Determination of Viscoelastic and Physicochemical Interactions of Dextran Type Exopolysaccharides (EPS) with Different Starch Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solutions for Three Distinct Dextrans
2.3. Rheological Analysis
2.3.1. Viscosity Determination
2.3.2. Amplitude Sweep
2.3.3. Frequency Sweep
2.3.4. Temperature Sweep
2.4. Preparation of Dextran-Starch Mixtures
Determination of Starch Gelation
2.5. FTIR Analysis
2.6. Statistical Analysis
3. Results and Discussion
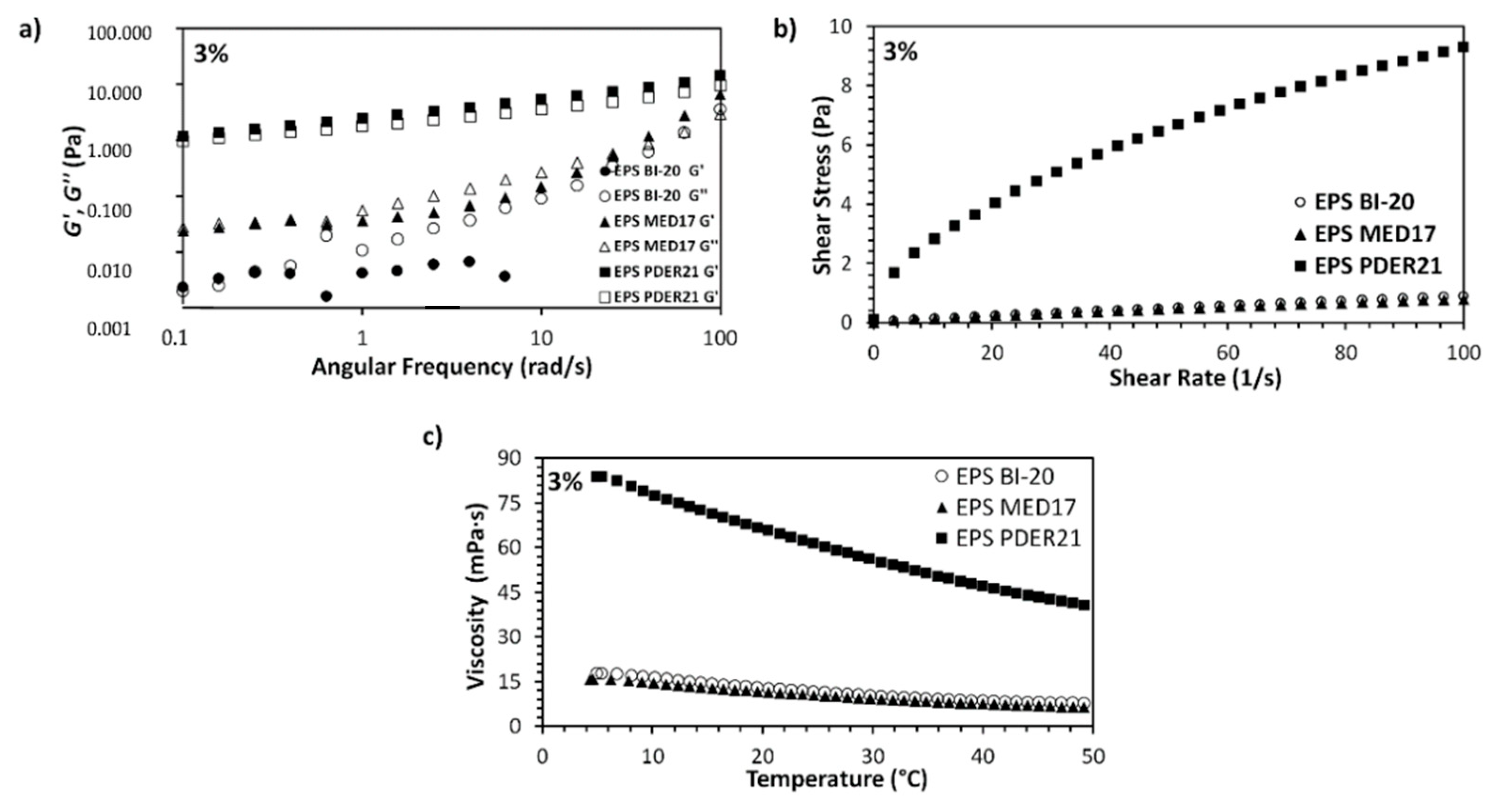
3.1. Rheological Properties of Dextran Solutions
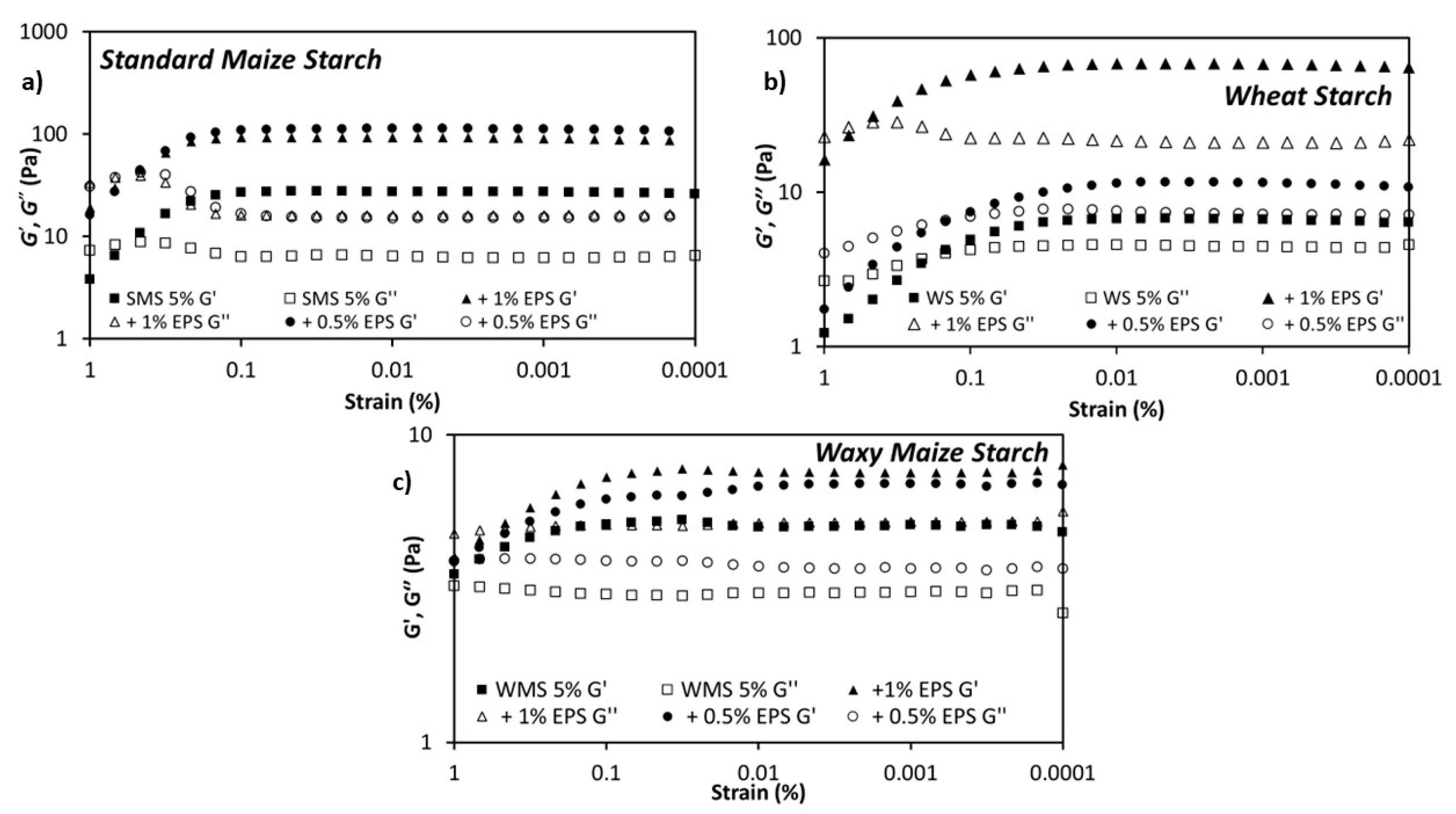
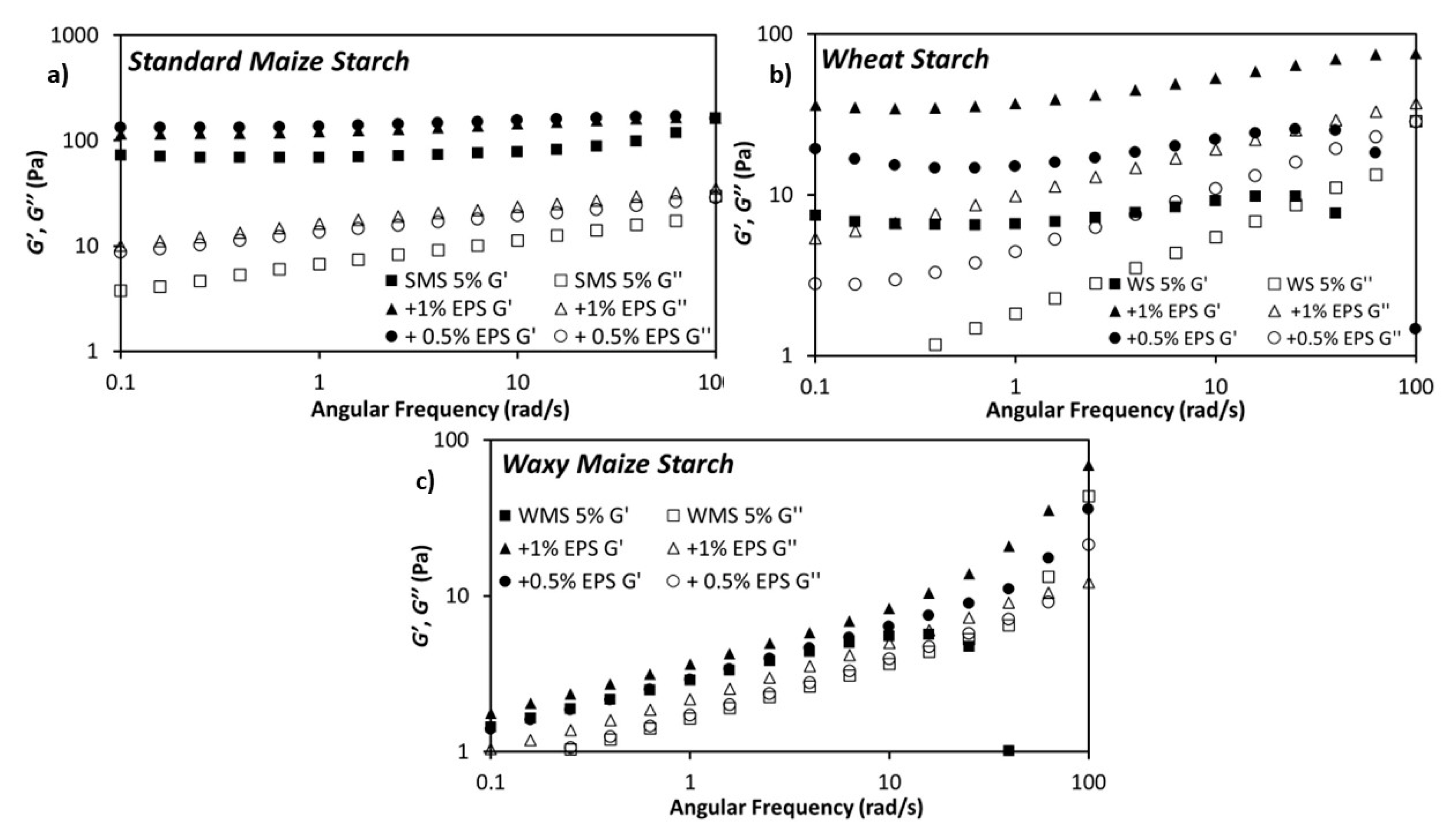
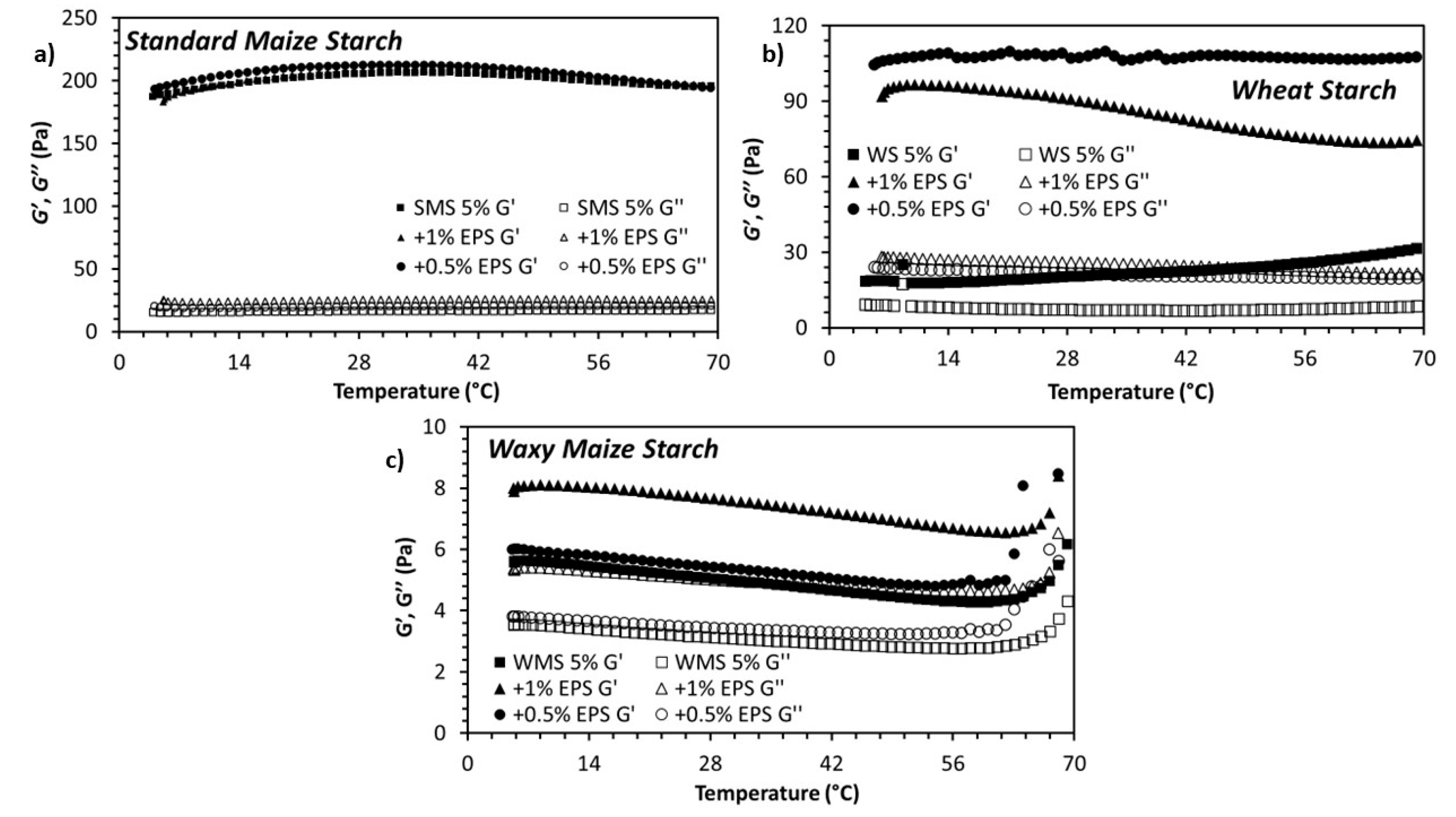
3.2. Rheological Properties of the Dextran-Starch Mixture
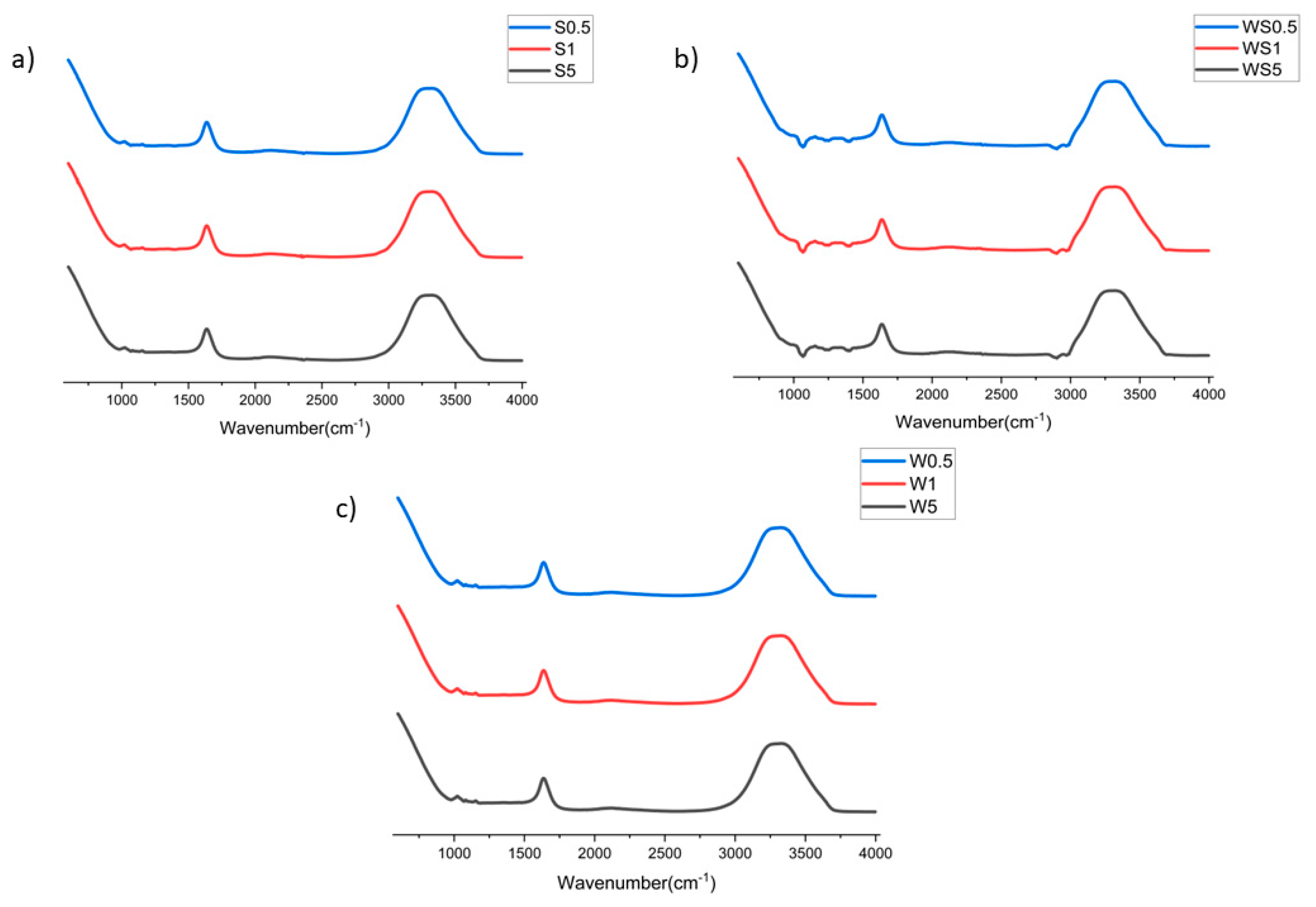
3.3. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mishra, S.; Rai, T. Morphology and Functional Properties of Corn, Potato and Tapioca Starches. Food Hydrocoll. 2006, 20, 557–566. [Google Scholar] [CrossRef]
- Wang, L.Z.; White, P.J. Structure and Properties of Amylose, Amylopectin and Intermediate Materials of Oat Starches. Cereal Chem. 1994, 71, 263–268. [Google Scholar]
- Karakelle, B.; Kian-Pour, N.; Toker, O.S.; Palabiyik, I. Effect of Process Conditions and Amylose/Amylopectin Ratio on the Pasting Behavior of Maize Starch: A Modeling Approach. J. Cereal Sci. 2020, 94. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch-Composition, Fine Structure and Architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Peidayesh, H.; Ahmadi, Z.; Khonakdar, H.A.; Abdouss, M.; Chodák, I. Baked Hydrogel from Corn Starch and Chitosan Blends Cross-linked by Citric Acid: Preparation and Properties. Polym. Adv. Technol. 2020, 31, 1256–1269. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch Modification through Environmentally Friendly Alternatives: A Review. Crit. Rev. Food Sci. Nutr. 2020, 61, 2482–2505. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Shang, P.L.; Sulaiman, S.; Ariffin, F.; Alias, A.K. A Review: Interaction of Starch/Non-Starch Hydrocolloid Blending and the Recent Food Applications. Food Biosci. 2017, 19, 110–120. [Google Scholar] [CrossRef]
- Razavi, S.M.A. Emerging Natural Hydrocolloids: Rheology and Functions, 1st ed.; Wiely: Hoboken, NJ, USA, 2019; pp. 1–52. [Google Scholar]
- Zheng, M.; Lin, Y.; Wu, H.; Zeng, S.; Zheng, B.; Zhang, Y.; Zeng, H. Water migration depicts the effect of hydrocolloids on the structural and textural properties of lotus seed starch. Food Chem. 2020, 315, 702–709. [Google Scholar] [CrossRef]
- Rahimi, G.; Hedayati, S.; Mazloomi, S.M. Studies on functional properties of wheat starch in the presence of Lepidium perfoliatum and Alyssum homolocarpum seed gums. J. Food Sci. 2021, 86, 699–704. [Google Scholar] [CrossRef]
- Shahzad, S.A.; Hussain, S.; Alamri, M.S.; Mohamed, A.A.; Ahmed, A.S.; Ibraheem, M.A.; Abdo Qasem, A.A. Use of Hydrocolloid Gums to Modify the Pasting, Thermal, Rheological, and Textural Properties of Sweet Potato Starch. Int. J. Polym. Sci. 2019, 2019, 6308591. [Google Scholar] [CrossRef]
- Song, J.Y.; Kwon, J.Y.; Choi, J.; Kim, Y.C.; Shin, M. Pasting Properties of Non-Waxy Rice Starch-Hydrocolloid Mixtures. Starch/Staerke 2006, 58, 223–230. [Google Scholar] [CrossRef]
- Kavitake, D.; Devi, P.B.; Shetty, P.H. Overview of Exopolysaccharides Produced by Weissella Genus–A Review. Int. J. Biol. Macromol. 2020, 164, 2964–2973. [Google Scholar] [CrossRef]
