures, as it is solvent-free, with lower detection limits, sensitivity, and good reproducibility, and it has proven its efficiency when coupled with GC techniques [33,34,35]. Moreover, SPME proved its utility for OCP extraction from aqueous samples. For instance, Jackson and Andrews [56] reported that the SPME of OCPs, separation (micro-bore 0.1 mm capillary column), and measurement (GC) from river water samples took less than 10 min.
The catalytic degradation of DDT in water by dehydrochlorination mediated by Pd-based nanoparticles and the monitoring of by-products were efficiently investigated by the SPME-GC-MS method, which provided high recovery (over 88.75%) and low detection limits (0.03 μg∙L−1) [57].
For the analysis of OCPs from the Aries River in Romania, Miclean and co-workers [58] adopted, for extraction, SPE, followed by headspace solid-phase microextraction (HS-SPME). The latter involves the exposure of the fiber in the headspace above the sample. Afterward, the quantification performed by GC with ECD showed values between 144.5 ng∙L−1 and 316.4 ng∙L−1 for γ-HCH, DDT, and its metabolites.
- Magnetic solid-phase extraction (MSPE) is another method based on the adsorption of the analyte of interest on a magnetic adsorbent, the advantage being that magnetic particles with nano size dimensions have a large specific surface area and consequently, a higher extraction capacity.
Nodeh and co-workers [59] synthesized new graphene-based silica-coated magnetic nanoparticles (Fe3O4@SiO2-G) used for the pre-concentration of OCPs from aqueous samples. This adsorbent has a large surface area, great adsorption capacity, and the presence of aromatic rings in its composition (which are able to interfere through π–π stacking with aromatic rings of OCPs), making it suitable for the extraction of benzene-based species. The investigation of this adsorbent’s properties revealed excellent recovery values (80.8–106.3%) at pH 6.5. Furthermore, Zhou and his team [60] used magnetic polyamidoamine dendrimers to adsorb OCPs from water samples.
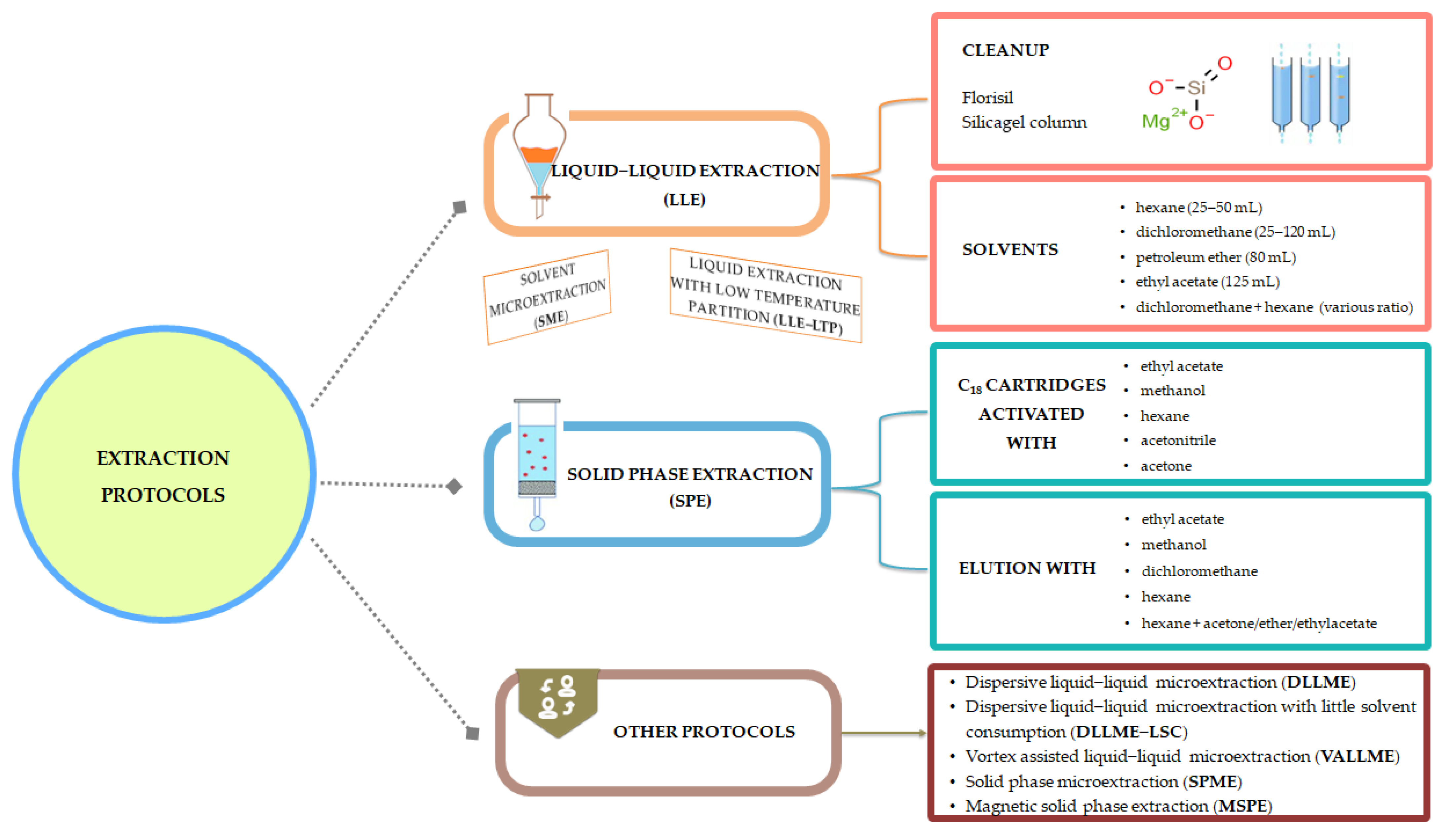
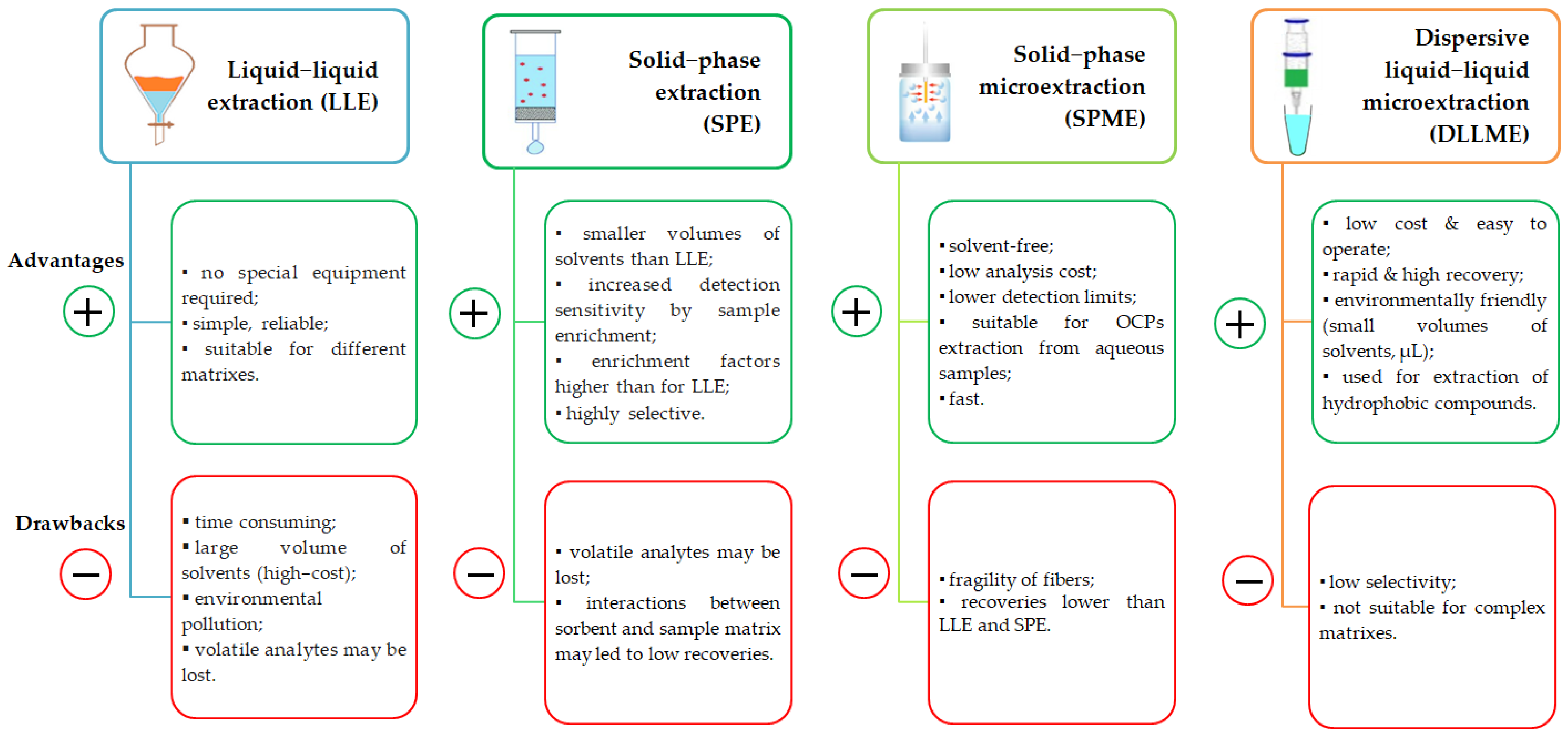
A brief presentation of the extraction protocols is presented in Figure 7; meanwhile, experimental details are provided in Table 2. Also, advantages and drawbacks for several extraction procedures can be found in Figure 8.


Table 2.
OCP extraction protocols and instrumentation.
Table 2.
