Mitigating Drought Stress Effects in Arid and Semi-Arid Agro-Ecosystems through Bioirrigation Strategies—A Review
Abstract
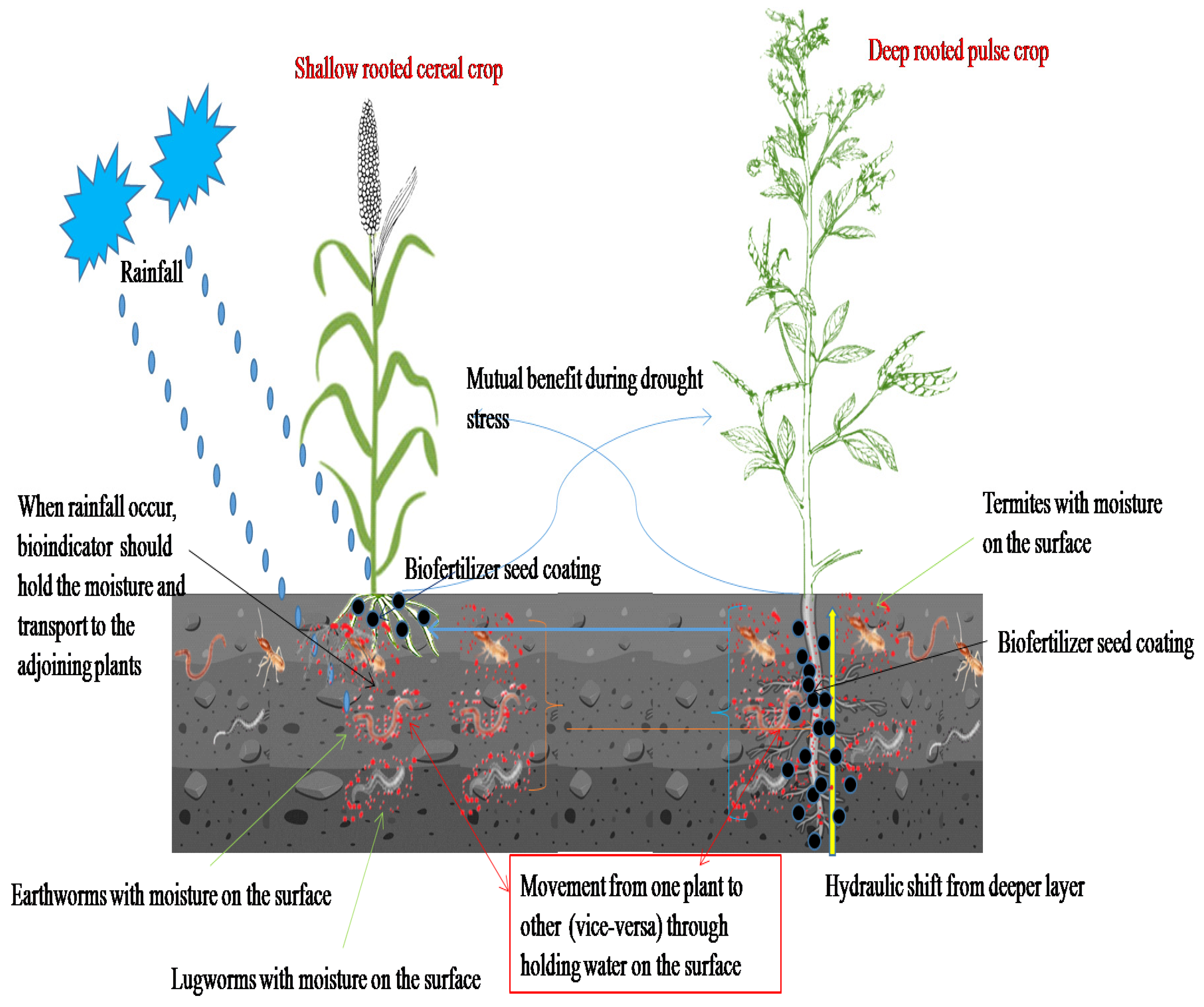
1. Introduction
| Bioirrigation Agent/Organism | Crop/Condition | Outcome | Reference |
|---|---|---|---|
| Lugworms (Arenicola marina) | Germany under sandy tidal bay | Lugworm activities prevent the sediments from developing sand to mud successively | [5] |
| Lugworms (Arenicola marina) | Strong bioindicator in coastal sediments | - | [36] |
| Diptera insect, Chironomusplumosus (Chironomidae) | Soil experiments | 2.5 times higher sediment respiration; widespread and abundant bioirrigator, building deep burrows in the sediment and pumping water through the burrow | [2] |
| Earthworms, Aporrectodea caliginosa; Lumbricus rubellus and Aporrectodea longa | Effects of irrigation and effluent distribution on the diversity of earthworm species in pastures | Sheep farms have larger earthworm populations and biomasses than dairy farms | [7] |
| Mites, Acariformes (Arachnida; Acari) Springtails (Arthropoda: Collembola) | Irrigated and non-irrigated conditions with treated municipal wastewater and dairy parlour washings on SRC willow plantations | The amount of earthworms and mites in the SRC willow plantation was considerably impacted by previous land use, with an increased abundance of earthworms under previously planted grasslands | [12] |
1.1. Need of Bioirrigation in Agriculture
1.2. Bioirrigation Potential in Agricultural Crops
1.3. Cropping Systems as Bioirrigation
1.4. Beneficial Microorganisms as Bioirrigation
| Region | Crop and Condition | PGPR Type | Mechanism | Observation | Reference |
|---|---|---|---|---|---|
| India | Mustard Field study | Bacillus casamancensis, Bacillus sp. MRD-17, and Bacillus aryabhattai NSRSSS-1 | Extracellular polymeric substances (EPS) | B. aryabhattai strain NSRSSS-1 demonstrated the maximum chlorophyll-a content after inoculation | [94] |
| India | Mustard Pot experiment | Bacillus sp. strain MR D17 & Bacillus cereus strain NA D7 | Significantly increased DREB2 and DREB1-2 gene expression; essential function for ABA-independent pathway in improving tolerance | Proline, amino acids, and sugar osmolyte accumulation is increased, which improves osmotic adjustment and RWC in the leaf tissues | [47] |
| India | Maize Pot/Lab experiment | Bacillus amyloliquefaciens, Bacillus licheniformis | Proline, carbohydrates, and free amino acids were all enhanced by osmoregulation, while electrolyte leakage was minimised | Sugar levels increased, and seedlings exhibited physiological reactions to counteract the deleterious consequences of drought stress | [46] |
| Sweden and Estonia | Wheat Pot/Lab experiment | Bacillus thuringiensis; Paenibacillus polymyxa | Biofilm of rhizosphere bacteria has protective role against drought stress. Volatiles emitted from the inoculated plants can provide stress resistance. | Changed root hairiness; higher net assimilation rate | [78] |
| Argentina | MaizePot/lab experiment | Azospirillum lipoferum | Gibberellins elevated ABA levels and reduced the effects of drought stress | Maize growth enhanced | [95] |