- Dertli, E.; Colquhoun, I.J.; Gunning, A.P.; Bongaerts, R.J.; Le Gall, G.; Bonev, B.B.; Mayer, M.J.; Narbad, A. Structure and Biosynthesis of Two Exopolysaccharides Produced by Lactobacillus johnsonii FI9785. J. Biol. Chem. 2013, 288, 31938–31951. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Zhang, L.; Wang, Z.; Li, L. Structural Characteristics and Antioxidant Properties of Exopolysaccharides Isolated from Soybean Protein Gel Induced by Lactic Acid Bacteria. LWT 2021, 150. [Google Scholar] [CrossRef]
- Dertli, E.; Yilmaz, M.T.; Tatlisu, N.B.; Toker, O.S.; Cankurt, H.; Sagdic, O. Effects of in Situ Exopolysaccharide Production and Fermentation Conditions on Physicochemical, Microbiological, Textural and Microstructural Properties of Turkish-Type Fermented Sausage (Sucuk). Meat Sci. 2016, 121, 156–165. [Google Scholar] [CrossRef]
- Gentès, M.C.; Turgeon, S.L.; St-Gelais, D. Impact of Starch and Exopolysaccharide-Producing Lactic Acid Bacteria on the Properties of Set and Stirred Yoghurts. Int. Dairy J. 2016, 55, 79–86. [Google Scholar] [CrossRef]
- Soumya, M.P.; Sasikumar, K.; Pandey, A.; Nampoothiri, K.M. Corrigendum to “Cassava Starch Hydrolysate as Sustainable Carbon Source for Exopolysaccharide Production by Lactobacillus plantarum”. Bioresour. Technol. Rep. 2020, 12, 85–88. [Google Scholar] [CrossRef]
- Du, R.; Pei, F.; Kang, J.; Zhang, W.; Wang, S.; Ping, W.; Ling, H.; Ge, J. Analysis of the Structure and Properties of Dextran Produced by Weissella confusa. Int. J. Biol. Macromol. 2022, 204, 187–195. [Google Scholar] [CrossRef]
- Buksa, K.; Kowalczyk, M.; Boreczek, J. Extraction, Purification and Characterisation of Exopolysaccharides Produced by Newly Isolated Lactic Acid Bacteria Strains and the Examination of Their Influence on Resistant Starch Formation. Food Chem. 2021, 362. [Google Scholar] [CrossRef]
