1. Introduction
Rare earths, despite the name, are not in fact rare in reserves on Earth, but rather are very rich, even more so than the reserves of commonly seen elements, such as copper, zinc, tin, etc. [
1]. Due to the limitations of science in the eighteenth century, it was difficult to purify rare earths during the smelting process, and people could only make some impure, earth-like oxides at that time, which caused rare earth elements to be rare in the perceived range and gave them their name [
2].
“Rare earth elements” is the collective name for the 17 metallic elements including the 15 lanthanides in the periodic table with atomic numbers ranging from 57 to 71, as well as scandium and yttrium, which have similar chemical properties. They belong to group III B of the periodic table and usually present a positive trivalent form. However, scandium is sometimes not included in the rare earth elements because of its weak symbiosis with other elements in nature and because scandium’s properties differ significantly from those of the other rare earth elements [
3].
As a rare earth mineral resource unique to China, ionic rare earth ores are a globally scarce mineral species. The mineralization conditions of ionic rare earth ores are very complex, and one of the following three conditions is indispensable: First, the original rock must contain rare earth minerals. Second, the rare earths must be hosted on weatherable rare earth minerals. Third, the rocks must be in a warm and humid area and be subject to biological, physical, and chemical interactions. In addition to this, the geological conditions of mineralization of ionic rare earth deposits are influenced by various factors such as rock conditions, tectonic conditions, and epigenetic conditions [
4,
5]. Compared to other types of rare earth ores, such as cerium fluorocarbon (65–75 wt.% REO), monazite (55–65 wt.% REO), and yttrium phosphate (25–60 wt.% REO), ionic rare earth ores have a very low rare earth content of 0.05–0.2 wt.% REO [
6,
7]. However, ionic rare earth ores are characterized by easy mining, low processing costs, and low radioactive element content, which greatly reduces their recovery costs.
During in situ leaching mining of rare earths, property losses and casualties caused by geological disasters often occur [
8]. The cited article points out that in situ leaching process may bring about some geological disasters, including landslides, collapses, mud rock flows, etc.
Figure 1 shows a test stope of a rare earth mine in southern Jiangxi, which is managed by Ganzhou Rare Earth Mining Company. Landslides have occurred on remote mountain tops; e.g.,
Figure 2 shows rare earth ore being mined in an area of southern Jiangxi, which is contracted by a private boss. The landslide surface extends from the foot of the mountain to the top of the mountain. Once a landslide or collapse occurs at the front edge of a slope, the rear edge will continuously slide or collapse along the landslide surface due to continuous liquid injection and rainfall, and the final result is a large-scale landslide. These landslides can peel off the clay layer from mountain surface. The clay layer, formed over thousands of years, is the life bed of vegetation. Once it is damaged, it will be difficult for vegetation to recover in a short time, and so mountains in rare earth mining areas become like scars. Therefore, landslides seriously damage the environment, making it difficult to restore vegetation, and making it difficult to recover some rare earth resources.
There are many factors that cause landslides or collapses in in situ leaching, including natural factors and human factors [
9]. Humans cannot control nature, but they can restrain themselves. The main controllable human factor is injection intensity [
10,
11]. There is a basic experience in mining areas: if the strength of the liquid injection is large, it very easily causes landslides; if the strength of the liquid injection is small, the mountain is very stable, and even cracks cannot be seen. After visiting rare earth mines in the south of Jiangxi Province, it was found that the mine owner, in order to maximize benefits, allowed more ore to flow out of mountain. Based on the principle of high-intensity liquid injection, supplemented by experience, when a crack is found somewhere in mountain, the amount of liquid injection should be reduced as soon as possible [
12,
13]. Therefore, it is of great significance to propose a calculation model for liquid injection strength to change the technical status quo of in situ leaching process based on experience and realize standardized mining [
14,
15].
The stability of a landslide is closely related to its permeability coefficient. The authors of [
16,
17] elaborated the relationship between the change in permeability coefficient and stability of landslide mass: for a landslide ore body with poor permeability, a change in permeability coefficient has no obvious impact on the stability of the landslide mass; For a landslide mass with good water permeability, a change in permeability coefficient has an obvious influence on the stability of the landslide mass. It can be seen that permeability change is an important factor causing landslides. Therefore, in order to ensure safety and stability of the slope in the mining process and to eliminate the waste of rare earth resources, this paper conducts an in-depth study on laws of permeability change during the leaching process of ionic rare earth ores, which provides a certain basis for slope stability in rare earth mining and mining process of rare earth ores.
