Gamma Attenuation and Mechanical Characteristics of a Lead/NBR/SBR Rubber Composite with Black Nanocarbon Reinforcement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Material Characterization
2.3. Mass Density Measurement
2.4. Mechanical Properties Measurement
3. Theoretical Background
4. Results and Discussion
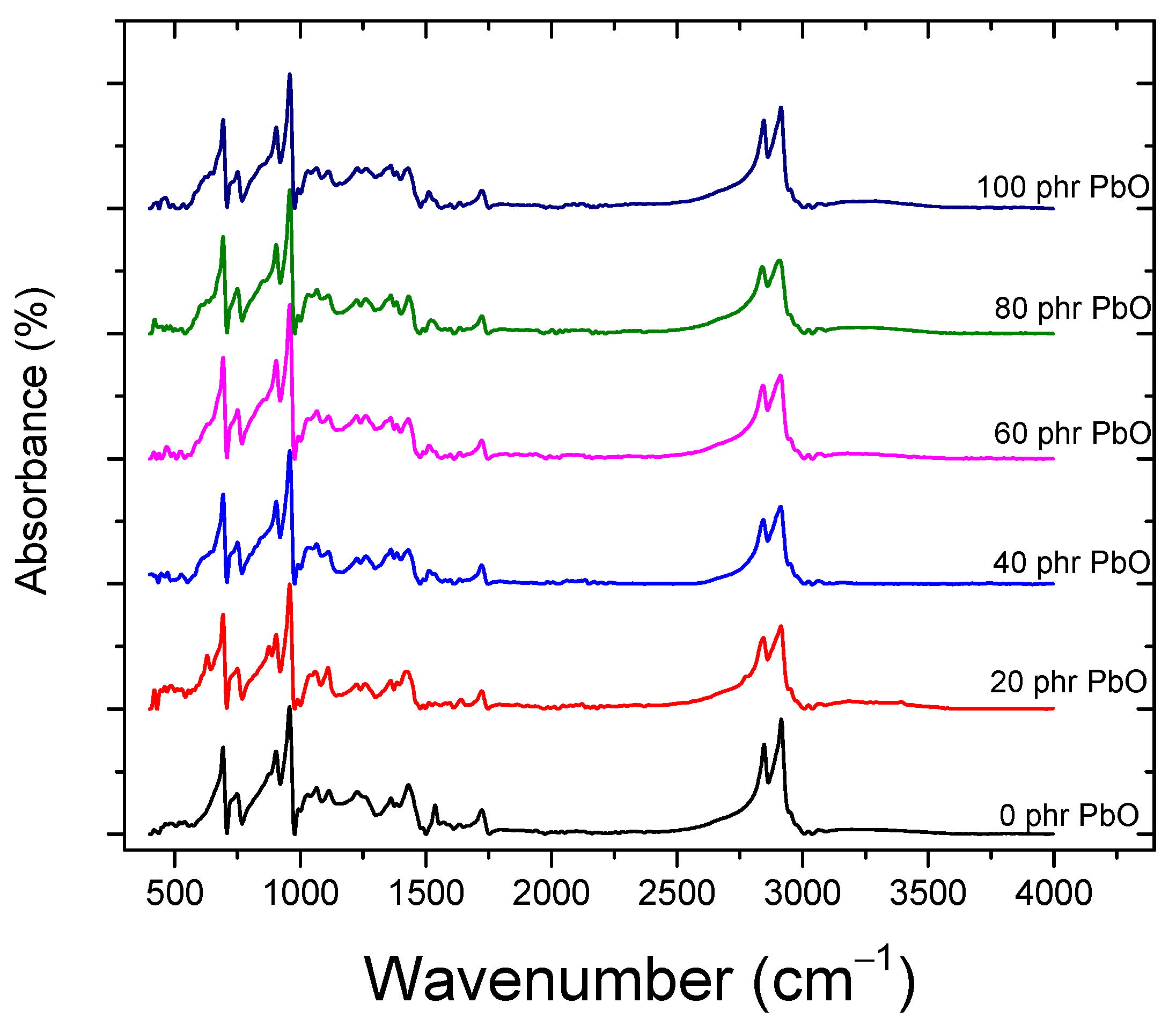
4.1. Fourier-Transform Infrared Spectroscopy (FTIR)