- Tang, X.; Liu, N.; Huang, W.; Cheng, X.; Wang, F.; Zhang, B.; Chen, J.; Jiang, H.; Omedi, J.O.; Li, Z. Syneresis Rate, Water Distribution, and Microstructure of Wheat Starch Gel during Freeze-Thaw Process: Role of a High Molecular Weight Dextran Produced by Weissella confusa QS813 from Traditional Sourdough. Cereal Chem. 2018, 95, 117–129. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; İspirli, H.; Taylan, O.; Taşdemir, V.; Sagdic, O.; Dertli, E. Characterisation and Functional Roles of a Highly Branched Dextran Produced by a Bee Pollen Isolate Leuconostoc mesenteroides BI-20. Food Biosci. 2022, 45. [Google Scholar] [CrossRef]
- Aburas, H.; İspirli, H.; Taylan, O.; Yilmaz, M.T.; Dertli, E. Structural and Physicochemical Characterisation and Antioxidant Activity of an α-D-Glucan Produced by Sourdough Isolate Weissella cibaria MED17. Int. J. Biol. Macromol. 2020, 161, 648–655. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Ispirli, H.; Taylan, O.; Alamoudi, M.; Dertli, E. Bioactive and Technological Properties of an α-D-Glucan Synthesized by Weissella cibaria PDER21. Carbohydr. Polym. 2022, 285. [Google Scholar] [CrossRef]
- Wei, Y.; Lin, Y.; Xie, R.; Xu, Y.; Yao, J.; Zhang, J. The Flow Behavior, Thixotropy and Dynamical Viscoelasticity of Fenugreek Gum. J. Food Eng. 2015, 166, 21–28. [Google Scholar] [CrossRef]
- Mohammed, Z.H.; Haque, A.; Richardson, R.K.; Morris, E.R. Promotion and Inhibition of Xanthan “weak-Gel” Rheology by Calcium Ions. Carbohydr. Polym. 2007, 70, 84–85. [Google Scholar] [CrossRef]
- Zhou, F.; Wu, Z.; Chen, C.; Han, J.; Ai, L.; Guo, B. Exopolysaccharides Produced by Rhizobium radiobacter S10 in Whey and Their Rheological Properties. Food Hydrocoll. 2014, 36, 362–368. [Google Scholar] [CrossRef]
- Rütering, M.; Schmid, J.; Gansbiller, M.; Braun, A.; Kleinen, J.; Schilling, M.; Sieber, V. Rheological Characterization of the Exopolysaccharide Paenan in Surfactant Systems. Carbohydr. Polym. 2018, 181, 719–726. [Google Scholar] [CrossRef]
