Effect of Carbide Slag Combined with Biochar on Improving Acidic Soil of Copper Sulfide Mines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Soil and Amendments
2.2. Soil Culture and Potting Test
2.3. Chemical Analysis
2.4. Statistical Analysis Methods
3. Results
3.1. Properties Possessed by Acidic Soil of Copper Sulfide Mines and Amendments
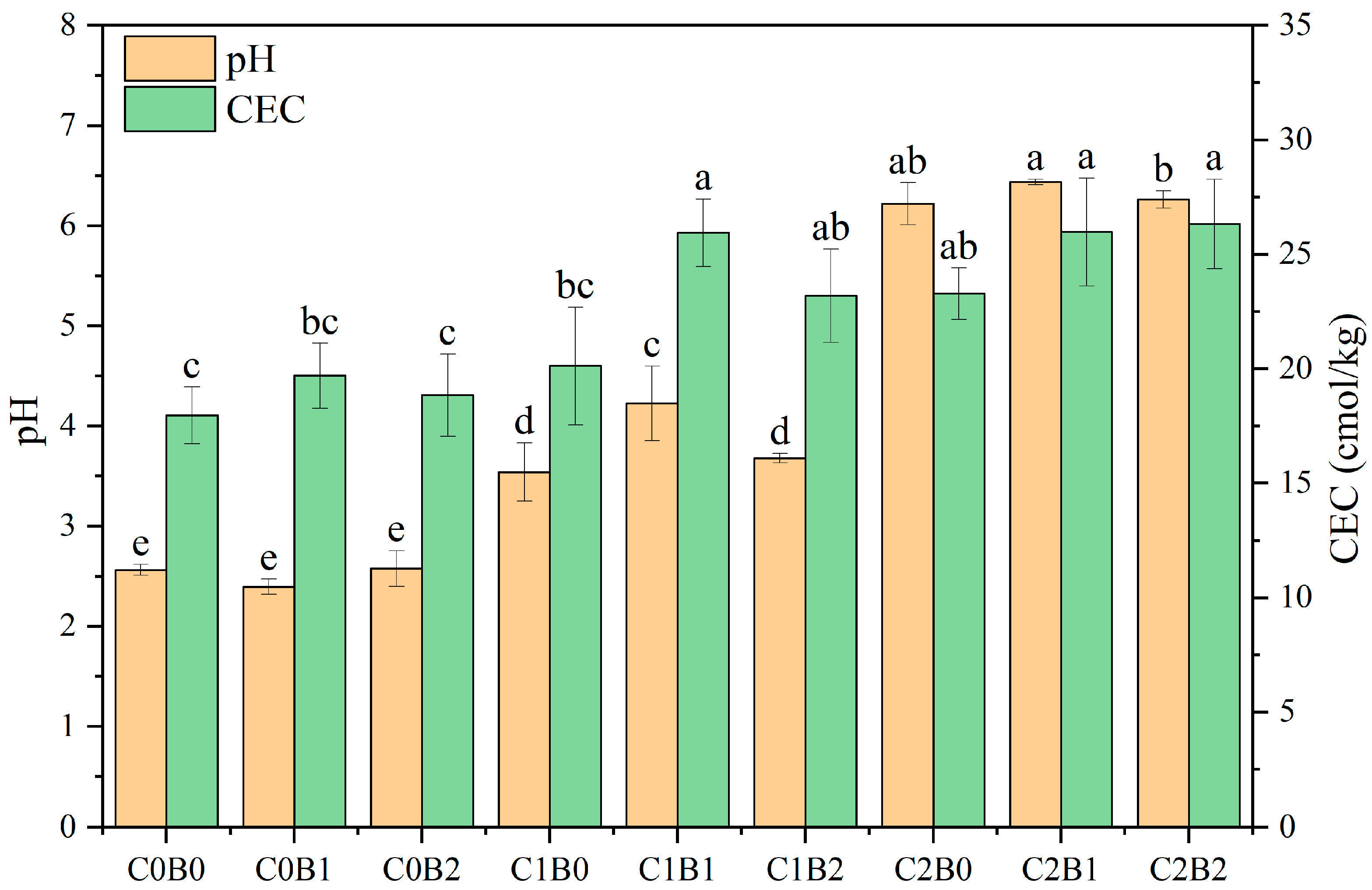
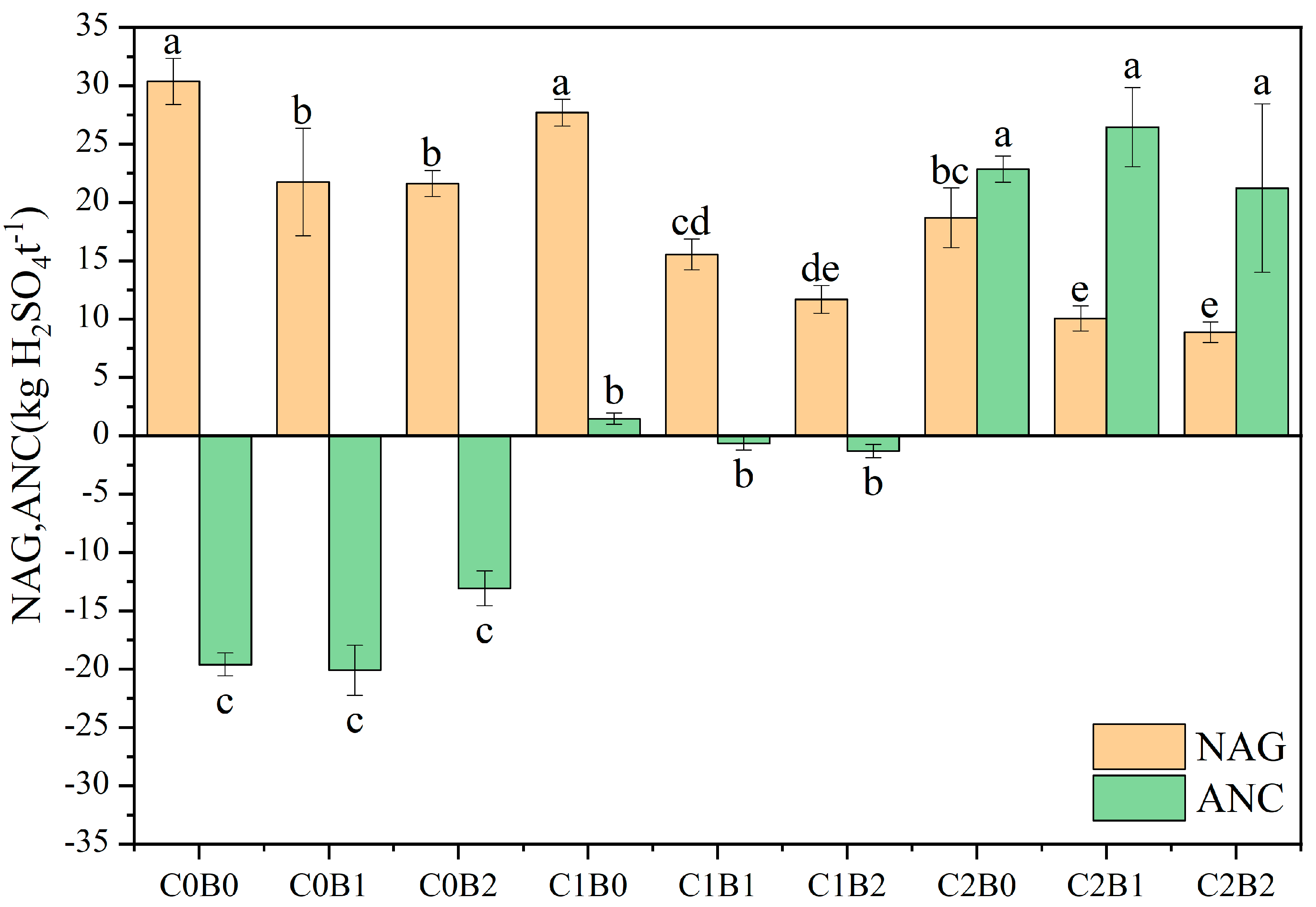
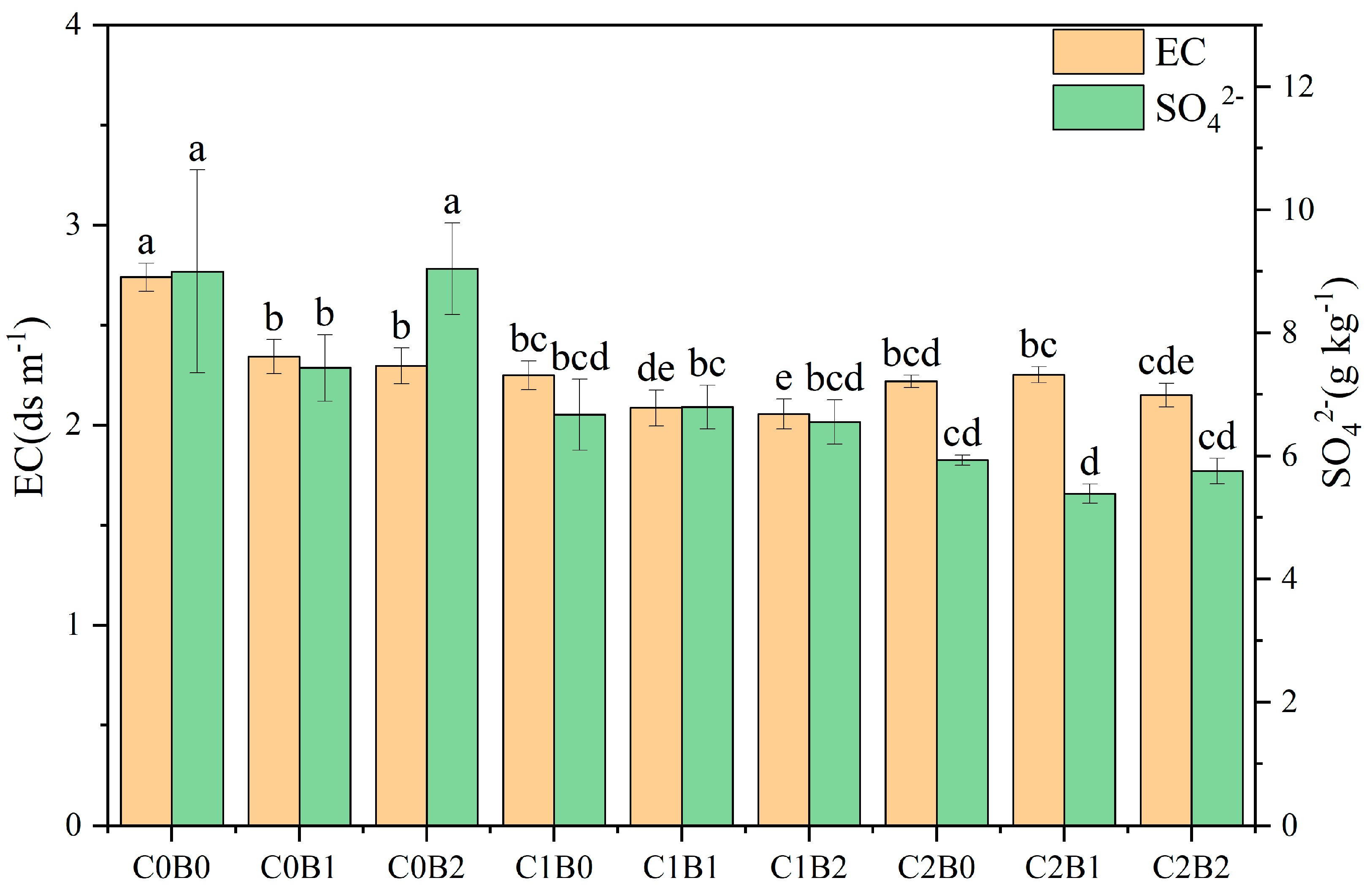
3.2. Changes in Physicochemical Properties Possessed by Acidic Soil of Copper Sulfide Mines
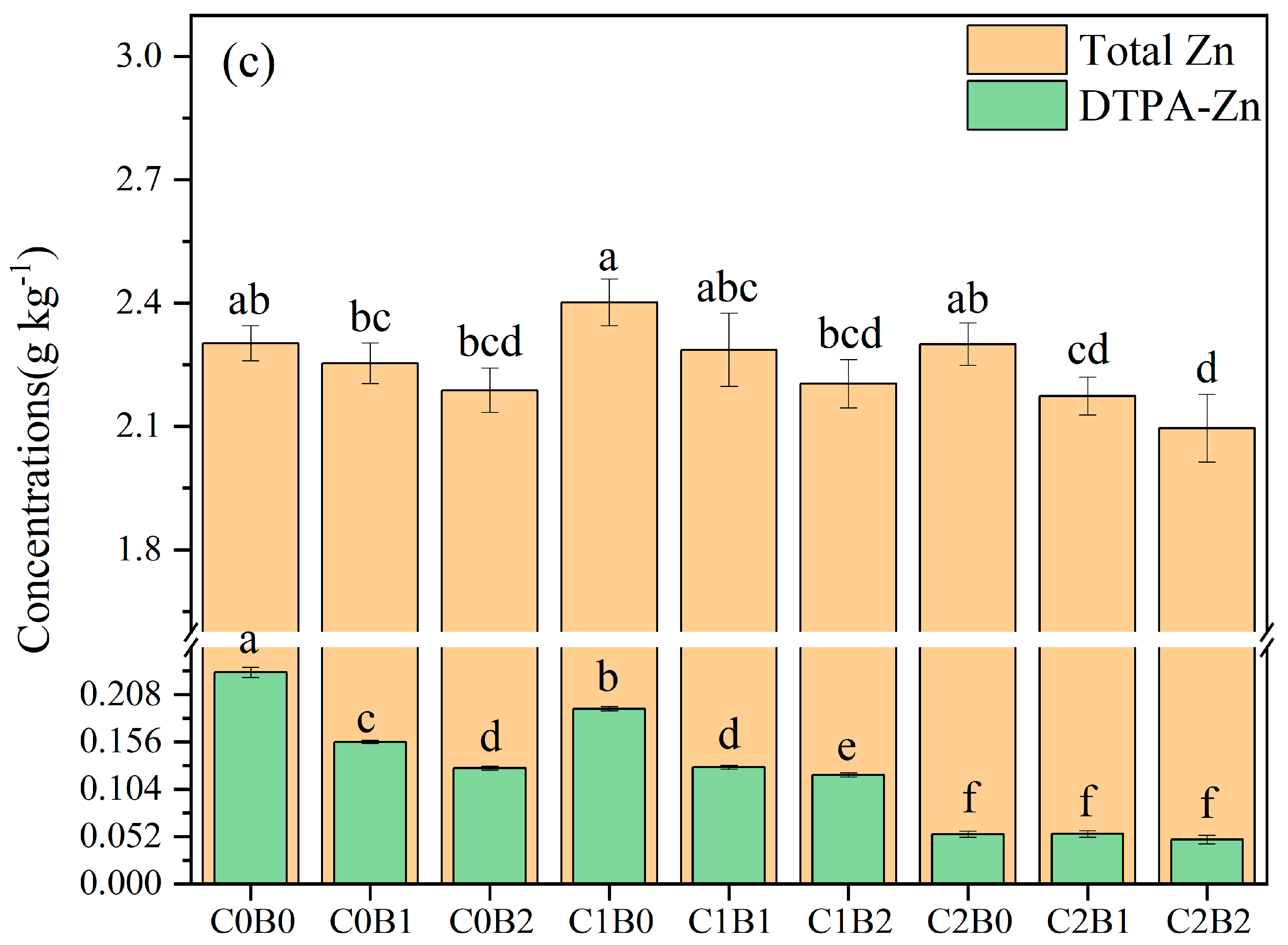
3.3. Changes in Heavy Metal Concentrations in Acidic Soils of Copper Sulfide Mines
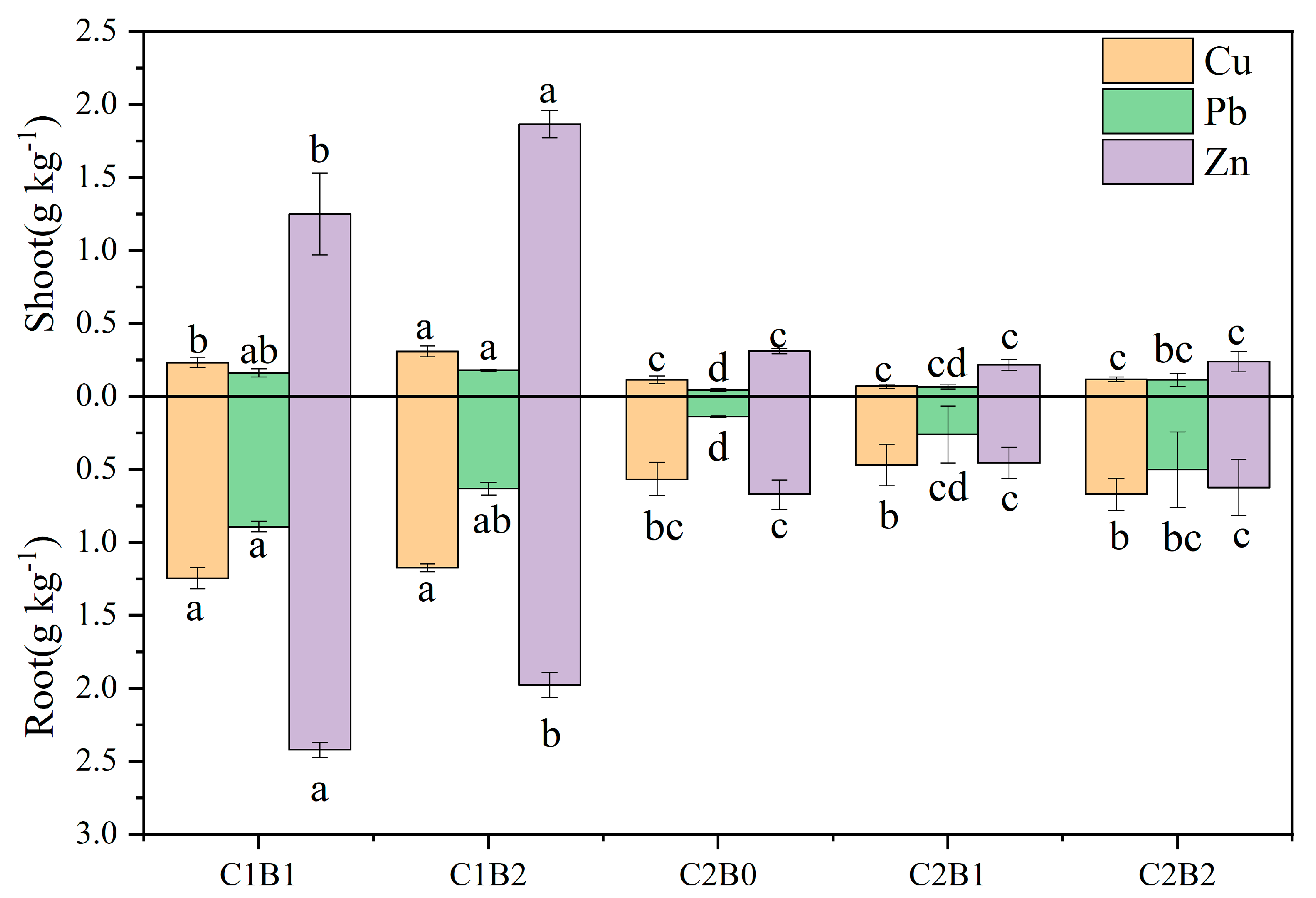
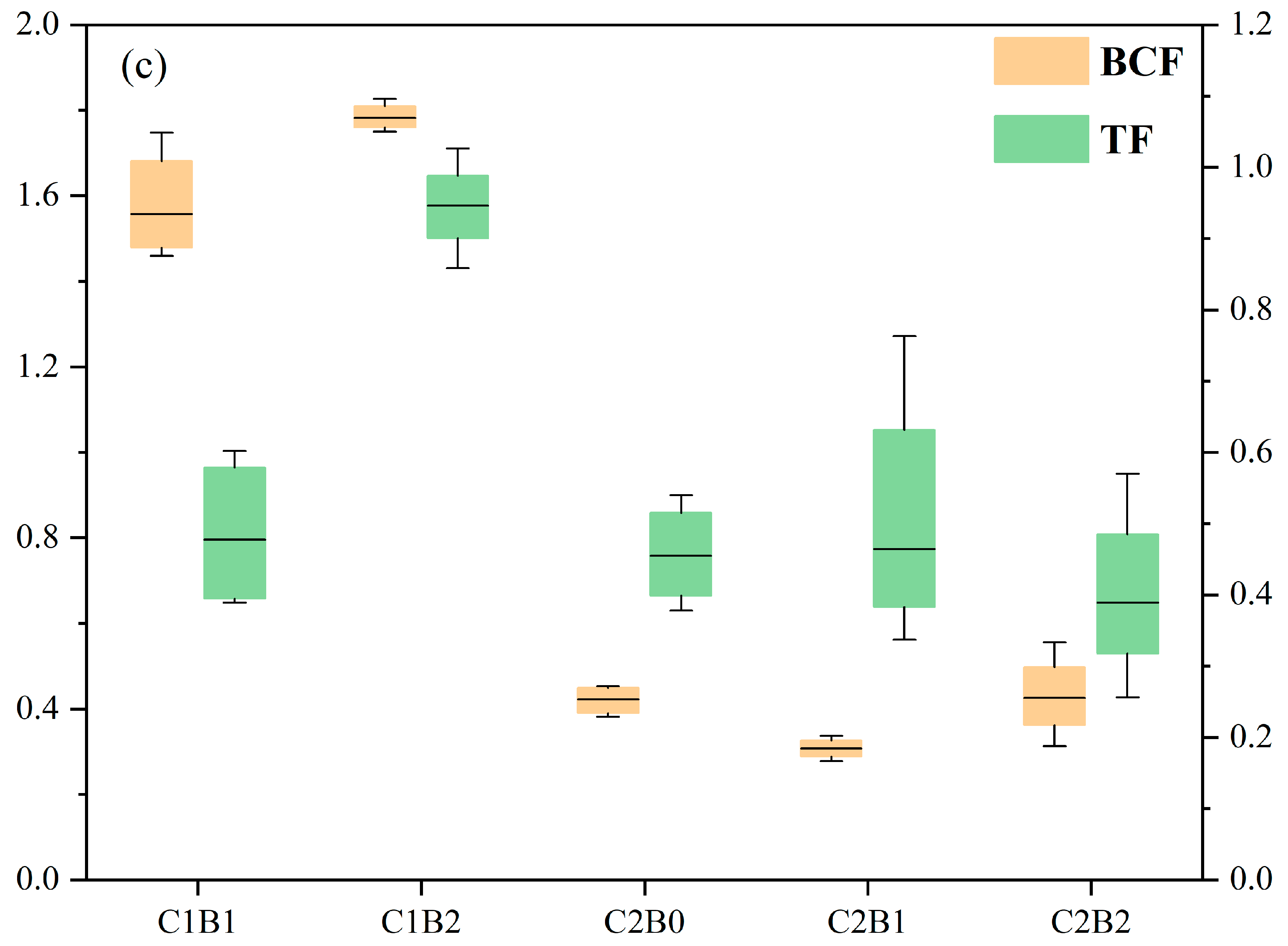
3.4. Accumulation and Transport of Heavy Metals in Ryegrass
4. Discussion
4.1. Effect of Amendments on Soil Acidity Potential
4.2. Effect of Amendments on the Toxicity of Heavy Metals in Soil
4.3. Transport and Accumulation of Heavy Metals in Ryegrass
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hilson, G.; Murck, B. Sustainable development in the mining industry: Clarifying the corporate perspective. Resour. Policy 2000, 26, 227–238. [Google Scholar] [CrossRef]
- Yousfi, R.; El Ouaer, Z.; Dahri, N.; Ouddane, B.; Rigane, H. Evaluating the Heavy Metals-Associated Ecological Risks in Soil and Sediments of a Decommissioned Tunisian Mine. Pol. J. Environ. Stud. 2019, 28, 2981–2993. [Google Scholar] [CrossRef] [PubMed]
- Klik, B.K.; Gusiatin, Z.M.; Kulikowska, D. Suitability of environmental indices in assessment of soil remediation with conventional and next generation washing agents. Sci. Rep. 2020, 10, 20586. [Google Scholar] [CrossRef] [PubMed]