3. Results
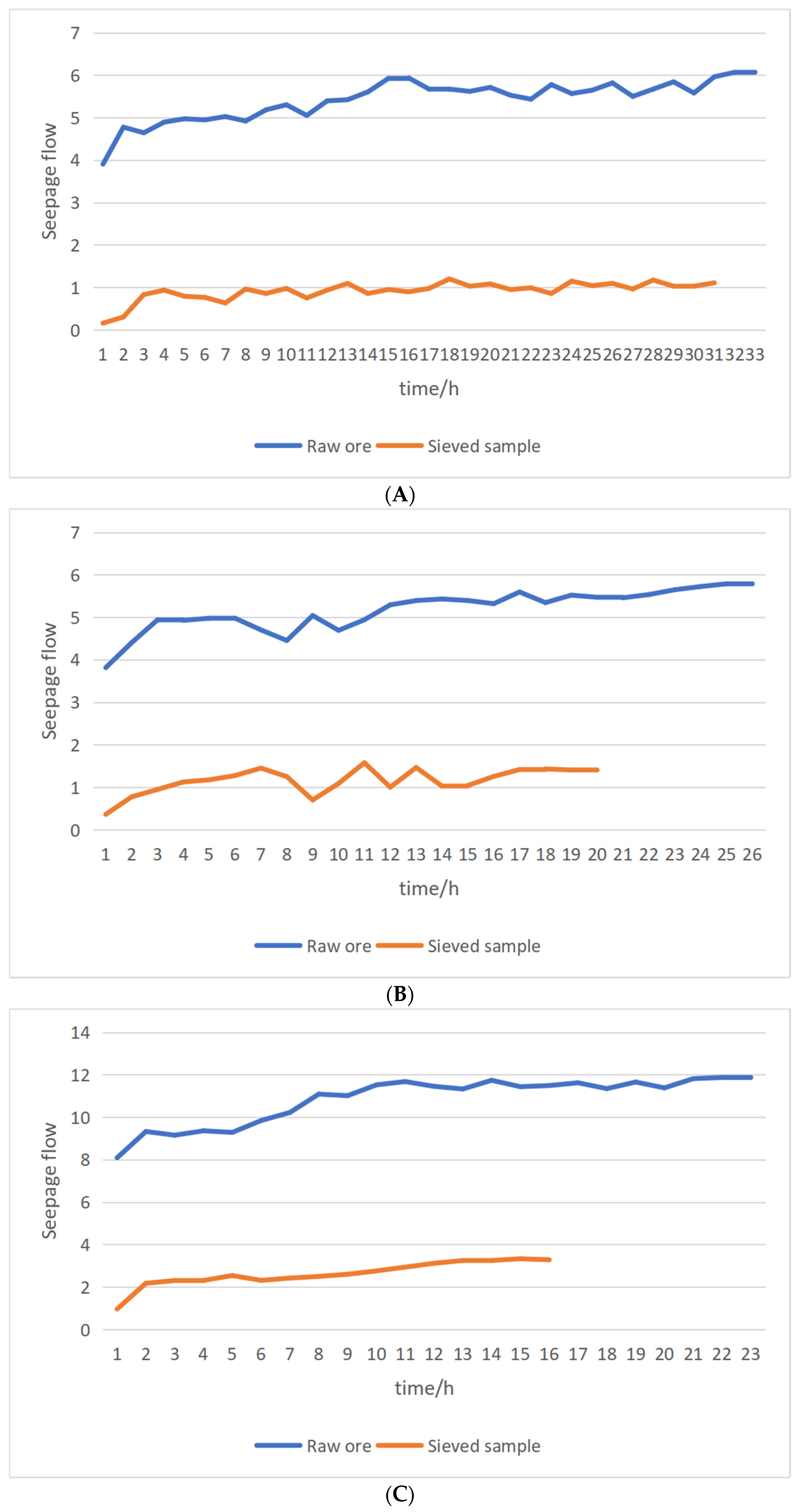
Figure 7 shows the curve of flow of raw ore and the screened sample with time during the water injection stage. It can be seen that the trend of seepage flow of raw ore and the screened sample is basically the same when water is injected. The trend can be divided into three stages: fast rising stage, slow rising stage, and stable stage. Although the trend is the same, the stability time and flow change amplitude are different. Since this test studies the process of water and solution forming stable seepage in soil samples, the relationship between flow rate and time is used to reflect the change of flow field during the water injection stage [
23]. It is assumed that if any two points j and j + 1 meet the condition of (
)/
< 1% within 24 h, then stable seepage has been formed in soil samples. The flow rate variation amplitude is defined as the difference between flow rate when the flow field is stable and the flow rate at initial time 11:
In Equation (9), and are flow when the flow field is stable and flow at initial time, respectively, in g/min.
The flow rate per minute measured in the test was plotted using the pole average method to obtain the flow rate versus time curve, and the above method was used to obtain
Table 2. The data in
Table 2 were analyzed from two aspects: stability time of flow field and flow change amplitude. As for stabilization time, it can be seen that when the hydraulic gradient was 1.25, the time needed for raw ore to reach seepage stability was 332 h, and the time needed for the screened sample to reach seepage stability was 311 h. The time needed for raw ore to reach seepage stability was about 20 h longer. When the hydraulic gradient was 2, the time needed for raw ore to reach seepage stability was 197.5 h, and the time needed for the screened sample to reach seepage stability was 121.5 h. The time needed for raw ore to reach seepage stability was about 50 h longer. When the hydraulic gradient was 3, the time needed for raw ore to reach seepage stability was 164 h, and the time needed for the screened sample to reach seepage stability was 92 h. The time needed for raw ore to reach seepage stability was about 70 h longer. With respect to the flow change range, it can be seen that the flow change range of raw ore was 2–4 times larger. The test data show that the soil sample grade has an important influence on its permeability coefficient. The greater the hydraulic gradient, the shorter the time needed to form a stable flow field. It took a longer time for raw ore to reach seepage stability.
For the sieved sample with a particle size range of 0.075~0.09 mm, because the pore throat was small and the particle diameter was larger than the pore throat, micro-particles cannot migrate from pores and so there was no micro particle migration during the seepage process of water in the sieved sample. The influence of the hydraulic gradient on permeability was analyzed by comparing the variation in the seepage flow rate of the screened samples with time under different hydraulic gradient conditions.
Figure 8 shows the change curve of seepage flow of the screened samples with different hydraulic gradients over time in the water injection stage.
