Volatile Fatty Acids Production by Acidogenic Fermentation of Wastewater: A Bibliometric Analysis
Abstract
1. Introduction
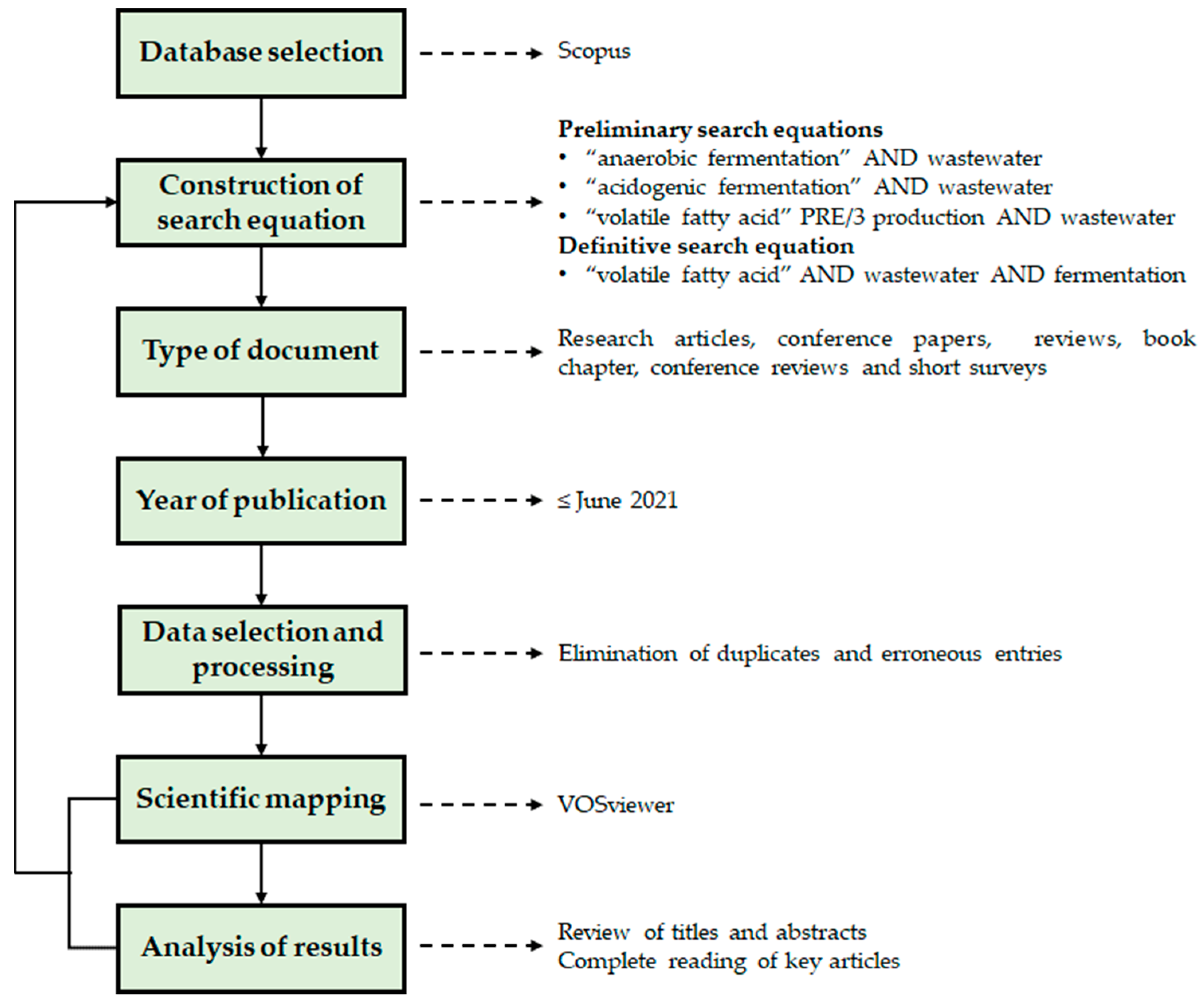
2. Materials and Methods
3. Results and Discussion
3.1. Worldwide Scientific Production Related to VFA and Wastewater Fermentation
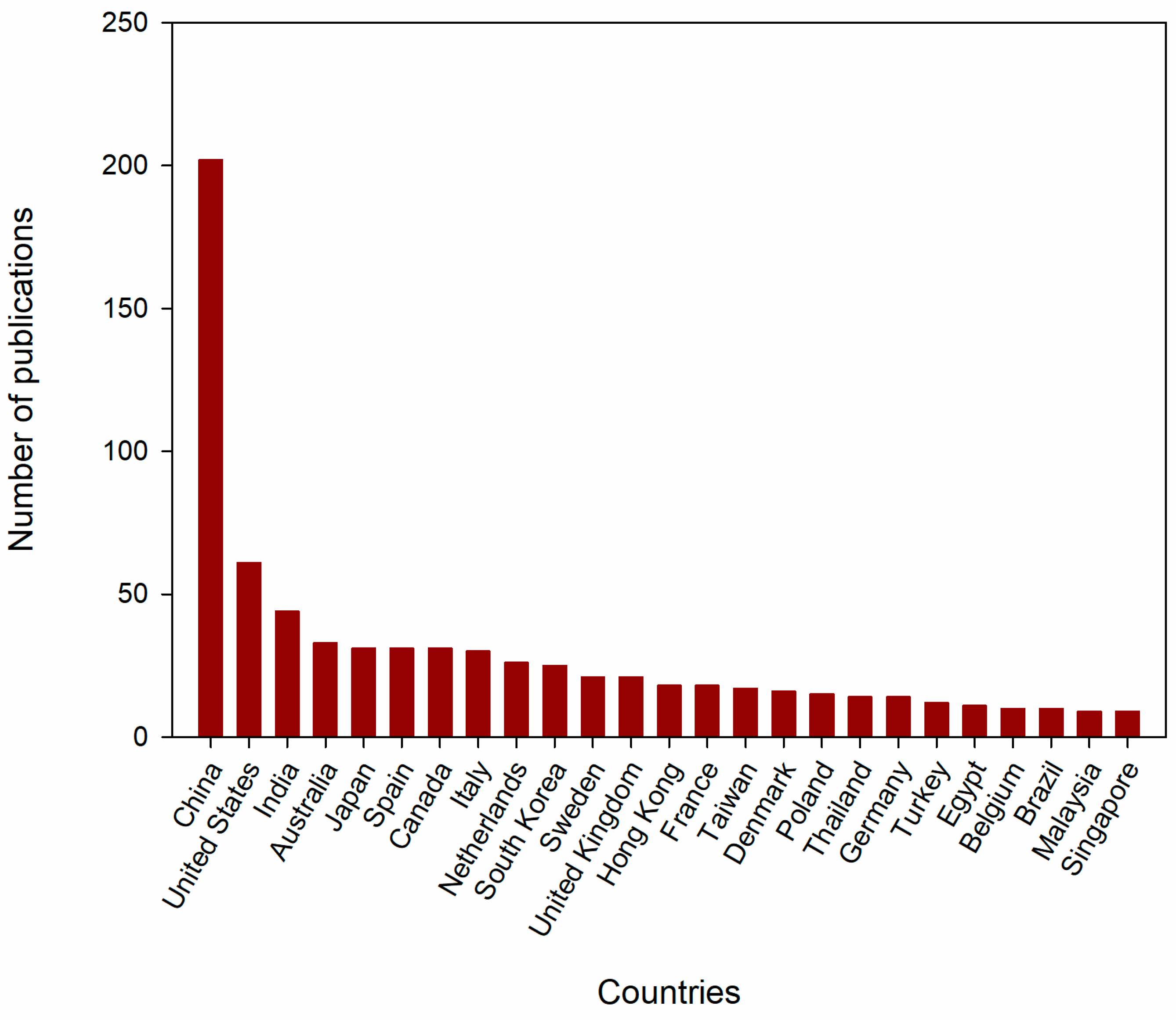
3.2. Most Productive Countries and Research Institutions Worldwide in the Scientific Literature on VFA and Wastewater Fermentation
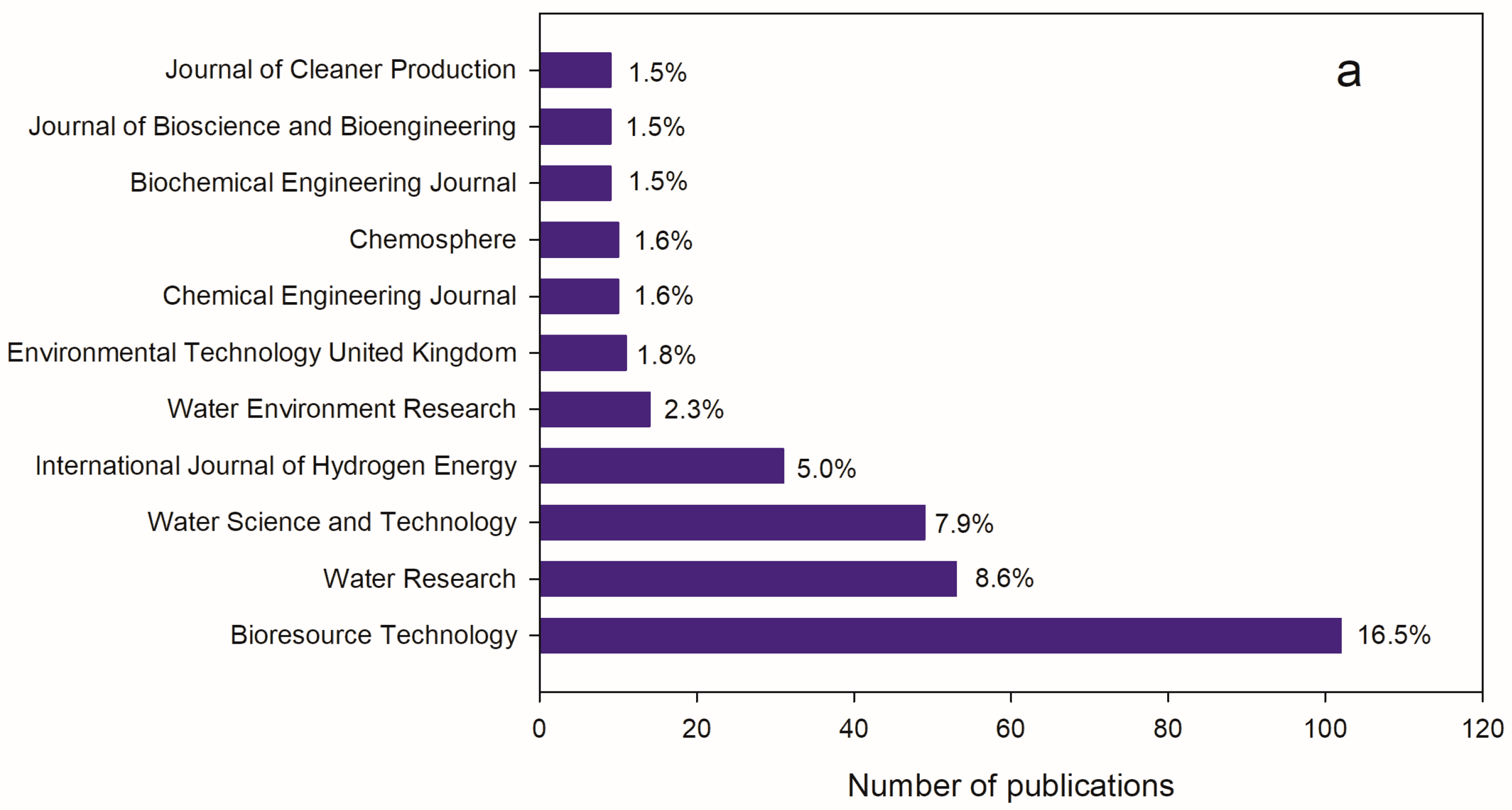
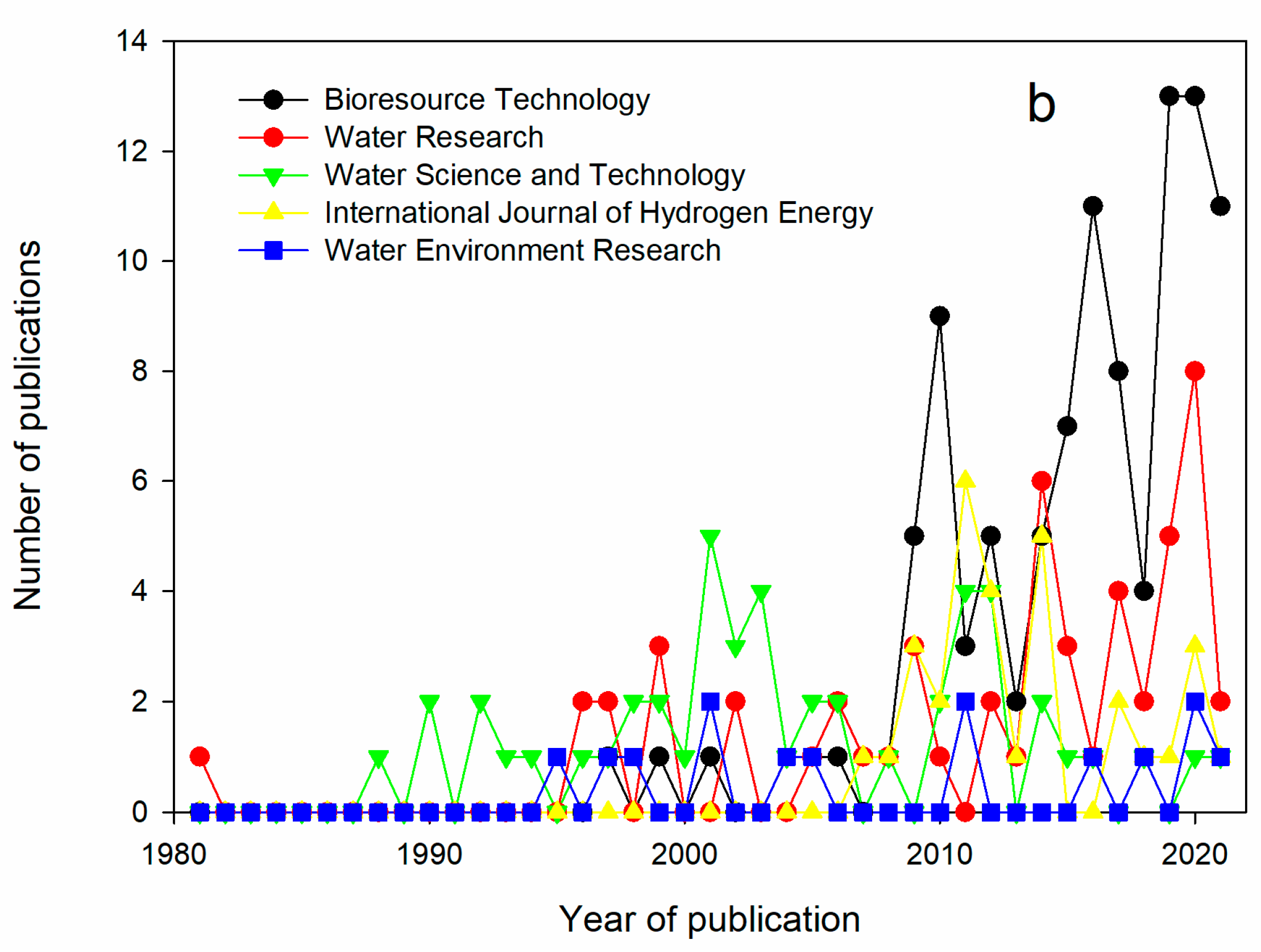
3.3. Main Journals Publishing on VFA and Wastewater Fermentation
3.4. Main Research Approaches on VFA Production
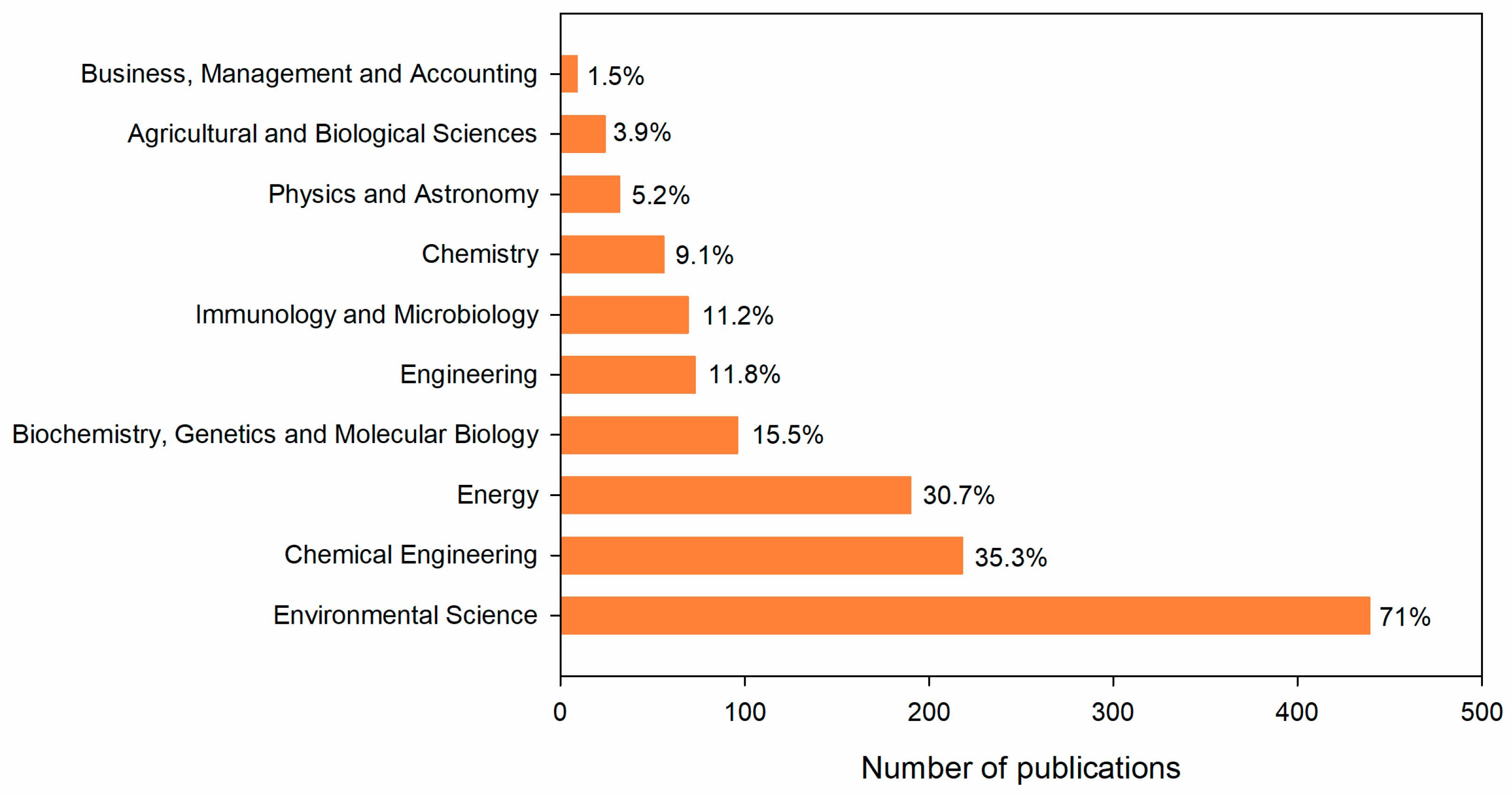
3.4.1. Subject Areas of VFA Production
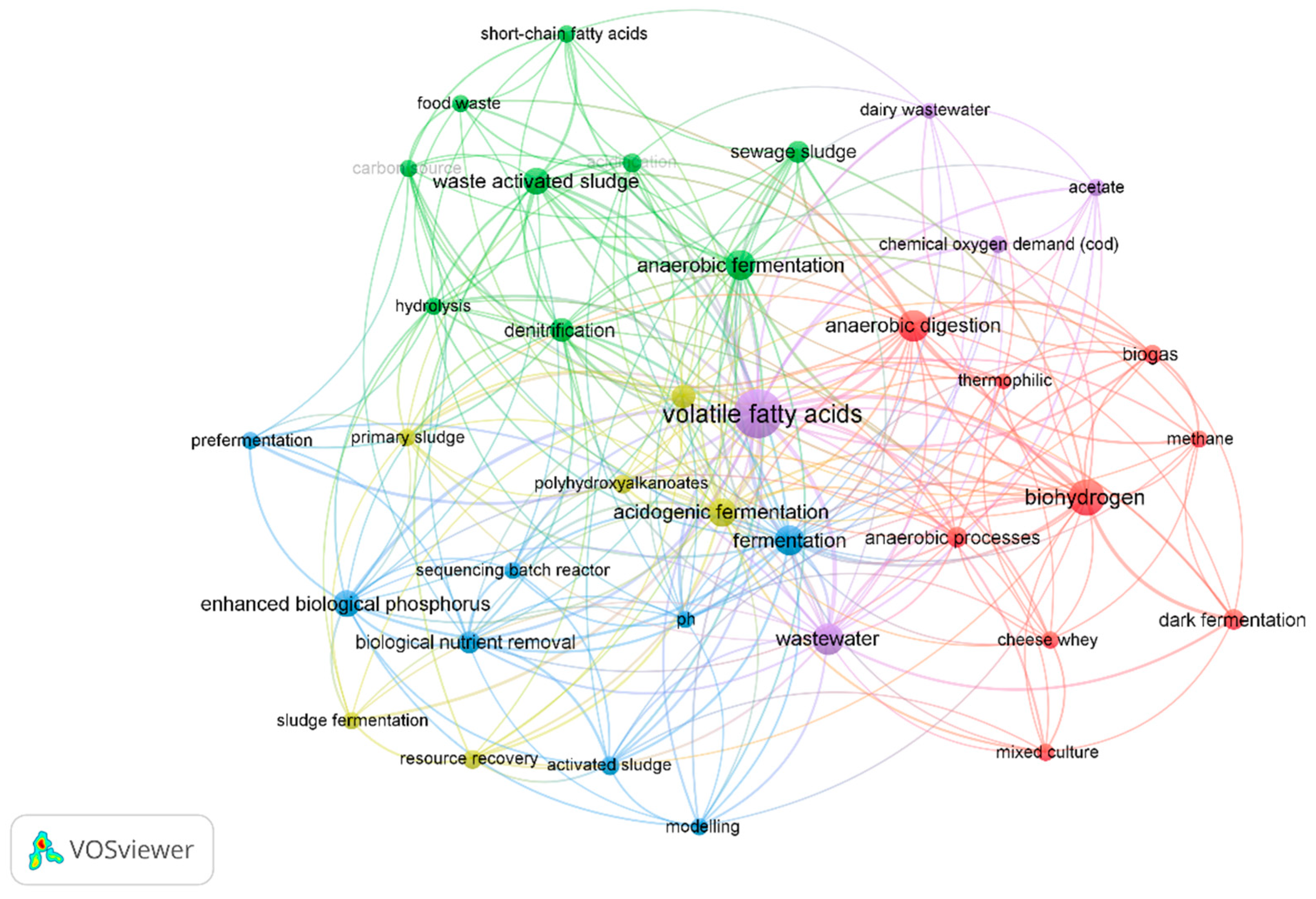
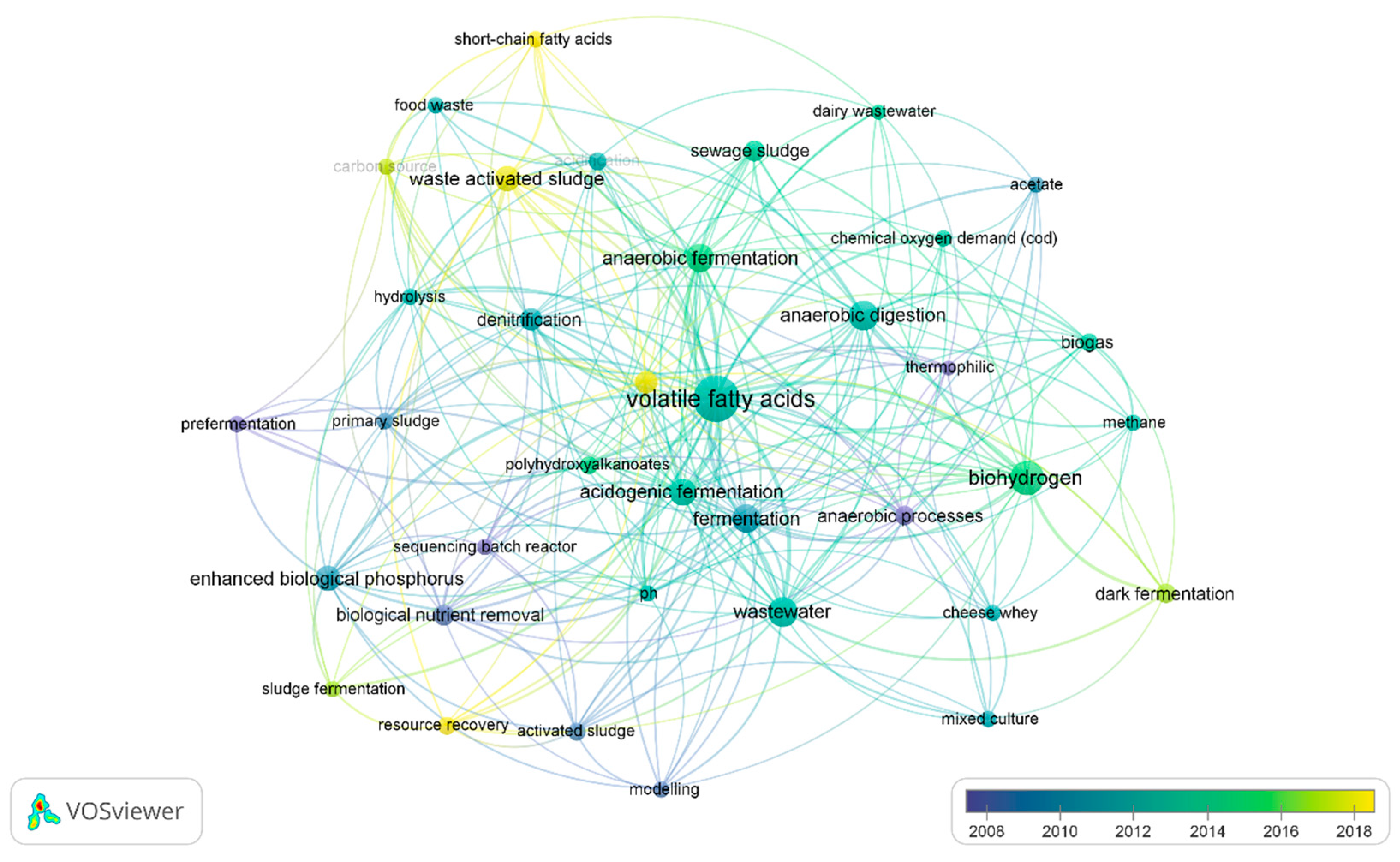
3.4.2. Bibliometric Networks of VFA and Wastewater Fermentation Studies
3.4.3. Operational Parameters of VFA Production by Wastewater Fermentation
- Effect of pH on VFA production by AF
- Effect of Hydraulic Retention Time on VFA production by AF
- Effect of Organic Loading Rate on VFA production by AF
- Effect of Temperature on VFA production by AF
- Effect of Inoculum on VFA production by AF
- Effect of Substrate Concentration on VFA production by AF
3.5. Future Perspectives and Challenges
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CRA. Análisis de Impacto Normativo. Regulación Frente a Tarifas Por Actividad Del Servicio-Tratamiento de Vertimientos. Documento Final; Comisión de Regulación de Agua Potable y Saneamiento Básico–CRA: Bogotá, Colombia, 2019. [Google Scholar]
- AIDIS. Uso Seguro Del Agua Para El Reúso; Tello Espinoza, P., Mijailova, P., Chamy, R., Eds.; Asociación Interamericana de Ingeniería Sanitaría y Ambiental (AIDIS): São Paulo, Brasil, 2016. [Google Scholar]
- Lema Rodicio, J.M. Oportunidades y Retos Para La Digestión Anaerobia. In Depuración de Aguas Residuales: Digestión Anaerobia; Chiva Vicent, S., Berlanga Clavijo, J.G., Martínez Cuenca, R., Climent Agustina, J., Eds.; Universitat Jaume I: Castelló, Spain, 2018; p. 256. ISBN 9788416546657. [Google Scholar]
- Bastidas-Oyanedel, J.R. Thermodynamic Based Modelling of Biohydrogen Production by Anaerobic Fermentation. Ph.D. Thesis, Université Montpellier 2, Montpellier, France, February 2011. [Google Scholar]
- de Sousa e Silva, A.; Morais, N.W.S.; Coelho, M.M.H.; Pereira, E.L.; dos Santos, A.B. Potentialities of Biotechnological Recovery of Methane, Hydrogen and Carboxylic Acids from Agro-Industrial Wastewaters. Bioresour. Technol. Reports 2020, 10, 100406. [Google Scholar] [CrossRef]
- Pavlostathis, S.G. Kinetics and Modeling of Anaerobic Treatment and Biotransformation Processes. In Comprehensive Biotechnology, 2nd ed.; Elsevier, B.V., Ed.; Georgia Institute of Technology: Atlanta, GA, USA, 2011; Volume 6, pp. 385–397. ISBN 9780080885049. [Google Scholar]