| Republic of Korea | Soybean Pot/lab experiment | Pseudomonas putida H-2–3 | Reprogramming the expression of chlorophyll, stress hormones, and antioxidants | Plant growth was enhanced by P. putida’s secretion of gibberellins. | [96] |
| Pakistan | Wheat Pot/lab experiment | Rhizobium leguminosarum (LR-30), Mesorhizobium ciceri (CR-30 and CR-39), and Rhizobium phaseoli (MR-2) | Wheat growth and drought tolerance index were all improved by IAA produced by the consortiums | Increased the seedlings’ root length to lessen the impact of the drought | [97] |
| Argentina | Maize Pot/lab experiment | Azospirillum sp. and Herbaspirillum sp. | Lower ZmVP14 gene expression, which is essential for the production of abscisic acid | Increased biomass production, higher amounts of carbon, nitrogen, and chlorophyll, and decreased levels of ethylene and abscisic acid | [98] |
| Canada | Pisum sativum Pot Exp. | Variovorax paradoxus 5C-2 | The enhanced increase in xylem abscisic acid | Symbiotic nitrogen-fixing bacteria boosted nodulation, preventing a drought-induced decline in nodulation and seed nitrogen content | [93] |
| China | Pisum sativum Pot Exp. | Variovorax paradoxus 5C-2 | ACC) deaminase (ACCd)-containing rhizobacterium | Enhanced shoot-to-root xylem fluxes of K by 2.1 times and by 1.8 times, respectively. | [99] |
| China | Alfalfa | Sinorhizobium meliloti 1021-Engineered strain | Cytokinin (ipt gene) excess production | Increased zeatin, nitrogenase, and antioxidant enzymes in leaves | [100] |
| USA | Squash | Glomus intraradices | Active aquaporins and AM colonisation | Under drought, the host plant’s root hydraulic conductivity (Lpr) increases or decreases | [91] |
| Spain | Phaseolus vulgaris | Glomus intraradices | Higher root plasma membrane aquaporins and root hydraulic characteristics (PIP) | Protein abundance, PIP gene expression, and root hydraulic conductance (L) | [92] |
1.5. Methods to Analyze the Effect of Bioirrigation Potential
1.6. Bioindicators Effect on Soil’s Physical and Chemical Properties
1.7. Bioindicators’ Effect on Biological Properties
1.8. Feasibility of Bioirrigation in Dryland Agriculture
1.9. Opportunities for Bioirrigation Potential
- There is a need to isolate benthic zone microbes, which are having higher water-holding capacity over the existing microbes, which are being utilized only for nutrient purpose;
- There is a need to identify and develop suitable microorganisms that have a higher bioirrigation potential to mitigate the drought stress in major cropping systems;
- Some available microbial inoculates are tolerant to harsh environments and there is a need to identify the method of application with the rapid enhancement of plant stress tolerance with higher water-holding capacity;
- There is a need for extensive studies on using beneficial microorganisms with the judicious use of available irrigation water under variable tillage conditions, which can lead to the production of eco-friendly cultivation practices;
- This enlists new possible options for using earthworms, termites, lugworms, etc., under diversified cropping systems in conjunction with microbes;
- They are wider options for breeders to breed efficient drought stress cultivars with efficient rooting patterns to extract moisture from deeper layers.
2. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koretsky, C.M.; Meile, C.; Van Cappellen, P. Quantifying bioirrigation using ecological parameters: A stochastic approach†. Geochem. Trans. 2002, 3, 17–30. [Google Scholar] [CrossRef]
- Baranov, V.; Lewandowski, J.; Romejn, P.; Krause, S. Bioirrigation impacts on sediment respiration and microbial metabolic activity. In Proceedings of the AGU, Fall Meeting, San Francisco, CA, USA, 14–18 December 2015; Volume 2015, p. H43C-1505. [Google Scholar]
- Singh, D.; Mathimaran, N.; Boller, T.; Kahmen, A. Deep-rooted pigeon pea promotes the water relations and survival of shallow-rooted finger millet during drought—Despite strong competitive interactions at ambient water availability. PLoS ONE 2020, 15, e0228993. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as Ecosystem Engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Volkenborn, N.; Hedtkamp, S.; van Beusekom, J.; Reise, K. Effects of bioturbation and bioirrigation by lugworms (Arenicola marina) on physical and chemical sediment properties and implications for intertidal habitat succession. Estuar. Coast. Shelf Sci. 2007, 74, 331–343. [Google Scholar] [CrossRef]
- Bouma, T.J.; Olenin, S.; Reise, K.; Ysebaert, T. Ecosystem engineering and biodiversity in coastal sediments: Posing hypotheses. Helgol. Mar. Res. 2009, 63, 95–106. [Google Scholar] [CrossRef]
- Manono, B.; Moller, H. Effects of stock type, irrigation and effluent dispersal on earthworm species composition, densities and biomasses in New Zealand pastures. Pedobiologia 2015, 58, 187–193. [Google Scholar] [CrossRef]
- Wiesebron, L.E.; Steiner, N.; Morys, C.; Ysebaert, T.; Bouma, T.J. Sediment Bulk Density Effects on Benthic Macrofauna Burrowing and Bioturbation Behavior. Front. Mar. Sci. 2021, 8, 707785. [Google Scholar] [CrossRef]
- Woodin, S.A.; Wethey, D.; Volkenborn, N. Infaunal Hydraulic Ecosystem Engineers: Cast of Characters and Impacts. Integr. Comp. Biol. 2010, 50, 176–187. [Google Scholar] [CrossRef]
- McCartain, L.D.; Townsend, M.; Thrush, S.F.; Wethey, D.S.; Woodin, S.A.; Volkenborn, N.; Pilditch, C.A. The effects of thin mud deposits on the behaviour of a deposit-feeding tellinid bivalve: Implications for ecosystem functioning. Mar. Freshw. Behav. Physiol. 2017, 50, 239–255. [Google Scholar] [CrossRef]
- Meysman, F.; Galaktionov, O.; Middelburg, J. Irrigation patterns in permeable sediments induced by burrow ventilation: A case study of Arenicola marina. Mar. Ecol. Prog. Ser. 2005, 303, 195–212. [Google Scholar] [CrossRef]
- Feighan, J. The Effect of Irrigation with Wastewaters on the Abundance of Bio-Indicators in Established Short Rotation Coppice Willow Plantations. Master’s Thesis, Institute of Technology, Sligo, Ireland, 2015. [Google Scholar]
- Beukema, J. Biomass and species richness of the macro-benthic animals living on the tidal flats of the Dutch Wadden Sea. Neth. J. Sea Res. 1976, 10, 236–261. [Google Scholar] [CrossRef]
- Reise, K. Sediment mediated species interactions in coastal waters. J. Sea Res. 2002, 48, 127–141. [Google Scholar] [CrossRef]
- Botto, F.; Iribarne, O. Contrasting Effects of Two Burrowing Crabs (Chasmagnathus granulata and Uca uruguayensis) on Sediment Composition and Transport in Estuarine Environments. Estuar. Coast. Shelf Sci. 2000, 51, 141–151. [Google Scholar] [CrossRef]
- Bolam, S.G.; Fernandes, T.F. Dense aggregations of Pygospio elegans (Claparède): Effect on macrofaunal community structure and sediments. J. Sea Res. 2003, 49, 171–185. [Google Scholar] [CrossRef]
- Chapin, F.; Matson, P.; Mooney, H. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2002. [Google Scholar]
- Jouquet, P.; Dauber, J.; Lagerlöf, J.; Lavelle, P.; Lepage, M. Soil invertebrates as ecosystem engineers: Intended and accidental effects on soil and feedback loops. Appl. Soil Ecol. 2006, 32, 153–164. [Google Scholar] [CrossRef]
- Dowd, M.; Grant, J.; Lu, L. Predictive modeling of marine benthic macrofauna and its use to inform spatial monitoring design. Ecol. Appl. 2014, 24, 862–876. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Scheu, S. Earthworms as drivers of the competition between grasses and legumes. Soil Biol. Biochem. 2008, 40, 2650–2659. [Google Scholar] [CrossRef]
- Flach, E.; Beukema, J. Density-governing mechanisms in populations of the lugworm Arenicola marina on tidal flats. Mar. Ecol. Prog. Ser. 1994, 115, 139–149. [Google Scholar] [CrossRef]
- Briones, M.J.I.; Ostle, N.J.; McNamara, N.P.; Poskitt, J. Functional shifts of grassland soil communities in response to soil warming. Soil Biol. Biochem. 2009, 41, 315–322. [Google Scholar] [CrossRef]