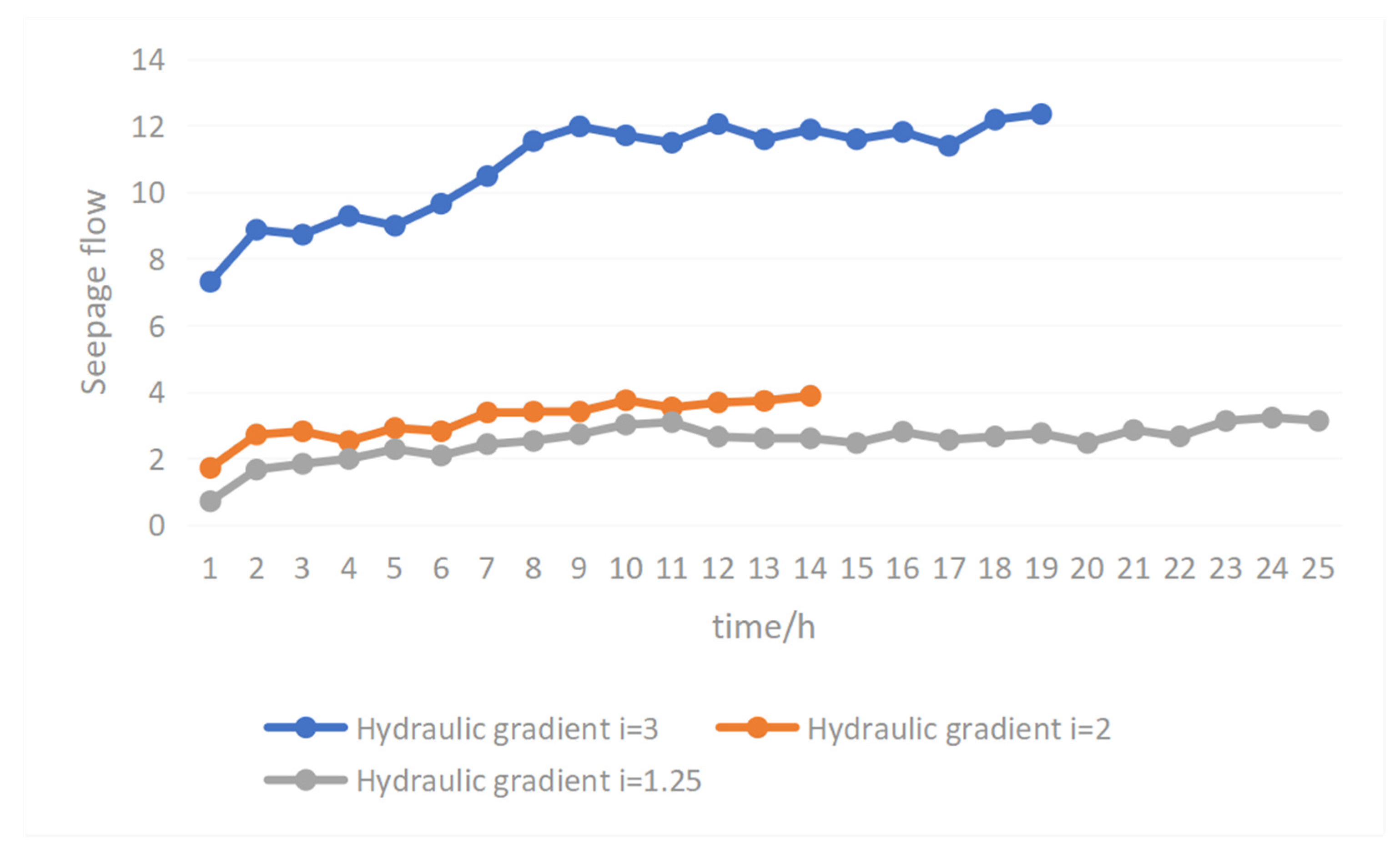
Figure 9 shows the change curve of raw ore seepage flow with time at different hydraulic gradients in the water injection stage. From the stability time analysis, it can be seen from
Figure 9 that the smaller the hydraulic gradient, the longer the time needed to reach stability of seepage. The time needed for raw ore with a hydraulic gradient of 1.25 to reach seepage stability was about 130 h longer than that with a hydraulic gradient of 2, and about 170 h longer than that with a hydraulic gradient of 3. This is because the smaller the hydraulic gradient, the smaller the seepage velocity, and the slower the migration of micro-particles. The smaller seepage the flow, the more difficult it is to achieve stability, and so the stability time is longer. From the analysis of the increased range of flow, the change range of raw ore flow was 2.97 when the hydraulic gradient was 1.25, 3.03 when the hydraulic gradient was 2, and 7.93 when the hydraulic gradient was 3. With an increase in hydraulic gradient, the range of flow also increased. This is because the larger the hydraulic gradient, the greater the flow velocity, which can take away micro-particles with a larger particle size range, making migration of micro-particles more obvious. This has a greater impact on seepage flow, and so the flow rate increases.