OCP extraction protocols and instrumentation.
| Sample Volume, L | Extraction | Cleanup | Instrumentation | Column | Recovery, % | Ref. |
|---|---|---|---|---|---|---|
| 0.5 | LLE procedure - 25 mL n-hexane (extraction); - Dehydration with Na2SO4. | - SupelcleanENVI_Florisil (0.5 g) SPE tubes; - Complete evaporation + re-dissolution in n-hexane. | GC equipped with 63Ni-ECD and HP 3396 integrator | Fused silica capillary column Ultra-2 (50 m × 0.2 mm i.d. × 0.33 μm film thickness) | 82–96 | [61] |
| 0.5 | LLE procedure - 25 mL n-hexane (extracting three times); - Dehydration with 0.5 g Na2SO4; - Evaporation to 1–2 mL at 50 °C. | GC equipped with 63Ni-ECD | 4% SE–30/6% QF column | - | [62] | |
| 0.5 | LLE procedure - Extraction with 50 mL dichloromethane in hexane (trice); - Drying on Na2SO4; - Concentration to 0.5 mL and redissolution in 5 mL hexane; - Concentration to 0.5 mL. | GC equipped with 63Ni-ECD | BP5 capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [63] | |
| 0.6 | LLE procedure - 80 mL petroleum ether for 10 min; - Organic phase was purified with 10 mL H2SO4 + Na2SO4 solution 2%; - Dehydration with Na2SO4; - Concentration to 1 mL. | GC equipped with μECD | Capillary column HP-5 (30 cm × 0.32 mm i.d. × 0.25 μm film thickness) | 89–97 | [37] | |
| 1.0 | LLE procedure - Extraction with 25 mL hexane (three times); - Dehydration with 0.5 g Na2SO4; - Evaporation to 1–2 mL at 50 °C. | GC equipped with 63Ni-ECD | 4% SSE–30/60% QF capillary column | - | [61] | |
| 1.0 | LLE procedure - 50 mL mixture of 15% dichloromethane and 85% n-hexane (three extractions); - Dehydration with Na2SO4; - Concentration to 2 mL. | Florisil column + elution with 200 mL 6%d iethylether in petroleum ether (5 mL∙min−1 flow rate) | GC | - | - | [64] |
| 1.0 | LLE procedure - 100 mL dichloromethane (extracting two times); - Drying over anhydrous MgSO4; - Concentration to 2 mL. | - Silica cartridges with 2 g of anhydrous Na2SO4 conditioned with 6 mL dichloromethane; - Extracts are loaded onto cartridges; - Extracts are concentrated to dryness and re-dissolved in 1 mL ethyl acetate. | GC equipped with 63Ni-ECD | capillary column coated with VF-5 30 m + 10 m EZ guard column × 0.25 mm i.d. × 0.25 μm film thickness) | 70–119 | [65] |
| 1.0 | LLE procedure - 25–30 mL dichloromethane was used for extraction; - Extract was concentrated to 4 mL. | - Chromatography column (alumina-to-silica gel ratio 1:2); - Elution with 20 mL mixture of dichloromethane and n-hexane (2:3, v/v); - Concentration to 0.5–1.0 mL. | GC-MS/MS | HP-5 MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 69.46 ± 32.81 (for TCmX); 82.14 ± 6.46 (for PCB 65); 79 ± 12.71 (for PCB155) | [6] |
| 1.0 | LLE procedure - 250 mL water sample was treated with 50 mL saturated NaCl solution and 30 mL dichloromethane; - Dehydration with anhydrous Na2SO4; - Extract was dried and re-dissolved in 2 mL hexane. | Purification with H2SO4. | GC equipped with 63Ni-ECD and confirmation by GC/MS | HP-5MS fused silica capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 83–110 | [38] |
| 1.0 | LLE procedure - Water sample was treated with 120 mL dichloromethane; - Organic phase was dried on anhydrous Na2SO4; - Aqueous phase was treated twice with dichloromethane (2 × 60 mL) and lower phase was extracted; - Extract was concentrated to 0.5 mL and reconstituted to cyclohexane: acetone (9:1, v/v); - Concentration to 1 mL. | - Silica gel grade 60 (10 g) and alumina with 3% H2O (5 g) packed in a glass column and topped with anhydrous Na2SO4; - Elution with 100 mL mixture of hexane and dichloromethane (1:1, v/v). | HRGC-HRMS | Rtx-Dioxin2 (40 m × 0.18 mm i.d. × 0.18 μm film thickness) | 82–107 | [66] |
| 1.0 | LLE procedure - Dichloromethane for extraction | - Silica gel chromatography; - Drying and dissolving in ethyl acetate. | GC equipped with 63Ni-ECD | SPB-5 [(5% phenyl)-methyl polysiloxane) capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [67] |
| LLE procedure - Extraction with hexane; - Drying on anhydrous Na2SO4. | - Florisil - Evaporation; - Dissolution in 1 mL hexane | GC equipped with μECD | DB 1 capillary column (30 m × 0.32 mm i.d. × 0.50 μm film thickness) | - | [68] | |
| 1.0 | LLE procedure - Extraction with hexane; - Drying on anhydrous Na2SO4; - Concentration. | GC equipped with 63Ni-ECD | DB-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [69] | |
| 1.0 | LLE procedure - Extraction with dichloromethane (thrice); - Concentration and drying on Na2SO4; - Extract is made up to 5 mL with hexane. | GC equipped with 63Ni-ECD | PE-17 fused silica capillary (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 62.14–125.20 | [70] | |
| 1.0 | LLE procedure - Extraction with dichloromethane (3 × 20 mL); - Concentration. | - Silica gel column; - Concentration to 2 mL. | GC equipped with 63Ni-ECD | DB-5 capillary column (5%-phenyl-95%-dimethylpolysiloxane) (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 76–95 | [29] |
| 1.0 | LLE procedure - Extraction with 30 mL dichloromethane; - Drying on anhydrous Na2SO4; - Concentration. | GC equipped with 63Ni-ECD | Elite GC DB-5 (60 m × 0.25 mm i.d.) | - | [71] | |
| 2.0 | LLE procedure - 25 mL dichloromethane for extraction (three times); - Dehydration with Na2SO4; - Concentration to 2–3 mL. | - Neutral alumina/silica gel (1:2, v/v) column; - Elution with 30 mL dichloromethane and n-hexane (2:3, v/v); - Concentration to 0.2 mL. | GC equipped with 63Ni-ECD | HP-5MS capillary column (30 m × 0.32 mm i.d. × 0.25 μm film thickness) | 70.8 ± 17 (TCmX); 86.6 ± 20.8 (PCB209) | [72] |
| - | LLE procedure - 25 mL water sample + 10% NaCl + 125 mL ethyl acetate; - Organic phase is evaporated until complete dryness; - Sample is reconstituted in 5 mL n-hexane. | GC equipped with 63Ni-ECD | Fused silica capillary column (25 m × 0.53 mm i.d. × 0.15 μm film thickness) | - | [36] | |
| 2.0–3.0 | LLE procedure - 500 mL water sample + 10–15 g NaCl + 3 × 50 mL 15% dichloromethane in hexane; - Dehydration with anhydrous Na2SO4; - Concentration to near-dryness; - Dichloromethane removal is performed by addition of 5 mL of hexane (thrice); - Final volume of 2 mL in hexane | GC equipped with 63Ni-ECD | SPB-5 of 5% diphenyl/95% dimethyl fused silica capillary column (30 m × 0.32 mm i.d. × 0.25 μm film thickness) | 80–111 | [73] | |
| 2.5 | LLE procedure - 1000 mL water samples mixed with 50 mL hexane; - Resultant organic phase is re-extracted twice with 50 mL hexane; - Drying on anhydrous Na2SO4; - Concentrated. | - Florisil column; - Elution with 10 mL hexane aliquots; - Evaporation to dryness and dissolution in 1 mL ethyl acetate. | GC equipped with 63Ni-ECD | Fused silica gel capillary column VF-5 ms (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 79–96 | [74] |
| - | LLE-LTP procedure - 4 mL water sample and 8 mL acetonitrile are added to a vial and placed in a freezer (−20 °C); - 2 mL organic phase is transferred to a falcon tube with anhydrous Na2SO4; - 1 mL extract is analyzed. | GC-MS in selective ion monitoring mode (SIM) | DB-5MS capillary column with 5% phenyl stationary phase and 95% methylpolysiloxane (30 m × 0.32 mm i.d. × 0.25 μm film thickness) | 80–110 | [41] | |
| - | DLLME procedure - 5 mL water sample is transferred to a 10 mL screw-cap glass test tube; - 0.5 mL acetone (dispenser solvent) + 13.5 μL CS2 (extraction solvent) are injected into sample. | GC equipped with 63Ni-ECD | Fused-silica BPX5 column (25 m × 0.25 mm i.d. × 0.25 μm film thickness) | 78.9–101.3 | [45] | |
| 0.5 | DLLME procedure - 5 mL sample is poured into a 10 mL falcon tube; - 10 μL tetrachloroethylene (extraction solvent) + 1000 μL acetone (dispenser solvent). | GC-MS | HP-5 column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [43] | |
| - | DLLME procedure - 10 mL sample is placed in a 15 mL glass test tube and spiked with each pesticide at 10 μg∙L−1; - 10 μL tetrachloroethylene (extraction solvent) and 1000 μL acetone (disperser solvent) are dropped into the sample solution; - 2 μL sample is injected into GC-MS for analysis | GC-MS | VF-5-Ms Factor-four Varian column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 56–113 | [44] | |
| 0.5 | SPE procedure - C18 cartridges are washed with 5 mL ethyl acetate, conditioned with 5 mL methanol, and washed with 2 × 5 mL ultrapure water; - Water samples are percolated through cartridges; - Elution with 6 mL ethyl acetate; - Drying on Na2SO4; - Concentration. | GC-μECD | DB-5MS column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 78–93 | [52] | |
| 1.0 | SPE procedure - C18 column is conditioned with 6 mL hexane and dried; - 6 mL water sample is passed through column after previously applying 12 mL methanol; - Eluate is evaporated to dryness and dissolved in 1 mL hexane. | GC equipped with 63Ni-ECD | Fused silica capillary column HP-5 (5%PhMe silicone; 10 m × 0.53 mm i.d. × 2.65 μm film thickness) | 91–93 | [49] | |
| 1.0 | SPE procedure - C18 cartridges are activated with 5 mL ethyl acetate and 10 mL methanol and washed with 10 mL ultrapure water; - Water samples are percolated through cartridges; - Elution with 5 mL methanol and 5 mL dichloromethane; - Drying on anhydrous Na2SO4; - Concentration to 0.7 mL. after dichloromethane is added. | GC-MS in EI mode | HP-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [53] | |
| 1.0 | SPE procedure - SPE cartridges are conditioned with 12 mL methanol and 12 mL deionized and purified water; - Water samples are passed through cartridges; - Cartridges are washed with 6.0 mL purified water; - Elution with 1.0 mL dichloromethane. | GC-MS | DB-5 capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 70.25–103.5 88.25–127.5 | [75] | |
| 1.0 | SPE procedure - Water samples are extracted using SPE disks; - 10 mL dichloromethane + ethyl acetate (1:1, v/v) as cleaning solvent; - Elution with 5 mL ethyl acetate (twice); - Dehydration with Na2SO4; - Extract is concentrated to 1 mL. | GC equipped with 63Ni-ECD | Fused silica capillary column coated with 5% diphenyl–95% dimethylsiloxane (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [76] | |
| 1.0 | SPE procedure - C18 column is washed with 20 mL methanol and 10 mL ultrapure water; - Elution with 10 mL hexane; - Concentration to 2 mL. | GC equipped with 63Ni-ECD | Fused silica capillary column HP-608 (30 m × 0.53 mm i.d. × 0.50 μm film thickness) | 28.65–121.57 | [54] | |
| 1.0 | SPE procedure - Water samples are passed through SPE cartridges previously rinsed with 5 mL ethyl acetate, 5 mL methanol, and 10 mL ultrapure water; - Elution twice with 10 mL ethyl acetate; - Dehydration using Na2SO4; - Concentration to 0.5 mL. | GC equipped with 63Ni-ECD | HP-5 capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 72.4–105 | [14] | |