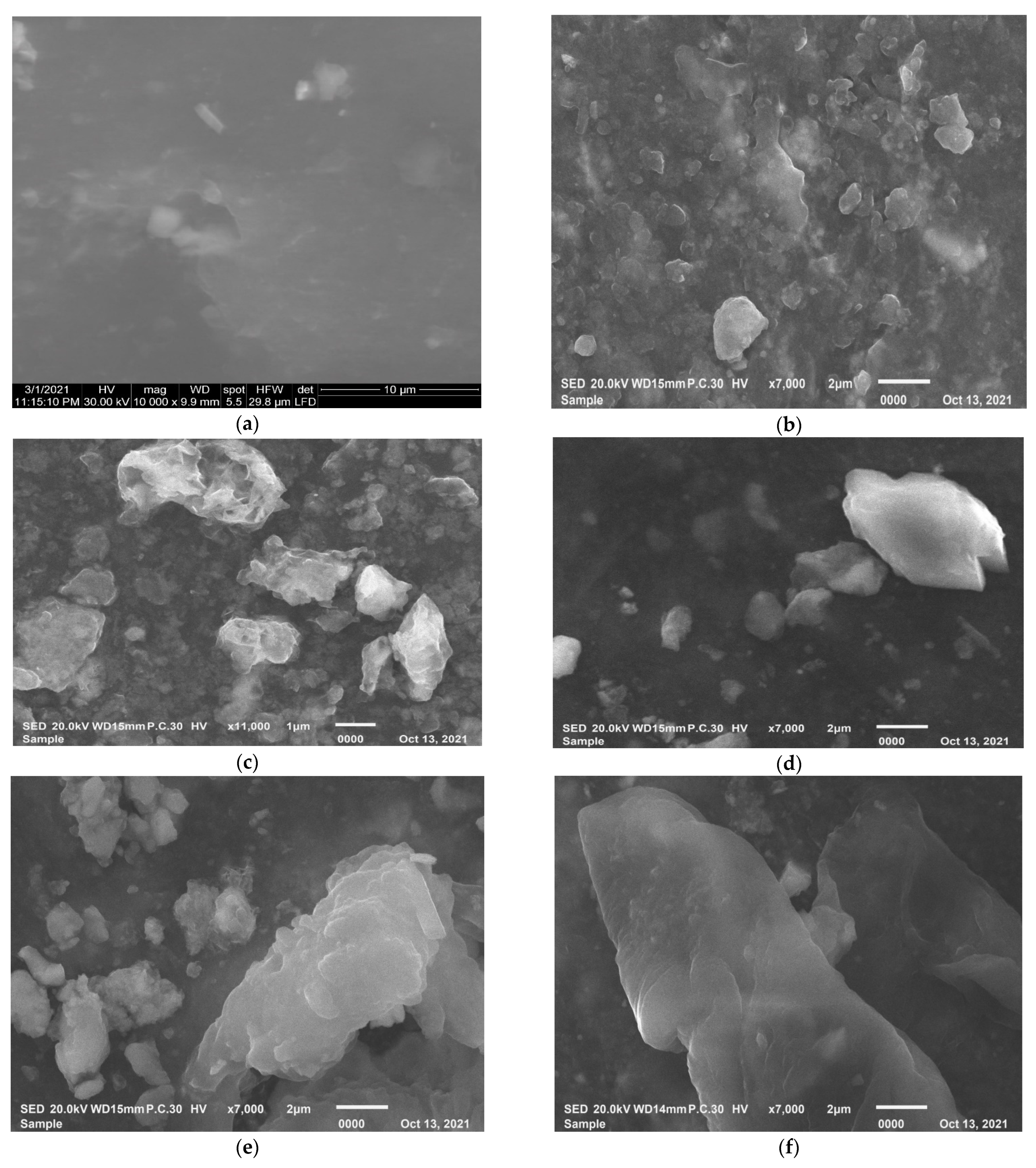
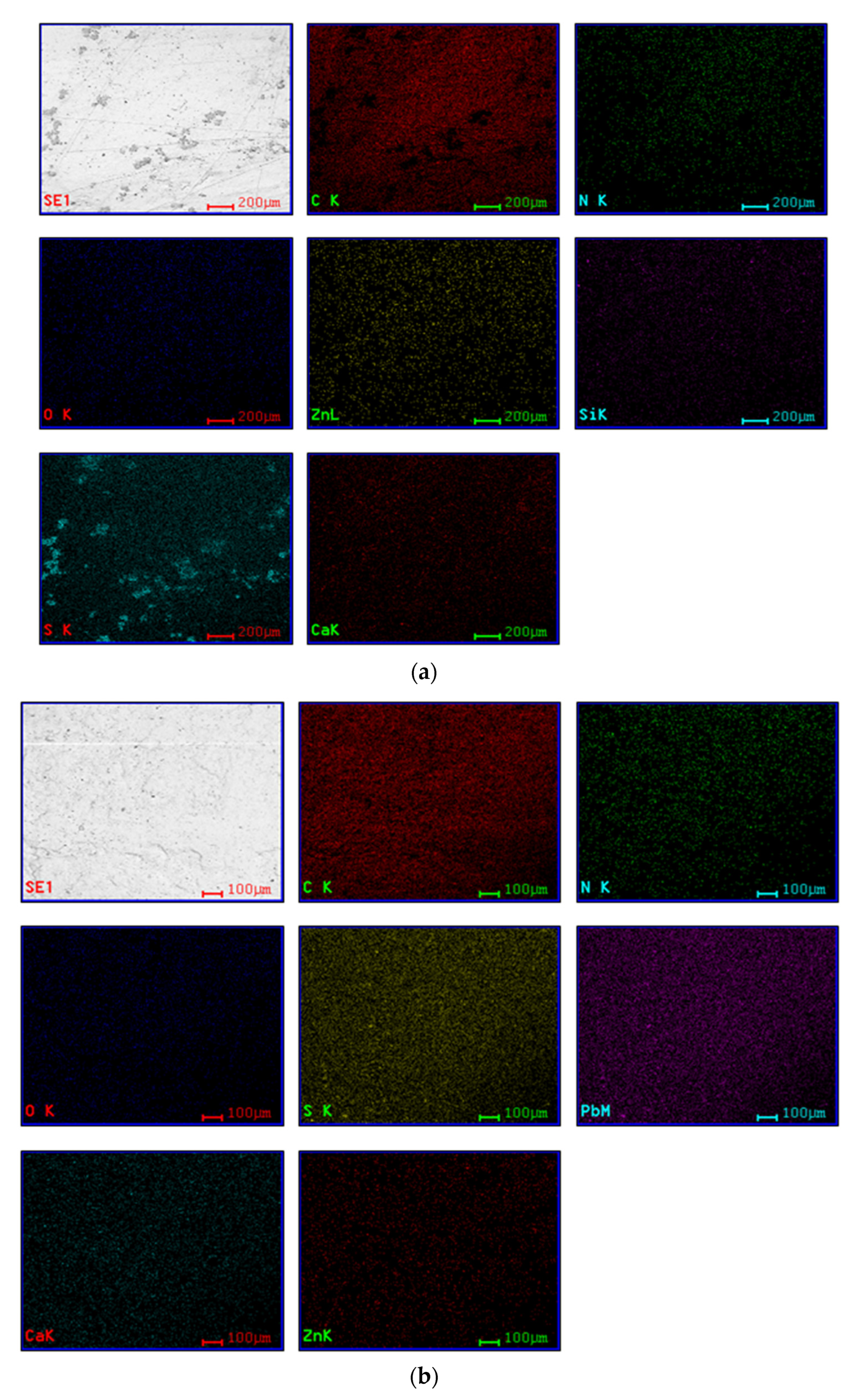