- Chernicharo, C.A.d.L. Anaerobic Reactors; IWA Publishing: Minas Gerais, Brazil, 2007; Volume 4, ISBN 9781843391647. [Google Scholar]
- Bastidas-Oyanedel, J.-R.; Bonk, F.; Thomsen, M.H.; Schmidt, J.E. Dark Fermentation Biorefinery in the Present and Future (Bio)Chemical Industry. Rev. Environ. Sci. Biotechnol. 2015, 14, 473–498. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-Based Volatile Fatty Acid Production and Recovery from Waste Streams: Current Status and Future Challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Valentino, F.; Morgan-Sagastume, F.; Campanari, S.; Villano, M.; Werker, A.; Majone, M. Carbon Recovery from Wastewater through Bioconversion into Biodegradable Polymers. N. Biotechnol. 2017, 37, 9–23. [Google Scholar] [CrossRef]
- Karadag, D.; Köroğlu, O.E.; Ozkaya, B.; Cakmakci, M.; Heaven, S.; Banks, C. A Review on Fermentative Hydrogen Production from Dairy Industry Wastewater. J. Chem. Technol. Biotechnol. 2014, 89, 1627–1636. [Google Scholar] [CrossRef]
- Cai, G.; Jin, B.; Monis, P.; Saint, C. Metabolic Flux Network and Analysis of Fermentative Hydrogen Production. Biotechnol. Adv. 2011, 29, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Malule, H.; López-Agudelo, V.A.; Gómez-Ríos, D. Candida Auris: A Bibliometric Analysis of the First Ten Years of Research (2009–2018). J. Appl. Pharm. Sci. 2020, 10, 12–21. [Google Scholar] [CrossRef]
- Ramírez-Malule, H.; Quiñones-Murillo, D.H.; Manotas-Duque, D. Emerging Contaminants as Global Environmental Hazards. A Bibliometric Analysis. Emerg. Contam. 2020, 6, 179–193. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- van Haandel, A.; van der Lubbe, J. Handbook of Biological Wastewater Treatment: Second Edition; IWA Publishing: London, UK, 2012; ISBN 9781780400808. [Google Scholar]
- van Haandel, A.; Marais, G. O Comportamento Do Sistema de Lodo Ativado: Teoria e Aplicações Para Projetos e Operação; Marais: Campina Grande, Brazil, 1999; ISBN 900847. [Google Scholar]
- Kamm, B.; Kamm, M. Principles of Biorefineries. Appl. Microbiol. Biotechnol. 2004, 64, 137–145. [Google Scholar] [CrossRef]
- Ahmed, I.; Zia, M.A.; Afzal, H.; Ahmed, S.; Ahmad, M.; Akram, Z.; Sher, F.; Iqbal, H.M.N. Socio-Economic and Environmental Impacts of Biomass Valorisation: A Strategic Drive for Sustainable Bioeconomy. Sustainability 2021, 13, 4200. [Google Scholar] [CrossRef]
- Kamm, B.; Kamm, M. Biorefinery-Systems. Chem. Biochem. Eng. Q. 2004, 18, 1–6. [Google Scholar]
- Leite, J.A.C.; Fernandes, B.S.; Pozzi, E.; Barboza, M.; Zaiat, M. Application of an Anaerobic Packed-Bed Bioreactor for the Production of Hydrogen and Organic Acids. Int. J. Hydrogen Energy 2008, 33, 579–586. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, L. The Scientometric Analysis of the Research on Microalgae-Based Wastewater Treatment. Environ. Sci. Pollut. Res. 2021, 28, 25339–25348. [Google Scholar] [CrossRef]