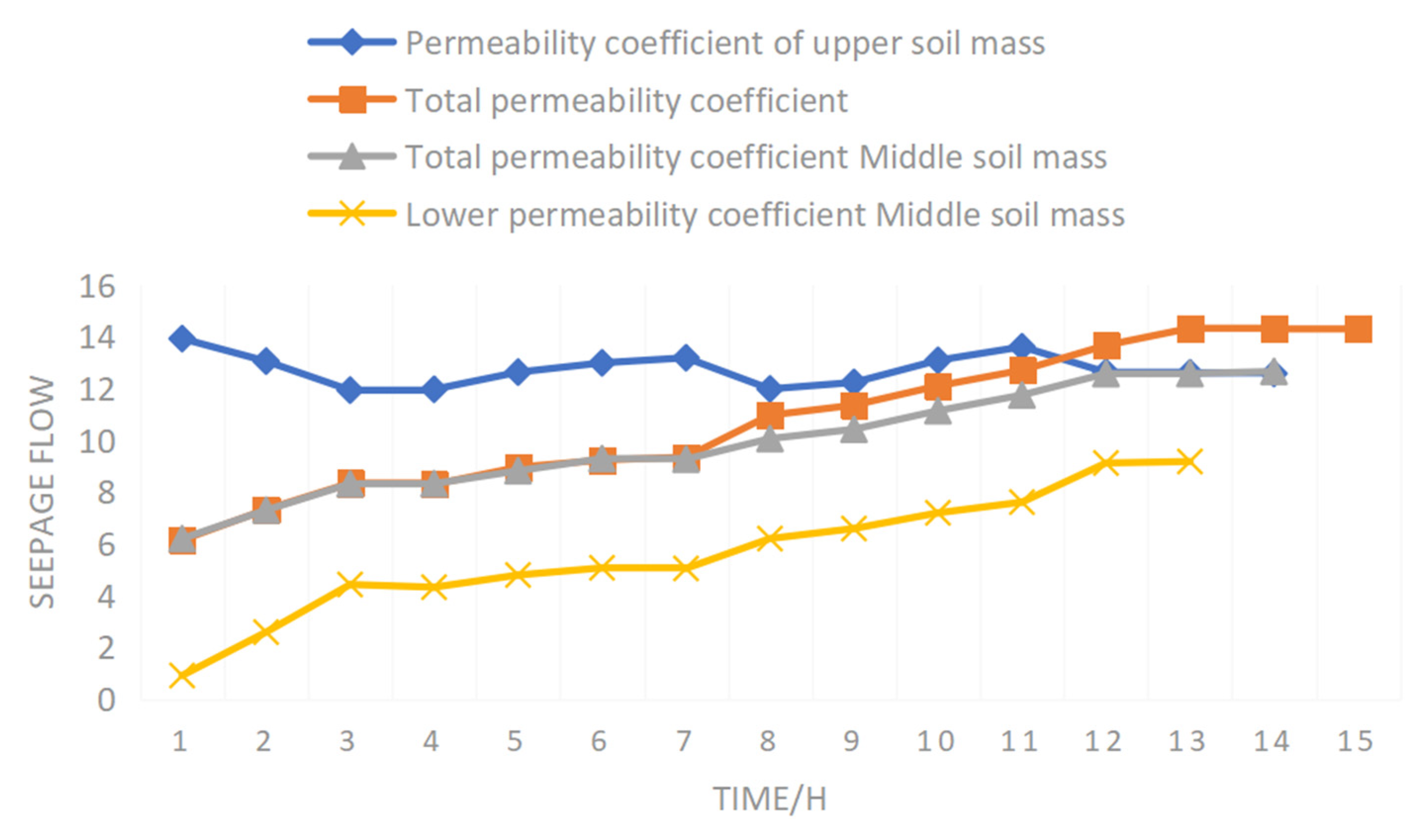
It can be seen from
Figure 10 that the trend in permeability coefficient was consistent across the upper, middle, and lower parts of the soil mass. It increased gradually at first, and then tended to be stable. The permeability coefficient of the upper soil mass was greater than the middle and lower parts of the soil mass. The main reason is that there was no condition for the migration of micro-particles in the screened sample. There was no migration of micro-particles in any part of the sample, which had no impact on the change law of the permeability coefficient of each part. The trend of the permeability coefficient was consistent. The hydraulic gradient of the upper soil mass was larger than the middle and lower two parts, and the flow velocity was faster, so the permeability coefficient of the upper soil mass was greater than that of the middle and lower two parts.
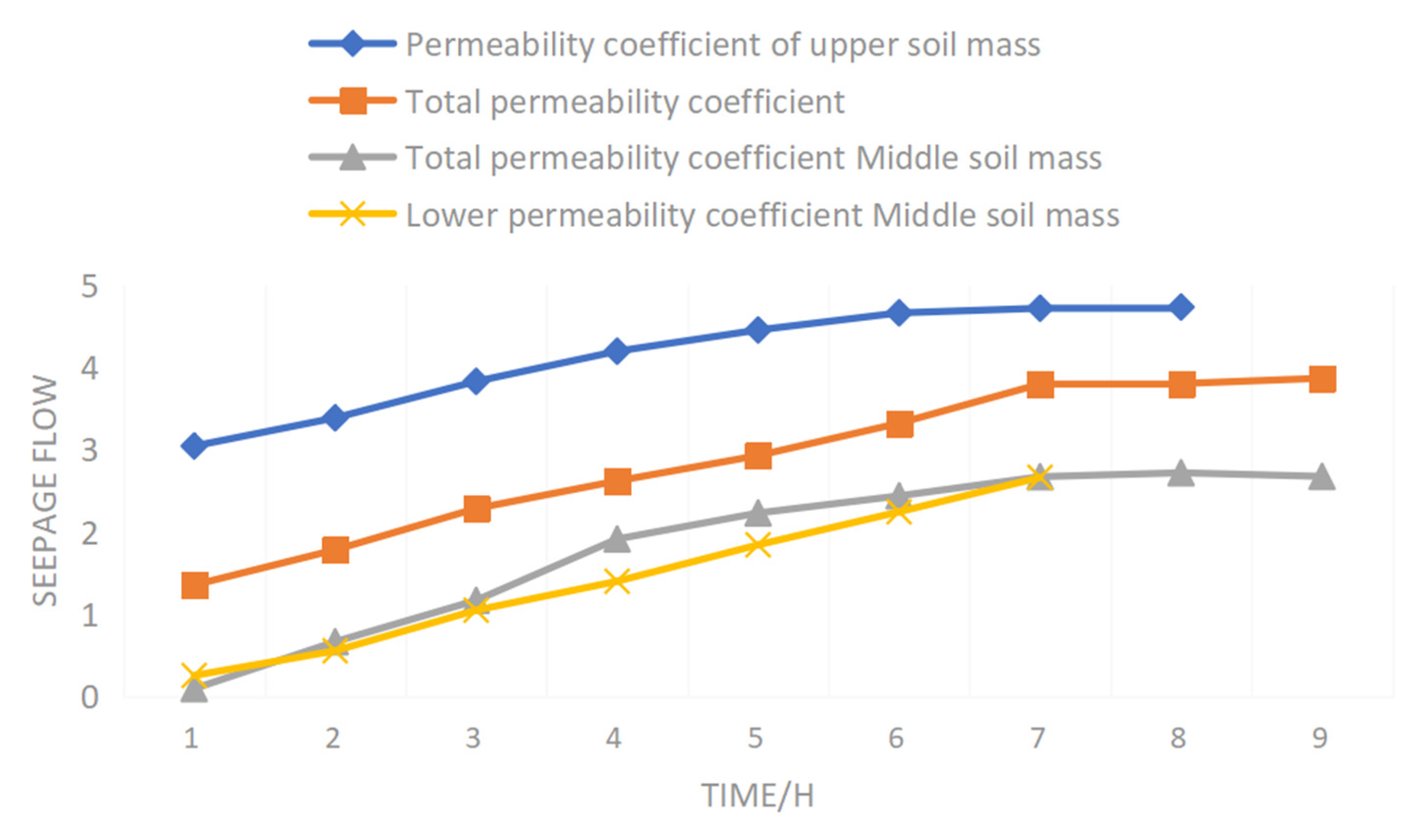
Figure 11 shows the change curve of the upper, middle and lower soil mass and total soil mass permeability coefficient with time when the hydraulic gradient was 1.25 during the clean water seepage process of raw ore.
