A Sustainable Approach towards the Restoration of Lead-Contaminated Soils through Nutrient-Doped Olive Waste-Derived Biochar Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Preparation
2.2. Synthesis of Silica Embedded Biochar
2.3. Characterization
2.4. Collection and Characterization of Soil
2.5. Incubation Trials
2.6. Statistical Analysis
3. Results and Discussion
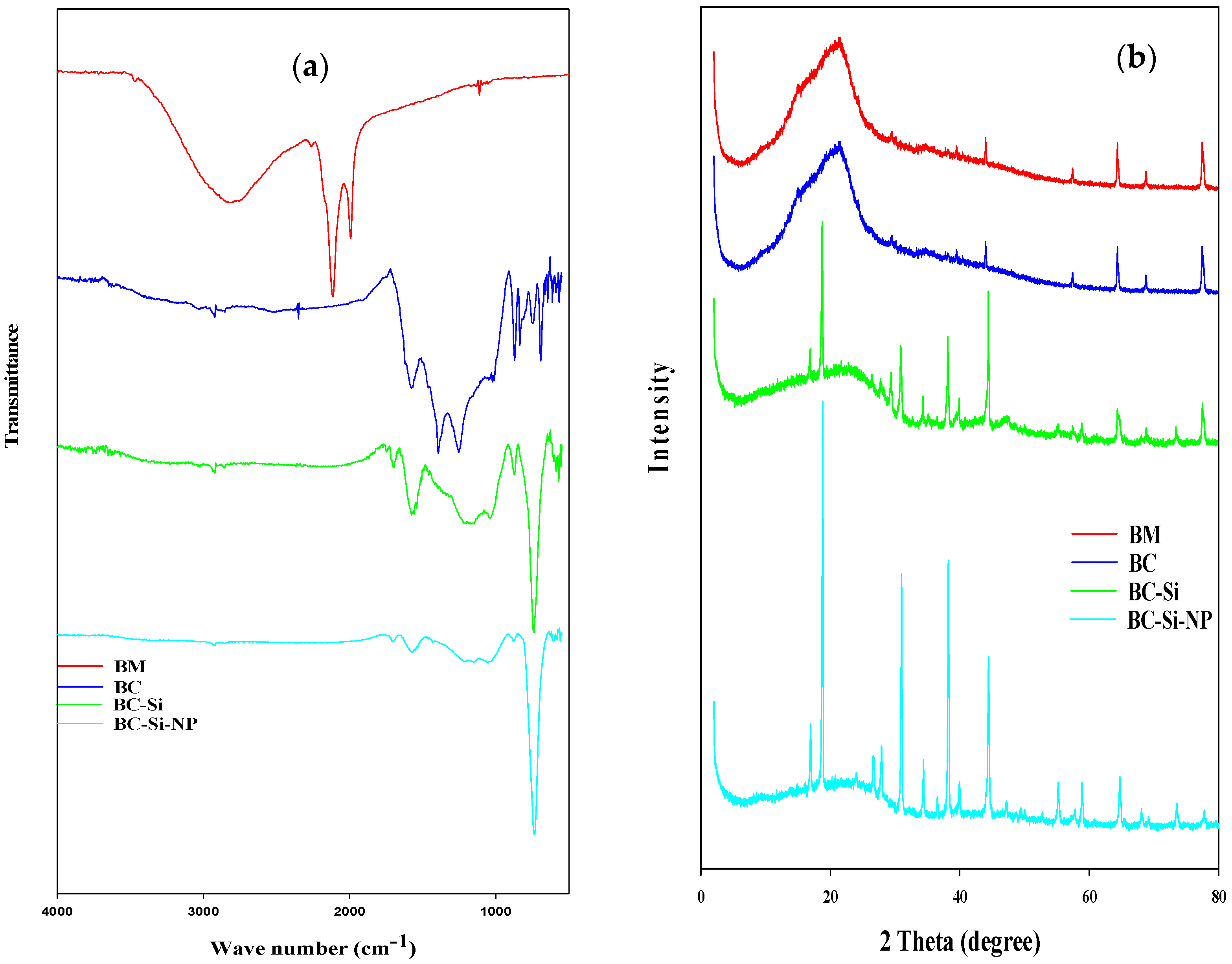
3.1. Characterization of Produced and Fabricate Biochars
3.2. Soil Characterization
3.2.1. Treatment Effects on Available Heavy Metals
3.2.2. Treatment Effects on Soil pH and EC
3.3. Kinetic Experiment
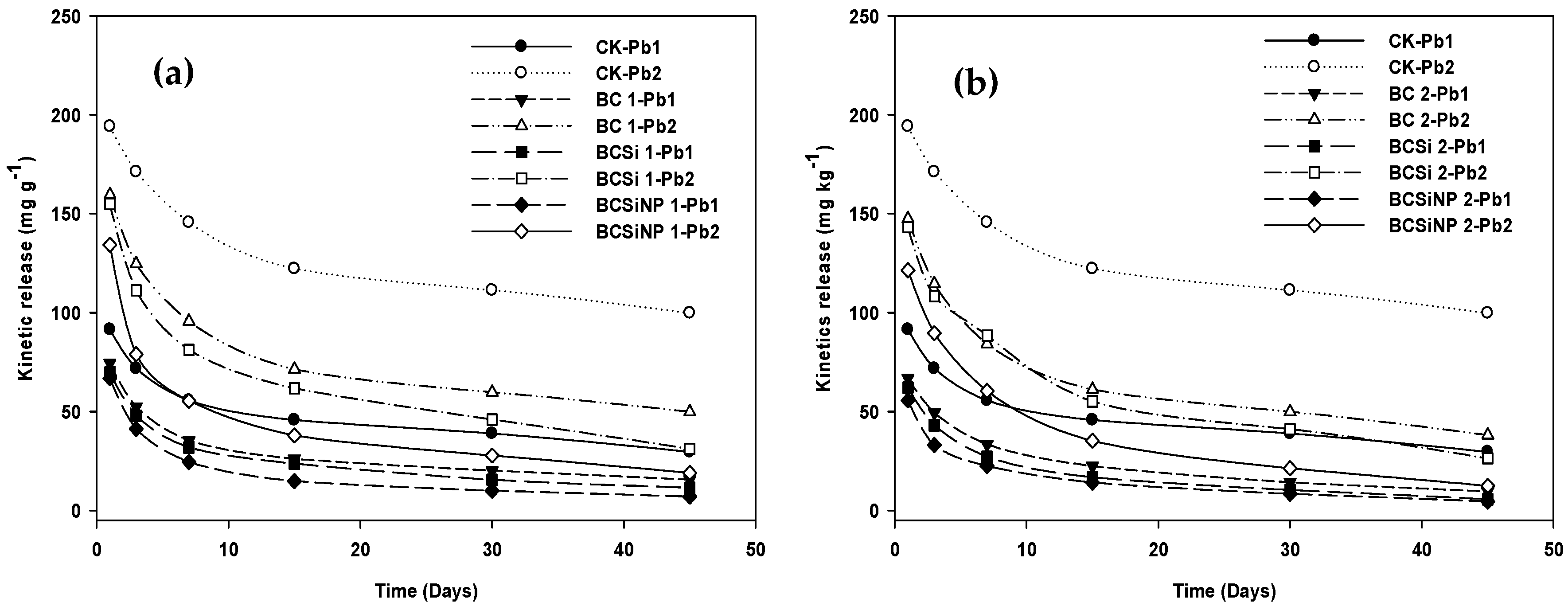
3.3.1. Kinetic Release of Pb
3.3.2. Adsorption Mechanism
3.4. Kinetics of Nutrient Release
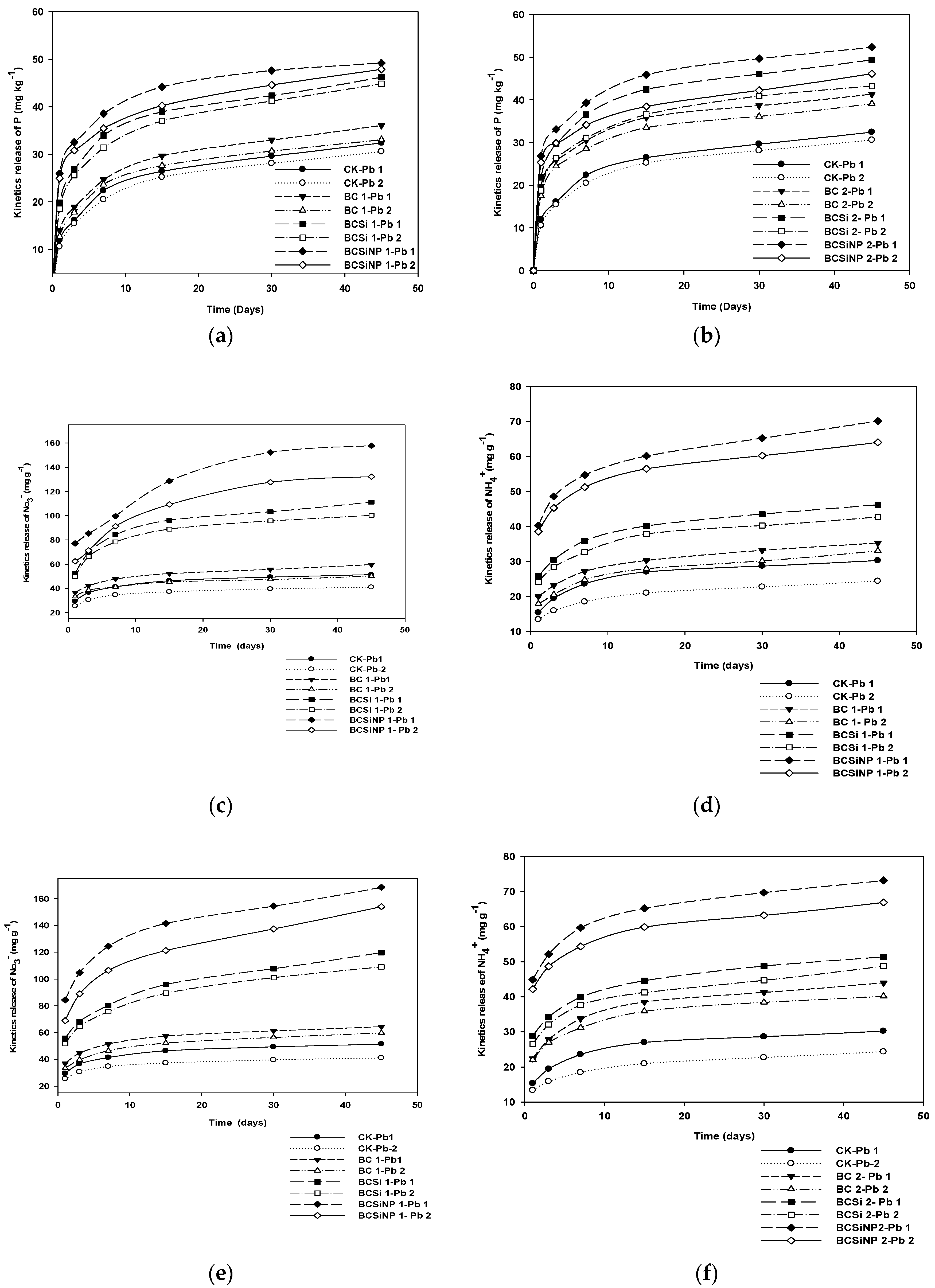
3.4.1. Kinetic Release of P and Total N (NO3− and NH4+)
3.4.2. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pepper, I.L.; Gerba, C.P.; Newby, D.T.; Rice, C.W. Soil: A public health threat or savior? Crit. Rev. Environ. Sci. Technol. 2009, 39, 416–432. [Google Scholar] [CrossRef]
- Swartjes, F.A. (Ed.) Dealing with Contaminated Sites: From Theory Towards Practical Application; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar]