- Callaham, M.; Richter, D.; Coleman, D.; Hofmockel, M. Long-term land-use effects on soil invertebrate communities in Southern Piedmont soils, USA. Eur. J. Soil Biol. 2006, 42, S150–S156. [Google Scholar] [CrossRef]
- Malmström, A.; Persson, T.; Ahlström, K.; Gongalsky, K.B.; Bengtsson, J. Dynamics of soil meso- and macrofauna during a 5-year period after clear-cut burning in a boreal forest. Appl. Soil Ecol. 2009, 43, 61–74. [Google Scholar] [CrossRef]
- Rossi, J.; Celini, L.; Mora, P.; Mathieu, J.; Lapied, E.; Nahmani, J.; Ponge, J.-F.; Lavelle, P. Decreasing fallow duration in tropical slash-and-burn agriculture alters soil macroinvertebrate diversity: A case study in southern French Guiana. Agric. Ecosyst. Environ. 2010, 135, 148–154. [Google Scholar] [CrossRef]
- Rao, C.S.; Lal, R.; Prasad, J.V.; Gopinath, K.A.; Singh, R.; Jakkula, V.S.; Sahrawat, K.L.; Venkateswarlu, B.; Sikka, A.K.; Virmani, S.M. Potential and Challenges of Rainfed Farming in India. Adv. Agron. 2015, 133, 113–181. [Google Scholar] [CrossRef]
- IWMI. Managing Water for Rainfed Agriculture; Issue 10, IWMI: Colombo, Sri Lanka, 2010. [Google Scholar]
- NRAA. National Rainfed Area Authority (NRAA), Ministry of Agriculture. Harnessing Opportunities in Rainfed Areas—Vision 2025. 2022. Available online: https://www.nraa-policy/article65758384.ece (accessed on 17 October 2022).
- Gopinath, K.A.; Rajanna, G.A.; Venkatesh, G.; Jayalakshmi, M.; Kumari, V.V.; Prabhakar, M.; Rajkumar, B.; Chary, G.R.; Singh, V.K. Influence of Crops and Different Production Systems on Soil Carbon Fractions and Carbon Sequestration in Rainfed Areas of Semiarid Tropics in India. Sustainability 2022, 14, 4207. [Google Scholar] [CrossRef]
- Rajanna, G.; Dass, A.; Suman, A.; Babu, S.; Venkatesh, P.; Singh, V.; Upadhyay, P.K.; Sudhishri, S. Co-implementation of tillage, irrigation, and fertilizers in soybean: Impact on crop productivity, soil moisture, and soil microbial dynamics. Field Crop. Res. 2022, 288, 108672. [Google Scholar] [CrossRef]
- Sharp, R.E.; Poroyko, V.; Hejlek, L.G.; Spollen, W.G.; Springer, G.K.; Bohnert, H.J.; Nguyen, H.T. Root growth maintenance during water deficits: Physiology to functional genomics. J. Exp. Bot. 2004, 55, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Ort, D.R. More than taking the heat: Crops and global change. Curr. Opin. Plant Biol. 2010, 13, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.; Schon, N.; Piercy, J.; Mackay, A.; Minor, M. Influence of summer irrigation on soil invertebrate populations in a long-term sheep irrigation trial at Winchmore (Canterbury). N. Z. J. Agric. Res. 2012, 55, 165–180. [Google Scholar] [CrossRef]
- Rajanna, G.; Dhindwal, A.; Nanwal, R. Effect of irrigation schedules on plant—Water relations, root, grain yield and water productivity of wheat [Triticum aestivum (L.) emend. Flori & Paol] under various crop establishment techniques. Cereal Res. Commun. 2017, 45, 166–177. [Google Scholar] [CrossRef]
- Kristensen, E. Impact of polychaetes (Nereis spp. and Arenicola marina) on carbon biogeochemistry in coastal marine sediments†. Geochem. Trans. 2001, 2, 92–104. [Google Scholar] [CrossRef]
- Rao, A.M.; Malkin, S.Y.; Montserrat, F.; Meysman, F.J. Alkalinity production in intertidal sands intensified by lugworm bioirrigation. Estuar. Coast. Shelf Sci. 2014, 148, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Turan, M.; Donmez, M.F. Mitigation of salt stress in radish (Raphanus Sativus L.) by plant growth promoting rhizobacteria. Roum. Biotechnol. Lett. 2008, 13, 3933–3943. [Google Scholar]
- Mao, L.; Zhang, L.; Li, W.; van der Werf, W.; Sun, J.; Spiertz, H.; Li, L. Yield advantage and water saving in maize/pea intercrop. Field Crop. Res. 2012, 138, 11–20. [Google Scholar] [CrossRef]
- Wang, G.; Ye, C.; Zhang, J.; Koziol, L.; Bever, J.D.; Li, X. Asymmetric facilitation induced by inoculation with arbuscular mycorrhizal fungi leads to overyielding in maize/faba bean intercropping. J. Plant Interact. 2019, 14, 10–20. [Google Scholar] [CrossRef]
- Harish, M.N.; Choudhary, A.K.; Bhupenchandra, I.; Dass, A.; Rajanna, G.A.; Singh, V.K.; Bana, R.S.; Varatharajan, T.; Verma, P.; George, S.; et al. Double zero-tillage and foliar-P nutrition coupled with bio-inoculants enhance physiological photosynthetic characteristics and resilience to nutritional and environmental stresses in maize–wheat rotation. Front. Plant Sci. 2022, 13, 959541. [Google Scholar] [CrossRef]
- Woitke, M.; Junge, H.; Schnitzler, W. Bacillus subtilis as growth promotor in hydroponically grown tomatoes under saline conditions. Acta Hortic. 2004, 659, 363–369. [Google Scholar] [CrossRef]