- Hakkou, R.; Benzaazoua, M.; Bussière, B. Acid Mine Drainage at the Abandoned Kettara Mine (Morocco): Environmental Characterization. Mine Water Environ. 2008, 27, 145–159. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, M.; Lin, H.; Gao, P.; Yang, Z.; Wang, D.; Sun, X.; Li, B.; Wang, Q.; Sun, W. Response of soil protozoa to acid mine drainage in a contaminated terrace. J. Hazard. Mater. 2021, 421, 126790. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Franks, D.; Boger, D.V.; Côte, C.M.; Mulligan, D.R. Sustainable development principles for the disposal of mining and mineral processing wastes. Resour. Policy 2011, 36, 114–122. [Google Scholar] [CrossRef]
- Waterland, L.R.; King, C.; Richards, M.K.; Thurnau, R.C. Incineration treatment of arsenic-contaminated soil. Remediat. J. 1991, 1, 227–237. [Google Scholar] [CrossRef]
- Piepel, G.F.; Shade, J.W. In Situ Vitrification and the Effects of Soil Additives-A Mixture Experiment Case Study. J. Am. Ceram. Soc. 1992, 75, 112–116. Available online: https://www.researchgate.net/publication/318447223_In-Situ_Vitrification_and_the_Effects_of_Soil_Additives-A_Mixture_Experiment_Case_Study (accessed on 15 November 2022). [CrossRef]
- Dermatas, D.; Meng, X. Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils. Eng. Geol. 2003, 70, 377–394. [Google Scholar] [CrossRef]