- Abadin, H.; Ashizawa, A.; Stevens, Y.W.; Llados, F.; Diamond, G.; Sage, G.; Citra, M.; Quinones, A.; Bosch, S.J.; Swarts, S.G. A framework to guide public health assessment decisions at lead sites. In Toxicological Profile for Lead; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2007. [Google Scholar]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Li, Z.; Alessi, D.S. Stabilization-based soil remediation should consider long-term challenges. Front. Environ. Sci. Eng. 2018, 12, 16. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Ahmad, M.; Rafique, M.I.; Akanji, M.A.; Al-Wabel, M.I.; Al-Swadi, H.A.; Al-Farraj, A.S. Silica modified biochar mitigates the adverse effects of salt and drought stress and improves safflower (Carthamus tinctorius L.) growth. J. Soils Sediments 2022, 23, 172–192. [Google Scholar] [CrossRef]
- Ho, S.H.; Wang, D.; Wei, Z.S.; Chang, J.S.; Ren, N.Q. Lead removal by a magnetic biochar derived from persulfate-ZVI treated sludge together with one-pot pyrolysis. Bioresour. Technol. 2018, 247, 463–470. [Google Scholar]
- Nzediegwu, C.; Naeth, M.A.; Chang, S.X. Lead (II) adsorption on microwave-pyrolyzed biochars and hydrochars depends on feedstock type and production temperature. J. Hazard. Mater. 2021, 412, 125255. [Google Scholar] [CrossRef]
- Moon, D.H.; Park, J.W.; Chang, Y.Y.; Ok, Y.S.; Lee, S.S.; Ahmad, M.; Koutsospyros, A.; Park, J.H.; Baek, K. Immobilization of lead in contaminated firing range soil using biochar. Environ. Sci. Pollut. Res. 2013, 20, 8464–8471. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Khan, M.I.R.; Fujita, M. Silicon mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassic napus L. S. Afr. J. Bot. 2018, 115, 50–57. [Google Scholar] [CrossRef]
- Hu, R.; Xiao, J.; Wang, T.; Gong, Y.; Chen, G.; Chen, L.; Tian, X. Highly concentrated amino-modified biochars using a plasma: Evolution of surface composition and porosity for heavy metal capture. Carbon 2020, 168, 515–527. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H.; Liu, Y.; Yang, S. Removal of phosphate from aque- ous solution b SiO2 –biochar nanocomposites prepared by pyrolysis of vermiculite treated algal biomass. RSC Adv. 2016, 6, 83534–83546. [Google Scholar] [CrossRef]
- ASTM D1762-84; Standard Methods for Chemical Analysis of Wood Charcoal. American Society for Testing and Materials (ASTM): Philadelphia, PA, USA, 1989.