4.2. Scanning Electron Microscope (SEM)
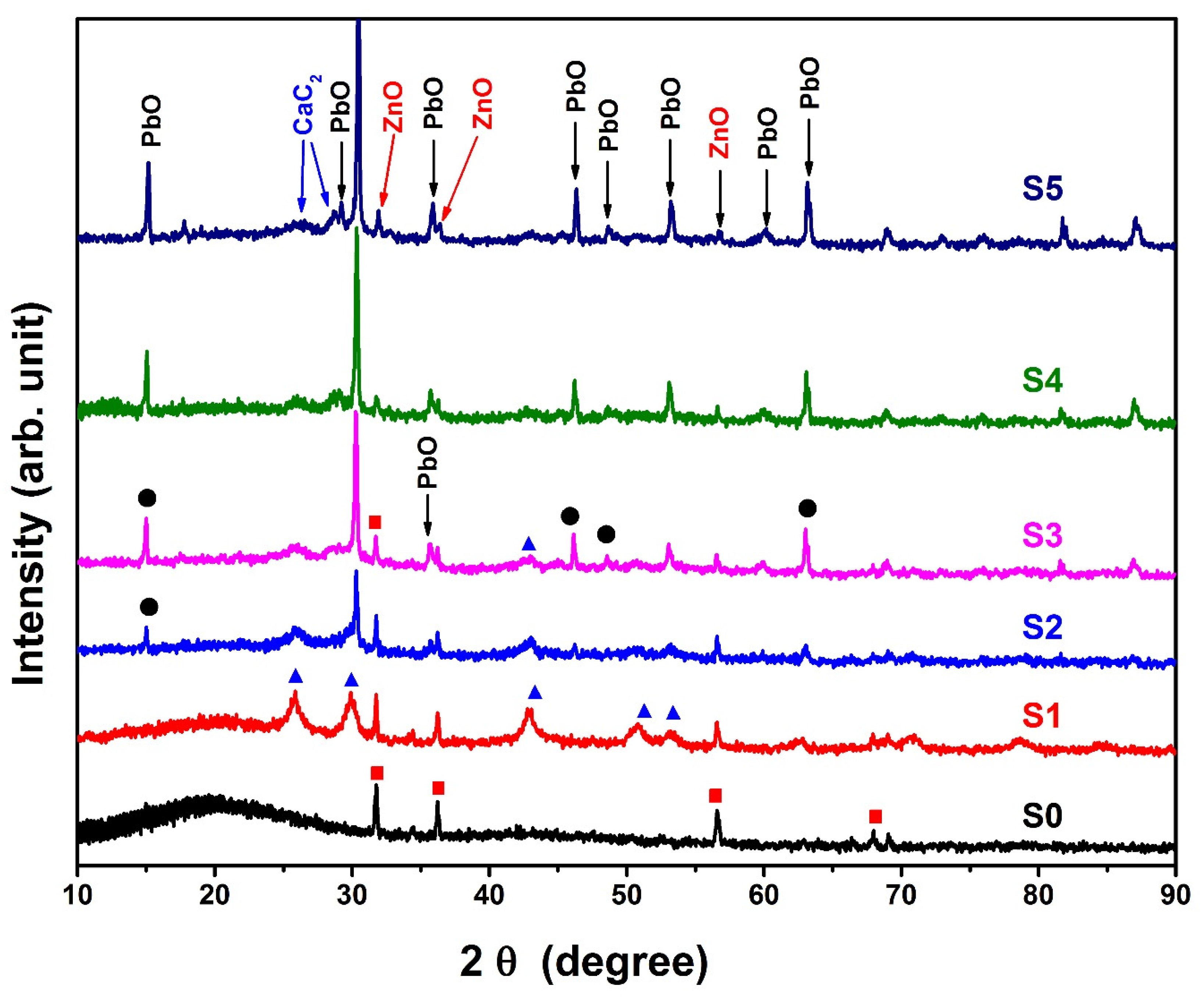
4.3. X-ray Diffraction (XRD)