- Brown, S.; Sprenger, M.; Maxemchuk, A.; Compton, H. Ecosystem Function in Alluvial Tailings after Biosolids and Lime Addition. J. Environ. Qual. 2005, 34, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Néel, C.; Bril, H.; Courtin-Nomade, A.; Dutreuil, J.-P. Factors affecting natural development of soil on 35-year-old sulphide-rich mine tailings. Geoderma 2003, 111, 1–20. [Google Scholar] [CrossRef]
- Fedje, K.K.; Yillin, L.; Strömvall, A.-M. Remediation of metal polluted hotspot areas through enhanced soil washing–Evaluation of leaching methods. J. Environ. Manag. 2013, 128, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Cameselle, C.; Chirakkara, R.A.; Reddy, K.R. Electrokinetic-enhanced phytoremediation of soils: Status and opportunities. Chemosphere 2013, 93, 626–636. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Mokolobate, M.S.; Haynes, R.J. Comparative liming effect of four organic residues applied to an acid soil. Biol. Fertil. Soils 2002, 35, 79–85. [Google Scholar]
- RoyChowdhury, A.; Sarkar, D.; Datta, R. A combined chemical and phytoremediation method for reclamation of acid mine drainage–impacted soils. Environ. Sci. Pollut. Res. 2019, 26, 14414–14425. [Google Scholar] [CrossRef]
- Yu, H.; Ma, J.; Chen, F.; Zhang, Q.; Wang, Y.; Bian, Z. Effective remediation of electronic waste contaminated soil by the combination of metal immobilization and phytoremediation. J. Environ. Chem. Eng. 2022, 10, 107410. [Google Scholar] [CrossRef]
- International Biochar Initiative (IBI). Standardized Product Definition and Product Testing Guidelines for Biochar that is Used in Soil; International Biochar Initiative (IBI): Princeton Junction, NJ, USA, 2012. [Google Scholar]
- Pandey, D.; Daverey, A.; Arunachalam, K. Biochar: Production, properties and emerging role as a support for enzyme immobilization. J. Clean. Prod. 2020, 255, 120267. [Google Scholar] [CrossRef]