- Heldman, D.R.; Lund, D.B.; Sabliov, C.M. Handbook of Food Engineering, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 2–138. ISBN 9781466563124. [Google Scholar]
- Yousefi, A.R.; Ako, K. Controlling the Rheological Properties of Wheat Starch Gels Using Lepidium perfoliatum Seed Gum in Steady and Dynamic Shear. Int. J. Biol. Macromol. 2020, 143, 928–936. [Google Scholar] [CrossRef]
- Danu, M.; Rotaru, I.; Ibǎnescu, C.; Hurduc, N.; Ibǎnescu, S.A.; Simionescu, B.C. Gluten Content Influence on Rheological Behavior of Starch-Gluten Networks. Environ. Eng. Manag. J. 2012, 11, 1883–1888. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, J.; Singh, H.; McCarthy, O.J. Starch-Cassia Gum Interactions: A Microstructure-Rheology Study. Food Chem. 2008, 111, 1–10. [Google Scholar] [CrossRef]
- Ronda, F.; Pérez-Quirce, S.; Angioloni, A.; Collar, C. Impact of Viscous Dietary Fibres on the Viscoelastic Behaviour of Gluten-Free Formulated Rice Doughs: A Fundamental and Empirical Rheological Approach. Food Hydrocoll. 2013, 32, 252–262. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, P.; Sui, Z.; Bao, J. Physicochemical Properties of Starches from Diverse Rice Cultivars Varying in Apparent Amylose Content and Gelatinisation Temperature Combinations. Food Chem. 2015, 172, 433–440. [Google Scholar] [CrossRef]
- Liu, W.; Wang, R.; Li, J.; Xiao, W.; Rong, L.; Yang, J.; Wen, H.; Xie, J. Effects of Different Hydrocolloids on Gelatinization and Gels Structure of Chestnut Starch. Food Hydrocoll. 2021, 120. [Google Scholar] [CrossRef]
- Arıcı, M.; Yıldırım, R.M.; Özülkü, G.; Yaşar, B.; Toker, O.S. Physicochemical and Nutritional Properties of Taro (Colocasia esculenta L. Schott) Flour as Affected by Drying Temperature and Air Velocity. LWT-Food Sci. Technol. 2016, 74, 434–440. [Google Scholar] [CrossRef]