- Niknejad, N.; Nazari, B.; Foroutani, S.; Hussin, A.R.B.C. A Bibliometric Analysis of Green Technologies Applied to Water and Wastewater Treatment. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Athanasopoulos, N.; Koutinas, A.A.; Papadimitriou, A. A Pilot Plant and Anaerobic Digestion Process for Raisin Finishing Wastewater Treatment. Biol. Wastes 1987, 22, 129–138. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kumar, S.; Lee, B.-D.; Kim, S.-H. Waste Based Hydrogen Production for Circular Bioeconomy: Current Status and Future Directions. Bioresour. Technol. 2020, 302, 122920. [Google Scholar] [CrossRef]
- Wu, J.H.; Lin, C.Y. Biohydrogen Production by Mesophilic Fermentation of Food Wastewater. Water Sci. Technol. 2004, 49, 223–228. [Google Scholar] [CrossRef]
- Dinopoulou, G.; Rudd, T.; Lester, J.N. Anaerobic Acidogenesis of a Complex Wastewater: I. The Influence of Operational Parameters on Reactor Performance. Biotechnol. Bioeng. 1988, 31, 958–968. [Google Scholar] [CrossRef]
- Kim, W.; Shin, S.G.; Lim, J.; Hwang, S. Effect of Temperature and Hydraulic Retention Time on Volatile Fatty Acid Production Based on Bacterial Community Structure in Anaerobic Acidogenesis Using Swine Wastewater. Bioprocess Biosyst. Eng. 2013, 36, 791–798. [Google Scholar] [CrossRef]
- Viturtia, A.M.; Mata-Alvarez, J.; Cecchi, F.; Fazzini, G. Two-Phase Anaerobic Digestion of a Mixture of Fruit and Vegetable Wastes. Biol. Wastes 1989, 29, 189–199. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xu, H.L.; Tay, J.H. A Hybrid Two-Phase System for Anaerobic Digestion of Food Waste. Water Sci. Technol. 2002, 45, 159–165. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Fu, B.; Guo, H.; Zhang, J.; Liu, H. Recovery of Volatile Fatty Acids from Sewage Sludge through Anaerobic Fermentation. Curr. Dev. Biotechnol. Bioeng. Resour. Recover. Wastes 2020, 151–175. [Google Scholar] [CrossRef]
- Rabinowitz, B.; Vassos, T.D.; Dawson, R.N.; Oldham, W.K. Upgrading Wastewater Treatment Plants for Biological Nutrient Removal. Water Sci. Technol. 1990, 22, 53–60. [Google Scholar] [CrossRef]
- Martin Ruel, S.; Comeau, Y.; Ginestet, P.; Héduit, A. Modeling Acidogenic and Sulfate-Reducing Processes for the Determination of Fermentable Fractions in Wastewater. Biotechnol. Bioeng. 2002, 80, 525–536. [Google Scholar] [CrossRef]
- Comeau, Y.; Lamarre, D.; Roberge, F.; Perrier, M.; Desjardins, G.; Hade, C.; Mayer, R. Biological Nutrient Removal from a Phosphorus-Rich Pre-Fermented Industrial Wastewater. Water Sci. Technol. 1996, 34, 169–177. [Google Scholar] [CrossRef]
- Münch, E.V.; Lant, P.; Newell, R.; Keller, J. Mathematical Modelling of Prefermenters-I. Model Development and Verification. Water Res. 1999, 33, 2757–2768. [Google Scholar] [CrossRef]
- Drewnowski, J.; Makinia, J. Modeling Hydrolysis of Slowly Biodegradable Organic Compounds in Biological Nutrient Removal Activated Sludge Systems. Water Sci. Technol. 2013, 67, 2067–2074. [Google Scholar] [CrossRef]