- Zahedi, H.; Abbasi, S. Effect of plant growth promoting rhizobacteria (PGPR) and water stress on phytohormones and polyamines of soybean. Indian J. Agric. Res. 2015, 49, 427–431. [Google Scholar] [CrossRef]
- Dale, V.H.; Beyeler, S.C. Challenges in the development and use of ecological indicators. Ecol. Indic. 2001, 1, 3–10. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Ghazvini, N.S.; Pazoki, A.; Tajali, F. Study the effect of Plant Growth promoting Rhizobacteria (PGPR) and Humic acid for some savory (Satureja hortensis L.) plant physiological traits under drought stress. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 182–185. [Google Scholar]
- Vardharajula, S.; Ali, S.Z.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Bandeppa, S.; Paul, S.; Thakur, J.K.; Chandrashekar, N.; Umesh, D.K.; Aggarwal, C.; Asha, A. Antioxidant, physiological and biochemical responses of drought susceptible and drought tolerant mustard (Brassica juncea L.) genotypes to rhizobacterial inoculation under water deficit stress. Plant Physiol. Biochem. 2019, 143, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Srinivasarao, C.; Venkateswarlu, B.; Lal, R.; Singh, A.K.; Kundu, S.; Vittal, K.P.R.; Sharma, S.K.; Sharma, R.A.; Jain, M.P.; Chary, G.R. Sustaining agronomic productivity and quality of a Vertisolic soil (Vertisol) under soybean–safflower cropping system in semi-arid central India. Can. J. Soil Sci. 2012, 92, 771–785. [Google Scholar] [CrossRef]
- Rana, D.S.; Dass, A.; Rajanna, G.A.; Kaur, R. Biotic and abiotic stress management in pulses. Indian J. Agron 2016, 61, S238–S248. [Google Scholar]
- Glick, B.R.; Liu, C.; Ghosh, S.; Dumbroff, E.B. Early development of canola seedlings in the presence of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. Soil Biol. Biochem. 1997, 29, 1233–1239. [Google Scholar] [CrossRef]
- Timmusk, S.; Wagner, E.G.H.; Gruau, C.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Clément, C.; Baillieul, F.; Aziz, A.; Stearns, J.C.; et al. The Plant-Growth-Promoting Rhizobacterium Paenibacillus polymyxa Induces Changes in Arabidopsis thaliana Gene Expression: A Possible Connection between Biotic and Abiotic Stress Responses. Mol. Plant-Microbe Interact. 1999, 12, 951–959. [Google Scholar] [CrossRef]
- Marulanda-Aguirre, A.; Azcón, R.; Ruiz-Lozano, J.M.; Aroca, R. Differential Effects of a Bacillus megaterium Strain on Lactuca sativa Plant Growth Depending on the Origin of the Arbuscular Mycorrhizal Fungus Coinoculated: Physiologic and Biochemical Traits. J. Plant Growth Regul. 2008, 27, 10–18. [Google Scholar] [CrossRef]
- Fleury, D.; Jefferies, S.; Kuchel, H.; Langridge, P. Genetic and genomic tools to improve drought tolerance in wheat. J. Exp. Bot. 2010, 61, 3211–3222. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Marôco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How Plants Cope with Water Stress in the Field? Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Beis, A.; Patakas, A. Differences in stomatal responses and root to shoot signalling between two grapevine varieties subjected to drought. Funct. Plant Biol. 2010, 37, 139–146. [Google Scholar] [CrossRef]
- Ionenko, I.; Anisimov, A. Effect of Water Deficit and Membrane Destruction on Water Diffusion in the Tissues of Maize Seedlings. Biol. Plant. 2001, 44, 247–252. [Google Scholar] [CrossRef]
- García-Mata, C.; LaMattina, L. Nitric Oxide Induces Stomatal Closure and Enhances the Adaptive Plant Responses against Drought Stress. Plant Physiol. 2001, 126, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Mishra, D.; Shekhar, S.; Singh, D.; Chakraborty, S.; Chakraborty, N. “Heat shock proteins and abiotic stress tolerance in plants” in Regulation of Heat Shock Protein Responses. In Heat Shock Proteins; Asea, A., Kaur, P., Eds.; Springer: Cham, Switzerland, 2018; Volume 13. [Google Scholar]
- Mishra, D.; Shekhar, S.; Chakraborty, S.; Chakraborty, N. Wheat 2-Cys peroxiredoxin plays a dual role in chlorophyll biosynthesis and adaptation to high temperature. Plant J. 2021, 105, 1374–1389. [Google Scholar] [CrossRef]
- Rane, J.; Singh, A.K.; Tiwari, M.; Prasad, P.V.V.; Jagadish, S.V.K. Effective Use of Water in Crop Plants in Dryland Agriculture: Implications of Reactive Oxygen Species and Antioxidative System. Front. Plant Sci. 2022, 12, 778270. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Dawson, T.E.; Richards, J.H. Hydraulic lift: Consequences of water efflux from the roots of plants. Oecologia 1998, 113, 151–161. [Google Scholar] [CrossRef]