- He, M.; Xiong, X.; Wang, L.; Hou, D.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Tsang, D.C. A critical review on performance indicators for evaluating soil biota and soil health of biochar-amended soils. J. Hazard. Mater. 2021, 414, 125378. [Google Scholar] [CrossRef]
- Jia, M.; Wang, F.; Bian, Y.; Jin, X.; Song, Y.; Kengara, F.O.; Xu, R.; Jiang, X. Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar. Bioresour. Technol. 2013, 136, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Yu, J.; Li, Z.; Wu, L.; Christie, P. Effects of biochar on the migration and transformation of metal species in a highly acid soil contaminated with multiple metals and leached with solutions of different pH. Chemosphere 2021, 278, 130344. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vila, A.; Covelo, E.F.; Forján, R.; Asensio, V. Recovering a copper mine soil using organic amendments and phytomanagement with Brassica juncea L. J. Environ. Manag. 2015, 147, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.A.M.; Liu, G.; Yousaf, B.; Mian, M.; Ali, M.U.; Ahmed, R.; Cheema, A.I.; Naushad, M. Contrasting effects of biochar and hydrothermally treated coal gangue on leachability, bioavailability, speciation and accumulation of heavy metals by rapeseed in copper mine tailings. Ecotoxicol. Environ. Saf. 2020, 191, 110244. [Google Scholar] [CrossRef]