| 1.0 | SPE procedure - C18-bonded silica cartridges are washed with 20 mL methanol and 10 mL ultrapure water; - Water sample is passed through cartridges; - Elution with 10 mL hexane; - Concentration to 0.2 mL | GC equipped with 63Ni-ECD | HP-608 fused capillary column (30 m × 0.53 mm i.d. × 0.50 μm film thickness) | 81.96–104.92 | [77] | |
| 1.0 | SPE procedure - Water sample is passed through C18 cartridge activated with 10 mL methanol and washed with 10 mL double-distilled water; - Elution with dichloromethane (3 × 10 mL); - Concentration to 5 mL | GC equipped with 63Ni-ECD | DB-5 capillary column (5%-phenyl-95%-dimethylpolysiloxane) (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 56–76 | [29] | |
| 1.0 | SPE procedure - C18 cartridge is washed with 6 mL ethyl acetate and 6 mL dichloromethane, and then washed with 10 mL methanol and 10 mL ultrapure water; - Water samples are percolated through cartridges; - Elution with 10 mL dichloromethane; - Drying on Na2SO4; - Concentration. | GC equipped with 63Ni-ECD GC-MS in selective ion monitoring mode (SIM) | HP-5 capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 74–113 | [27] | |
| 1.0 | SPE procedure - FlorisilSPE cartridges are conditioned with 5 mL hexane, 5 mL methanol, and 5 mL ultrapure water; - Elution with 10 mL ethyl acetate; - Drying on Na2SO4; - Concentration to 1 mL. | GC-MS | HP-5 capillary column (30 m × 0.32 mm i.d. × 0.25 μm film thickness) | 76.11–108.32 | [78] | |
| 1.0 | SPE procedure - C18 cartridges are washed with 5 mL ethyl acetate, conditioned with 5 mL methanol, and washed with 2 × 5 mL ultrapure water; - Water samples are percolated through cartridges; - Elution with 6 mL ethyl acetate; - Drying on Na2SO4; - Concentration to 1 mL. | GC equipped with 63Ni-ECD | DB-5 fused capillary column (30 m × 0.32 mm i.d. × 0.25 μm film thickness) | 76–87 | [79] | |
| 2.0 | SPE + SPME procedure - Water samples are passed through C18 columns previously washed with methanol and ultrapure water; - Elution with 10 mL hexane; - Eluate is collected in SPME vials containing 0.10 g NaCl. | GC equipped with μECD | DB-1 capillary column (30 m × 0.32 mm i.d. × 3 μm film thickness) | 36.5–112.2 | [58] | |
| 2.5 | SPE procedure - Water sample is passed through C18 Sep-Pak cartridges conditioned with 5 mL acetonitrile and 5 mL methanol; - Elution with ethyl acetate. | GC equipped with 63Ni-ECD | Glass column (2 m × 2 mm i.d.) with 1:1 mixture of 10% OV-101 and 15% OV-210 on ChromosorbWHP | 42–102 | [50] | |
| 2.5 | SPE procedure - 250 mL water sample is passed through C18 Sep-Pak cartridges conditioned with 10 mL mixture of acetonitrile + dichloromethane (1:1, v/v); - Elution with 3 mL acetone, 3 mL hexane + acetone (1:1, v/v), and 3 mL hexane; - Evaporation to dryness; - Residue is dissolved in 1 mL cyclohexane. | GC-MS/MS | Fused-silica untreated capillary column connected to Factor Four capillary column VF-5 ms (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 70–110 | [51] | |
| 2.5 | SPE procedure - Dichloromethane for extraction. | Celite–activated charcoal column. | GC equipped with 63Ni-ECD | Glass column with 1:1 mixture of 10% OV-101 and 15% OV-210 on ChromosorbWHP | [80] | |
| 5.0 | SPE procedure - Water sample is drawn through C18 column conditioned with 3 × 3 mL hexane:acetone, 3 mL methanol, and 3 mL distilled water; - Retained species are dissolved in hexane:acetone mixture; - Concentration to 2 mL. | Alumina–silicic acid column (2 g alumina and 3 g silicic acid) - Elution with 20 mL dichloromethane; - Concentration to 1 mL. | GC equipped with 63Ni-ECD | DB 1702 column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 74–96 | [16] |
| 5.0 | SPE procedure - Samples are passed through cartridges conditioned with 10 mL methanol + 2 × 5 mL deionized water; - Elution with 6 mL dichloromethane; - Dehydration with Na2SO4; - Concetration to 0.4 mL. | GC equipped with μECD GC coupled to MS detector in selected ion mode | Capillary column HP-5MS (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 86.7–116.4 | [48] | |
| - | SPE procedure - Samples passed through C18 column; - Elution with 5 mL hexane + ether (4.5:0.5, v/v); - Concentration to 100 μL and then dilution to 1 mL (with hexane). | GC equipped with 63Ni-ECD | DB-101 fused-silica capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [81] | |
| - | SPE procedure - 100 mL sample is passed through C18 SPE laminar disk; - Elution with 10 mL ethyl acetate and 3 mL hexane; - Drying on Na2SO4 followed by concentration. | GC equipped with 63Ni-ECD GC-MS | Methyl–phenyl–cyanopropyl silicone fused silica capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 96–104 | [82] |
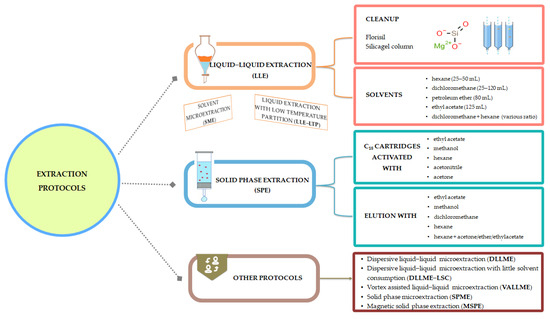
Figure 7.
Synthetic representation of extraction protocols.
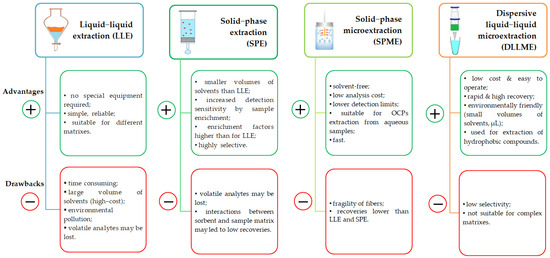
Figure 8.
A brief presentation of the advantages and disadvantages of the main extraction procedures.
4.3. Removal of Interfering Species-Cleanup
The cleanup of the extract is a procedure that occurs before the quantification of samples with a complex matrix. In the case of water samples, the cleanup procedure is not required for relatively clean samples (groundwater and drinking water). A column of Florisil, silica gel, alumina, or charcoal [6,61,65,72,80] may be used for this step, if needed. In addition, it is possible that several OCPs are found in the sample; therefore, a crude separation into subcategories could be performed by using various types of column chromatography [83].
Huang and co-workers [72] investigated the presence of OCPs in groundwater from the Zigui karst area in China. After the LLE procedure, a neutral alumina–silica gel column (v/v, 1:2) was used for cleanup, followed by OCP elution with a mixture of dichloromethane/n-hexane (2:3) and concentrated to 0.2 mL prior to GC analysis. The results showed that 24 OCPs were detected in spring water (300–32,200 ng∙L−1) and river water (318–2250 ng∙L−1). The same extraction procedure for OCPs from water collected from the Mediterranean Sea and the River Nile of Rosetta was used by El-Alfy and his team [64]. The cleanup procedure was performed by passing the extract through a Florisil column and, afterward, elution was performed with diethyl ether. The OCP levels were below the detection limit of the method used. This outcome was related to the moment when the water was sampled, namely in the summer, when OCPs may hydrolyze more easily and volatilize. Another team [65] studied the presence of OCPs in drinking water from a cocoa Farm in Ghana. For cleanup, the team used silica cartridges through which concentrated OCPs were passed. For elution, they used dichloromethane. The detected OCPs were lindane, α-endosulfan, endosulfan sulfate, dieldrin, and p,p’-DDT.
4.4. Preconcentration or Enrichment
The preconcentration of a sample is usually required before cleanup and it involves solvent removal. In the case of OCPs, this process is performed by evaporation or filtration under pressure through a membrane.
Mirzaei and Rakh [84] reported a preconcentration method for OCPs from water samples. The method consists of joining of SPE to DLLME based on a solidification of floating organic drop (DLLME-SFO), which provided an ultra-enrichment factor (8280–28,221) for nine OCPs. In addition, the matrices of water samples did not influence the performance of the SPE-DLLME-SFO method; the recoveries were between 72% and 112%.
4.5. Detection and Quantification Methods of OCPs and Theirmetabolites
In the case of pesticides, the methods used are categorized as chromatographic, spectrophotometric, electrochemical, biological, and radiochemical, with the latter being rarely used.
The following chromatographic methods are worth mentioning: paper chromatography, thin-layer chromatography (TLC), gas–liquid chromatography, liquid-column chromatography, capillary electrophoresis, and supercritical fluid chromatography (SFC).
OCPs are usually determined by gas chromatographic techniques (GC) using electron capture detectors [61,63,69]. These techniques are accurate, but time-consuming and expensive.
In today’s studies, for the determination of OCPs, researchers predominantly use fused silica capillary columns with lengths of 25 to 50 m, an internal diameter of0.15 to 0.25 mm, and a film thickness higher than 0.15 mm to prevent on-column degradation [85].
El-Gawad [86] reported the validation of an optimized method for the determination of 18 OCPs by gas chromatography operating with a quadrupole mass detector (GC-QMS). The validation was applied to the OCPs quantification in freshwater from the Ismailia Canal in Egypt.
Ali et al. [87] developed a GC method preceded by SPE for the assessment of various OCPs in water from the Hindon River in India. The extraction recoveries for the investigated OCPs were between 89.5% and 98.1%. The optimization of the GC conditions was conducted by using different columns, gas flow rate variations, and temperatures.
In addition to the so-called traditional methods for OCP quantification, there are other methods that involve the use of different sensors. For instance, an electrochemical sensor based on Fe3O4 nanostructures decorated with indium tin oxide has proven specificity for OCPs based on the ability of chlorine atoms from OCPs to interact with Fe3O4 nanoparticles [88]. Another sensor, based on gold nanoparticles, was reported [89] due to its ability to detect endosulfan and catalyze the decomposition of endosulfan into non-toxic products.
The detection of chlordane, heptachlor, lindane, and mirex in marine water can be performed by cyclodextrin-promoted fluorescence modulation, which is characterized by low micromolar detection limits, selectivity, and the possibility to be used for different water samples with different salinity degrees [90]. In addition, this method can be implemented in the manufacturing of portable detection devices.
Surface-enhanced Raman spectroscopy (SERS) is a valuable method for OCP detection, but there is still a need to amplify the signal of OCPs. Many strategies to overcome this issue were reviewed by Moldovan and her team [91]. Furthermore, Li and co-workers [92] reported a SERS procedure for the detection of 4,4′-DDT, α-endosulfan, and chlordane in farmland, river, and fishpond water with recoveries between 90.20% and 109.4%.
The detection of lindane in an aqueous environment by using a polymer-modified electrode was achieved by Noori et al. [93]. The measurement time was as short as 20 s and the stability of the electrode for repeated use stretched for over a week. All these are excellent attributes for field measurements.
4.6. Confirmatory Techniques
To obtain a reliable result with a given technique, the analyst should use an additional technique to confirm the results, because in some cases, the interfering compounds may generate false-positive results. In the case of OCPs, the primary technique is GC equipped with ECD, and the confirmatory technique is mainly GC-MS.