It can be seen from
Figure 11 that the trend in the permeability coefficient of the upper soil mass is obviously different from that of the middle and lower soil mass and total soil mass. The permeability coefficient of the upper soil mass increases rapidly to peak value at first, then decreases gradually, and finally tends to be stable. The permeability coefficient of the middle and lower soil mass and total soil mass increases rapidly at first, then increases slowly, and finally tends to be stable. The permeability coefficient of the upper soil mass is greater than the middle and lower parts of the soil mass, while the permeability coefficient of the middle soil mass is less than the lower soil mass. At the stage of flow stability, the permeability coefficient of the lower soil mass is greater.
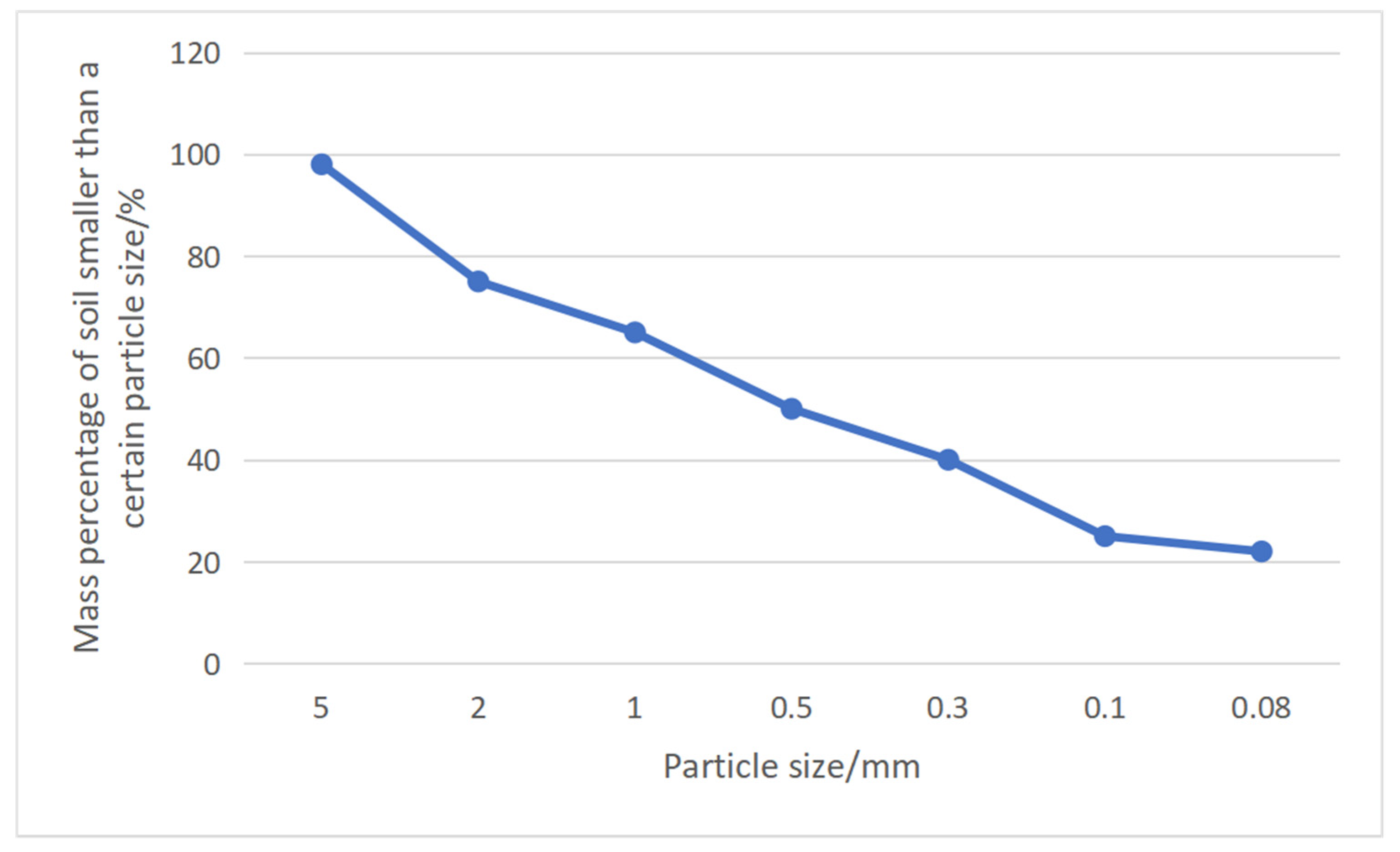
The median particle size has an important influence on analyzing the migration characteristics of particles in porous media. As for median particle size, it can be seen from
Figure 12 that the median particle size of soil mass before testing was 15.5 m, and the median particle size of the upper soil mass after testing was 20.4 μm. The median particle size of the middle soil mass after testing was 17.5 μm. After testing, the median particle size of the lower soil mass was 22.3 μm. It can be seen that the median particle size of each part of the soil mass after testing was larger. The median particle size of the lower soil mass was the largest, followed by the upper soil mass, and the median particle size of the middle soil mass was the smallest. The main reason is that, due to the short distance of micro-particle migration, micro-particle migration in the upper soil mass forms a micro-particle enrichment area in the upper soil mass, and only a small part migrates to middle soil mass, while the lower soil mass migrates to the bottom and seeps from the bottom due to micro-particle migration. The test data show that there is micro-particle migration in each part of the sample during testing, and micro-particle migration is more obvious in the lower soil mass, followed by the upper soil mass, and is less obvious in the middle soil mass.