- Karim, A.A.; Nadiha, M.Z.; Chen, F.K.; Phuah, Y.P.; Chui, Y.M.; Fazilah, A. Pasting and Retrogradation Properties of Alkali-Treated Sago (Metroxylon sagu) Starch. Food Hydrocoll. 2008, 22, 1044–1053. [Google Scholar] [CrossRef]
- Chaisawang, M.; Suphantharika, M. Pasting and Rheological Properties of Native and Anionic Tapioca Starches as Modified by Guar Gum and Xanthan Gum. Food Hydrocoll. 2006, 20, 641–649. [Google Scholar] [CrossRef]
- Huang, C.-C. Physicochemical, Pasting and Thermal Properties of Tuber Starches as Modified by Guar Gum and Locust Bean Gum. Int. J. Food Sci. Technol. 2009, 44, 50–57. [Google Scholar] [CrossRef]
- Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Ibraheem, M.A.; Qasem, A.A.A.; Shahzad, S.A.; Ababtain, I.A. Use of Gum Cordia (Cordia myxa) as a Natural Starch Modifier; Effect on Pasting, Thermal, Textural, and Rheological Properties of Corn Starch. Foods 2020, 9, 909. [Google Scholar] [CrossRef]
- von Borries-Medrano, E.; Jaime-Fonseca, M.R.; Aguilar-Méndez, M.A. Tapioca Starch-Galactomannan Systems: Comparative Studies of Rheological and Textural Properties. Int. J. Biol. Macromol. 2019, 122, 1173–1183. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; You, X.; Fang, F.; Li, B. Impacts of Guar and Xanthan Gums on Pasting and Gel Properties of High-Amylose Corn Starches. Int. J. Biol. Macromol. 2020, 146, 1060–1068. [Google Scholar] [CrossRef]
- Lutfi, Z.; Nawab, A.; Alam, F.; Hasnain, A.; Haider, S.Z. Influence of Xanthan, Guar, CMC and Gum Acacia on Functional Properties of Water Chestnut (Trapa bispinosa) Starch. Int. J. Biol. Macromol. 2017, 103, 220–225. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, Structural and Functional Properties of Native and Irradiated Starch: A Review. J. Food Sci. Technol. 2019, 56, 513–523. [Google Scholar] [CrossRef]