For example, Gao and his colleagues [48] detected lindane, p,p’-DDT, and heptachlor oxide in surface water from China by GC equipped with μECD. Peak confirmation was achieved by GC coupled with an MS detector in SIM. The obtained results indicated that, from the investigated compounds, lindane was more frequently detected, more precisely in 83.9% of samples, with a mean concentration of 31.3 ng∙L−1. It was followed by p,p’-DDT, which was detected at 63.1% from samples with a mean value of 14.6 ng∙L−1.
Mahmoud and co-workers [38] monitored the levels of DDT and its metabolites in water collected from regions that are close to an abandoned DDT factory. The extraction of OCPs was performed via an LLE procedure; meanwhile, quantification was performed by GC with ECD and confirmed by GC-MS. The recovery values were 83% to 110% and the DDTs detected in water ranged between 0.59 and 3.01 μg∙L−1. The levels of p,p’-DDT, which were higher than those of o,p’-DDT, indicated pollution with technical-grade DDT (65–80% p,p’-DDT) from the factory.
5. OCP Levels Detected in Water from Different Sources
Despite OCPs being banned for many years, they are still found in various products and environments: water [1,6,14,16,29,36,37,38,39,45,48,49,50,62,70,75,78,87], soil [92,94,95,96], vegetables [97,98], meat [99,100], dairy products [101], and honey-bees [102]. This is related to their long life and insolubility in water.
An analysis of the literature on the levels of OCPs and their metabolites found in surface waters, groundwaters, and rainwaters is summarized in Table 3, Table 4, Table 5, Table 6 and Table 7 (OCPs were sorted into categories of HCHs, DDTs, Drins, Chls, Sulphs, and other OCPs), Table 8, and Table 9, respectively. The presence of reported OCPs is associated with past practices, accidental pollution, or continued fraudulent/illegal use.
The highest average HCHs levels were found in surface water from India, in the River Ganges (α-HCH was 190 ng∙L−1, γ-HCH was 260 ng∙L−1) [70]. High values were also found in the water of the Densu River in Ghana (10–1070 ng∙L−1) [74] and the Küçuk Menderes River in Turkey (159–198 ng∙L−1) [63].
Furthermore, high levels of DDT and its metabolites were identified in ditch and pond water from Bangladesh, as follows: p,p’-DDT was in the range of 190–1540 ng∙L−1, p,p’-DDE was in the range of 200–650 ng∙L−1, and p,p’-DDD was in the range of 210–830 ng∙L−1.This shows the effect of pollution caused by the random spread of pesticides after a DDT factory closure [38]. Even higher DDTs concentrations (1200–3250 ng∙L−1) were detected in the Jukskei River, South Africa, and sewage and waste dump sites around the river were suspected as possible sources [29].
Concerning the levels of Drins, those in water samples from the River Ganges, India, stand out as the average dieldrin level was 1670 ng∙L−1 [70]. Wide ranges of variations in the aldrin concentrations were reported for the Küçuk Menderes River (17–1790 ng∙L−1) [63] and the Kusadasi Dilek National Park (ND-2180 ng∙L−1) [16].
The highest average levels of HCHs, DDT, and its metabolites in groundwater were found also in India (Table 8). There have been studies that demonstrate the relationship between the consumption of OCP-contaminated groundwater and the occurrence of type 2 diabetes mellitus [71].
The literature survey evidenced the presence of OCPs, mainly HCHs and DDTs, in rainwater from India (Table 9).








Table 3.
HCHs concentrations (ng∙L−1) in surface water.
Table 3.
HCHs concentrations (ng∙L−1) in surface water.
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| α-HCH | China | Shaying River | 2.0 | 0.7–3.3 | [37] |
| Songhua River | 4.18 | 2.27–6.91 | [81] | ||
| Jiuxi Valley | 1.45 | 0.12–5.24 | [14] | ||
| Jiuxi Valley | 7.17 | 4.31–13.0 | [14] | ||
| Surface water | - | 1.01–3.86 | [27] | ||
| Weihe River | 1.10 | 0.04–2.87 | [78] | ||
| Qiantang River | - | <0.08–72.24 | [79] | ||
| Greece | Surface water | - | ND–440 | [54] | |
| India | River Ganges, Kanpur | 190 | - | [70] | |
| Romania | River water | 5.80 | - | [103] | |
| Arieș River | 8.34; 24.10 | - | [58] | ||
| Danube, Jiu River, Olt River | - | <1–5 | [68] | ||
| Turkey | Mid-Black Sea region | - | ND–7.0 | [76] | |
| Küçuk Menderes River | - | 20–24 | [63] | ||
| KusadasiDilek National Park | 52 | ND–120 | [16] | ||
| β-HCH | China | Shaying River | 3.1 | 1.0–7.5 | [37] |
| Songhua River | 10.18 | <0.001–18.31 | [81] | ||
| Jiuxi Valley | 0.968 | 0.509–2.98 | [14] | ||
| Jiuxi Valley | 3.84 | 2.25–11.5 | [14] | ||
| Surface water | - | 0.29–3.44 | [27] | ||
| Weihe River | 4.50 | 0.28–21.10 | [78] | ||
| Qiantang River | - | <0.16–19.99 | [79] | ||
| Greece | Surface water | - | ND–401 | [54] | |
| Romania | Arieș River | 9.61; 46.90 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1–6 | [68] | ||
| Turkey | Mid-Black Sea region | - | ND–17.4 | [76] | |
| Küçuk Menderes River | - | 101–121 | [63] | ||
| Kusadasi Dilek National Park | 11 | ND–68 | [16] | ||
| γ-HCH | China | Shaying River | 5.2 | 1.8–9.3 | [37] |
| Songhua River | 4.89 | 0.001–5.94 | [81] | ||
| Jiuxi Valley | 1.08 | 0.844–1.84 | [14] | ||
| Jiuxi Valley | 3.16 | 2.42–5.02 | [14] | ||
| Surface water | - | 1.92–6.99 | [27] | ||
| Weihe River | 3.85 | 0.13–24.55 | [78] | ||
| Qiantang River | - | <0.08–173.11 | [79] | ||
| Ghana | Densu River | - | 20–100 | [74] | |
| Greece | Surface waters | ND–81 | [54] | ||
| Iliki Lake | 15 | - | [50] | ||
| Pinios River | - | 2–12 | [80] | ||
| India | River Ganges of Kanpur | 260 | - | [70] | |
| Philippines | Pampanga River | 29 | - | [67] | |
| Romania | Arieș River | 13.8; 125.1 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1 | [68] | ||
| Moara Domneasca pond | 11.5 | 8–18 | [69] | ||
| Turkey | Mid-Black Sea region | - | ND–4.2 | [76] | |
| Küçuk Menderes River | - | 159–198 | [63] | ||
| KusadasiDilek National Park | 20 | ND–92 | [16] | ||
| δ-HCH | China | Shaying River | 6.2 | 1.8–12.5 | [37] |
| Songhua River | 8.78 | <0.001–16.53 | [81] | ||
| Jiuxi Valley | 0.198 | 0.142–0.312 | [14] | ||
| Jiuxi Valley | 0.598 | 0.485–0.756 | [14] | ||
| Weihe River | 14.32 | 0.14–157.89 | [78] | ||
| Qiantang River | - | <0.08–46.26 | [79] | ||
| Ghana | Densu River | - | 10–1070 | [74] | |
| Greece | Surface water | - | ND–189 | [54] | |
| Romania | Arieș River | 2.07; 3.60 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1 | [68] | ||
| Turkey | Mid-Black Sea region | - | ND–250.5 | [76] | |
| Küçuk Menderes River | - | ND–16 | [63] | ||
| KusadasiDilek National Park | 44 | ND–54 | [16] | ||
| ΣHCH | China | Yangtze River | 0.44 | 0.17–1.14 | [104] |
| Beiluo River | 0.34 | 0.090–0.61 | [6] | ||
| Jiuxi Valley | 3.69 | 1.71–8.12 | [14] | ||
| Jiuxi Valley | 14.8 | 9.94–23.5 | [14] | ||
| Pearl River Delta | - | 0.84–12.23 | [105] | ||
| Weihe River | 19.85 | 2.41–178.18 | [78] | ||
| Qiantang River | - | 0.74–202.8 | [79] | ||
| Greece | Surface water | - | ND–421 | [54] | |
| Romania | Danube, Jiu River, Olt River | - | 1–9 | [68] | |
ND = not detected.

Table 4.
DDTs concentrations (ng∙L−1) in surface water.
Table 4.
DDTs concentrations (ng∙L−1) in surface water.
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| p,p’-DDT | Bangladesh | Ditch and pond water | - | 190–1540 | [38] |
| China | Songhua River | 11.78 | <0.001–14.120 | [81] | |
| Yangtze River | 13.4 | - | [48] | ||
| Huaihe River | 41.3 | - | [48] | ||
| Pearl River | 10.1 | - | [48] | ||
| Jiuxi Valley | 1.11 | 0.715–1.84 | [14] | ||
| Jiuxi Valley | 1.48 | 0.849–2.12 | [14] | ||
| Surface water | - | ND–3.17 | [27] | ||
| Weihe River | 0.77 | 0.10–4.50 | [78] | ||
| Qiantang River | - | <0.08–24.70 | [79] | ||
| Ghana | Densu River | - | 10–20 | [74] | |
| Greece | Surface water | - | ND–35 | [54] | |
| Philippines | Pampanga River | - | 37–40 | [67] | |
| Romania | Arieș River | 35.70; 42.60 | - | [58] | |
| Danube, Jiu River, Olt River | - | <2 | [68] | ||
| Turkey | Küçuk Menderes River | - | 21–50 | [63] | |
| Kusadasi Dilek National Park | 6 | ND–18 | [16] | ||
| o,p’-DDT | China | Songhua River | 7.47 | <0.001–10.81 | [81] |
| Jiuxi Valley | 0.904 | 0.247–1.860 | [14] | ||
| Jiuxi Valley | 1.45 | 0.708–4.13 | [14] | ||
| Weihe River | 4.68 | 0.04–115.53 | [78] | ||
| Qiantang River | - | <0.08–10.88 | [79] | ||
| Romania | Arieș River | 1.84; 4.25 | - | [58] | |
| Danube, Jiu River, Olt River | - | <9 | [68] | ||
| p,p’-DDE | Bangladesh | Ditch and pond water | - | 200–650 | [38] |
| China | Songhua River | 13.11 | <0.001–17.45 | [81] | |
| Jiuxi Valley | 0.161 | <0.05–0.246 | [14] | ||
| Jiuxi Valley | 0.283 | 0.0724–0.622 | [14] | ||
| Surface water | - | ND–0.65 | [27] | ||
| Weihe River | 0.43 | 0.03–2.17 | [78] | ||
| Qiantang River | - | <0.08–93.40 | [79] | ||
| Ghana | Densu River | - | ND–20 | [74] | |
| Greece | Surface waters | - | ND–64 | [54] | |
| Romania | Arieș River | 10.80; 15.00 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1 | [68] | ||
| Turkey | Uluabat Lake | 0.113 | 0.022–0.237 | [61] | |
| Kusadasi Dilek National Park | 3 | ND–44 | [16] | ||
| o,p’-DDE | China | Weihe River | 0.37 | 0.03–1.05 | [78] |
| p,p’-DDD | Bangladesh | Ditch and pond water | - | 210–830 | [38] |
| China | Songhua River | - | <0.001–0.014 | [81] | |
| Jiuxi Valley | 0.0612 | 0.04–0.128 | [14] | ||
| Jiuxi Valley | 0.12 | <0.04–0.316 | [14] | ||
| Surface water | - | ND–2.41 | [27] | ||
| Weihe River | 0.98 | 0.04–4.32 | [78] | ||
| Qiantang River | - | <0.08–13.56 | [79] | ||
| Greece | Surface waters | - | ND–112 | [54] | |
| Romania | Arieș River | 9.07; 11.06 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1 | [68] | ||
| Turkey | Mid-Black Sea region | - | ND–8.9 | [76] | |
| Küçuk Menderes River | - | 23–52 | [63] | ||
| Kusadasi Dilek National Park | 5 | ND–43 | [16] | ||
| o,p’-DDD | China | Weihe River | 0.15 | 0.01–1.00 | [78] |
| Romania | Arieș River | 6.41; 8.07 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1 | [68] | ||
| ΣDDT | China | Yangtze River | 0.52 | 0–0.98 | [104] |
| Beiluo River | 1.39 | 0.50–4.48 | [6] | ||
| Jiuxi Valley | 2.23 | 1.19–3.31 | [14] | ||
| Jiuxi Valley | 3.34 | 1.90–6.23 | [14] | ||
| Weihe River | 7.37 | 0.94–116.83 | [78] | ||
| Qiantang River | - | 0.40–97.54 | [79] | ||
| Romania | Danube, Jiu River, Olt River | - | <1–4 | [68] | |
| South Africa | Jukskei River | - | 1200–3250 | [29] | |
| Turkey | Meriç Delta | - | ND–1010 | [62] | |
ND = not detected.