- Garcia-Aguirre, J.; Aymerich, E.; de Goñi, J.G.M.; Esteban-Gutiérrez, M. Selective VFA Production Potential from Organic Waste Streams: Assessing Temperature and PH Influence. Bioresour. Technol 2017, 244, 1081–1088. [Google Scholar] [CrossRef]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic Fermentation of Industrial Wastewaters: Effects of Chemostat Retention Time and PH on Volatile Fatty Acids Production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]
- Niz, M.Y.K.; Formagini, E.L.; Boncz, M.À.; Paulo, P.L. Acidogenic Fermentation of Cassava Wastewater for Volatile Fatty Acids Production. Int. J. Environ. Waste Manag. 2020, 25, 245–261. [Google Scholar] [CrossRef]
- Hasan, S.D.M.; Giongo, C.; Fiorese, M.L.; Gomes, S.D.; Ferrari, T.C.; Savoldi, T.E. Volatile Fatty Acids Production from Anaerobic Treatment of Cassava Waste Water: Effect of Temperature and Alkalinity. Environ. Technol. 2015, 36, 2637–2646. [Google Scholar] [CrossRef]
- Amorim, N.C.S.; Amorim, E.L.C.; Kato, M.T.; Florencio, L.; Gavazza, S. The Effect of Methanogenesis Inhibition, Inoculum and Substrate Concentration on Hydrogen and Carboxylic Acids Production from Cassava Wastewater. Biodegradation 2018, 29, 41–58. [Google Scholar] [CrossRef]
- da Silva, D.B.; Fenandes, B.S.; da Silva, A.J. Effect of Initial PH and Substrate Concentration on the Lactic Acid Production from Cassava Wastewater Fermentation by an Enriched Culture of Acidogenic Microorganisms. Water Environ. Res. 2021, 93, 1925–1933. [Google Scholar] [CrossRef]
- Coelho, M.M.H.; Morais, N.W.S.; Pereira, E.L.; Leitão, R.C.; dos Santos, A.B. Potential Assessment and Kinetic Modeling of Carboxylic Acids Production Using Dairy Wastewater as Substrate. Biochem. Eng. J. 2020, 156, 107502. [Google Scholar] [CrossRef]
- Arslan, D.; Steinbusch, K.J.J.; Diels, L.; Hamelers, H.V.M.; Strik, D.P.B.T.B.; Buisman, C.J.N.; De Wever, H. Selective Short-Chain Carboxylates Production: A Review of Control Mechanisms to Direct Mixed Culture Fermentations. Crit. Rev. Environ. Sci. Technol. 2016, 46, 592–634. [Google Scholar] [CrossRef]
- Steinbusch, K.J.J.; Hamelers, H.V.M.; Plugge, C.M.; Buisman, C.J.N. Biological Formation of Caproate and Caprylate from Acetate: Fuel and Chemical Production from Low Grade Biomass. Energy Environ. Sci. 2011, 4, 216–224. [Google Scholar] [CrossRef]
- Elain, A.; Le Grand, A.; Corre, Y.M.; Le Fellic, M.; Hachet, N.; Le Tilly, V.; Loulergue, P.; Audic, J.L.; Bruzaud, S. Valorisation of Local Agro-Industrial Processing Waters as Growth Media for Polyhydroxyalkanoates (PHA) Production. Ind. Crops Prod. 2016, 80, 1–5. [Google Scholar] [CrossRef]
- Karaca, S.; Yagci, N.; Randall, C.W. Polyhydroxyalkanoate Production Using Enriched Biomass and Acidogenic Fermentation Products of Dairy Wastewater and Organic Food Waste. Desalin. Water Treat. 2021, 215, 388–397. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigun, O. Anaerobic Acidogenesis of Dairy Wastewater: The Effects of Variations in Hydraulic Retention Time with No PH Control. J. Chem. Technol. Biotechnol. 2004, 79, 755–760. [Google Scholar] [CrossRef]