- Hamidi, S.; Pakzoki, A.; Asli, D.E. Effect of Drought stress, plant growth promoting Rhizobacteria (PGPR) and Humic acid on some physiological and agronomic traits in Shahriyar Herb Cilantro. Adv. Bio Res. 2015, 6, 123–128. [Google Scholar]
- Sekiya, N.; Araki, H.; Yano, K. Applying hydraulic lift in an agroecosystem: Forage plants with shoots removed supply water to neighboring vegetable crops. Plant Soil 2011, 341, 39–50. [Google Scholar] [CrossRef]
- Hallam, J.; Berdeni, D.; Grayson, R.; Guest, E.; Holden, J.; Lappage, M.G.; Prendergast-Miller, M.; Robinson, D.A.; Turner, A.; Leake, J.R.; et al. Effect of earthworms on soil physico-hydraulic and chemical properties, herbage production, and wheat growth on arable land converted to ley. Sci. Total. Environ. 2020, 713, 136491. [Google Scholar] [CrossRef] [PubMed]
- Deleon, E.; Bauder, T.A.; Wardle, E.; Fonte, S.J. Conservation tillage supports soil macrofauna communities, infiltration, and farm profits in an irrigated maize-based cropping system of Colorado. Soil Sci. Soc. Am. J. 2020, 84, 1943–1956. [Google Scholar] [CrossRef] [PubMed]
- Keplin, B.; Broll, G. Earthworm coenoses in wet grassland of Northwest-Germany.Effects of restoration management on a histosol and a gleysol. In Wetlands in Central Europe Soil Organisms, Soil Ecological Processes and Trace Gas Emissions; Springer: Berlin/Heidelberg, Germany, 2010; pp. 11–34. [Google Scholar]
- Kristensen, E.; Penha-Lopes, G.; Delefosse, M.; Valdemarsen, T.; Quintana, C.O.; Banta, G.T. What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar. Ecol. Prog. Ser. 2012, 446, 285–302. [Google Scholar] [CrossRef]
- Volkenborn, N.; Polerecky, L.; Wethey, D.S.; Woodin, S.A. Oscillatory porewater bioadvection in marine sediments induced by hydraulic activities of Arenicola marina. Limnol. Oceanogr. 2010, 55, 1231–1247. [Google Scholar] [CrossRef]
- Hirota, I.; Sakuratani, T.; Sato, T.; Higuchi, H.; Nawata, E. A split-root apparatus for examining the effects of hydraulic lift by trees on the water status of neighbouring crops. Agrofor. Syst. 2004, 60, 181–187. [Google Scholar] [CrossRef]
- Ludwig, F.; Dawson, T.E.; Prins, H.H.T.; Berendse, F.; de Kroon, H. Below-ground competition between trees and grasses may overwhelm the facilitative effects of hydraulic lift. Ecol. Lett. 2004, 7, 623–631. [Google Scholar] [CrossRef]
- Richards, J.H.; Caldwell, M.M. Hydraulic lift: Substantial nocturnal water transport between soil layers by Artemisia tridentata roots. Oecologia 1987, 73, 486–489. [Google Scholar] [CrossRef]
- Sekiya, N.; Yano, K. Do pigeon pea and sesbania supply groundwater to intercropped maize through hydraulic lift?—Hydrogen stable isotope investigation of xylem waters. Field Crop. Res. 2004, 86, 167–173. [Google Scholar] [CrossRef]
- Saharan, K.; Schütz, L.; Kahmen, A.; Wiemken, A.; Boller, T.; Mathimaran, N. Finger Millet Growth and Nutrient Uptake Is Improved in Intercropping with Pigeon Pea Through “Biofertilization” and “Bioirrigation” Mediated by Arbuscular Mycorrhizal Fungi and Plant Growth Promoting Rhizobacteria. Front. Environ. Sci. 2018, 6, 46. [Google Scholar] [CrossRef]
- Sharma, A.; Guled, M.B. Effect of set-furrow cultivation in pigeonpea + pearlmillet and pigeonpea + sesame intercropping systems in shallow black soil under rainfed conditions. Karnataka J. Agric. Sci. 2011, 24, 643–650. [Google Scholar]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, U. Drought-Tolerance of Wheat Improved by Rhizosphere Bacteria from Harsh Environments: Enhanced Biomass Production and Reduced Emissions of Stress Volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S. Rhizobacterial Application for Sustainable Water Management on the Areas of Limited Water Resources. Irrig. Drain. Syst. Eng. 2012, 1, 111. [Google Scholar] [CrossRef]
- Kim, Y.C.; Glick, B.; Bashan, Y.; Ryu, C.M. Enhancement of plant drought tolerance by microbes. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin, Germany, 2013. [Google Scholar]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef]
- Natarajan, M. Biofertilization and “Bioirrigation” for Sustainable Mixed Cropping of Pigeon Pea and Finger Millet (The BIOFI project-H43C-1505). University of Basel Research Institute of Organic Agriculture (FiBL): Frick, Switzerland, 2015; unpublished. [Google Scholar]
- Timmusk, S.; Nevo, E. Plant root associated biofilms. In Bacteria in Agrobiology Plant Nutrient Management; Maheshwari, D.K., Ed.; Springer: Berlin, Germany, 2011; Volume 3, pp. 285–300. [Google Scholar]
- Timmusk, S.; Timmusk, K.; Behers, L. Rhizobacterial plant drought stress tolerance enhancement. J. Food Secur. 2013, 1, 6–9. [Google Scholar] [CrossRef]