- Hsieh, C.F.; Liu, W.; Whaley, J.K.; Shi, Y.C. Structure and Functional Properties of Waxy Starches. Food Hydrocoll. 2019, 94, 238–254. [Google Scholar] [CrossRef]
- Alam, F.; Nawab, A.; Lutfi, Z.; Haider, S.Z. Effect of Non-Starch Polysaccharides on the Pasting, Gel, and Gelation Properties of Taro (Colocasia esculenta) Starch. Starch-Stärke 2020, 73. [Google Scholar] [CrossRef]
- Dangi, N.; Yadav, B.S.; Yadav, R.B. Pasting, Rheological, Thermal and Gel Textural Properties of Pearl Millet Starch as Modified by Guar Gum and Its Acid Hydrolysate. Int. J. Biol. Macromol. 2019, 139, 387–396. [Google Scholar] [CrossRef]
- Weber, F.H.; Clerici, M.T.P.S.; Collares-Queiroz, F.P.; Chang, Y.K. Interaction of Guar and Xanthan Gums with Starch in the Gels Obtained from Normal, Waxy and High-Amylose Corn Starches. Starch-Stärke 2009, 61, 28–34. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Yousefi, A.; Ako, K. Steady/Dynamic Rheological Characterization and FTIR Study on Wheat Starch-Sage Seed Gum Blends. Food Hydrocoll. 2021, 111. [Google Scholar] [CrossRef]
- Xiong, J.; Li, Q.; Shi, Z.; Ye, J. Interactions between Wheat Starch and Cellulose Derivatives in Short-Term Retrogradation: Rheology and FTIR Study. Food Res. Int. 2017, 100, 858–863. [Google Scholar] [CrossRef]
| Starch Type (5%) | EPS Content (%) | Peak Viscosity (cP) | Peak Time (min) | Holding Strength Viscosity (cP) | Holding Strength Time (min) | Breakdown Viscosity (cP) | Final Viscosity (cP) | Setback from Peak (cP) |
|---|---|---|---|---|---|---|---|---|
| Standard maize starch | 0 | 3625 B | 3.43 C | 456 C | 17.3 B | 3170 C | 575.7 B | 3049 C |
| 0.5 | 8832 A | 4.83 B | 549 B | 20.8 A | 8283 A | 474.8 C | 8357 A | |