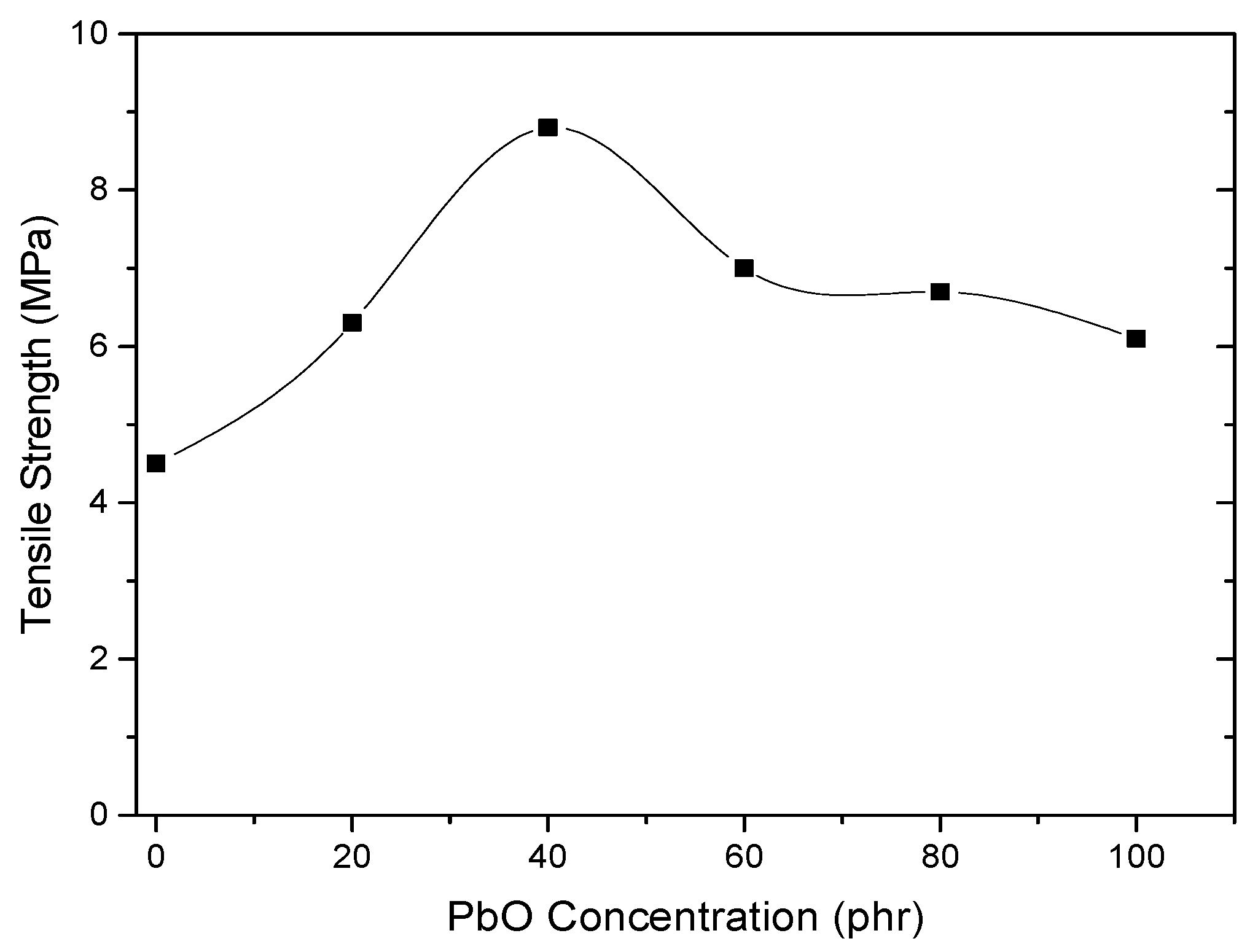
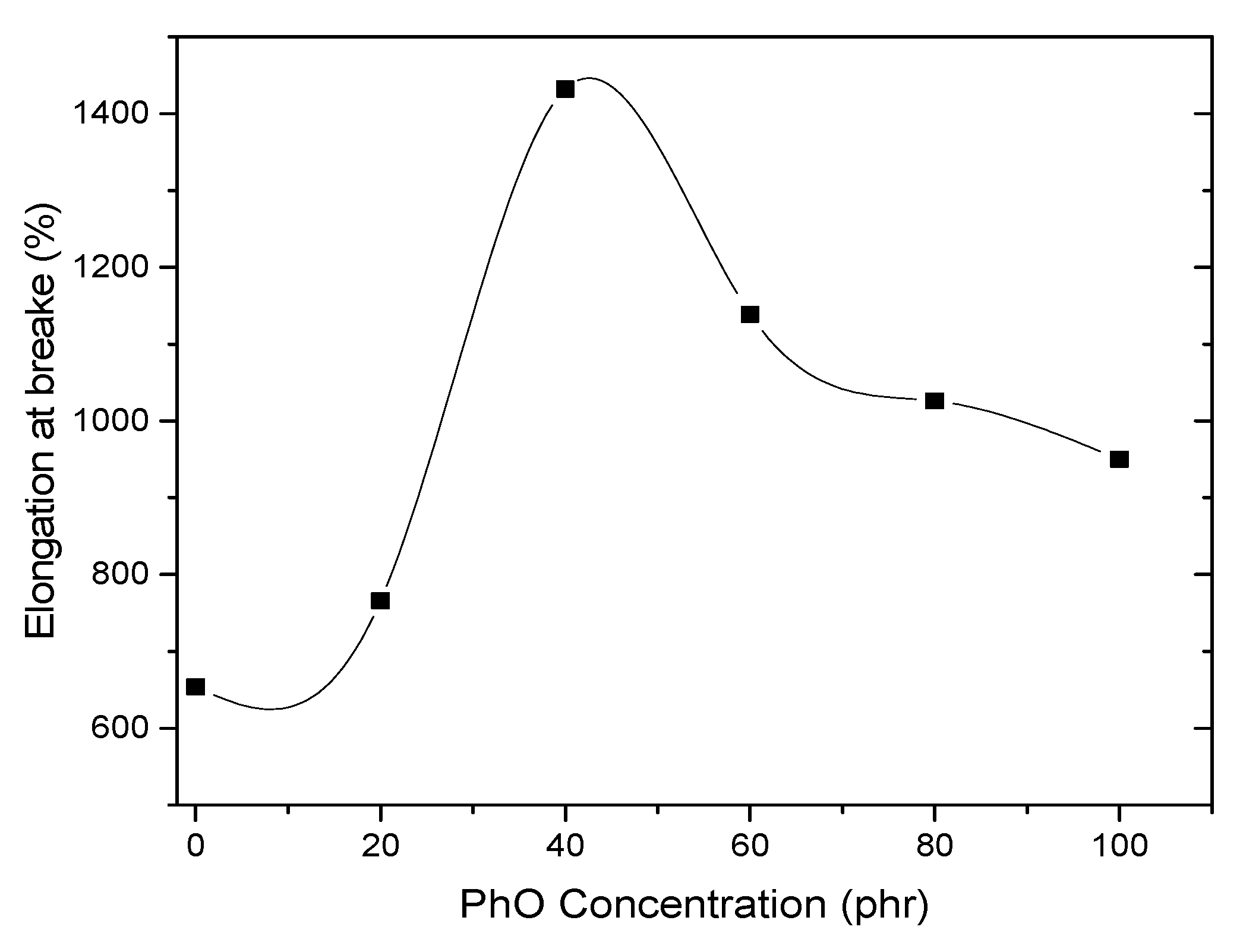