- Choi, G.; Kim, J.; Lee, C. Effect of Low PH Start-up on Continuous Mixed-Culture Lactic Acid Fermentation of Dairy Effluent. Appl. Microbiol. Biotechnol. 2016, 100, 10179–10191. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Yu, H.Q. Effect of HRT on Mesophilic Acidogenesis of Dairy Wastewater. J. Environ. Eng. 2000, 126, 1145–1149. [Google Scholar] [CrossRef]
- de Sousa e Silva, A.; Tavares Ferreira, T.J.; Sales Morais, N.W.; Lopes Pereira, E.; Bezerra dos Santos, A. S/X Ratio Impacts the Profile and Kinetics of Carboxylic Acids Production from the Acidogenic Fermentation of Dairy Wastewater. Environ. Pollut. 2021, 287, 1–7. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Volatile Fatty Acids Production during Mixed Culture Fermentation–The Impact of Substrate Complexity and PH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Gameiro, T.; Sousa, F.; Silva, F.C.; Couras, C.; Lopes, M.; Louros, V.; Nadais, H.; Capela, I. Olive Oil Mill Wastewater to Volatile Fatty Acids: Statistical Study of the Acidogenic Process. Water. Air. Soil Pollut. 2015, 226, 115. [Google Scholar] [CrossRef]
- Fra-Vázquez, A.; Pedrouso, A.; Val del Rio, A.; Mosquera-Corral, A. Volatile Fatty Acid Production from Saline Cooked Mussel Processing Wastewater at Low PH. Sci. Total Environ. 2020, 732, 139337. [Google Scholar] [CrossRef] [PubMed]
- Vergine, P.; Sousa, F.; Lopes, M.; Silva, F.; Gameiro, T.; Nadais, H.; Capela, I. Synthetic Soft Drink Wastewater Suitability for the Production of Volatile Fatty Acids. Process Biochem. 2015, 50, 1308–1312. [Google Scholar] [CrossRef]
- Mato, T.; Ben, M.; Kennes, C.; Veiga, M.C. Valuable Product Production from Wood Mill Effluents. Water Sci. Technol. 2010, 62, 2294–2300. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Hwang, K.; Shin, S.G.; Lee, S.; Hwang, S. Effect of High Temperature on Bacterial Community Dynamics in Anaerobic Acidogenesis Using Mesophilic Sludge Inoculum. Bioresour. Technol. 2010, 101, S17–S22. [Google Scholar] [CrossRef]
- Yang, K.; Oh, C.; Hwang, S. Optimizing Volatile Fatty Acid Production in Partial Acidogenesis of Swine Wastewater. Water Sci. Technol. 2004, 50, 169–176. [Google Scholar] [CrossRef]
- Silva, A.J.; Pozzi, E.; Foresti, E.; Zaiat, M. The Influence of the Buffering Capacity on the Production of Organic Acids and Alcohols from Wastewater in Anaerobic Reactor. Appl. Biochem. Biotechnol. 2015, 175, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mu, Y.; Yu, H.Q. Response Surface Analysis to Evaluate the Influence of PH, Temperature and Substrate Concentration on the Acidogenesis of Sucrose-Rich Wastewater. Biochem. Eng. J. 2005, 23, 175–184. [Google Scholar] [CrossRef]