Table 5.
Drins concentrations (ng∙L−1) in surface waters.
Table 5.
Drins concentrations (ng∙L−1) in surface waters.
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| Aldrin | China | Shaying River | 2.3 | 1.0–3.8 | [37] |
| Jiuxi Valley | 0.736 | <0.05–1.62 | [14] | ||
| Jiuxi Valley | 1.28 | 0.213–2.45 | [14] | ||
| surface water | 1.81 | 0.78–4.74 | [27] | ||
| Weihe River | 0.54 | 0.11–2.00 | [78] | ||
| Qiantang River | - | <0.08–103.9 | [79] | ||
| Ghana | Densu River | - | ND–20 | [74] | |
| Greece | Surface waters | - | ND–104 | [54] | |
| Philippines | Pampanga River | 29 | - | [67] | |
| Romania | Arieș River | 9.75; 12.60 | - | [58] | |
| Danube, Jiu River, Olt River | - | <2–2 | [68] | ||
| Turkey | Meriç Delta | - | ND–40 | [62] | |
| Küçuk Menderes River | - | 17–1790 | [63] | ||
| Kusadasi Dilek National Park | 3 | ND–2180 | [16] | ||
| Dieldrin | China | Shaying River | 4.6 | - | [37] |
| Qiantang River | - | <0.15–42.06 | [79] | ||
| Ghana | Densu River | - | ND–20 | [74] | |
| Greece | Surface waters | - | ND–39 | [54] | |
| India | River Ganges of Kanpur | 1671 | - | [70] | |
| Philippines | Pampanga River | - | 28–29 | [67] | |
| Romania | Arieș River | 0.103; 0.421 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1 | [68] | ||
| Turkey | Meriç Delta | - | ND–10 | [62] | |
| Mid-Black Sea region | - | ND–1.8 | [76] | ||
| Küçuk Menderes River | - | 8–5117 | [63] | ||
| Kusadasi Dilek National Park | 8 | ND–30 | [16] | ||
| Endrin | China | Shaying River | 6.2 | 1.0–11.3 | [37] |
| Jiuxi Valley | 0.063 | <0.04–0.110 | [14] | ||
| Jiuxi Valley | 0.126 | <0.04–0.196 | [14] | ||
| Surface waters | 2.22 | 0.53–4.39 | [27] | ||
| Weihe River | 4.74 | 0.37–26.28 | [78] | ||
| Qiantang River | - | <0.10–28.46 | [79] | ||
| Ghana | Densu River | - | 10–30 | [74] | |
| Turkey | Küçuk Menderes River | - | 14–191 | [63] | |
| Kusadasi Dilek National Park | 8 | ND–32 | [16] | ||
| Surface waters | 5.37 | 0.45–11.75 | [27] | ||
| Ghana | Densu River | - | 30–150 | [74] | |
| Greece | Surface waters | - | ND-80 | [54] | |
| Philippines | Pampanga River | - | 417–1341 | [67] | |
| Turkey | Küçuk Menderes River | - | 43–138 | [63] | |
| Kusadasi Dilek National Park | 9 | ND–9 | [16] | ||
| Endrin ketone | Ghana | Densu River | - | ND–20 | [74] |
| Turkey | Küçuk Menderes River | - | 41–56 | [63] | |
| ΣEndrin | China | Beiluo River | 2.31 | 0.15–20.91 | [6] |
ND = not detected.

Table 6.
Chls concentrations (ng∙L−1) in surface waters.
Table 6.
Chls concentrations (ng∙L−1) in surface waters.
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| Heptachlor | China | Shaying River | 8.3 | 1.3–17.9 | [37] |
| Jiuxi Valley | 0.426 | <0.04–1.75 | [14] | ||
| Jiuxi Valley | 0.316 | 0.0634–1.23 | [14] | ||
| Surface waters | - | 1.12–2.32 | [27] | ||
| Weihe River | 0.89 | 0.05–3.22 | [78] | ||
| Qiantang River | - | <0.15–88.34 | [79] | ||
| Ghana | Densu River | - | ND–40 | [74] | |
| Greece | Surface waters | - | ND–20 | [54] | |
| Philippines | Pampanga River | 28 | - | [67] | |
| Romania | Arieș River | 2.44; 3.16 | - | [58] | |
| Turkey | Küçuk Menderes River | - | 46–181 | [63] | |
| Heptachlor epoxide | China | Shaying River | 1.4 | 0.6–1.9 | [37] |
| China | Qiantang River | - | <0.08–111.8 | [79] | |
| Turkey | Mid-Black Sea region | - | ND–21.4 | [76] | |
| Küçuk Menderes River | - | 110–297 | [63] | ||
| Philippines | Pampanga River | - | 19–70 | [67] | |
| Turkey | Meriç Delta | - | ND–200 | [62] | |
ND = not detected.

Table 7.
Sulphs and other OCP concentrations (ng∙L−1) in surface waters.
Table 7.
Sulphs and other OCP concentrations (ng∙L−1) in surface waters.
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| α-Endosulfan | China | Shaying River | 8.5 | 2.1–40.7 | [37] |
| Surface waters | - | ND–1.62 | [27] | ||
| Ghana | Densu River | - | ND–40 | [74] | |
| Romania | Danube, Jiu River, Olt River | - | <1 | [68] | |
| Turkey | Küçuk Menderes River | - | 3–7 | [63] | |
| Kusadasi Dilek National Park | 11 | ND–30 | [16] | ||
| Surface waters | - | ND–1.85 | [27] | ||
| Philippines | Pampanga River | - | 40–64 | [67] | |
| Romania | Danube, Jiu River, Olt River | - | <1 | [68] | |
| Turkey | Küçuk Menderes River | - | 23–25 | [63] | |
| Kusadasi Dilek National Park | 3 | ND–39 | [16] | ||
| Endosulfan sulfate | Greece | Surface waters | - | ND–58 | [54] |
| Turkey | Kusadasi Dilek National Park | 5 | ND–7 | [16] | |
| Küçuk Menderes River | - | 19–121 | [63] | ||
| ΣEndosulfan | China | Surface waters | 1.37 | 0.067–9.25 | [6] |
| Surface waters | 3.05 | ND–9.23 | [27] | ||
ND = not detected.

Table 8.
OCPs concentrations (ng∙L−1) in groundwater.
Table 8.
OCPs concentrations (ng∙L−1) in groundwater.
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| α-HCH | China | Taihu Lake region, shallow groundwater | 5.85 | 0.92–41.77 | [52] |
| India | Kanpur, groundwater | 189 | - | [70] | |
| Delhi, tap water | 4 | 0–12 | [71] | ||
| Romania | Fountain | 6 | - | [103] | |
| β-HCH | China | Taihu Lake region, shallow groundwater | 14.53 | 0.94–107.6 | [52] |
| India | Delhi, tap water | 30 | 22–52 | [71] | |
| γ-HCH | China | Taihu Lake region, shallow groundwater | 8.77 | 1.38–56.05 | [52] |
| Ghana | Well water | 30 | - | [65] | |
| India | Kanpur, groundwater | 303; 471 | - | [70] | |
| Hyderabad, well water | - | 680–1380 | [77] | ||
| Delhi, tap water | 650 | 0–1690 | [71] | ||
| Philippines | Groundwater | 30 | - | [67] | |
| Romania | Tap water | 4 | - | [103] | |
| δ-HCH | China | Taihu Lake region, shallow groundwater | 14.56 | 3.69–80.58 | [52] |
| p,p’-DDT | China | Taihu Lake region, shallow groundwater | 4.64 | ND–60.28 | [52] |
| Ghana | Well water | 40 | - | [65] | |
| India | Hyderabad, well water | - | 150–190 | [77] | |
| Delhi, tap water | 79 | 62–142 | [71] | ||
| o,p’-DDT | India | Delhi, tap water | 820 | 520–1440 | [71] |
| p,p’-DDE | China | Taihu Lake region, shallow groundwater | 46.77 | 0.72–453.5 | [52] |
| India | Delhi, tap water | 78 | 48–126 | [71] | |
| o,p’-DDE | India | Delhi, tap water | 4 | 0–87 | [71] |
| p,p’-DDD | China | Taihu Lake region, shallow groundwater | 1.04 | ND–6.95 | [52] |
| India | Delhi, tap water | 3 | 0–20 | [71] | |
| Aldrin | China | Taihu Lake region, shallow groundwater | 27.59 | ND–356.5 | [52] |
| Dieldrin | Ghana | Well water | 30 | - | [65] |
| Philippines | Groundwater | - | 28–29 | [67] | |
| Endrin | China | Taihu Lake region, shallow groundwater | 11.53 | ND–129.2 | [52] |
| Endrin aldehyde | Philippines | Groundwater | - | 504–998 | [67] |
| China | Taihu Lake region, shallow groundwater | 6.15 | ND–78.79 | [52] | |
| α-Endosulfan | Ghana | Well water | 30 | - | [65] |
| India | Hyderabad, well water | - | 1340–2140 | [77] | |
| β-Endosulfan | India | Hyderabad, well water | - | 210–870 | [77] |
| Endosulfan sulfate | Ghana | Well water | 30 | - | [65] |
| Heptachlor | China | Taihu Lake region, shallow groundwater | 21.98 | 4.97–189.2 | [52] |
| Ghana | Well water | 20 | - | [65] | |
| Philippines | groundwater | 20 | - | [67] | |
| Heptachlor epoxide | China | Taihu Lake region, shallow groundwater | 12.31 | ND–47.12 | [52] |
| Philippines | Groundwater | 22 | - | [67] | |
ND = not detected.

Table 9.
OCPs concentrations (μg∙L−1) in rainwater.
Table 9.
OCPs concentrations (μg∙L−1) in rainwater.