- Richard, L.A. Diagnosis and Improvement of Saline and Alkali Soils Agriculture Handbook; US Department of Agri: Washington, DC, USA, 1954; Volume 60.
- SEPA (State Environmental Protection Agency). Water and Waste Water Monitoring Analysis Method; China Environmental Science Press: Beijing, China, 2002.
- Soltanpour, P.N.; Schwab, A.P. A new soil test for simultaneous extraction of macro- and micronutrients in alkaline soils. Commun. Soil Sci. Plant Anal. 1977, 8, 195–207. [Google Scholar] [CrossRef]
- Sparks, D.L. Methods of Soil Analysis; Soil Society of American: Madison, WI, USA, 1996. [Google Scholar]
- Walkley, A.J.; Black, I.A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book Co., Inc.: New York, NY, USA, 1997; pp. 400–428. [Google Scholar]
- Usman, A.R.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrol. 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liu, R. Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Chia, C.H.; Gong, B.; Joseph, S.D.; Marjo, C.E.; Munroe, P.; Rich, A.M. Imaging of mineral-enriched biochar by FTIR, Raman and SEM–EDX. Vib. Spectrosc. 2012, 62, 248–257. [Google Scholar] [CrossRef]
- Yuney, K.; Oladipo, A.A.; Gazi, M.; Younis, D.Z. CuO coated olive cake nanocomposites for rapid phenol removal and effective discoloration of high strength olive mill wastewater. Chemosphere 2020, 253, 126703. [Google Scholar] [CrossRef] [PubMed]
- Kraiem, T.; Hassen, A.B.; Belayouni, H.; Jeguirim, M. Production and characterization of bio-oil from the pyrolysis of waste frying oil. Environ. Sci. Pollut. Res. 2017, 24, 9951–9961. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.S.; Jain, C.K.; Yadav, A.K.; Vishwavidyalaya, G.K. Preparation and characterization of plant based low cost adsorbents. J. Glob. Biosci. 2015, 4, 1824–1829. [Google Scholar]
- Aissaoui, M.H.; Trabelsi, A.B.H.; Bensidhom, G.; Ceylan, S.; Leahy, J.J.; Kwapinski, W. Sustainable Valorization of Olive Pomace Waste to Renewable Biofuels, Biomaterials and Biochemicals via Pyrolysis Process: Experimental and Numerical Investigation. Res. Sq. 2021; preprint. [Google Scholar] [CrossRef]
- Losacco, D.; Campanale, C.; Tumolo, M.; Ancona, V.; Massarelli, C.; Uricchio, V.F. Evaluating the Influence of Nitrogen Fertilizers and Biochar on Brassica oleracea L. var. botrytis by the Use of Fourier Transform Infrared (FTIR) Spectroscopy. Sustainability 2022, 14, 11985. [Google Scholar] [CrossRef]
- Gámiz, B.; Hall, K.; Spokas, K.A.; Cox, L. Understanding activation effects on low-temperature biochar for optimization of herbicide sorption. Agronomy 2019, 9, 588. [Google Scholar] [CrossRef]
- Patel, M.; Chaubey, A.K.; Pittman, C.U.; Mohan, D. Aqueous ibuprofen sorption by using activated walnut shell biochar: Process optimization and cost estimation. Environ. Sci. Adv. 2022, 1, 530–545. [Google Scholar] [CrossRef]
- He, Y.; Jia, X.; Zhou, S.; Chen, J.; Zhang, S.; Li, X.; Huang, Y.; Wågberg, T.; Hu, G. Separatable MoS2 loaded biochar/CaCO3/Alginate gel beads for selective and efficient removal of Pb (II) from aqueous solution. Sep. Purif. Technol. 2022, 303, 122212. [Google Scholar] [CrossRef]