- Hartley, W.; Dickinson, N.M.; Riby, P.; Lepp, N.W. Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ. Pollut. 2009, 157, 2654–2662. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.-W. Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.S.; Pei, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Sun, R.; Li, Y.; Zhao, J.; Liu, C.; Lu, C. CO2 capture using carbide slag modified by propionic acid in calcium looping process for hydrogen production. Int. J. Hydrogen Energy 2013, 38, 13655–13663. [Google Scholar] [CrossRef]
- Meng, J.; Zhong, L.; Wang, L.; Liu, X.; Tang, C.; Chen, H.; Xu, J. Contrasting effects of alkaline amendments on the bioavailability and uptake of Cd in rice plants in a Cd-contaminated acid paddy soil. Environ. Sci. Pollut. Res. 2018, 25, 8827–8835. [Google Scholar] [CrossRef]
- Sudmeyer, R. Climate in the Pilbara; Department of Agriculture and Food: Western Australia, Perth, Australia, 2016. [Google Scholar]
- Shu, W.; Ye, Z.; Lan, C.; Zhang, Z.; Wong, M. Acidification of lead/zinc mine tailings and its effect on heavy metal mobility. Environ. Int. 2001, 26, 389–394. [Google Scholar] [CrossRef]
- Meng, Y.-N.; Li, T.-P.; Shi, Z.; Cai, J.-P.; Xu, Z.-W.; Jiang, Y. Effects of fertilization and water addition on soil acid neutralizing capacity in an old-field grassland. J. Appl. Ecol. 2020, 31, 1579–1586. [Google Scholar]
- Bao, S. Soil and Agricultural Chemistry Analysis China. Available online: https://www.researchgate.net/publication/301822463_Soil_and_agricultural_chemistry_analysis (accessed on 15 November 2022).
- Rayment, G.E.; Lyons, D.J. New, Comprehensive Soil Chemical Methods Book for Australasia. Commun. Soil Sci. Plant Anal. 2012, 43, 412–418. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytostabilization of Mine Tailings in Arid and Semiarid Environments—An Emerging Remediation Technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Meng, J.; Huang, Y.; Dai, Z.; Tang, C.; Xu, J. Effects of carbide slag, lodestone and biochar on the immobilization, plant uptake and translocation of As and Cd in a contaminated paddy soil. Environ. Pollut. 2020, 266, 115194. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-L.; Qiu, Y.; Xu, X.; Yang, F.; Wang, Z.; Feng, J.; Wang, J. EDTA-enhanced phytoremediation of heavy metals from sludge soil by Italian ryegrass (Lolium perenne L.). Ecotoxicol. Environ. Saf. 2020, 191, 110185. [Google Scholar] [CrossRef]
- Curtin, D.; Trolove, S. Predicting pH buffering capacity of New Zealand soils from organic matter content and mineral characteristics. Soil Res. 2013, 51, 494–502. [Google Scholar] [CrossRef]
- Plante, B.; Bussière, B.; Benzaazoua, M. Static tests response on 5 Canadian hard rock mine tailings with low net acid-generating potentials. J. Geochem. Explor. 2012, 114, 57–69. [Google Scholar] [CrossRef]
- Yang, S.X.; Li, J.T.; Yang, B.; Liao, B.; Zhang, J.T.; Shu, W.S. Effectiveness of amendments on re-acidification and heavy metal immobilization in an extremely acidic mine soil. J. Environ. Monit. 2011, 13, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.; Bennett, A.; Newton, A.; White, P.; McKenzie, B.; George, T.; Pakeman, R.; Bailey, J.; Fornara, D.; Hayes, R. Liming impacts on soils, crops and biodiversity in the UK: A review. Sci. Total. Environ. 2018, 610–611, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Caliani, J.; Barba-Brioso, C. Metal immobilization in hazardous contaminated minesoils after marble slurry waste application. A field assessment at the Tharsis mining district (Spain). J. Hazard. Mater. 