Sustainable Process to Recover Metals from Waste PCBs Using Physical Pre-Treatment and Hydrometallurgical Techniques
Abstract
1. Introduction
2. Materials and Methodology
2.1. Raw Material and Chemical Reagents
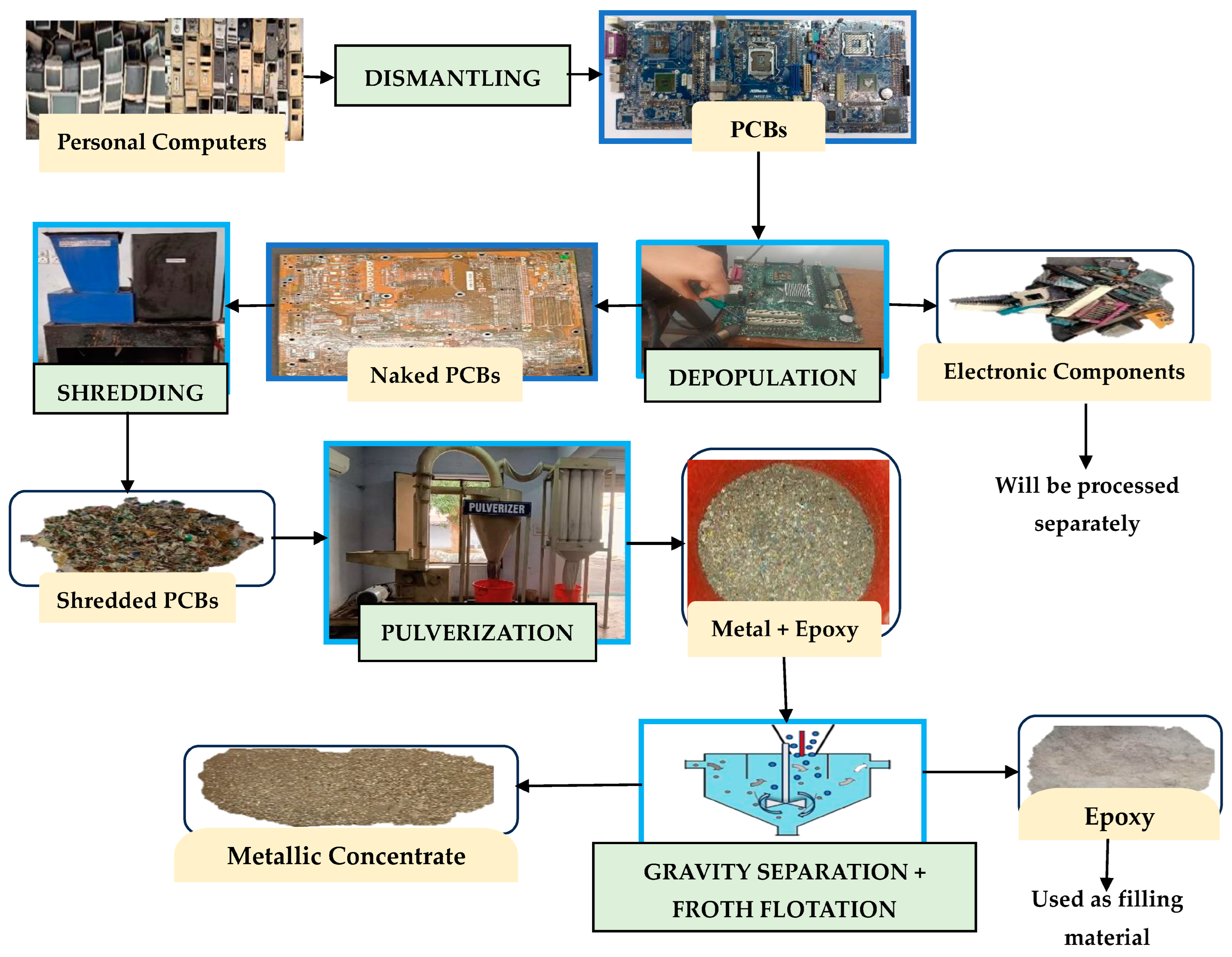
2.2. Pre-Treatment Processes
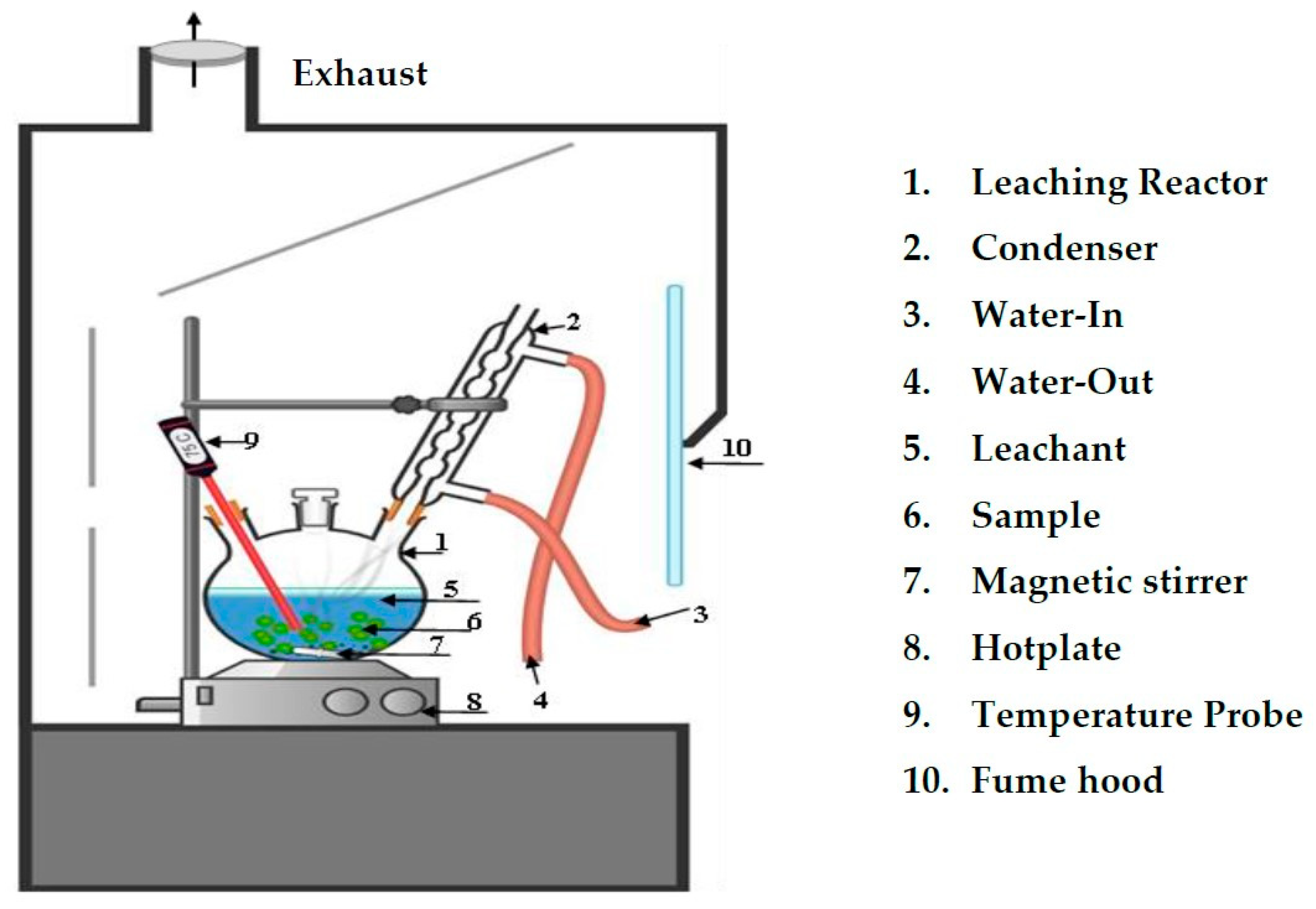
2.3. Methodology
2.4. Analytical Procedure