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| α-HCH | India | Delhi | - | ND–0.062 | [105] |
| Hisar | - | ND–0.2 | [73] | ||
| Tanzania | Kibaha Coast Region | - | 0.001–100 | [66] | |
| β-HCH | India | Hisar | - | ND–0.1 | [73] |
| Tanzania | Kibaha Coast Region | - | 0.001–99 | [66] | |
| γ-HCH | India | Delhi | - | ND–0.2 | [73] |
| Tanzania | Kibaha Coast Region | - | 0.01–3.1 | [66] | |
| δ-HCH | India | Hisar | - | ND–0.1 | [73] |
| Tanzania | Kibaha Coast Region | - | ND–5.4 | [66] | |
| ΣHCH | India | Hisar | ND–0.4 | [73] | |
| Tanzania | Kibaha Coast Region | - | 0.01–170 | [66] | |
| p,p’-DDT | India | Hisar | - | ND–7 | [73] |
| Tanzania | Kibaha Coast Region | - | 0.003–3000 | [66] | |
| Tanzania | Kibaha Coast Region | - | 0.001–230 | [66] | |
| Tanzania | Kibaha Coast Region | - | 0.0001–95 | [66] | |
| o,p’-DDE | India | Hisar | - | ND–3 | [73] |
| Tanzania | Kibaha Coast Region | - | 0.0001–7 | [66] | |
| Hisar | - | ND–2 | [73] | ||
| o,p’-DDD | Tanzania | Kibaha Coast Region | - | ND–5 | [66] |
| ΣDDT | India | Hisar | - | ND–7.06 | [73] |
| Tanzania | Kibaha Coast Region | - | 0.01–3200 | [66] | |
| Aldrin | Tanzania | Kibaha Coast Region | - | ND–0.044 | [66] |
| Dieldrin | Tanzania | Kibaha Coast Region | - | ND–1.3 | [66] |
| Endrin | Tanzania | Kibaha Coast Region | - | ND–0.2 | [66] |
| α-Endosulfan | Tanzania | Kibaha Coast Region | - | ND–0.2 | [66] |
| β-Endosulfan | Tanzania | Kibaha Coast Region | - | ND–0.03 | [66] |
| Endosulfan sulphate | India | Hisar | - | ND–0.4 | [73] |
| ΣEndosulfan | India | Hisar | - | ND–3.02 | [73] |
| Heptachlor | India | Hisar | - | ND–0.02 | [73] |
| Tanzania | Kibaha Coast Region | - | ND–0.2 | [66] | |
| Methoxychlor | Tanzania | Kibaha Coast Region | - | ND–0.1 | [66] |
ND = not detected.
6. Conclusions and Future Prospects
Despite the OCP bans in many countries, OCPs and their metabolites are still present in the environment and they pose an actual problem. They have even been identified in Arctic areas. The incessant application in the past and the fraudulent application today have resulted in the pollution of water bodies.
Considering that OCPs and their metabolites are still found in water, and generally in the environment, efforts today are geared toward the development of extraction and preconcentration methods as a starting point before analysis. Many extraction protocols have been reviewed, from LLE or SPE to refined ones, such as VALLME, SPME, and MSPE. The literature available includes studies showing that GC-ECD is the most used technique for measuring the levels of OCPs and their metabolites, while GC-MS is used as a confirmatory technique.
Analysis of the literature has evidenced the presence of OCPs, mainly DDTs, HCHs, endosulfan, aldrin, dieldrin, endrin, and heptachlor, in surface water, groundwater, and rainwater. The locations where they are found at high levels are mostly Asian countries. According to our knowledge, India is the sole producer of DDT and, globally, is the largest consumer to prevent vector-borne diseases.
It is quite likely that scientists will continue to find new techniques and to improve existing ones to identify and quantify the levels of OCPs in different environments, especially because of their implications on human health (diabetes, different types of cancer, etc.) and because the presence of OCPs presence in the environment seems to be a continuous issue, despite monitoring programs and legal policies.
Author Contributions
Conceptualization, R.M.M. and G.V.S.; methodology, G.V.S.; software, M.A.S.; formal analysis, R.M.M. and G.V.S.; investigation, G.V.S.; data curation, R.M.M.; writing—original draft preparation, G.V.S. and M.A.S.; writing—review and editing—R.M.M. and G.V.S.; supervision—R.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACE | Activated carbon extraction |
| DDE | Dichlorodiphenyldichloroethylene |
| DDT | Dichlorodiphenyltrichloroethane |
| DLLME | Droplet liquid–liquid microextraction |
| DLLME-LSC | Dispersive liquid–liquid microextraction with little solvent consumption |
| DLLME-SFO | Dispersive liquid–liquid microextraction based on a solidification of floating organic drop |
| ECD | Electron capture detector |
| EI | Electron impact |
| GC | Gas chromatography |
| GC-MS | Gas chromatography–mass spectrometry |
| GC-QMS | Gas chromatography operating with quadrupole mass detector |
| HCB | Hexachlorobenzene |
| HCH | Hexachlorocyclohexane |
| HRGC-HRMS | High-resolution gas chromatography coupled with high-resolution mass spectrometry |
| HS-SPME | Headspace solid-phase microextraction |
| LLE | Liquid–liquid extraction |
| LLE-LTP | Liquid–liquid extraction with low-temperature partition |
| MDLs | Method detection limits |
| MSPE | Magnetic solid phase extraction |
| OCPs | Organochlorine pesticides |
| PCP | Pentachlorophenol |
| PeCB | Pentachlorobenzene |
| POPs | Persistent organic pollutants |
| SERS | Surface-enhanced Raman spectroscopy |
| SFC | Supercritical fluid chromatography |
| SME | Solvent microextraction |
| SPME | Solid-phase microextraction |
| SPE | Solid-phase extraction |
| TCmX | 2,4,5,6-tetrachloro-m-xylene |
| TLC | Thin-layer chromatography |
| VALLME | Vortex-assisted liquid–liquid microextraction |
| μECD | Micro-cell electron capture detector |
References
- Grung, M.; Lin, Y.; Zhang, H.; Steen, A.O.; Huang, J.; Zhang, G. Pesticide levels and environmental risk in aquatic environments in China—A review. Environ. Int. 2015, 81, 87–97. [Google Scholar] [CrossRef]
- Nayak, P.; Solanki, H. Pesticides and Indian agriculture—A review. Int. J. Res. Granthaalayah 2021, 9, 250–263. [Google Scholar] [CrossRef]
- Rusiecki, J.A.; McAdam, J.; Denic-Roberts, H.; Sjodin, A.; Davis, M.; Jones, R.; Hoang, T.D.; Ward, M.H.; Ma, S.; Zhang, Y. Organochlorine pesticides and risk of papillary thyroid cancer in U.S. military personnel: A nested case-control study. Environ. Health 2024, 23, 28. [Google Scholar] [CrossRef]
- Akoto, O.; Oppong-Otoo, J.; Osie-Fosu, P. Carcinogenic and non-carcinogenic risk of organochlorine pesticide residues in processed cereal-based complementary foods for infants and young children in Ghana. Chemosphere 2015, 132, 193–199. [Google Scholar] [CrossRef]
- Attaullah, M.; Yousuf, M.; Shaukat, S.; Anjum, S.I.; Ansari, M.J.; Buneri, I.D.; Tahir, M.; Amin, M.; Ahmad, N.; Khan, S.U. Serum organochlorine pesticides residues and risk cancer: A case-control study. Saudi J. Biol. Sci. 2018, 25, 1284–1290. [Google Scholar] [CrossRef]
- Guo, J.; Chen, W.; Wu, M.; Qu, C.; Sun, H.; Guo, J. Distribution, sources, and risk assessment of organochlorine pesticides in water from Beiluo River, Loess Plateau, China. Toxics 2023, 11, 496. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1021 of the European Parliament and of the Council of 20 June 2019 on Persistent Organic Pollutants. Available online: https://eur-lex.europa.eu/eli/reg/2019/1021/oj (accessed on 2 May 2024).
- Stockholm Convention. All POPs Listed in the Stockholm Convention. Available online: https://www.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx (accessed on 2 May 2024).
- The 12 Initial POPs under the Stockholm Convention. Available online: http://chm.pops.int/TheConvention/ThePOPs/The12InitialPOPs/tabid/296/Default.aspx (accessed on 2 May 2024).
- United Nations Environment Programme (UNEP). Why Do Persistent Organic Pollutants Matter? UNEP—UN Environment Programme. Available online: https://www.unep.org/topics/chemicals-and-pollution-action/pollution-and-health/persistent-organic-pollutants-pops/why (accessed on 2 May 2024).
- Bigot, M.; Curran, M.A.J.; Moy, A.D.; Muir, D.C.G.; Hawker, D.W.; Cropp, R.; Dachs, J.; Teixeira, C.F.; Nash Bengtson, S.M. Brief communication: Organochlorine pesticides in an archived firm core from Law Dome, East Antarctica. Cryosphere 2016, 10, 2533–2539. [Google Scholar]
- Directive 2008/105/EC of the European Parliament and the Council of 16 December 2008 on the Environmental Quality Standards in the Field of Water Policy. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/eli/dir/2008/105/oj (accessed on 2 May 2024).
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32020L2184 (accessed on 2 May 2024).
- Syed, J.H.; Malik, R.N.; Liu, D.; Xu, Y.; Wang, Y.; Li, J.; Zhang, G.; Jones, K.C. Organochlorine pesticides in air and soil and estimated air-soil exchange in Punjab, Pakistan. Sci. Total Environ. 2013, 444, 491–497. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, G.; Liu, Z. Organochlorine pesticides in surface water of Jiuxi Valley, China: Distribution, source analysis, and risk evaluation. J. Chem. 2020, 2020, 5101936. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Turgut, C.; Atatanir, L.; Cutright, T. Evaluation of pesticide contamination in Dilek National Park, Turkey. Environ. Monit. Assess. 2010, 170, 671–679. [Google Scholar] [CrossRef]
- PPDB: Pesticide Properties DataBase. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/1642.htm (accessed on 2 May 2024).
- ChemicalBook. Available online: https://www.chemicalbook.com/ (accessed on 2 May 2024).
- National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 2 May 2024).
- Stockholm Convention. Chemical Listed in Annex A. Available online: https://chm.pops.int/Implementation/Alternatives/AlternativestoPOPs/ChemicalslistedinAnnexA/tabid/5837/Default.aspx (accessed on 2 May 2024).