- Sornhiran, N.; Aramrak, S.; Prakongkep, N.; Wisawapipat, W. Silicate minerals control the potential uses of phosphorus-laden mineral-engineered biochar as phosphorus fertilizers. Biochar 2022, 4, 1–17. [Google Scholar] [CrossRef]
- Zhang, J.; Lü, F.; Zhang, H.; Shao, L.; Chen, D.; He, P. Multiscale visualization of the structural and characteristic changes of sewage sludge biochar oriented towards potential agronomic and environmental implication. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; Melo, I.C.N.D.; Melo, L.C.; Magriotis, Z.M.; Sanchez-Monedero, M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, 0176884. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Singh, S.; Xu, X.; Park, K.; Qi, Y.; Cheong, S.W.; Vanderbilt, D.; Rabe, K.M.; Musfeldt, J.L. Vibrational fingerprints of ferroelectric HfO2. Npj Quantum Mater. 2022, 7, 1–8. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, M.; Usman, A.R.; Al-Faraj, A.S.; Abduljabbar, A.; Ok, Y.S.; Al-Wabel, M.I. Date palm waste-derived biochar composites with silica and zeolite: Synthesis, characterization and implication for carbon stability and recalcitrant potential. Environ. Geochem. Health 2019, 41, 1687–1704. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Shen, J.; Ni, Z.; Tang, J.; Song, S.; Chen, J.; Zhao, L. Electrochemically created roughened lead plate for electrochemical reduction of aqueous CO2. Catal. Commun. 2015, 72, 38–42. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Usman, A.R.A.; Al-Farraj, A.S.; Ok, Y.S.; Abduljabbar, A.; Al-Faraj, A.I.; Sallam, A.S. Date palm waste biochars alter a soil respiration, microbial biomass carbon, and heavy metal mobility in contaminated mined soil. Environ. Geochem. Health 2019, 41, 1705–1722. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Ahmad, M.; Usman, A.R.; Al-Farraj, A.S. Designing chitosan based magnetic beads with conocarpus waste-derived biochar for efficient sulfathiazole removal from contaminated water. Saudi J. Biol. Sci. 2021, 28, 6218–6229. [Google Scholar] [CrossRef]
- Rafique, M.I.; Usman, A.R.; Ahmad, M.; Sallam, A.; Al-Wabel, M.I. In situ immobilization of Cr and its availability to maize plants in tannery waste–contaminated soil: Effects of biochar feedstock and pyrolysis temperature. J. Soils Sediments 2020, 20, 330–339. [Google Scholar] [CrossRef]
- Li, S.; Barreto, V.; Li, R.; Chen, G.; Hsieh, Y.P. Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2018, 133, 136–146. [Google Scholar] [CrossRef]
- Masud, M.M.; Jiu-Yu, L.I.; Ren-Kou, X.U. Use of alkaline slag and crop residue biochars to promote base saturation and reduce acidity of an acidic ultisol. Pedosphere 2014, 24, 791–798. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Wang, L.; Butterly, C.R.; Wang, Y.; Herath, H.M.S.K.; Xi, Y.G.; Xiao, X.J. Effect of crop residue biochar on soil acidity amelioration in strongly acidic tea garden soils. Soil Use Manag. 2014, 30, 119–128. [Google Scholar] [CrossRef]