3. Results and Discussion
3.1. Leaching Studies
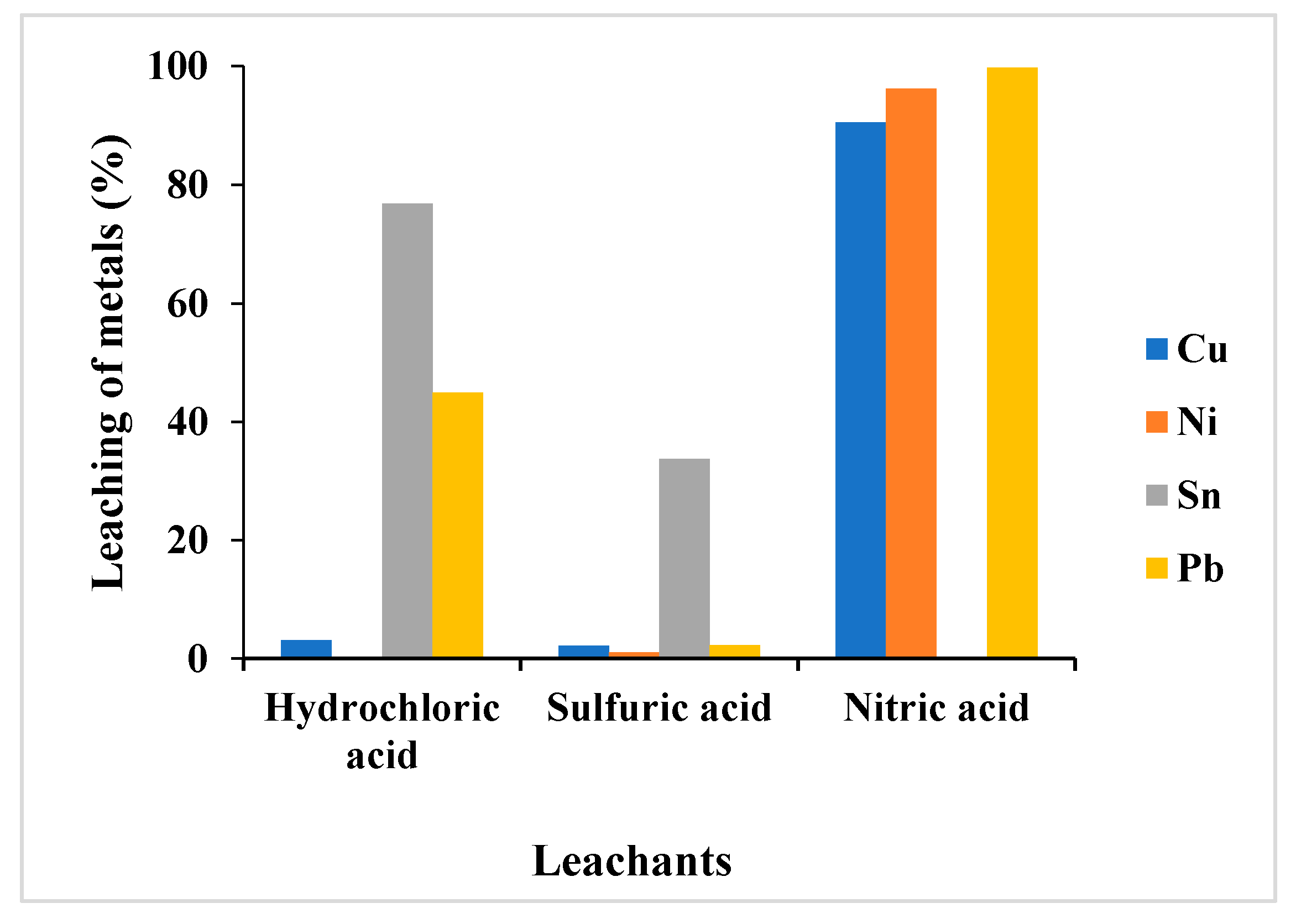
3.1.1. Selection of Leachant
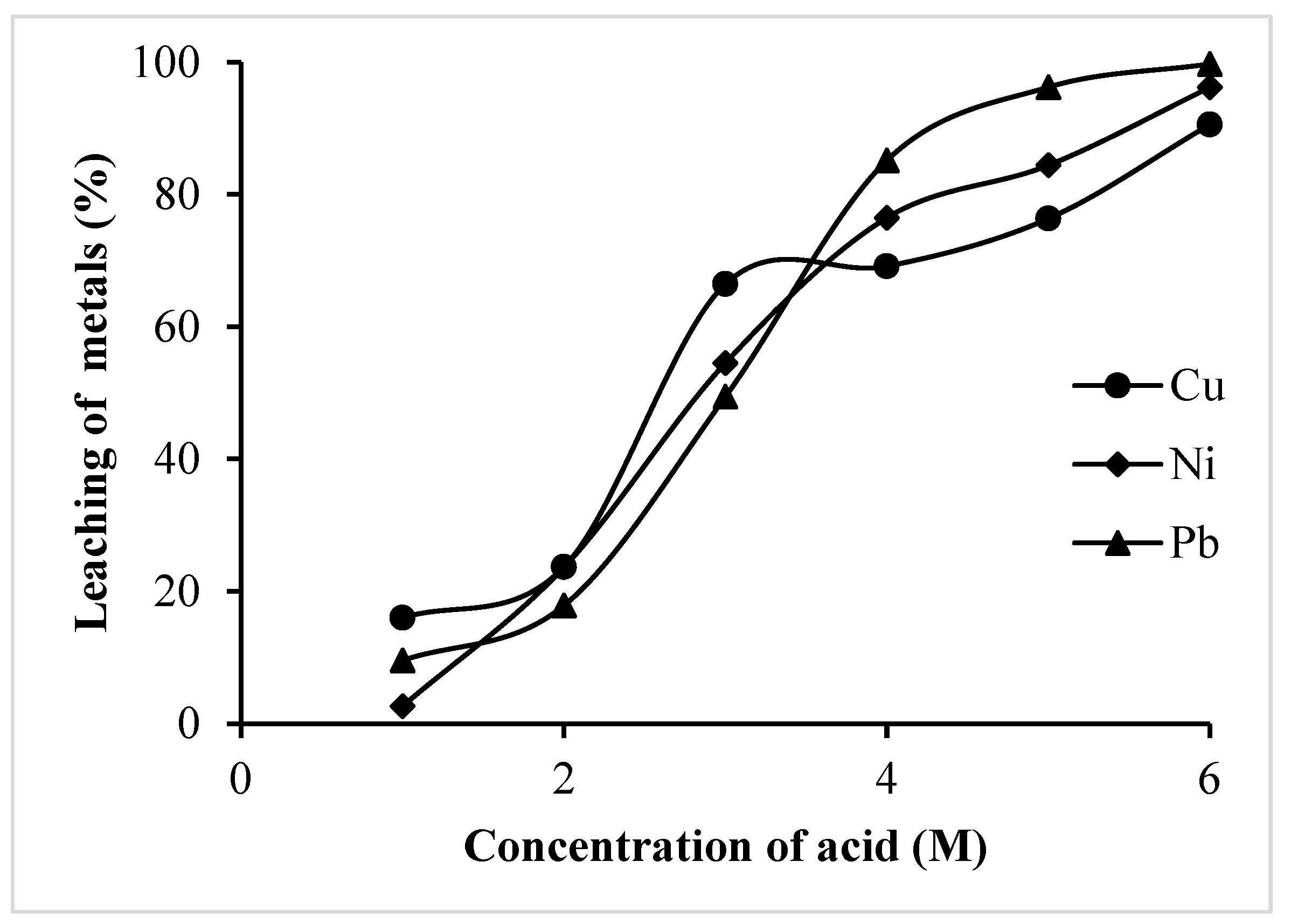
3.1.2. Effect of Leachant Concentration
3.1.3. Effect of Temperature
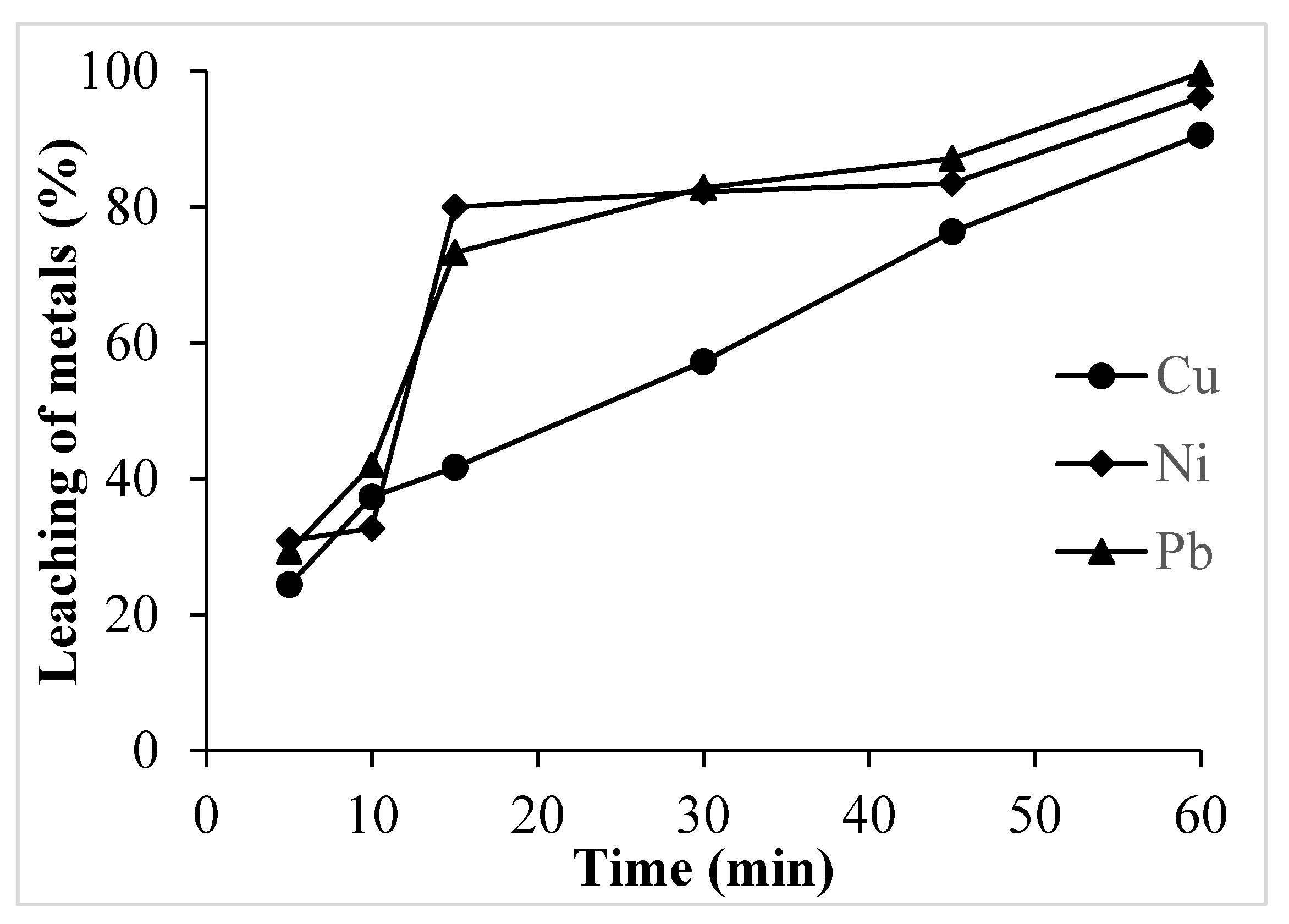
3.1.4. Effect of Time
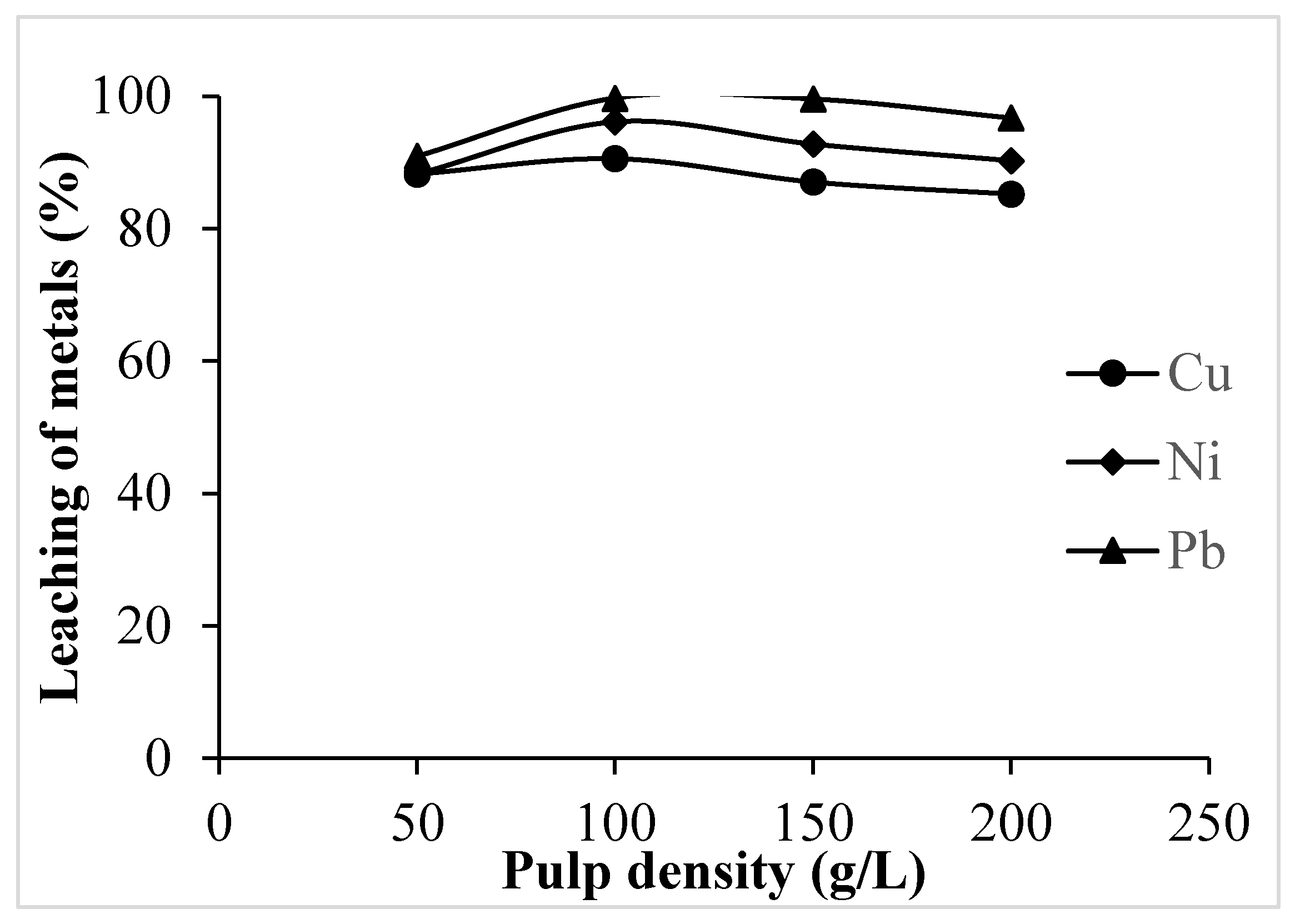
3.1.5. Effect of Pulp Density
3.2. Characterization Studies