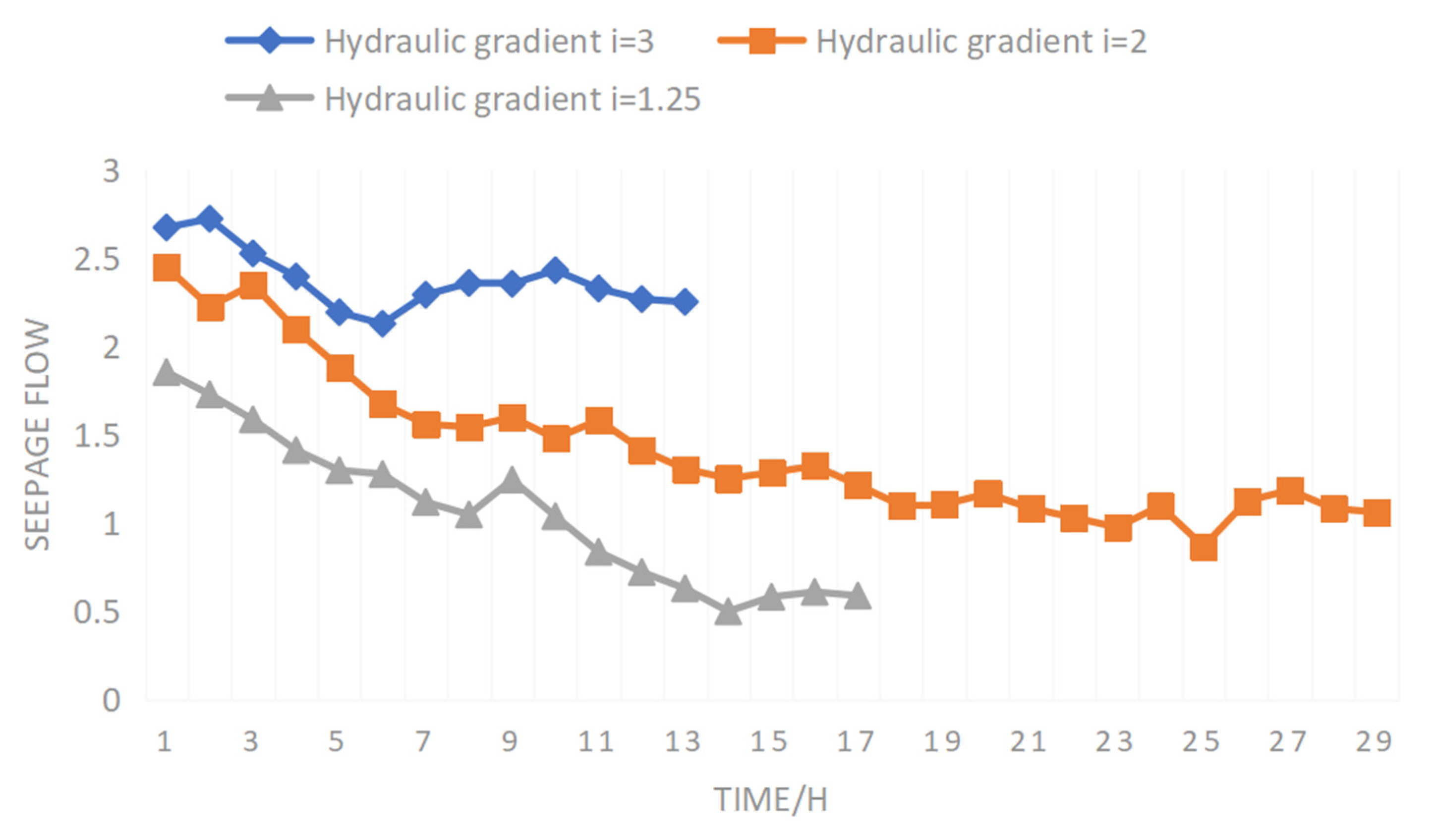
Figure 13 shows the curve of permeability coefficients of the sieved samples according to solution stage with time. It can be seen from
Figure 13 that, during the ore leaching process, the trend in the permeability coefficient of the screened sample can be divided into two stages: a slow reduction stage and a stable stage. The permeability coefficient of the screened sample decreased in a period of time after injection of sulfuric acid according to the solution. The main reason is that the permeability coefficient changed due to ion adsorption and exchange reaction. In sulfuric acid injection according to solution stage, rare earth particles have negative charges on the surface, which generates an electric field, and sulfuric acid is adsorbed according to root ions in the solution. The water film thickness increases, seepage pore diameter decreases, seepage path increases, and the permeability coefficient decreases. As root ions are more active than rare earth ions, when the adsorption amount of root ions reaches a certain value, an ion exchange reaction will occur, and trivalent rare earth ions will be resolved by exchange. The lower the ion valence, the greater the thickness of water film. When low valence rare earth ions are replaced by root ions, water film thickness will continue to increase, making the permeability coefficient continue to decrease. Because the particle size of screened samples is relatively uniform and similar, the change in permeability coefficient caused by micro-particle migration can be ignored, and so the permeability coefficient of the screened samples is gradually reduced.