- de Caprariis, B.; De Filippis, P.; Hernandez, A.D.; Petrucci, E.; Petrullo, A.; Scarsella, M.; Turchi, M. Pyrolysis wastewater treatment by adsorption on biochars produced by poplar biomass. J. Environ. Manage. 2017, 197, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.P.; Lu, C.; Su, F. Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar] [CrossRef]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Yang, G.; Zhang, X.; Qi, H.; Ma, J.; Xiao, Y.; et al. Adsorption characteristics of Pb (II) using biochar derived from spent mushroom substrate. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Ding, W.; Dong, X.; Ime, I.M.; Gao, B.; Ma, L.Q. Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars. Chemosphere 2014, 105, 68–74. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions-A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef]
- Morales, M.M.; Comerford, N.B.; Behling, M.; de Abreu, D.C.; Guerrini, I.A. Biochar chemistry in a weathered tropical soil: Kinetics of phosphorus sorption. Agriculture 2021, 11, 295. [Google Scholar] [CrossRef]
- Cui, H.J.; Wang, M.K.; Fu, M.L.; Ci, E. Enhancing phosphorus availability in phosphorus-fertilized zones by reducing phosphate adsorbed on ferrihydrite using rice straw-derived biochar. J. Soils Sediments 2011, 11, 1135–1141. [Google Scholar] [CrossRef]
- Chintala, R.; Schumacher, T.E.; McDonald, L.M.; Clay, D.E.; Malo, D.D.; Papiernik, S.K.; Clay, S.A.; Julson, J.L. Phosphorus sorption and availability from biochars and soil/B iochar mixtures. Clean (Weinh) 2014, 42, 626–634. [Google Scholar]
- Rupa, T.R.; Tomar, K.P.; Srinivasa Rao, C.H.; Subba Rao, A. Kinetics of phosphate sorption-desorption as influenced by soil pH and electrolytes. Agrochimica 2001, 45, 124–133. [Google Scholar]
- Kammann, C.I.; Schmidt, H.P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.W.; Conte, P.; Joseph, S. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 1–13. [Google Scholar]
- Conte, P.; Marsala, V.; De Pasquale, C.; Bubici, S.; Valagussa, M.; Pozzi, A.; Alonzo, G. Nature of water-biochar interface interactions. Gcb Bioenergy. 2013, 5, 116–121. [Google Scholar] [CrossRef]
- Hagemann, N.; Kammann, C.I.; Schmidt, H.P.; Kappler, A.; Behrens, S. Nitrate capture and slow release in biochar amended compost and soil. PloS ONE 2017, 12, 0171214. [Google Scholar] [CrossRef] [PubMed]
- Jellali, S.; El-Bassi, L.; Charabi, Y.; Uaman, M.; Khiari, B.; Al-Wardy, M.; Jeguirim, M. Recent advancements on biochars enrichment with ammonium and nitrates from wastewaters: A critical review on benefits for environment and agriculture. J. Environ. Manage. 2022, 305, 114368. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wang, B.; Feng, Q.; Zhang, X.; Chen, M. Co-adsorption performance and mechanism of nitrogen and phosphorus onto eupatorium adenophorum biochar in water. Bioresour. Technol. 2021, 340, 125696. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Reichel, R.; Brüggemann, N. Fenton oxidation of biochar improves retention of cattle slurry nitrogen. J. Environ. Qual. 2022, 51, 1319–1326. [Google Scholar] [CrossRef]
| Sr. No. | Biochar Type | Biochar Application (%) | Pb Application (mg kg−1 Soil) | Treatments |
|---|---|---|---|---|
| 1 | Control | 0 | 100 | CK-Pb1 |
| 2 | Control | 0 | 200 | CK-Pb2 |
| 3 | BC | 1 | 100 | BC1-Pb1 |
| 4 | BC | 2 | 100 | BC2-Pb1 |
| 5 | BC | 1 | 200 | BC1-Pb2 |
| 6 | BC | 2 | 200 | BC2-Pb2 |