- Ruel, S.M.; Comeau, Y.; Héduit, A.; Deronzier, G.; Ginestet, P.; Audic, J.M. Operating Conditions for the Determination of the Biochemical Acidogenic Potential of Wastewater. Water Res. 2002, 36, 2337–2341. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A Review of the Production and Applications of Waste-Derived Volatile Fatty Acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Gallert, C.; Winter, J. Bacterial Metabolism in Wastewater Treatment Systems. In Environmental Biotechnology: Concepts and Applications; Jördening, H.J., Winter, J., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 1–48. ISBN 3527305858. [Google Scholar]
- Liu, H.; Chen, Y.; Wu, J. Municipal Wastewater Biological Nutrient Removal Driven by the Fermentation Liquid of Dairy Wastewater. Environ. Technol. 2017, 38, 2639–2649. [Google Scholar] [CrossRef]
- Atasoy, M.; Cetecioglu, Z. The Effects of PH on the Production of Volatile Fatty Acids and Microbial Dynamics in Long-Term Reactor Operation. J. Environ. Manage. 2022, 319. [Google Scholar] [CrossRef]
- Barana, A.C.; Cereda, M.P. Cassava Wastewater (Manipueira) Treatment Using a Two-Phase Anaerobic Biodigestor. Ciência e Tecnol. Aliment. 2000, 20, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Xie, L.; Chen, Y.G.; Zhou, Q. VFAs Production Potential of Brewery Industry Wastewater and Starch Wastewater. Adv. Mater. Res. 2013, 777, 225–231. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Kornaros, M. Effect of PH on the Anaerobic Acidogenesis of Agroindustrial Wastewaters for Maximization of Bio-Hydrogen Production: A Lab-Scale Evaluation Using Batch Tests. Bioresour. Technol. 2014, 162, 218–227. [Google Scholar] [CrossRef]
- Lv, N.; Cai, G.; Pan, X.; Li, Y.; Wang, R.; Li, J.; Li, C.; Zhu, G. PH and Hydraulic Retention Time Regulation for Anaerobic Fermentation: Focus on Volatile Fatty Acids Production/Distribution, Microbial Community Succession and Interactive Correlation. Bioresour. Technol. 2022, 347, 126310. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrère, H.; Steyer, J.P. Hydrogen Production from Agricultural Waste by Dark Fermentation: A Review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of Anaerobic Digestion for Volatile Fatty Acids, Hydrogen or Methane Production: A Critical Review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Ben, M.; Mato, T.; Lopez, A.; Vila, M.; Kennes, C.; Veiga, M.C. Bioplastic Production Using Wood Mill Effluents as Feedstock. Water Sci. Technol. 2011, 63, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Liu, Y.; Nghiem, L.D.; Chang, S.W.; Nguyen, D.D.; Zhang, S.; Luo, G.; Jia, H. Optimization of Hydraulic Retention Time and Organic Loading Rate for Volatile Fatty Acid Production from Low Strength Wastewater in an Anaerobic Membrane Bioreactor. Bioresour. Technol. 2019, 271, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Azbar, N.; Tuba, F.C.; Keskin, T.; Korkmaz, K.S.; Syed, H.M. Continuous Fermentative Hydrogen Production from Cheese Whey Wastewater under Thermophilic Anaerobic Conditions. Int. J. Hidrogen Energy 2009, 34, 7441–7447. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile Fatty Acids Production from Food Waste: Effects of PH, Temperature, and Organic Loading Rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Infantes, D.; Gonzáles del Campo, A.; Villaseñor, J.; Fernández, F.J. Kinetic Model and Study of the Influence of PH, Temperature and Undissociated Acids on Acidogenic Fermentation. Biochem. Eng. J. 2012, 66, 66–72. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Nghiem, L.D.; Hai, F.I.; Deng, L.J.; Wang, J.; Wu, Y. Optimization of Process Parameters for Production of Volatile Fatty Acid, Biohydrogen and Methane from Anaerobic Digestion. Bioresour. Technol. 2016, 219, 738–748. [Google Scholar] [CrossRef]
- Yu, H.Q.; Fang, H.H.P. Anaerobic Acidification of a Synthetic Wastewater in Batch Reactors at 55 °C. Water Sci. Technol. 2002, 46, 153–157. [Google Scholar] [CrossRef]
- Yin, X.; Li, J.; Shin, H.D.; Du, G.; Liu, L.; Chen, J. Metabolic Engineering in the Biotechnological Production of Organic Acids in the Tricarboxylic Acid Cycle of Microorganisms: Advances and Prospects. Biotechnol. Adv. 2015, 33, 830–841. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, H.Q. From Wastewater to Bioenergy and Biochemicals via Two-Stage Bioconversion Processes: A Future Paradigm. Biotechnol. Adv. 2011, 29, 972–982. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, C.J.; Simiqueli, A.P.R.; de Lima, F.A.; da Silva, J.B.; de Andrade, L.M.; Fai, A.E.C. Cassava Wastewater as Substrate in Biotechnological Processes. In Handbook on Cassava: Production, Potential Uses and Recent Advances; Klein, C., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 171–200. ISBN 9781536103076. [Google Scholar]
- González-Cabaleiro, R.; Lema, J.M.; Rodríguez, J. Metabolic Energy-Based Modelling Explains Product Yielding in Anaerobic Mixed Culture Fermentations. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Llanes, J.; Pagés-Díaz, J.; Kalogirou, E.; Contino, F. Development and Application in Aspen Plus of a Process Simulation Model for the Anaerobic Digestion of Vinasses in UASB Reactors: Hydrodynamics and Biochemical Reactions. J. Environ. Chem. Eng. 2020, 8, 103540. [Google Scholar] [CrossRef]
- Bhatt, A.H.; Ren, Z.; Tao, L. Value Proposition of Untapped Wet Wastes: Carboxylic Acid Production through Anaerobic Digestion. iScience 2020, 23, 101221. [Google Scholar] [CrossRef] [PubMed]



| Position | Research Institution | Number of Publications |
|---|---|---|
| 1 | Tongji University | 31 |
| 2 | Harbin Institute of Technology | 26 |
| 3 | Ministry of Education China | 25 |
| 4 | Indian Institute of Chemical Technology | 24 |
| 5 | Chinese Academy of Sciences | 19 |
| 6 | The University of Queensland | 18 |
| 7 | Beijing University of Technology | 16 |
| 8 | The University of Hong Kong | 14 |
| 9 | Tsinghua University | 14 |
| 10 | University of Science and Technology of China | 13 |
| 11 | Jiangnan University | 13 |
| Type of Wastewater | Substrate Concentration (mgCOD /L) | Optimum pH | Total Acids (mg/L) | Acid Composition | Degree of Acidification | Reference |