Due to the relatively uniform and similar particle size of the screening samples, the pores in the soil body were relatively uniform. In the test, the screening sample pore ratio was 0.8, the soil body was dense, and the percolation pore size was small. At the same time, sulfuric acid infiltrated into the soil body, root ions were adsorbed in the bound water layer and exchanged with of rare earth ions, so that the thickness of the soil body bound water film increased and the percolation pore size decreased. At this time, there are two possible situations after the reduction in size of the bound water pore: one is that the solution can continue to flow after the reduction in size of the percolation pore, and the other is the reduction in the percolation pore size leads to the overlap of the bound water layer and blocks the pore.
For stability time, it can be seen from
Table 3 that seepage stability time was 200 h when the hydraulic gradient was 1.25, 321.5 h when the hydraulic gradient was 2, and 149 h when the hydraulic gradient was 3. The data show that when the hydraulic gradient was 1.25 or 2, the time needed for seepage to reach stability increased with the increase in the hydraulic gradient. Ion adsorption and ion exchange reactions have obvious effects on the permeability coefficient. When the hydraulic gradient was 3, the seepage stability time decreased. This is because when the hydraulic gradient was 1.25 or 2, the flow velocity was small, the seepage force was small, and water molecules at the edge of the combined water layer were mainly subject to electrostatic attraction without flowing, so the solution could only flow through the first pore. At low flow velocity, with an increase in hydraulic gradient, the ion adsorption and exchange reactions were more obvious, the water film was thicker, the permeability coefficient was smaller, and it was more difficult for seepage to achieve stability. Therefore, the larger the hydraulic gradient, the longer the seepage stability time. When the hydraulic gradient was 3, the seepage velocity was large and the seepage force played a large role. The water molecules at the edge of the combined water layer flow under seepage force, so the solution could not only seep through the first type of pores, but also seep through some of the second types of pores. As the seepage pores of the sample section increased, the flow rate of the solution flowing through the sample section increased, the permeability coefficient increased, and the time needed for seepage to reach stability is short. Therefore, when the hydraulic gradient was 3, the seepage stability time was small. See
Table 3.
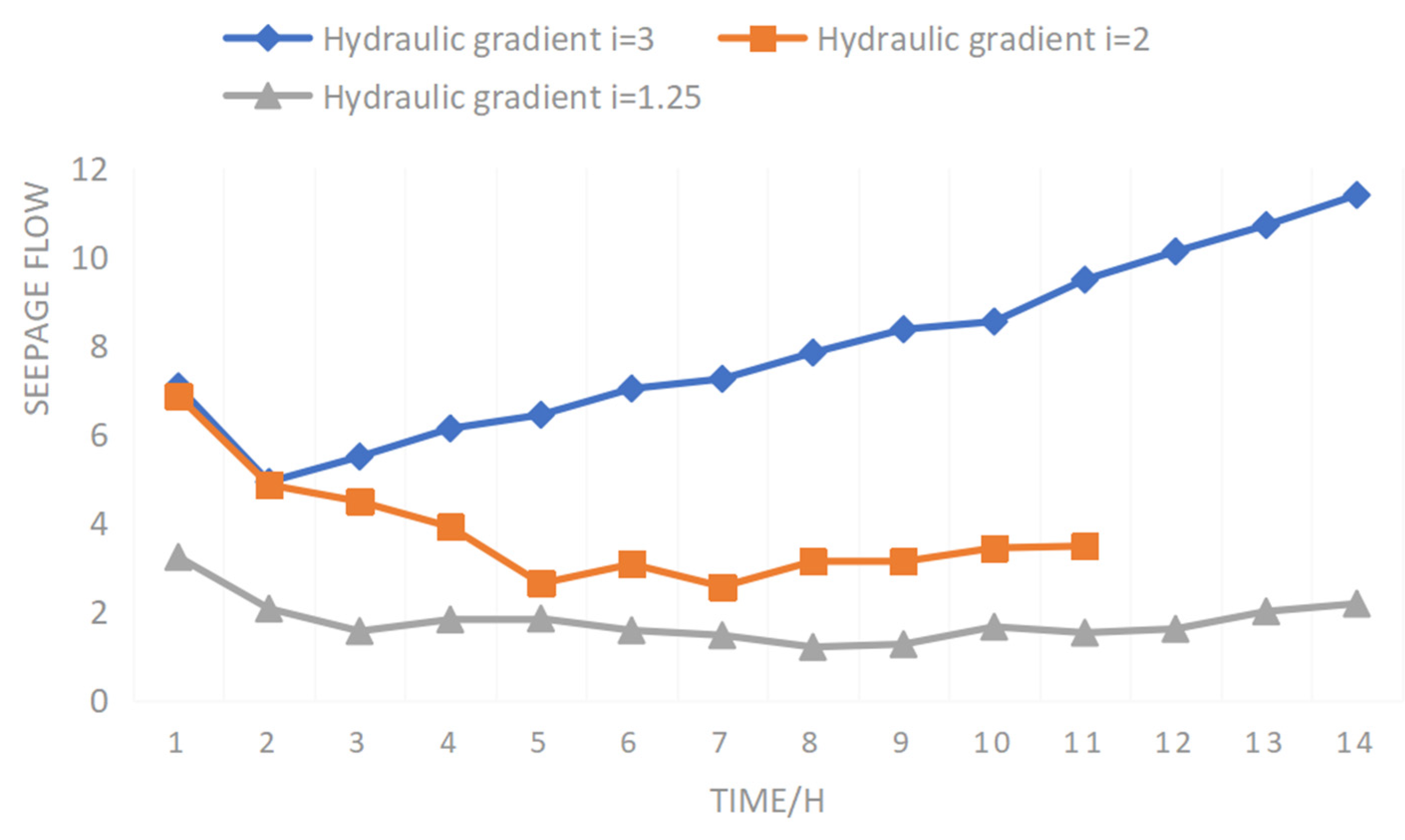
Figure 14 shows the change curve of the permeability coefficient of raw ore with time according to solution stage of sulfuric acid injection. It can be seen from
Figure 13 that during the ore leaching process, there is a difference between the permeability coefficient of raw ore and that of the screened samples. The permeability coefficient of raw ore can be divided into three stages: rapid reduction, slow rise, and steady seepage flow. When the hydraulic gradient was 3, the test had been conducted for 252 h, and the permeability coefficient of raw ore changed only in two stages: rapid reduction and continuous rise.