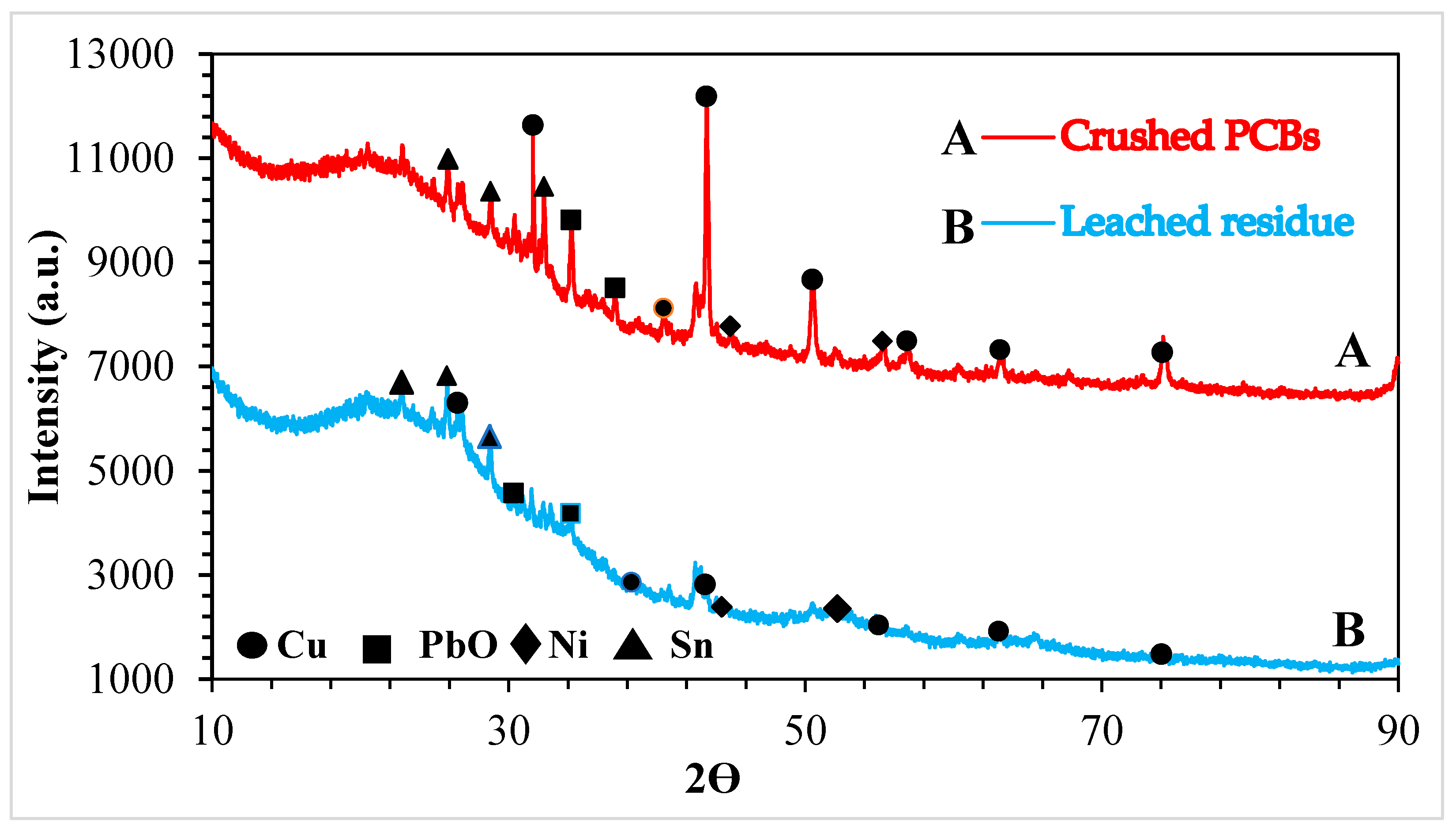
3.2.1. X-ray Powder Diffraction (XRD)
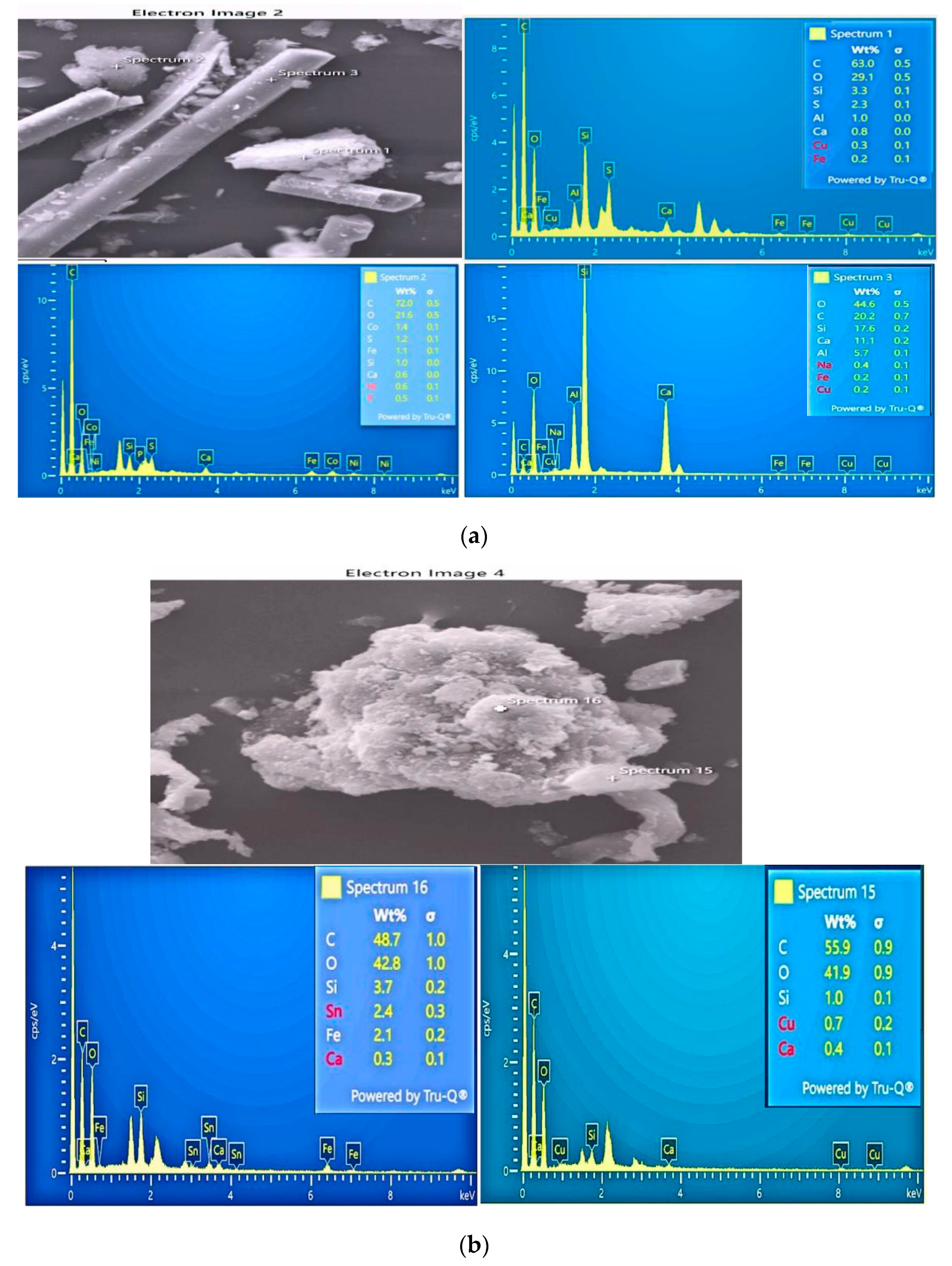
3.2.2. Scanning Electron Microscopy (SEM-EDS)
3.3. Scientific Validation of Leaching
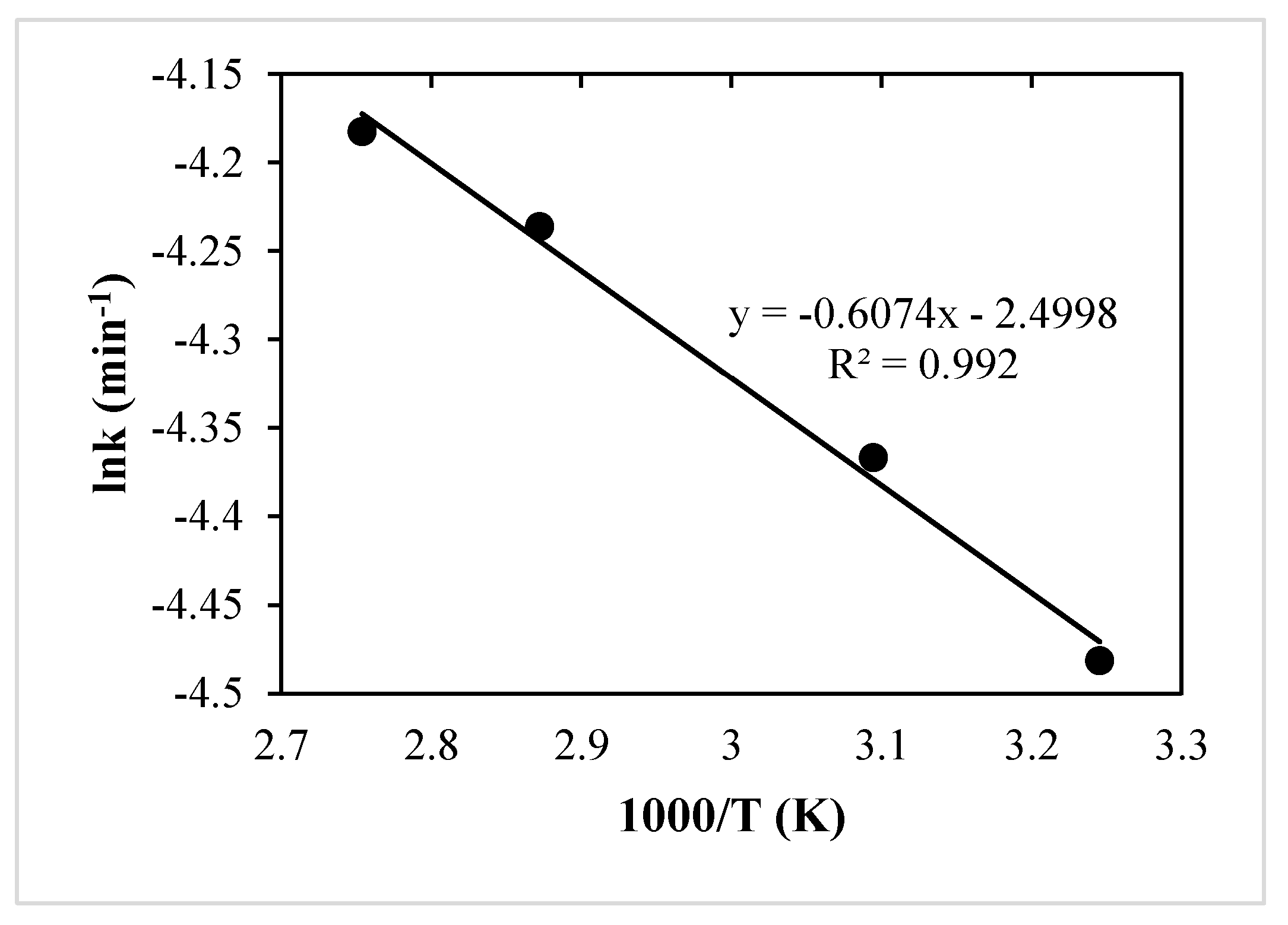
3.4. Arrhenius Equation
4. Conclusions
- Metal liberation was found to be ~99.90%; however, with the removal of