|---|---|---|---|---|---|---|
| Meat extract | 2920 | 7.0 | NA | Acetic acid (39%) Propionic acid (35%) | 40% | [27] |
| Cheese whey | 4590 | 5.25–5.5 | 2270 | Acetic acid (43%) Butyric acid (42%) Propionic acid (15%) | 83% | [38] |
| Pulp and paper | 7740 | 5.5–6.0 | 3960 | Butyric acid (78%) Acetic acid (9%) Propionic acid (6%) Others (7%) | 76% | [38] |
| Cassava processing | 6040 to 7600 | 5.50–6.09 | 5080 to 5760 | Butyric acid (87%) | 84.10% | [39] |
| Cassava processing | 8865 | 5.9 | 4000 | Butyric acid (42%) Acetic acid (35%) Propionic acid (20%) | 63% | [40] |
| Cassava processing | 50,000 (glucose) | 6.5 | 40,000 (lactic acid) | Lactic acid Acetic acid Propionic acid | NA Note: the paper was oriented only to the production of lactic acid. | [42] |
| Dairy wastewater | 111,450 ± 1937 | 8 | 20,585 | Acetic acid (17.5%) Butyric acid (61.1%) Propionic acid (9.3%) Valeric acid (12.0%) | NA | [64] |
| Cheese processing | 20,000 | 5.0 | 3688 | Propionic acid (24%) Acetic acid (21%) Valeric acid (20%) Butyric acid (17%) | 93% | [65] |
| Cassava Processing | 1000–25,440 | 5.5–6.0 | 200 to 4520 | Lactic Acid (82%) Butyric Acid (6%) Acetic Acid (5%) Small concentrations of tartaric, succinic, propionic, iso-butyric and iso-valeric acids were detected. | NA | [66] |
| Cassava vinasse | 36,000 | 8.0 | 24,000 | Butyric acid (40%) Acetic acid (36.5%) Propionic acid (20%) | 71% | [67] |
| Starch | 19,400 | 8.0 | 8600 | Acetic acid (68.3%) Propionic acid (15.5%) Butyric acid (13.8%) | 68% | [67] |
| Agro-industrial (oil mill, cheese whey and cow manure slurry—55:40:5 v/v/v) | 95,000 | 6.5 | 13,430 | Butyric acid Propionic acid Acetic acid Ethanol Lactic acid | NA | [68] |
| Synthetic wastewater (with glucose) DQO:N:P—300:5:1 | 4000 | 6.0 | 2139.11 as mgHAc/L | Acetic acid (49.88%) Butyric acid Propionic acid | 56.64% | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Ledesma, L.M.; Ramírez-Malule, H.; Rodríguez-Victoria, J.A. Volatile Fatty Acids Production by Acidogenic Fermentation of Wastewater: A Bibliometric Analysis. Sustainability 2023, 15, 2370. https://doi.org/10.3390/su15032370
Sanchez-Ledesma LM, Ramírez-Malule H, Rodríguez-Victoria JA. Volatile Fatty Acids Production by Acidogenic Fermentation of Wastewater: A Bibliometric Analysis. Sustainability. 2023; 15(3):2370. https://doi.org/10.3390/su15032370
Chicago/Turabian StyleSanchez-Ledesma, Lina Marcela, Howard Ramírez-Malule, and Jenny Alexandra Rodríguez-Victoria. 2023. "Volatile Fatty Acids Production by Acidogenic Fermentation of Wastewater: A Bibliometric Analysis" Sustainability 15, no. 3: 2370. https://doi.org/10.3390/su15032370
APA StyleSanchez-Ledesma, L. M., Ramírez-Malule, H., & Rodríguez-Victoria, J. A. (2023). Volatile Fatty Acids Production by Acidogenic Fermentation of Wastewater: A Bibliometric Analysis. Sustainability, 15(3), 2370. https://doi.org/10.3390/su15032370