| 7 | BCSi | 1 | 100 | BCSi1-Pb1 |
| 8 | BCSi | 2 | 100 | BCSi2-Pb1 |
| 9 | BCSi | 1 | 200 | BCSi1-Pb2 |
| 10 | BCSi | 2 | 200 | BCSi2-Pb2 |
| 11 | BCSiNP | 1 | 100 | BCSiNP1-Pb1 |
| 12 | BCSiNP | 2 | 100 | BCSiNP2-Pb1 |
| 13 | BCSiNP | 1 | 200 | BCSiNP1-Pb2 |
| 14 | BCSiNP | 2 | 200 | BCSiNP2-Pb2 |
| Material | Yield (%) | Moisture (%) | Mobile Matter (%) | Ash (%) | Resident Matter (%) | pH (1:25) | EC (dSm−1) |
|---|---|---|---|---|---|---|---|
| BM | - | 6.83 ± 0.04 | 69.96 ± 2.54 | 5.07 ± 2.17 | 18.14 ± 0.41 | 5.88 ± 0.06 | 0.87 ± 0.01 |
| BC | 33 | 3.04 ± 0.74 | 14.21 ± 4.36 | 12.68 ± 4.16 | 70.07 ± 0.94 | 9.62 ± 0.02 | 2.23 ± 0.22 |
| BCSi | - | 2.85 ± 0.24 | 18.37 ± 0.59 | 10.17 ± 0.04 | 68.61 ± 0.87 | 3.59 ± 0.06 | 1.68 ± 0.03 |
| BC-Si-NP | - | 2.41 ± 0.46 | 12.26 ± 4.55 | 30.19 ± 12.75 | 55.13 ± 8.66 | 2.77 ± 0.04 | 2.26 ± 0.08 |
| Property | pH | EC | OM | CEC | Sand | Silt | Clay | Texture Class | CaCO3 | Available P | Nitrate NO3− | Available K | Available Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | -- | dS m−1 | % | cmol kg−1 | % | % | % | -- | % | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 |
| soil | 7.21 ± 0.56 | 2.41 ± 0.19 | 0.62 ± 0.02 | 21.19 ± 2.89 | 63.19 ± 3.03 | 33.41 ± 2.16 | 3.39 ± 1.15 | Sandy loam | 8.45 ± 0.88 | 9.16 ± 1.75 | 2.88 ± 0.03 | 78.00 ± 4.55 | 25.81 ± 2.01 |
| First-Order | Pseudo Second-Order | |||||
|---|---|---|---|---|---|---|
| Adsorbents | k1 | R2 | k2′ | qe | h | R2 |
| CK-Pb1 | −0.02 | 0.76 | −0.01 | 03.02 | −10.48 | 0.97 |
| CK-Pb2 | −0.01 | 0.79 | −0.01 | 09.11 | −56.88 | 0.99 |
| BC1-Pb1 | −0.03 | 0.67 | −0.02 | 15.54 | −04.70 | 0.97 |
| BC2-Pb1 | −0.04 | 0.75 | −0.02 | 09.76 | −02.28 | 0.94 |
| BC1-Pb2 | −0.02 | 0.73 | −0.01 | 49.50 | −18.59 | 0.99 |
| BC2-Pb2 | −0.03 | 0.75 | −0.01 | 38.26 | −12.08 | 0.97 |
| BCSi1-Pb1 | −0.04 | 0.69 | −0.02 | 11.47 | −02.97 | 0.96 |
| BCSi2-Pb1 | −0.05 | 0.71 | −0.03 | 05.91 | −01.18 | 0.91 |
| BCSi1-Pb2 | −0.03 | 0.74 | −0.01 | 31.72 | −08.30 | 0.95 |
| BCSi2-Pb2 | −0.04 | 0.80 | −0.01 | 26.96 | −06.51 | 0.94 |
| BCSiNP1-Pb1 | −0.05 | 0.62 | −0.03 | 54.01 | −01.67 | 0.96 |
| BCSiNP2-Pb1 | −0.05 | 0.67 | −0.04 | 57.78 | −00.95 | 0.91 |
| BCSiNP1-Pb2 | −0.04 | 0.63 | −0.01 | 49.26 | −04.87 | 0.95 |
| BCSiNP2-Pb2 | −0.05 | 0.75 | −0.02 | 52.81 | −02.63 | 0.92 |
| First-Order | Pseudo Second-Order | |||||
|---|---|---|---|---|---|---|
| Adsorbents | k1 | R2 | k2′ | qe | h | R2 |
| CK-Pb1 | 0.02 | 0.82 | 0.01 | 33.97 | 10.38 | 0.99 |
| CK-Pb2 | 0.02 | 0.81 | 0.01 | 32.19 | 09.57 | 0.99 |
| BC1-Pb1 | 0.02 | 0.83 | 0.02 | 37.67 | 12.13 | 0.99 |
| BC2-Pb1 | 0.01 | 0.80 | 0.01 | 42.54 | 19.63 | 0.99 |
| BC1-Pb2 | 0.02 | 0.79 | 0.02 | 34.51 | 12.08 | 0.98 |
| BC2-Pb2 | 0.01 | 0.79 | 0.02 | 40.19 | 17.54 | 0.99 |
| BCSi1-Pb1 | 0.02 | 0.79 | 0.01 | 47.64 | 19.04 | 0.98 |
| BCSi2-Pb1 | 0.01 | 0.78 | 0.02 | 50.97 | 21.92 | 0.99 |
| BCSi1-Pb2 | 0.01 | 0.83 | 0.01 | 46.51 | 16.72 | 0.99 |
| BCSi2-Pb2 | 0.02 | 0.80 | 0.01 | 44.89 | 18.27 | 0.98 |
| BCSiNP1-Pb1 | 0.02 | 0.76 | 0.02 | 50.71 | 29.40 | 0.99 |
| BCSiNP2-Pb1 | 0.01 | 0.81 | 0.02 | 53.90 | 26.84 | 0.99 |
| BCSiNP1-Pb2 | 0.01 | 0.85 | 0.01 | 49.11 | 22.79 | 0.98 |