With respect to the change range of permeability coefficient, it can be seen from
Table 4 that when the hydraulic gradient was 1.25, the change range of the permeability coefficient of raw ore was 2.22; when the hydraulic gradient was 2, the change range of the permeability coefficient of raw ore was 0.75; when the hydraulic gradient was 3, it was not stable within 252 h. The data show that with an increase in the hydraulic gradient, the change range of permeability coefficient decreased. When the hydraulic gradient was 3, seepage did not reach stability within 252 h, and the permeability coefficient increased. This is because with an increase in the hydraulic gradient, the seepage flow rate increased, migration capacity of micro-particles increased, and it was difficult for the micro-particle enrichment area to migrate due to the reduction in pore diameter. However, micro-particles at the bottom of the sample rushed out from the bottom of the sample under the action of dynamic water pressure, which increased the permeability coefficient. The increased permeability coefficient counteracted the reduction in permeability coefficient due to part of the ion adsorption and ion exchange reactions, and the change range of the permeability coefficient decreased. When the hydraulic gradient was 3, due to large seepage velocity, micro-particles with larger particle size range could be taken away, which made migration of micro-particles more obvious. The micro-particles with larger particle size continuously rushed out from the bottom of the sample, making the permeability coefficient always increase, and the sample did not become stable within 252 h.
See
Table 5 for changes in stability time and permeability coefficient of flow field of raw ore and screened sample injected with sulfuric acid according to solution stage under different hydraulic gradients. Comparing seepage stability time of the screened sample with that of raw ore, it can be seen that when the hydraulic gradient was 1.25, the time needed for raw ore to reach seepage stability was 268 h, the time needed for the screened sample to reach seepage stability was 200 h, and the time needed for raw ore to reach seepage stability was about 70 h longer than the screened sample. When the hydraulic gradient was 2, the time needed for raw ore to reach flow field stability was 340.5 h, and the time needed for the screened sample to reach seepage stability was 321.5 h. The time needed for raw ore to reach seepage stability was about 20 h longer. When the hydraulic gradient was 3, raw ore did not become stable within 252 h, and the time needed for the screened sample to reach seepage stability was 149 h. The test data show that under different hydraulic gradient conditions, the time needed for raw ore to reach seepage stability was longer. This is because raw ore soil mass is uneven, and there are micro-particle enrichment areas and micro-particle loss areas. After the sulfuric acid solution was injected, the amount of root ions adsorbed in different areas is different, the rate of the ion exchange reaction is different, and the change speed of the permeability coefficient in different areas is different, so it is more difficult to achieve stability, that is, flow field stability time is longer.
4. Discussion
Ionic rare earth ore is an important strategic mineral resource that adsorbs on the surface of clay minerals such as erosite, illite, and kaolinite in the form of hydrated ions or hydroxyl hydrated ions and can exist stably [
24]. Its complete allotment, low radioactivity ratio, high comprehensive utilization value, and use in the high-tech industry with a shortage of medium and heavy rare earth elements, mean it has now become the focus of widespread attention at home and abroad, and is now classified as a strategic key metal resource of restricted mining and protection type in China [
25].
Ionic rare earth ores currently use the in situ leaching process, where ion exchange between the ore surface and leaching agent occurs and is accompanied by the breaking and regeneration of hydrogen bonds [
26,
27]. However, numerous studies have shown that ionic rare earth ores are prone to transport, stripping, and migration precipitation of microfine particles in the in situ leaching process [
28,
29]. The leaching process is complicated by the hydration mechanism between the leaching agent and the ore surface, and the inter-particle bridge bond is prone to breakage due to the presence of multiple forces and dispersion in the ion adsorption and exchange process [
30,
31], resulting in the migration and rearrangement of microfine particles. In the pore throat, the clogging phenomenon is produced by precipitation at the pore throat. As a result, the permeability and pore structure of the ore body are changed to some extent, which affects the leaching efficiency of rare earths. Therefore, it is important to carry out research on the migration law and optimal regulation of microfine particles in the ionic rare earth ore leaching process.
In this paper, we conducted percolation tests of water on rare earth ore samples and column leaching tests of sulfuric acid solution on ionic rare earth ore and sieved rare earth ore samples with particle size ranging from 0.075 mm to 0.09 mm. After water injection and stabilization, the sulfuric acid solution was injected. Based on the theory of Darcy’s law, the variation of permeability coefficient with time during the seepage process was studied, with the aim of providing some data references for the slope stability and rare earth mining process in rare earth mining. Meanwhile, based on the electric double layer theory, the mechanism and law of microparticle migration are explained theoretically, and the influence of ion adsorption and exchange reactions on permeability during the ore leaching process and its law are explained [
32,
33].