- Mantelin, S. Plant growth-promoting bacteria and nitrate availability: Impacts on root development and nitrate uptake. J. Exp. Bot. 2004, 55, 27–34. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: Emergence of a biochemical role of a family of plant dehydration proteins. Physiol. Plant. 1996, 97, 795–803. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M. Induction of plant tolerance to semi-arid environments by beneficial soil microorganisms—A review. In Climate Change, Intercropping, Pest Control and Beneficial Microorganisms; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 121–135. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.-F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef]
- Han, H.; Supanjani, S.; Lee, K. Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptáme and growth of pepper and cucumber. Plant Soil Environ. 2006, 52, 130–136. [Google Scholar] [CrossRef]
- Kalra, A. Organic cultivation of Medicinal and aromatic plants. A hope for sustainability and quality enhancement. In Journal of Organic Production of Medicinal, Aromatic and Dye-Yielding Plants; FAO: Rome, Italy, 2003; p. 198. [Google Scholar]
- Auge, R.M.; Toler, H.D.; Sams, C.E.; Nasim, G. Hydraulic conductance and water potential gradients in squash leaves showing mycorrhiza-induced increases in stomatal conductance. Mycorrhiza 2008, 18, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R.; Porcel, R.; Ruiz-Lozan, J.M. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef]
- Belimov, A.A.; Dodd, I.C.; Hontzeas, N.; Theobald, J.C.; Safronova, V.I.; Davies, W.J. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol. 2009, 181, 413–423. [Google Scholar] [CrossRef]
- Vikram, K.; Meena, S.; Kumar, S.; Ranjan, R.; Nivetha, N.; Paul, S. Influence of medium-term application of rhizobacteria on mustard yield and soil properties under different irrigation systems. Rhizosphere 2022, 24, 100608. [Google Scholar] [CrossRef]
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 2009, 87, 455–462. [Google Scholar] [CrossRef]
- Kang, S.-M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.-J.; Park, J.-M.; Kim, B.-R.; Shin, D.-H.; Lee, I.-J. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.B.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. Exo polysaccharides producing rhizobia ameliorate drought stress in wheat. Int. J. Agric. Biol. 2014, 16, 3–13. [Google Scholar]
- Cura, J.A.; Franz, D.R.; Filosofía, J.E.; Balestrasse, K.B.; Burgueño, L.E. Inoculation with Azospirillum sp. and Herbaspirillum sp. Bacteria Increases the Tolerance of Maize to Drought Stress. Microorganisms 2017, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Chen, L.; Belimov, A.A.; Shaposhnikov, A.I.; Gong, F.; Meng, X.; Hartung, W.; Jeschke, D.W.; Davies, W.J.; Dodd, I.C. Multiple impacts of the plant growth-promoting rhizobacterium Variovorax paradoxus 5C-2 on nutrient and ABA relations of Pisum sativum. J. Exp. Bot. 2012, 63, 6421–6430. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.-L.; Luo, L. Effects of Engineered Sinorhizobium meliloti on Cytokinin Synthesis and Tolerance of Alfalfa to Extreme Drought Stress. Appl. Environ. Microbiol. 2012, 78, 8056–8061. [Google Scholar] [CrossRef]
- Lamande, M.; Hallaire, V.; Curmi, P.; Pérès, G.; Cluzeau, D. Changes of pore morphology, infiltration and earthworm community in a loamy soil under different agricultural managements. Catena 2003, 54, 637–649. [Google Scholar] [CrossRef]
- Blouin, M.; Hodson, M.; Delgado, E.A.; Baker, G.L.; Brussaard, L.; Butt, K.; Dai, J.; Dendooven, L.; Peres, G.; Tondoh, J.E.; et al. A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef]
- Hallam, J.; Holden, J.; Robinson, D.A.; Hodson, M.E. Effects of winter wheat and endogeic earthworms on soil physical and hydraulic properties. Geoderma 2021, 400, 115126. [Google Scholar] [CrossRef]
- Bastardie, F.; Cluzeau, D. Assessment of earthworm contribution to soil hydrology: A laboratory method to measure water diffusion through burrow walls. Biol. Fertil. Soils 2005, 41, 124–128. [Google Scholar] [CrossRef]
- Smagin, A.; Prusak, A.V. The effect of earthworm coprolites on the soil water retention curve. Eurasian Soil Sci. 2008, 41, 618–622. [Google Scholar] [CrossRef]
- Tang, X.; Jiang, Z.; Jiang, S.; Wang, P.; Xiang, C. Effect of Organic Matter and Maturity on Pore Size Distribution and Gas Storage Capacity in High-Mature to Post-Mature Shales. Energy Fuels 2016, 30, 8985–8996. [Google Scholar] [CrossRef]