- Popek, E. Environmental Chemical Pollutants. In Sampling and Analysis of Environmental Chemical Pollutants, 2nd ed.; Popek, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 13–69. [Google Scholar] [CrossRef]
- EL-Saeid, M.H.; BaQais, A.; Alshabanat, M. Study of the Photocatalytic Degradation of Highly Abundant Pesticides in Agricultural Soils. Molecules 2022, 27, 634. [Google Scholar] [CrossRef]
- Raina, V.; Hauser, A.; Buser, H.R.; Rentsch, D.; Sharma, P.; Lal, R.; Holliger, C.; Poiger, T.; Müller, M.; Kohler, H.-P. Hydroxylated metabolites of β- and δ-hexachlorocyclohexane: Bacterial formation, stereochemical configuration, and occurrence in groundwater at a former production site. Environ. Sci. Technol. 2007, 41, 4292–4298. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, Z.; Pang, S.; Bhatt, P.; Chen, S. Insights into the biodegradation of lindane (γ-hexachlorocyclohexane) using a microbial system. Front. Microbiol. 2020, 11, 522. [Google Scholar] [CrossRef]
- Sparling, D. Organochlorine Pesticides. In Ecotoxicology Essentials. Environmental Contaminants and Their Biological Effects on Animals and Plants; Elsevier: Amsterdam, The Netherlands, 2016; pp. 69–107. [Google Scholar] [CrossRef]
- Wei, L.; Yang, Y.; Li, Q.; Wang, J. Composition, distribution, and risk assessment of organochlorine pesticides in drinking water sources in South China. Water Qual. Expo. Health 2015, 7, 8–97. [Google Scholar] [CrossRef]
- Wolfe, L.; Zepp, R.; Paris, D.; Baughman, G.; Hollis, R. Methoxychlor and DDT degradation in water: Rates and products. Environ. Sci. Technol. 1977, 11, 1077–1081. [Google Scholar] [CrossRef]
- Sibali, L.; Okonkwo, J.; Zvinowanda, C. Determination of DDT and metabolites in surface water and sediment using LLE, SPE, ACE and SE. Bull. Environ. Contam. Toxicol. 2009, 83, 885–891. [Google Scholar] [CrossRef]
- Zacharia, J. Degradation Pathways of Persistent Organic Pollutants (POPs) in the Environment. In Persistent Organic Pollutants; Donyinah, S.K., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Rathore, H.S. Methods of and problems in analyzing pesticide residues in the environment. In Handbook of Pesticides. Methods of Pesticide Residue Analysis; Nollet, L., Rathore, H., Eds.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2010; pp. 8–44. [Google Scholar]
- Lee, C.-C.; Liu, Y.-S.; Tseng, C.-H.; Chen, J.-Y.; Juang, F.-S.; Chang, Y.-Y.; Hsu, H.-C. Pesticide Residue Testing System for Fruits and Vegetables by Color Identification Technology. In Proceedings of the 2019 IEEE International Conference on Consumer Electronics—Taiwan (ICCE-TW), Yilan, Taiwan, 20–22 May 2019; pp. 1–2. [Google Scholar] [CrossRef]
- Nasiri, M.; Ahmadzadeh, H.; Amiri, A. Sample preparation and extraction methods for pesticides in aquatic environments: A review. TrAC 2020, 123, 115772. [Google Scholar] [CrossRef]
- Ohoro, C.R.; Wepener, V. Review of scientific literature on available methods of assessing organochlorine pesticides in the environment. Heliyon 2023, 9, e22142. [Google Scholar] [CrossRef]
- Campanale, C.; Massareli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The monitoring of pesticides in water matrices and the analytical criticalities: A review. TrAC 2021, 144, 116423. [Google Scholar] [CrossRef]
- Jan, R.; Shah, J.; Khawaja, M.; Gul, K. DDT residue in soil and water in and around abandoned DDT manufacturing factory. Environ. Monit. Assess. 2009, 155, 31–38. [Google Scholar] [CrossRef]
- Bai, Y.; Ruan, X.; van der Hoek, J.P. Residues of organochlorine pesticides (OCPs) in aquatic environment and risk assessment along Shaying River, China. Environ. Geochem. Health 2018, 40, 2525–2538. [Google Scholar] [CrossRef]
- Al Mahmud, M.N.U.; Khalil, F.; Rahman, M.; Mamun, M.I.R.; Shoeb, M.; El-Atu, A.M.A.; Park, J.-H.; Shin, H.-C.; Nahar, N.; Shim, J.-H. Analysis of DDT and its metabolites in soil and water samples obtained in the vicinity of a closed-down factory in Bangladesh using various extraction methods. Environ. Monit. Assess. 2015, 187, 743. [Google Scholar] [CrossRef]
- Fatoki, O.S.; Awofolu, R.O. Methods for selective determination of persistent organochlorine pesticide residues in water and sediments by capillary gas chromatography and electron-capture detection. J. Chromatogr. A 2003, 983, 225–236. [Google Scholar] [CrossRef]
- De Jager, L.; Andrews, A. Development of a rapid screening technique for organochlorine pesticides using solvent microextraction (SME) and fast gas chromatography. Analyst 2000, 125, 1943–1948. [Google Scholar] [CrossRef]
- Mesquita, T.; Santos, R.; Cacique, A.; De Sa, L.; Silveiro, F.; Pinho, G. Easy and fast extraction methods to determine organochlorine pesticides in sewage sludge, soil, and water samples based at low temperature. J. Environ. Sci. Health B 2018, 53, 199–206. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Hosseini, M.-R.M.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Jorfi, S.; Poormohammadi, A.; Maraghi, E.; Almasi, H. Monitoring and health risk assessment of organochlorine pesticides in Karun River and drinking water Ahvaz city, South West of Iran. Toxin Rev. 2022, 41, 361–369. [Google Scholar] [CrossRef]
- Cortada, C.; Vidal, L.; Pastor, R.; Santiago, N.; Canals, A. Determination of organochlorine pesticides in water samples by dispersive liquid-liquid microextraction coupled to gas chromatography-mass spectrometry. Anal. Chim. Acta 2009, 649, 218–221. [Google Scholar] [CrossRef]
- Faraji, H.; Helalizadeh, M. Determination of organochlorine pesticides in river water using dispersive liquid-liquid microextraction and gas chromatography-electron capture detection. Intern. J. Environ. Anal. Chem. 2010, 90, 869–879. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Huang, S.-D. Dispersive liquid-liquid microextraction with little solvent consumption combined with gas chromatography-mass spectrometry for the pre-treatment of organochlorine pesticides in aqueous samples. J. Chromatogr. A 2009, 1216, 5171–5175. [Google Scholar] [CrossRef]
- Ozcan, S. Viable and rapid determination of organochlorine pesticides in water. Clean-Soil Air Water 2010, 38, 457–465. [Google Scholar] [CrossRef]
- Gao, J.; Liu, L.; Liu, X.; Lu, J.; Zhou, H.; Huang, S. Occurrence and distribution of organochlorine pesticides—Lindane, p,p’-DDT and heptachlor epoxide—In surface water of China. Environ. Int. 2008, 34, 1097–1103. [Google Scholar] [CrossRef]
- Badach, H.; Nazimek, T.; Kaminski, R.; Turski, W. Organochlorine pesticides concentration in the drinking water from regions of extensive agriculture in Poland. Ann. Agric. Environ. Med. 2000, 7, 25–28. [Google Scholar]
- Miliadis, G.E. Determination of pesticide residues in natural waters of Greece by solid phase extraction and gas chromatography. Bull. Environ. Contam. Toxicol. 1994, 52, 25–30. [Google Scholar] [CrossRef]
- Ruiz-Gil, L.; Romero-Gonzalez, R.; Garrido-Frenich, A.; Martinez Vidal, J. Determination of pesticides in water samples by solid phase extraction and gas chromatography tandem mass spectrometry. J. Sep. Sci. 2008, 31, 151–161. [Google Scholar] [CrossRef]
- Wu, C.; Luo, Y.; Gui, T.; Huang, Y. Concentrations and potential health hazards of organochlorine pesticides in shallow groundwater of Taihu Lake region, China. Sci. Total Environ. 2014, 470–471, 1047–1055. [Google Scholar] [CrossRef]
- Chen, C.; Li, T.; Zou, W.; Chen, S.; Zhang, K.; Ma, L. Spatial distribution and sources of organochlorine pesticides in surface waters of Shanghai, China. SN Appl. Sci. 2020, 2, 1739. [Google Scholar] [CrossRef]
- Golfinopoulos, S.; Nikolaou, A.; Kostopoulou, M.; Xilourgidis, N.; Vagi, M.; Lekkas, D. Organochlorine pesticides in the surface waters of Northern Greece. Chemosphere 2003, 50, 507–516. [Google Scholar] [CrossRef]
- Ozcan, S.; Tor, A.; Aydin, M.E. Application of magnetic nanoparticles to residue analysis of organochlorine pesticides in water samples by GC/MS. J. AOAC Int. 2012, 95, 1343–1349. [Google Scholar] [CrossRef]
- Jackson, G.; Andrews, R.J. New fast screening method for organochlorine pesticides in water by using solid-phase microextraction with fast gas chromatography and a pulsed-discharge electron capture detector. Analyst 1998, 123, 1085–1090. [Google Scholar] [CrossRef]
- Mendes, L.D.; Bernardi, G.; Elias, W.C.; de Oliveira, D.; Domingos, J.B.; Carasek, E. A green approach to DDT degradation and metabolite monitoring in water comparing the hydrodechlorimation efficiency of Pd, Au-on-Pd and Cu-on-Pd nanoparticle catalysis. Sci. Total Environ. 2021, 760, 143403. [Google Scholar] [CrossRef]
- Miclean, M.; Senila, L.; Cadar, O.; Roman, M.; Kovacs, M.H. Determination of organochlorine pesticides in Aries River water near industrial dump using SPE/HS-SPME/GC-ECD method. Agric.-Sci. Pract. 2015, 1–2, 123–127. [Google Scholar]
- Nodeh, H.R.; Ibrahim, W.A.W.; Kamboh, M.A.; Sanagi, M.M. Dispersive graphene-based silica coated magnetic nanoparticles as a new adsorbent for preconcentration of chlorination pesticides from environmental water. RSC Adv. 2015, 5, 76424–76434. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, Y.; Sun, Y.; Sheng, X.; Tong, Y.; Guo, J.; Zhou, B.; Zhao, J. Magnetic polyamidoamine dendrimers for magnetic separation and sensitive determination of organochlorine pesticides from water samples by high-performance liquid chromatography. J. Environ. Sci. 2021, 102, 64–73. [Google Scholar] [CrossRef]
- Barlas, N.; Çok, I.; Akbulut, N. The contamination levels of organochlorine pesticides in water and sediment samples in Uluabat Lake, Turkey. Environ. Monit. Assess. 2006, 118, 383–391. [Google Scholar] [CrossRef]
- Erkmen, B.; Kolankaya, D. Determination of organochlorine pesticide residues in water, sediment, and fish samples from the Meriç Delta, Turkey. Int. J. Environ. Anal. Chem. 2006, 86, 161–169. [Google Scholar] [CrossRef]
- Turgut, C. The contamination with organochlorine pesticides and heavy metals in surface water in Küçük Menderes River in Turkey, 2000–2002. Environ. Int. 2003, 29, 29–32. [Google Scholar] [CrossRef]