| BCSiNP2-Pb2 | 0.01 | 0.88 | 0.02 | 47.01 | 21.97 | 0.99 |
| First-Order | Pseudo Second-Order | |||||
|---|---|---|---|---|---|---|
| Adsorbents | k1 | R2 | k2′ | qe | h | R2 |
| CK-Pb1 | 0.01 | 0.75 | 0.01 | 52.48 | 35.50 | 0.99 |
| CK-Pb2 | 0.01 | 0.84 | 0.02 | 41.70 | 34.22 | 0.99 |
| BC1-Pb1 | 0.01 | 0.79 | 0.01 | 60.46 | 38.72 | 0.99 |
| BC2-Pb1 | 0.01 | 0.82 | 0.01 | 65.69 | 41.80 | 0.99 |
| BC1-Pb2 | 0.02 | 0.82 | 0.02 | 50.69 | 41.21 | 0.99 |
| BC2-Pb2 | 0.01 | 0.78 | 0.02 | 61.03 | 34.08 | 0.99 |
| BCSi1-Pb1 | 0.02 | 0.90 | 0.01 | 114.16 | 54.14 | 0.99 |
| BCSi2-Pb1 | 0.01 | 0.75 | 0.02 | 123.43 | 42.34 | 0.99 |
| BCSi1-Pb2 | 0.01 | 0.87 | 0.01 | 103.13 | 56.64 | 0.99 |
| BCSi2-Pb2 | 0.02 | 0.90 | 0.01 | 112.81 | 42.61 | 0.99 |
| BCSiNP1-Pb1 | 0.02 | 0.90 | 0.02 | 167.05 | 53.32 | 0.99 |
| BCSiNP2-Pb1 | 0.01 | 0.86 | 0.02 | 172.36 | 76.50 | 0.99 |
| BCSiNP1-Pb2 | 0.01 | 0.88 | 0.01 | 139.23 | 49.15 | 0.99 |
| BCSiNP2-Pb2 | 0.01 | 0.88 | 0.02 | 158.20 | 54.51 | 0.99 |
| First-Order | Pseudo Second-Order | |||||
|---|---|---|---|---|---|---|
| Adsorbents | k1 | R2 | k2′ | qe | h | R2 |
| CK-Pb1 | 0.01 | 0.76 | 0.02 | 31.05 | 16.89 | 0.99 |
| K-Pb2 | 0.01 | 0.84 | 0.02 | 24.90 | 12.72 | 0.99 |
| BC1-Pb1 | 0.01 | 0.85 | 0.01 | 36.06 | 19.04 | 0.99 |
| BC2-Pb1 | 0.01 | 0.79 | 0.01 | 45.11 | 22.99 | 0.99 |
| BC1-Pb2 | 0.01 | 0.86 | 0.01 | 33.62 | 15.76 | 0.99 |
| BC2-Pb2 | 0.01 | 0.79 | 0.01 | 41.20 | 24.00 | 0.99 |
| BCSi1-Pb1 | 0.01 | 0.83 | 0.01 | 47.23 | 25.89 | 0.99 |
| BCSi2-Pb1 | 0.01 | 0.83 | 0.01 | 52.61 | 29.13 | 0.99 |
| BCSi1-Pb2 | 0.01 | 0.82 | 0.01 | 43.65 | 24.60 | 0.99 |
| BCSi2-Pb2 | 0.01 | 0.84 | 0.01 | 49.51 | 25.41 | 0.99 |
| BCSiNP1-Pb1 | 0.01 | 0.84 | 0.01 | 71.27 | 40.34 | 0.99 |
| BCSiNP2-Pb1 | 0.01 | 0.80 | 0.01 | 74.40 | 51.87 | 0.99 |
| BCSiNP1-Pb2 | 0.01 | 0.82 | 0.01 | 65.04 | 42.07 | 0.99 |
| BCSiNP2-Pb2 | 0.01 | 0.82 | 0.01 | 67.63 | 48.75 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usama, M.; Rafique, M.I.; Ahmad, J.; Ahmad, M.; Al-Wabel, M.I.; Al-Farraj, A.S.F. A Sustainable Approach towards the Restoration of Lead-Contaminated Soils through Nutrient-Doped Olive Waste-Derived Biochar Application. Sustainability 2023, 15, 2606. https://doi.org/10.3390/su15032606
Usama M, Rafique MI, Ahmad J, Ahmad M, Al-Wabel MI, Al-Farraj ASF. A Sustainable Approach towards the Restoration of Lead-Contaminated Soils through Nutrient-Doped Olive Waste-Derived Biochar Application. Sustainability. 2023; 15(3):2606. https://doi.org/10.3390/su15032606
Chicago/Turabian StyleUsama, Muhammad, Muhammad I. Rafique, Jahangir Ahmad, Munir Ahmad, Mohammad I. Al-Wabel, and Abdullah S. F. Al-Farraj. 2023. "A Sustainable Approach towards the Restoration of Lead-Contaminated Soils through Nutrient-Doped Olive Waste-Derived Biochar Application" Sustainability 15, no. 3: 2606. https://doi.org/10.3390/su15032606
APA StyleUsama, M., Rafique, M. I., Ahmad, J., Ahmad, M., Al-Wabel, M. I., & Al-Farraj, A. S. F. (2023). A Sustainable Approach towards the Restoration of Lead-Contaminated Soils through Nutrient-Doped Olive Waste-Derived Biochar Application. Sustainability, 15(3), 2606. https://doi.org/10.3390/su15032606