| 1.0 | 8625 A | 12.90 A | 4305 A | 16.7 B | 4320 B | 4707 A | 3918 B | |
| Wheat starch | 0 | 3477 C | 2.13 B | 183 B | 17.1 B | 3294 B | 327.1 B | 3150 B |
| 0.5 | 3753 B | 3.70 A | 1596 A | 10.0 C | 2156 C | 2166 A | 1587 C | |
| 1.0 | 6596 A | 0.87 C | 186 B | 19.1 A | 6410 A | 234.4 C | 6361 A | |
| Waxy maize starch | 0 | 2740 C | 5.70 A | 1730 C | 17.1 B | 1010 C | 1768 C | 972.6 C |
| 0.5 | 5149 B | 5.85 A | 3239 A | 15.0 C | 1910 B | 3212 A | 1937 B | |
| 1.0 | 5994 A | 6.23 A | 2640 B | 21.2 A | 3354 A | 2506 B | 3488 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gokcan, H.; Ozmen, D.; Yildirim Yalcin, M.; Dertli, E.; Toker, O.S.; Sujka, M. Determination of Viscoelastic and Physicochemical Interactions of Dextran Type Exopolysaccharides (EPS) with Different Starch Samples. Sustainability 2023, 15, 4934. https://doi.org/10.3390/su15064934
Gokcan H, Ozmen D, Yildirim Yalcin M, Dertli E, Toker OS, Sujka M. Determination of Viscoelastic and Physicochemical Interactions of Dextran Type Exopolysaccharides (EPS) with Different Starch Samples. Sustainability. 2023; 15(6):4934. https://doi.org/10.3390/su15064934
Chicago/Turabian StyleGokcan, Hande, Duygu Ozmen, Meral Yildirim Yalcin, Enes Dertli, Omer Said Toker, and Monika Sujka. 2023. "Determination of Viscoelastic and Physicochemical Interactions of Dextran Type Exopolysaccharides (EPS) with Different Starch Samples" Sustainability 15, no. 6: 4934. https://doi.org/10.3390/su15064934
APA StyleGokcan, H., Ozmen, D., Yildirim Yalcin, M., Dertli, E., Toker, O. S., & Sujka, M. (2023). Determination of Viscoelastic and Physicochemical Interactions of Dextran Type Exopolysaccharides (EPS) with Different Starch Samples. Sustainability, 15(6), 4934. https://doi.org/10.3390/su15064934