2010, 181, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Tahervand, S.; Jalali, M. Sorption, desorption, and speciation of Cd, Ni, and Fe by four calcareous soils as affected by pH. Environ. Monit. Assess. 2016, 188, 322. [Google Scholar] [CrossRef]
- Appel, C.; Ma, L. Concentration, pH, and Surface Charge Effects on Cadmium and Lead Sorption in Three Tropical Soils. J. Environ. Qual. 2002, 31, 581–589. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, R.; Ji, S.; Xie, L.; Zhang, H. Effects of biochar derived from sewage sludge and sewage sludge/cotton stalks on the immobilization and phytoavailability of Pb, Cu, and Zn in sandy loam soil. J. Hazard. Mater. 2021, 419, 126468. [Google Scholar] [CrossRef]
- Soria, R.I.; Rolfe, S.A.; Betancourth, M.P.; Thornton, S.F. The relationship between properties of plant-based biochars and sorption of Cd(II), Pb(II) and Zn(II) in soil model systems. Heliyon 2020, 6, e05388. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Nandillon, R.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Biochar effect associated with compost and iron to promote Pb and As soil stabilization and Salix viminalis L. growth. Chemosphere 2019, 222, 810–822. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]



| S | C | B | |
|---|---|---|---|
| pH | 2.43 ± 0.08 | 13.21 ± 0.22 | 9 ± 0.12 |
| TOC (g kg−1) | 14.67 ± 0.39 | 4.65 ± 0.29 | 35.74 ± 0.95 |
| Na2O (%) | 0.25 | 0.49 | 0.79 |
| Al2O3 (%) | 14.84 | 0.98 | 20.48 |
| SiO2 (%) | 27.41 | 2.35 | 57.31 |
| SO3 (%) | 5.19 | 0.47 | 1.27 |