- El-Alfy, M.; Hasballah, A.; El-Hamid, H.T.A.; El-Zeiny, A.M. Toxicity assessment of heavy metals and organochlorine pesticides in freshwater and marine environments, Rosetta area, Egypt using multiple approaches. Sustain. Environ. Res. 2019, 29, 19. [Google Scholar] [CrossRef]
- Fosu-Mensah, B.; Okoffo, E.; Darko, G.; Gordon, C. Assessment of organochlorine pesticide residues in soils and drinking water sources from cocoa farms in Ghana. SpringerPlus 2016, 5, 869. [Google Scholar] [CrossRef]
- Mahugija, J.A.M.; Henkelmann, B.; Schramm, K.-W. Levels and patterns of organochlorine pesticides and their degradation products in rainwater in Kibaha Coast Region, Tanzania. Chemosphere 2015, 118, 12–19. [Google Scholar]
- Navarrete, I.; Tee, K.A.; Unson, J.R.; Hallare, A. Organochlorine pesticide residues in surface water and groundwater along Pampanga River, Philippines. Environ. Monit. Assess. 2018, 190, 289. [Google Scholar] [CrossRef]
- Roman, C.; Miclean, M.; Roman, M.; Senila, L.; Cadar, O.; Levei, E. Pollution indices for assessment of organochlorine pesticides contamination in Danube water and sediments, Calafat-Turnu Magurele sector, Romania. Stud. UBB Ambient. 2014, LXI, 129–137. [Google Scholar]
- Sandu, M.A.; Madjar, R.M.; Preda, M.; Vîrsta, A.; Stavrescu-Bedivan, M.-M.; Vasile Scăețeanu, G. Assessment of water quality and parasitofauna, and a biometric analysis of the prussian carp of the Romanian lentic ecosystem in Moara Domnească, Ilfov County. Water 2023, 15, 3978. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Sharma, A.K.; Sanghi, R. Organochlorine and organophosphorus pesticide residues in ground water and surface waters of Kanpur, Uttar Pradesh, India. Environ. Int. 2005, 31, 113–120. [Google Scholar] [CrossRef]
- Tyagi, S.; Siddarth, M.; Mishra, B.K.; Banerjee, B.D.; Urfi, A.J.; Madhu, S.V. High levels of organochlorine pesticides in drinking water as a risk factor for type 2 diabetes: A study in north India. Environ. Pollut. 2021, 271, 116287. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Xiong, S.; Zeng, F.; Bu, J.; Zhang, B.; Liu, W.; Zhou, H.; Qi, S.; Xu, L.; et al. Rapid transport of organochlorine pesticides (OCPs) in multimedia environment from karst area. Sci. Total Environ. 2021, 775, 145698. [Google Scholar] [CrossRef]
- Kumari, B.; Madan, V.K.; Kathpal, T.S. Pesticide residues in rain water from Hisar, India. Environ. Monit. Assess. 2007, 133, 467–471. [Google Scholar] [CrossRef]
- Kuranchie-Mensah, H.; Atiemo, M.S.; Palm, L.M.N.-D.; Blankson-Arthur, S.; Tutum, A.O.; Fosu, P. Determination of organochlorine pesticide residue in sediment and water from Densu river basin, Ghana. Chemosphere 2012, 86, 286–292. [Google Scholar] [CrossRef]
- De Figueiredo, L.; Chiavelli, L.; Da Costa, W. Determination of concentration levels of organochlorine pesticides in water from Mandacaru Stream in Maringa-Parana-Brazil employing gas-chromatography-mass spectrometry. Anal. Lett. 2013, 46, 1597–1606. [Google Scholar] [CrossRef]
- Geyikçi, F.; Büyükgüngör, H. Monitoring of organochlorine pesticides in the surface waters from Mid-Black Sea region, Turkey. Environ. Monit. Assess. 2011, 173, 127–137. [Google Scholar] [CrossRef]
- Shukla, G.; Kumar, A.; Bhanti, M.; Joseph, P.E.; Taneja, A. Organochlorine pesticide contamination of ground water in the city of Hyderabad. Environ. Int. 2006, 32, 244–247. [Google Scholar] [CrossRef]
- Wang, D.; Yang, S.; Wang, G.; Gao, L.; Wang, Y.; Jiang, Q.; Chen, Y. Residues and distributions of organochlorine pesticides in China’s Weihe River. Pol. J. Environ. Stud. 2016, 25, 1285–1292. [Google Scholar] [CrossRef]
- Zhou, R.; Zhu, L.; Yang, K.; Chen, Y. Distribution of organochlorine pesticides in surface water and sediments from Qiantang River, East China. J. Hazard. Mat. A 2006, 137, 68–75. [Google Scholar] [CrossRef]
- Miliadis, G.E. Organochlorine and organophosphorus pesticide residues in the water of the Pinios River, Greece. Bull. Environ. Contam. Toxicol. 1995, 54, 837–840. [Google Scholar] [CrossRef]
- Cai, S.R.; Sun, K.; Dong, S.Y.; Wang, Y.M.; Wang, S.; Jia, L. Assessment of organochlorine pesticide residues in water, sediment, and fish of the Songhua River, China. Environ. Forensics 2014, 15, 352–357. [Google Scholar] [CrossRef]
- Concha-Grana, E.; Turnes-Carou, M.I.; Muniategui-Lorenzo, S.; Lopez-Mahia, P.; Prada-Rodriguez, D.; Fernandez-Fernandez, E. Evaluation of HCH isomers and metabolites in soils, leachates, river water and sediments of a highly contaminated area. Chemosphere 2006, 64, 588–595. [Google Scholar] [CrossRef]
- Mikherjee, I.; Gopal, M. Chromatographic techniques in the analysis of organochlorine pesticide residues. J. Chromatogr. A 1996, 754, 33–42. [Google Scholar] [CrossRef]
- Mirzaei, M.; Rakh, M. Preconcentration of organochlorine pesticides in aqueous samples by dispersive liquid-liquid microextraction based on solidification of floating organic drop after SPE with multiwalled carbon nanotubes. J. Sep. Sci. 2014, 37, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Mirmigkou, S.; de Boer, J. DDT and metabolites. In Dioxin and Related Compounds. The Handbook of Environmental Chemistry; Alaee, M., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 49, pp. 355–378. [Google Scholar] [CrossRef]
- El-Gawad, H.A. Validation method of organochlorine pesticides residues in water using gas chromatography-quadruple mass. Water Sci. 2016, 30, 96–107. [Google Scholar] [CrossRef]
- Ali, I.; Singh, P.; Rawat, M.S.M.; Badoni, A. Analysis of organochlorine pesticides in the Hindon River water, India. J. Environ. Prot. Sci. 2008, 2, 47–53. [Google Scholar]
- Alnahdi, H.; Mousa, R.M.A.; El-Said, W. Development of organochlorine pesticide electrochemical sensor based on Fe3O4 nanoparticles@indium tin oxide electrode. Electroanalysis 2022, 35, 2202100659. [Google Scholar] [CrossRef]
- Goel, P.; Arora, M. Fabrication of chemical sensor for organochlorine pesticide detection using colloidal gold nanoparticles. MRS Commun. 2018, 8, 1000–1007. [Google Scholar] [CrossRef]
- DiScenza, D.; Lynch, J.; Miller, J.; Verderame, M.; Levine, M. Detection of organochlorine pesticides in contaminated marine environments via cyclodextrin-promoted fluorescence modulation. ACS Omega 2017, 2, 8591–8599. [Google Scholar] [CrossRef]
- Moldovan, R.; Iacob, B.-C.; Farcău, C.; Bodoki, E.; Oprean, R. Strategies for SERS detection of organochlorine pesticides. Nanomaterials 2021, 11, 304. [Google Scholar] [CrossRef]
- Li, C.; Qi, X.; Wang, Y.; Meng, Q.; Li, W.; Liu, L.; Zheng, Y.; Cui, H. Organochlorine pesticides in soil–groundwater–plant system in a famous agricultural production area in China: Spatial distribution, source identification and migration prediction. Water 2023, 15, 4147. [Google Scholar] [CrossRef]
- Noori, J.S.; Mortensen, J.; Geto, A. Rapid and sensitive quantification of the pesticide lindane by polymer modified electrochemical sensor. Sensors 2021, 21, 393. [Google Scholar] [CrossRef] [PubMed]
- Tzanetou, E.N.; Karasali, H. A comprehensive review of organochlorine pPesticide monitoring in agricultural soils: The silent threat of a conventional agricultural past. Agriculture 2022, 12, 728. [Google Scholar] [CrossRef]
- Chen, W.; Zeng, F.; Liu, W.; Bu, J.; Hu, G.; Xie, S.; Yao, H.; Zhou, H.; Qi, S.; Huang, H. Organochlorine Pesticides in Karst Soil: Levels, Distribution, and Source Diagnosis. Int. J. Environ. Res. Public Health 2021, 18, 11589. [Google Scholar] [CrossRef]
- Pănescu, V.-A.; Bocoș-Bințințan, V.; Herghelegiu, M.-C.; Coman, R.-T.; Berg, V.; Lyche, J.L.; Beldean-Galea, M.S. Pollution Assessment with Persistent Organic Pollutants in Upper Soil of a Series of Rural Roma Communities in Transylvania, Romania, Its Sources Apportionment, and the Associated Risk on Human Health. Sustainability 2024, 16, 232. [Google Scholar] [CrossRef]
- Galani, Y.J.H.; Houbraken, M.; Wumbei, A.; Djeugap, J.F.; Fotio, D.; Gong, Y.Y.; Spanoghe, P. Contamination of foods from Cameroon with residues of 20 halogenated pesticides, and health risk of adult human dietary exposure. Int. J. Environ. Res. Public Health 2021, 18, 5043. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Cui, Z.; Wang, Y.; Zhang, J. Characteristics and residual health risk of organochlorine pesticides in fresh Vegetables in the suburb of Changchun, Northeast China. Int. J. Environ. Res. Public Health 2022, 19, 12547. [Google Scholar] [CrossRef] [PubMed]
- Kartalović, B.; Mastanjević, K.; Novakov, N.; Vranešević, J.; LjubojevićPelić, D.; Puljić, L.; Habschied, K. Organochlorine Pesticides and PCBs in Traditionally and Industrially Smoked Pork Meat Products from Bosnia and Herzegovina. Foods 2020, 9, 97. [Google Scholar] [CrossRef]
- Đokić, M.; Nekić, T.; Varenina, I.; Varga, I.; SolomunKolanović, B.; Sedak, M.; Čalopek, B.; Kmetič, I.; Murati, T.; Vratarić, D. Distribution of pesticides and polychlorinated biphenyls in food of animal origin in Croatia. Foods 2024, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Đokić, M.; Nekić, T.; Varenina, I.; Varga, I.; SolomunKolanović, B.; Sedak, M.; Čalopek, B.; Vratarić, D.; Bilandžić, N. Pesticides and polychlorinated biphenyls in milk and dairy products in Croatia: A health risk assessment. Foods 2024, 13, 1155. [Google Scholar] [CrossRef] [PubMed]
- Quiralte, D.; Zarzo, I.; Fernandez-Zamudio, M.-A.; Barco, H.; Soriano, J.M. Urban Honey: A review of its physical, chemical, and biological parameters that connect it to the environment. Sustainability 2023, 15, 2764. [Google Scholar] [CrossRef]
- Ferencz, L.; Balog, A. A pesticide survey in soil, water and foodstuffs from Central Romania. Carpathian J. Earth Environ. Sci. 2010, 5, 111–118. [Google Scholar]
- Jin, X.; Liu, Y.; Qiao, X.; Guo, R.; Liu, C.; Wang, X.; Zhao, X. Risk assessment of organochlorine pesticides in drinking water source of the Yangtze River. Ecotoxicol. Environ. Saf. 2019, 182, 109390. [Google Scholar] [CrossRef]
- Malik, A.; Singh, V.; Singh, K. Occurrence and distribution of persistent trace organics in rainwater in an urban region (India). Bull. Environ. Contam. Toxicol. 2007, 7, 639–645. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).