- Amer, A.M.; Suarez, C.; Valverde, F.; Carranza, R.; Matute, L.; Delfini, G. Saturated Hydraulic Conductivity Changes with Time and Its Prediction at SAR and Salinity in Quevedo Region Soils. J. Water Resour. Prot. 2014, 6, 1561. [Google Scholar] [CrossRef]
- van Groenigen, J.W.; Lubbers, I.M.; Vos, H.M.J.; Brown, G.G.; De Deyn, G.B.; van Groenigen, K.J. Earthworms increase plant production: A meta-analysis. Sci. Rep. 2014, 4, 6365. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. Eur. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Deru, J.; Schilder, H.; Van der Schoot, J.R.; Van Eekeren, N.; Roldán-Ruiz, I.; Baert, J.; Reheul, D. No Trade-Off between Root Biomass and Aboveground Production in Lolium Perenne; Springer International Publishing: Cham, Switzerland, 2016; pp. 289–292. [Google Scholar]
- Wyngaarden, S.L.; Gaudin, A.C.; Deen, W.; Martin, R.C. Expanding Red Clover (Trifolium pratense) Usage in the Corn–Soy–Wheat Rotation. Sustainability 2015, 7, 15487–15509. [Google Scholar] [CrossRef]
- Bodner, G.; Leitner, D.; Kaul, H.-P. Coarse and fine root plants affect pore size distributions differently. Plant Soil 2014, 380, 133–151. [Google Scholar] [CrossRef]
- Zangiabadi, M.; Gorji, M.; Shorafa, M.; Khorasani, S.K.; Saadat, S. Effect of soil pore size distribution on plant-available water and least limiting water range as soil physical quality indicators. Pedosphere 2017, 30, 253–262. [Google Scholar] [CrossRef]
- Aller, R.C.; Yingst, J.Y.; Ullman, W.J. Comparative biogeochemistry of water in intertidal Onuphis (Polychaeta) and Upoqebia (Crustacea) burrows: Temporal patterns and causes. J. Mar. Res. 1983, 41, 571–604. [Google Scholar] [CrossRef]
- Dobbs, F.; Guckert, J. Callianassa trilobata (Crustacea: Thalassinidea) influences abundance of meiofauna and biomass, composition, and physiologic state of microbial communities within its burrow. Mar. Ecol. Prog. Ser. 1988, 45, 69–79. [Google Scholar] [CrossRef]
- Halverson, L.J. Role of alginate in bacterial biofilms. In Alginates: Biology and Applications; Rehm, B.H.A., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 136–141. [Google Scholar]
- Donati, I.; Paoletti, S. Material properties of alginates. In Alginates: Biology and Applications; Rehm, B.H.A., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 1–54. [Google Scholar]
- Hay, I.D.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial alginate production, modification and its applications. Microb. Biotechnol. 2013, 6, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Gashash, E.A.; Osman, N.A.; Alsahli, A.A.; Hewait, H.M.; Ashmawi, A.E.; Alshallash, K.S.; El-Taher, A.M.; Azab, E.S.; Abd El-Raouf, H.S.; Ibrahim, M.F.M. Effects of Plant-Growth-Promoting Rhizobacteria (PGPR) and Cyanobacteria on Botanical Characteristics of Tomato (Solanum lycopersicon L.) Plants. Plants 2022, 11, 2732. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.R.; Brunetti, C.; Khalid, A.; Della Rocca, G.; Raio, A.; Emiliani, G.; DE Carlo, A.; Mahmood, T.; Centritto, M. Drought response of Mucuna pruriens (L.) DC. inoculated with ACC deaminase and IAA producing rhizobacteria. PLoS ONE 2018, 13, e0191218. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Khan, M.S.; El-Beltagi, H.S.; Umar, S.; Lee, J. Bioprospecting Plant Growth Promoting Rhizobacteria for Enhancing the Biological Properties and Phytochemical Composition of Medicinally Important Crops. Molecules 2022, 27, 1407. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Smith, D.M.S.; Lambin, E.F.; Turner, B.L.; Mortimore, M.; Batterbury, S.P.; Downing, T.E.; Dowlatabadi, H.; Fernández, R.J.; Herrick, J.E.; et al. Global Desertification: Building a Science for Dryland Development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajanna, G.A.; Suman, A.; Venkatesh, P. Mitigating Drought Stress Effects in Arid and Semi-Arid Agro-Ecosystems through Bioirrigation Strategies—A Review. Sustainability 2023, 15, 3542. https://doi.org/10.3390/su15043542
Rajanna GA, Suman A, Venkatesh P. Mitigating Drought Stress Effects in Arid and Semi-Arid Agro-Ecosystems through Bioirrigation Strategies—A Review. Sustainability. 2023; 15(4):3542. https://doi.org/10.3390/su15043542
Chicago/Turabian StyleRajanna, Gandhamanagenahalli A., Archna Suman, and Paramesha Venkatesh. 2023. "Mitigating Drought Stress Effects in Arid and Semi-Arid Agro-Ecosystems through Bioirrigation Strategies—A Review" Sustainability 15, no. 4: 3542. https://doi.org/10.3390/su15043542
APA StyleRajanna, G. A., Suman, A., & Venkatesh, P. (2023). Mitigating Drought Stress Effects in Arid and Semi-Arid Agro-Ecosystems through Bioirrigation Strategies—A Review. Sustainability, 15(4), 3542. https://doi.org/10.3390/su15043542