| CaO (%) | 2.41 | 93.92 | 4.54 |
| P2O5 (%) | 0.29 | 0.09 | 0.20 |
| K2O (%) | 1.25 | 0.09 | 2.95 |
| SO3 (%) | 5.19 | 0.47 | 1.27 |
| Fe2O3 (%) | 42.79 | 0.49 | 10.81 |
| CuTotal (mg kg−1) | 2233.5 ± 30.3 | 48.7 ± 2.2 | 17.1 ± 2.6 |
| ZnTotal | 2367.8 ± 23.3 | 70.3 ± 0.9 | 29.1 ± 1.5 |
| PbTotal | 2736.7 ± 17.0 | ND | 90.3 ± 1.1 |
| CrTotal | 32.5 ± 1.4 | ND | 26.5 ± 2.8 |
| TiTotal | 38.2 ± 6.5 | ND | ND |
| Treatment | Carbide Slag (w/w) | Biochar (w/w) |
|---|---|---|
| COB0 | 0 | 0 |
| C0B1 | 0 | 5% |
| C0B2 | 0 | 10% |
| C1B0 | 1% | 0 |
| C1B1 | 1% | 5% |
| C1B2 | 1% | 10% |
| C2B0 | 2% | 0 |
| C2B1 | 2% | 5% |
| C2B2 | 2% | 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Xue, J.; Zhang, X.; Wang, X.; Huang, J.; Dai, X. Effect of Carbide Slag Combined with Biochar on Improving Acidic Soil of Copper Sulfide Mines. Sustainability 2023, 15, 3206. https://doi.org/10.3390/su15043206
Han H, Xue J, Zhang X, Wang X, Huang J, Dai X. Effect of Carbide Slag Combined with Biochar on Improving Acidic Soil of Copper Sulfide Mines. Sustainability. 2023; 15(4):3206. https://doi.org/10.3390/su15043206
Chicago/Turabian StyleHan, Huaqin, Jinchun Xue, Xiao Zhang, Xiaojuan Wang, Jiaxing Huang, and Xun Dai. 2023. "Effect of Carbide Slag Combined with Biochar on Improving Acidic Soil of Copper Sulfide Mines" Sustainability 15, no. 4: 3206. https://doi.org/10.3390/su15043206
APA StyleHan, H., Xue, J., Zhang, X., Wang, X., Huang, J., & Dai, X. (2023). Effect of Carbide Slag Combined with Biochar on Improving Acidic Soil of Copper Sulfide Mines. Sustainability, 15(4), 3206. https://doi.org/10.3390/su15043206