epoxy resins or plastics, metal liberation reached 99.99%.
- The optimum conditions for the recovery or dissolution of 90.58% Cu, 96.19% Ni, and 99.72% Pb were a nitric acid concentration of 6 M, a temperature of 75 °C, a pulp density of 100 g/L, and a time of 60 min. The higher concentration of HNO3 and elevated temperature were chosen to achieve optimal leaching efficiency within a reasonable timeframe during the present research.
- The film diffusion control dense constant-size small particle (all geometries) model (XB = kc.t) fit well with the leaching kinetics for Cu.
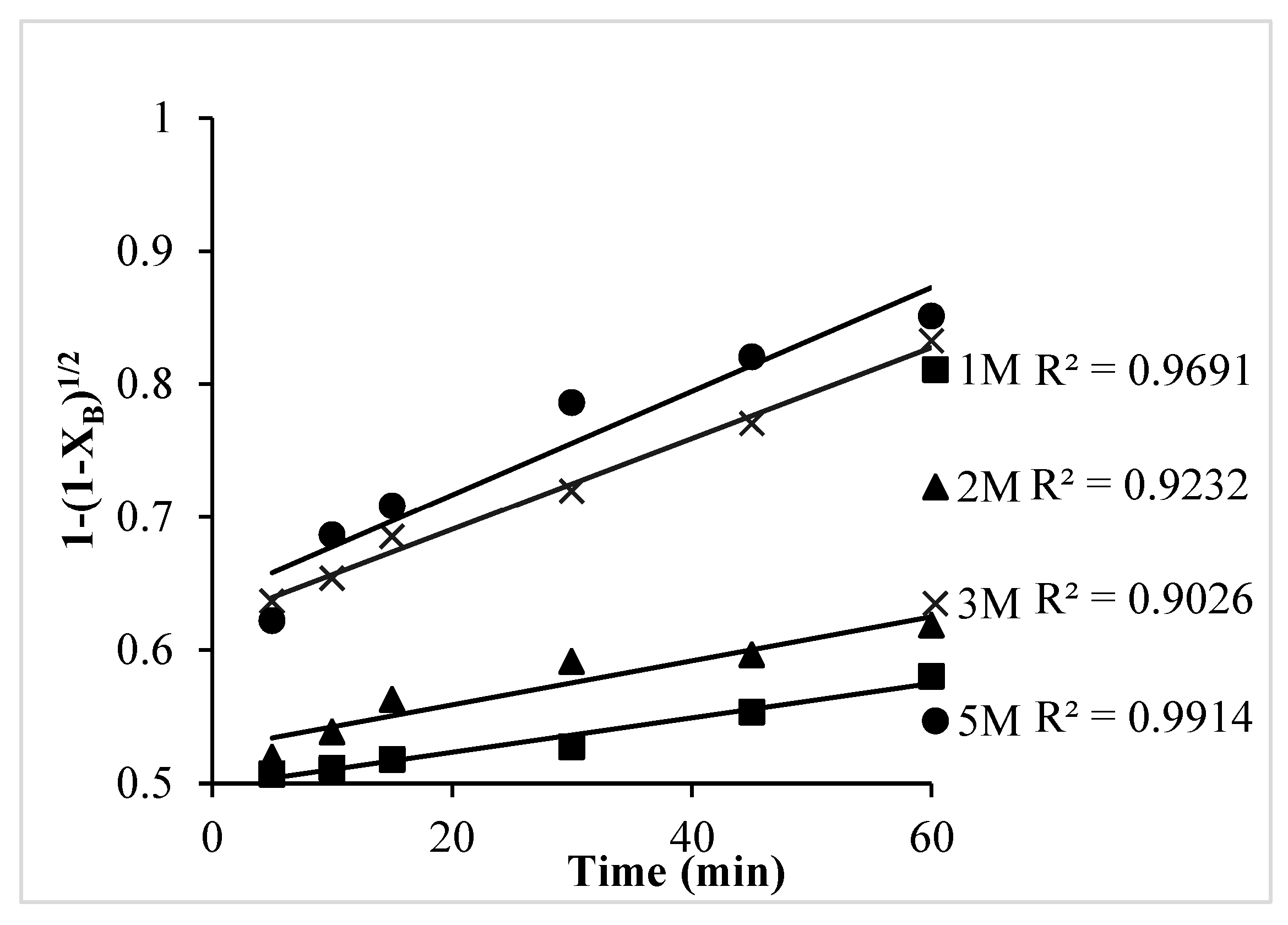
- The chemical reaction control dense constant-size model (1 − (1 − XB)1/2) fit well with the leaching kinetics for Ni.
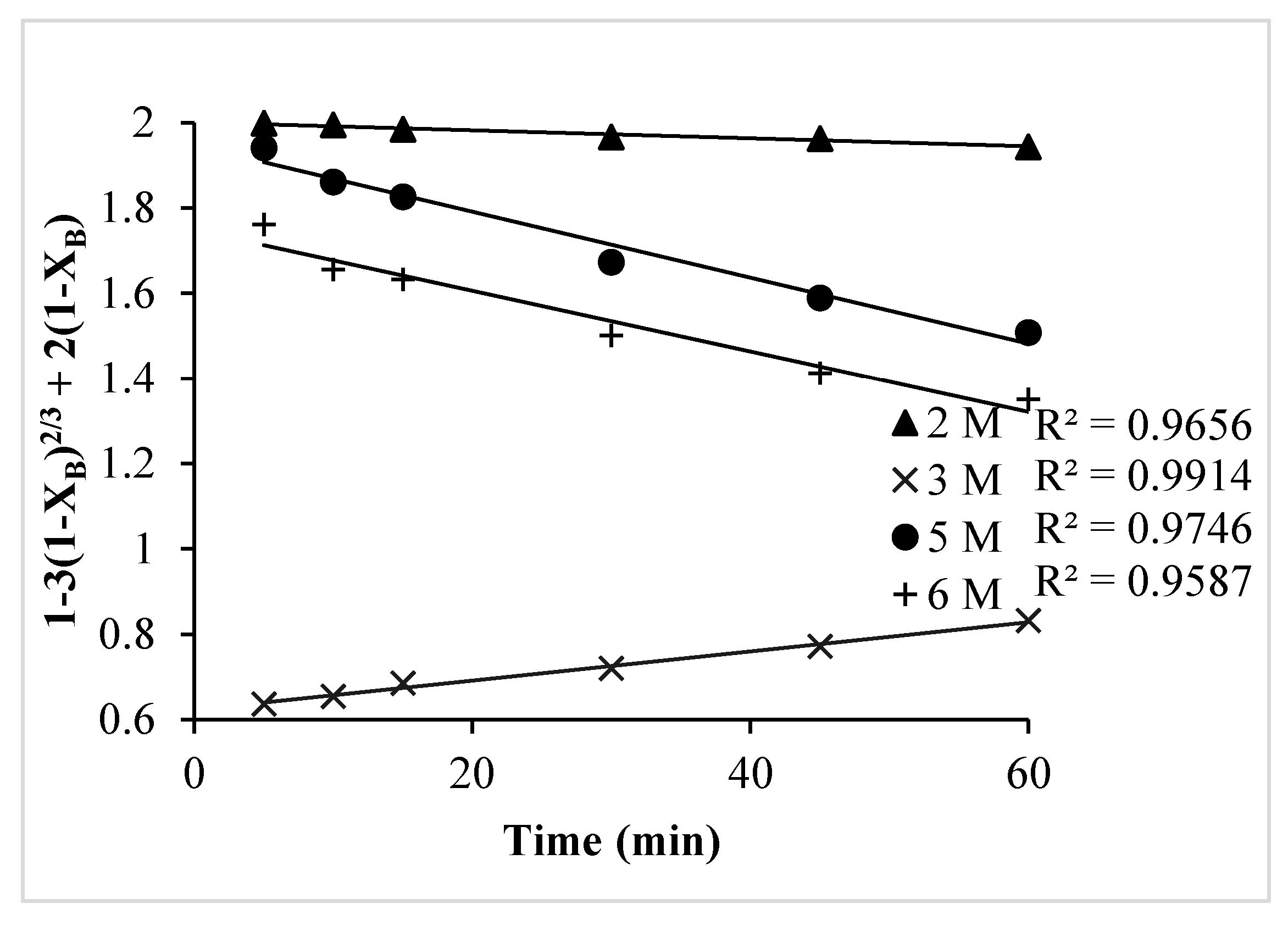
- The ash diffusion control dense constant-size model (1 − 3(1 − XB)2/3 + 2(1 − XB)) fit well with the leaching kinetics for Pb.
- The activation energy for Cu was 19.42 kJ/mol.
- Nitric leaching in a closed-loop system can result in high metal recovery rates and a larger percentage of the target metal could be leached without emitting noxious gases. As nitric acid is a strong oxidizing agent, it facilitates faster metal dissolution. Efficiency is crucial for economic and environmental reasons, as it reduces waste and increases the overall yield of valuable metals by considering implementation and mitigation measures during the leaching process.
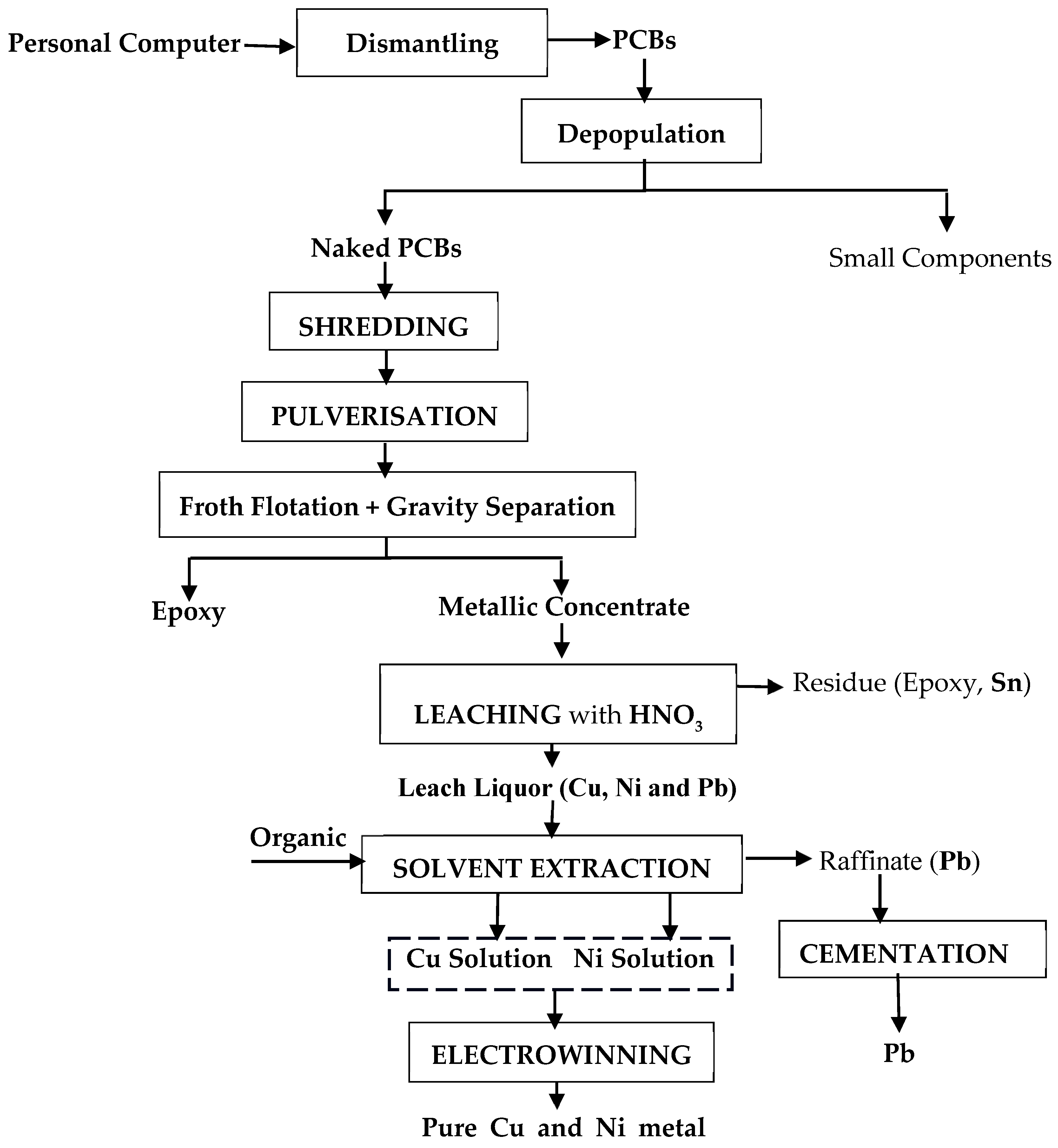
- The generated leach liquid could be further processed using solvent extraction, cementation, and electrowinning processes to obtain purified metal products. The flow diagram of this process is shown in Figure 14.
- The process is viable and eco-friendly at the laboratory scale and has potential for commercial exploitation. The leached residues and effluents produced during the experiment will be properly treated and can be reused using standard procedures before their final disposal into the environment.
- Complete recycling will not only reduce the loss of valuable metals, but will also aid in the establishment of an organized sector for e-waste recycling, taking into account environmental regulations and raising public awareness about the loss of valuable metals caused by the dumping of such waste into the environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gande, V.V.; Vats, S.; Bhatt, N.; Pushpavanam, S. Sequential recovery of metals from waste printed circuit boards using a zero-discharge hydrometallurgical process. Clean. Eng. Technol. 2021, 4, 100143. [Google Scholar] [CrossRef]
- Ahirwar, R.; Tripathi, A.K. E-waste management: A review of recycling process, environmental and occupational health hazards, and potential solutions. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100409. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Y.; Wu, Y.; Guo, F. Metal recovery from waste printed circuit boards: A review for current status and perspectives. Resour. Conserv. Recycl. 2020, 157, 104787. [Google Scholar] [CrossRef]
- Chancerel, P.; Rotter, S. Recycling-oriented characterization of small waste electrical and electronic equipment. Waste Manag. 2009, 29, 2336–2352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, J.; Gao, P.; Ding, Y. A review on hydrometallurgical recovery of precious and base metals from waste printed circuit boards. J. Hazard. Mater. 2020, 400, 123051. [Google Scholar]
- Rocchetti, L.; Amato, A.; Beolchini, F. Printed circuit board recycling: A patent review. J. Clean. Prod. 2018, 178, 814–832. [Google Scholar] [CrossRef]
- Udayakumar, S.; Razak, M.I.B.A.; Ismail, S. Recovering valuable metals from Waste Printed Circuit Boards (WPCB): A short review. Mater. Today Proc. 2022, 66, 3062–3070. [Google Scholar] [CrossRef]
- Le, H.L.; Jeong, J.; Lee, J.C.; Pandey, B.D.; Yoo, J.-M.; Huyunh, T.H. Hydrometallurgical Process for Copper Recovery from Waste Printed Circuit Boards (PCBs). Miner. Process. Extr. Met. Rev. 2011, 32, 90–104. [Google Scholar] [CrossRef]
- Cui, H.; Anderson, C.G. Literature Review of Hydrometallurgical Recycling of Printed Circuit Boards (PCBs). J. Adv. Chem. Eng. 2016, 6, 142. [Google Scholar] [CrossRef]
- Barragan, J.A.; Ponce de León, C.; Alemán Castro, J.R.; Peregrina-Lucano, A.; Gómez-Zamudio, F.; Larios-Durán, E.R. Copper and Antimony Recovery from Electronic Waste by Hydrometallurgical and Electrochemical Techniques. ACS Omega 2020, 5, 12355–12363. [Google Scholar] [CrossRef]
- Elbashier, E.; Mussa, A.; Hafiz, M.; Hawari, A.H. Recovery of rare earth elements from waste streams using membrane processes: An overview. Hydrometallurgy 2021, 204, 105706. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Mayyas, M.; Maroufi, S.; Sahajwalla, V. Direct transformation of waste printed circuit boards into high surface area t-SnO2 for photocatalytic dye degradation. J. Environ. Chem. Eng. 2019, 7, 103133. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, S.; Xiao, K.; Hu, J.; Hou, H.; Liu, B.; Yang, J. A cost-effective strategy for metal recovery from waste printed circuit boards via crushing pretreatment combined with pyrolysis: Effects of particle size and pyrolysis temperature. J. Clean. Prod. 2021, 280, 124505. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Tuncuk, A.; Panda, S. Intensified acidophilic bioleaching of multi-metals from waste printed circuit boards (WPCBs) of spent mobile phones. J. Chem. Technol. Biotechnol. 2020, 95, 2272–2285. [Google Scholar] [CrossRef]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Passadoro, M.; Ferella, F.; Pellei, G.; Vegliò, F. Recovery of Metals from Printed Circuit Boards by Gold-REC 1 Hydrometallurgical Process. Sustainability 2023, 15, 7348. [Google Scholar] [CrossRef]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives—A review. Resour. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Gámez, S.; Garcés, K.; de la Torre, E.; Guevara, A. Precious metals recovery from waste printed circuit boards using thiosulfate leaching and ion exchange resin. Hydrometallurgy 2019, 186, 1–11. [Google Scholar] [CrossRef]
- Gulliani, S.; Volpe, M.; Messineo, A.; Volpe, R. Recovery of metals and valuable chemicals from waste electric and electronic materials: A critical review of existing technologies. R. Soc. Chem. RSC Sustain. 2023, 1, 1085–1108. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Optimization of process parameters for the selective leaching of copper, nickel and isolation of gold from obsolete mobile phone PCBs. Clean. Eng. Technol. 2021, 4, 100180. [Google Scholar] [CrossRef]
- Lee, J.C.; Kumar, M.; Kim, M.S.; Jeong, J.; Yoo, K. Leaching of metals from waste printed circuit boards (WPCBs) using sulfuric and nitric acids. Environ. Eng. Manag. J. 2014, 13, 2601–2607. [Google Scholar] [CrossRef]
- Birloaga, I.; De Michelis, I.; Ferella, F.; Buzatu, M.; Vegliò, F. Study on the influence of various factors in the hydrometallurgical processing of waste printed circuit boards for copper and gold recovery. Waste Manag. 2013, 33, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Yazici, E.Y.; Deveci, H.A. Extraction of metals from waste printed circuit boards (WPCBs) in H2SO4 CuSO4 NaCl solutions. Hydrometallurgy 2013, 139, 30–38. [Google Scholar] [CrossRef]
- Behnamfard, A.; Mehdi, M.; Veglio, F. Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation. Waste Manag. 2013, 33, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Bas, A.D.; Deveci, H.; Yazici, E.Y. Treatment of manufacturing scrap TV boards by nitric acid leaching. Sep. Purif. Technol. 2014, 130, 151–159. [Google Scholar] [CrossRef]
- Park, Y.J.; Fray, D.J. Recovery of high purity precious metals from printed circuit boards. J. Hazard. Mater. 2009, 164, 1152–1158. [Google Scholar] [CrossRef]
- Ashiq, A.; Kulkarni, J.; Vithanage, M. Hydrometallurgical Recovery of Metals from E-waste, Electron. Waste Manag. Treat. Technol. 2019, 225–246. [Google Scholar] [CrossRef]
- Akcil, A.; Erust, C.; Gahan, C.S.; Ozgun, M.; Sahin, M.; Tuncuk, A. Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants–A review. Waste Manag. 2015, 45, 258–271. [Google Scholar] [CrossRef]
- Birloaga, I.; Coman, V.; Kopacek, B.; Vegliò, F. An advanced study on the hydrometallurgical processing of waste computer printed circuit boards to extract their valuable content of metals. Waste Manag. 2014, 34, 2581–2586. [Google Scholar] [CrossRef]
- Li, J.; Miller, J.D. Reaction kinetics of gold dissolution in acid thiourea solution using ferric sulfate as oxidant. Hydrometallurgy 2007, 89, 279–288. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Kawakita, H.; Ohto, K.; Inoue, K.; Alam, S. Selective Recovery of Precious Metals from Acidic Leach Liquor of Circuit Boards of Spent Mobile Phones Using Chemically Modified Persimmon Tannin Gel. Ind. Eng. Chem. Res. 2012, 51, 11901–11913. [Google Scholar] [CrossRef]
- Tesfaye, F.; Lindberg, D.; Hamuyuni, J. Valuable Metals and Energy Recovery from Electronic Waste Streams. Energy Technol. 2017, 103–116. [Google Scholar] [CrossRef]
- Liu, G.; Pan, D.; Wu, Y.; Yuan, H.; Yu, L.; Wang, W. An integrated and sustainable hydrometallurgical process for enrichment of precious metals and selective separation of copper, zinc, and lead from a roasted sand. Waste Manag. 2021, 132, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Xiu, F.-R.; Weng, H.; Qi, Y.; Yu, G.; Zhang, Z.; Zhang, F.-S.; Chen, M. A novel recovery method of copper from waste printed circuit boards by supercritical methanol process: Preparation of ultrafine copper materials. Waste Manag. 2017, 60, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, T.; Liu, J.; Wen, J.; Gong, S. A review of current progress of supercritical fluid technologies for e-waste treatment. Environ. Sci. Pollut. Res. 2021, 28, 44622–44637. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, F.-S. Degradation of brominated flame retardant in computer housing plastic by supercritical fluids. J. Hazard. Mater. 2012, 205–206, 156–163. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhang, F.S. A green process for copper recovery from waste printed circuit boards. Adv. Mater. Res. 2014, 878, 374–379. [Google Scholar] [CrossRef]
- Jadhao, P.; Chauhan, G.; Pant, K.; Nigam, K. Greener approach for the extraction of copper metal from electronic waste. Waste Manag. 2015, 57, 102–112. [Google Scholar] [CrossRef]
- Chauhan, G.; Pant, K.K.; Nigam, K.D.P. Environmental Science Processes & Impacts Chelation technology: A promising green approach for resource management and waste minimization. Environ. Sci. Process. Impacts 2014, 17, 12–40. [Google Scholar] [CrossRef]
- Altansukh, B.; Haga, K.; Ariunbolor, N.; Kawamura, S.; Shibayama, A. Leaching and Adsorption of Gold from Waste Printed Circuit Boards Using Iodine-Iodide Solution and Activated Carbon. Eng. J. 2016, 20, 29–40. [Google Scholar] [CrossRef]
- Chaurasia, A.; Singh, K.; Mankhand, T. Extraction of tin and copper by acid leaching of PCBs. Int. J. Metall. Eng. 2013, 2, 243–248. [Google Scholar] [CrossRef]
- Hsu, E.; Barmak, K.; West, A.C.; Park, A.-H.A. Advancements in the treatment and processing of electronic waste with sustainability: A review of metal extraction and recovery technologies. Green Chem. 2019, 21, 919–936. [Google Scholar] [CrossRef]
- Kang, K.D.; Ilankoon, I.M.S.K.; Dushyantha, N.; Chong, M.N. Assessment of Pre-Treatment Techniques for Coarse Printed Circuit Boards (PCBs) Recycling. Minerals 2021, 11, 1134. [Google Scholar] [CrossRef]
- Wang, L.; Song, S.; Gao, H.; Wang, L.; Yang, S.; Liu, C. The optimization and characterization of the recycling utilization of raffinate in the copper leaching process. J. Mater. Res. Technol. 2020, 9, 2214–2222. [Google Scholar] [CrossRef]
- Missen, R.W.; Mims, C.A.; Saville, B.A.; Missen, R.W. Introduction to Chemical Reaction Engineering and Kinetics; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]


| Leaching Details | Targeted Metals | Remarks | References |
|---|---|---|---|
| Sulfuric acid leaching with oxidizing agent H2O2 | Cu | Pros: Higher leaching efficiency, reduced corrosivity, and enables selective leaching. Cons:
| [22,23,24] |
| Aqua regia | Au, Ag | Pros: Specificity for metal leaching. Cons:
| [21,25,26] |
| Cyanide leaching | Au, Ag, Pd, Pt | Pros: Highly efficient to recover gold. Cons: Toxicity, regulatory challenges, and limited selectivity. | [27,28] |
| Thiourea leaching CS(NH2)2 | Au, Ag | Pros: High selectivity for gold and silver. Cons:
| [22,29,30] |
| Thiosulphate leaching | Au, Ag | Pros: Less toxicity, lower environmental impact, and non-corrosivity. Cons:
| [22,28,29,31,32] |
| Halide leaching | Au | Pros: Higher recovery of base metal, as well as precious metal. Cons: Emission of toxic gases, such as chlorine gas. | [19,33] |
| Supercritical methanol (SCM) process | Cu | Pros: Ease of implementation and no additional reducing agents required. Cons:
| [34,35,36,37] |
| Cuprous Chloride synthesis | Cu | Pros: Applicability of non-corrosive acids during the process. Cons: Limited to copper recovery. | [23,38] |
| Chelation technology | Cu | Pros: Effective for extracting toxic metals. Cons: Expensive and lacks selectivity. | [39,40] |
| Iodine leaching | Precious metals | Pros: Iodine/iodide is a superior substitute for chlorine/chloride due to its rapid kinetics, non-toxicity, and high selectivity for precious metals. Cons: Elevated costs and high consumption rates of iodine prevent industrialization. | [41] |
| Nitric acid leaching | Cu, Pb, Sn | Pros: Dissolution percent is higher and versatile in extracting more metals. Cons:
| [1,25,42] |
| HNO3 leaching (our work) | Cu, Pb, Ni | Fast kinetic reaction due to the powerful oxidizing agent, facilitating the dissolution of various metals, resulting in maximum recovery and less time consumption. Closed-loop system curbs NOx formation and helps to reuse chemicals by reducing the overall environmental impact. | Present Research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Panda, R.; Prasad, R.; Alorro, R.D.; Jha, M.K. Sustainable Process to Recover Metals from Waste PCBs Using Physical Pre-Treatment and Hydrometallurgical Techniques. Sustainability 2024, 16, 418. https://doi.org/10.3390/su16010418
Kumari S, Panda R, Prasad R, Alorro RD, Jha MK. Sustainable Process to Recover Metals from Waste PCBs Using Physical Pre-Treatment and Hydrometallurgical Techniques. Sustainability. 2024; 16(1):418. https://doi.org/10.3390/su16010418
Chicago/Turabian StyleKumari, Suruchi, Rekha Panda, Ranjit Prasad, Richard Diaz Alorro, and Manis Kumar Jha. 2024. "Sustainable Process to Recover Metals from Waste PCBs Using Physical Pre-Treatment and Hydrometallurgical Techniques" Sustainability 16, no. 1: 418. https://doi.org/10.3390/su16010418
APA StyleKumari, S., Panda, R., Prasad, R., Alorro, R. D., & Jha, M. K. (2024). Sustainable Process to Recover Metals from Waste PCBs Using Physical Pre-Treatment and Hydrometallurgical Techniques. Sustainability, 16(1), 418. https://doi.org/10.3390/su16010418