4.4. Mechanical Properties
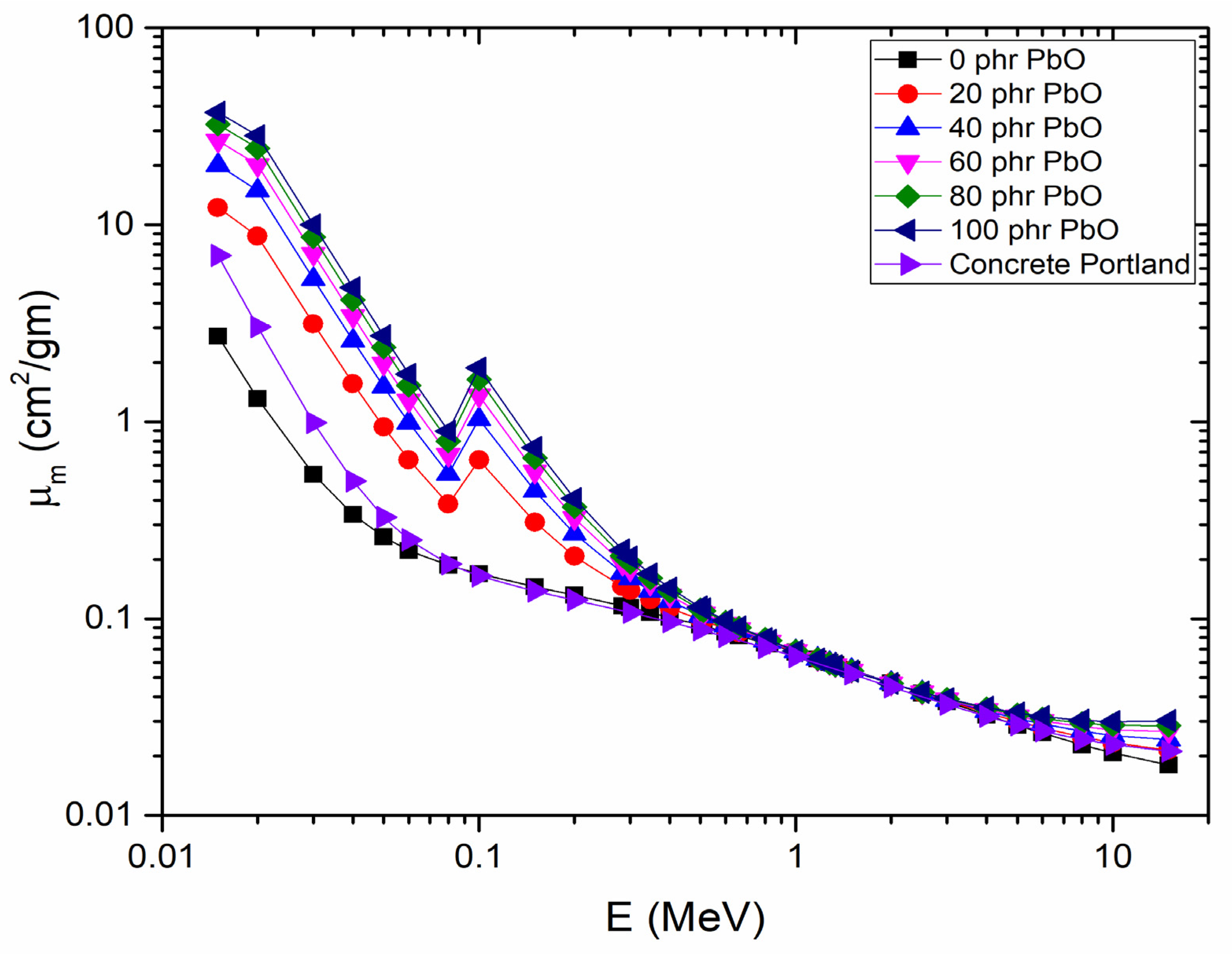
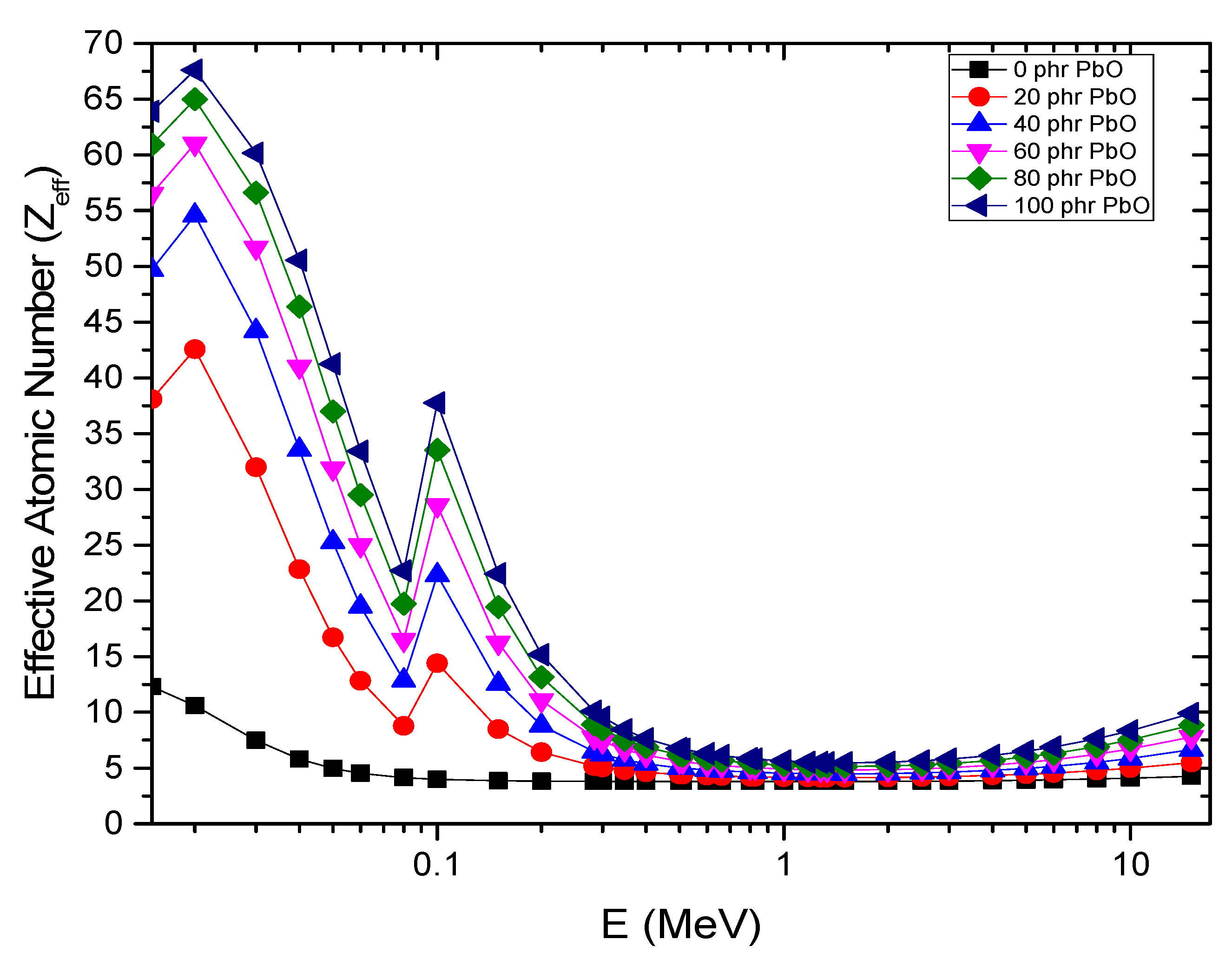
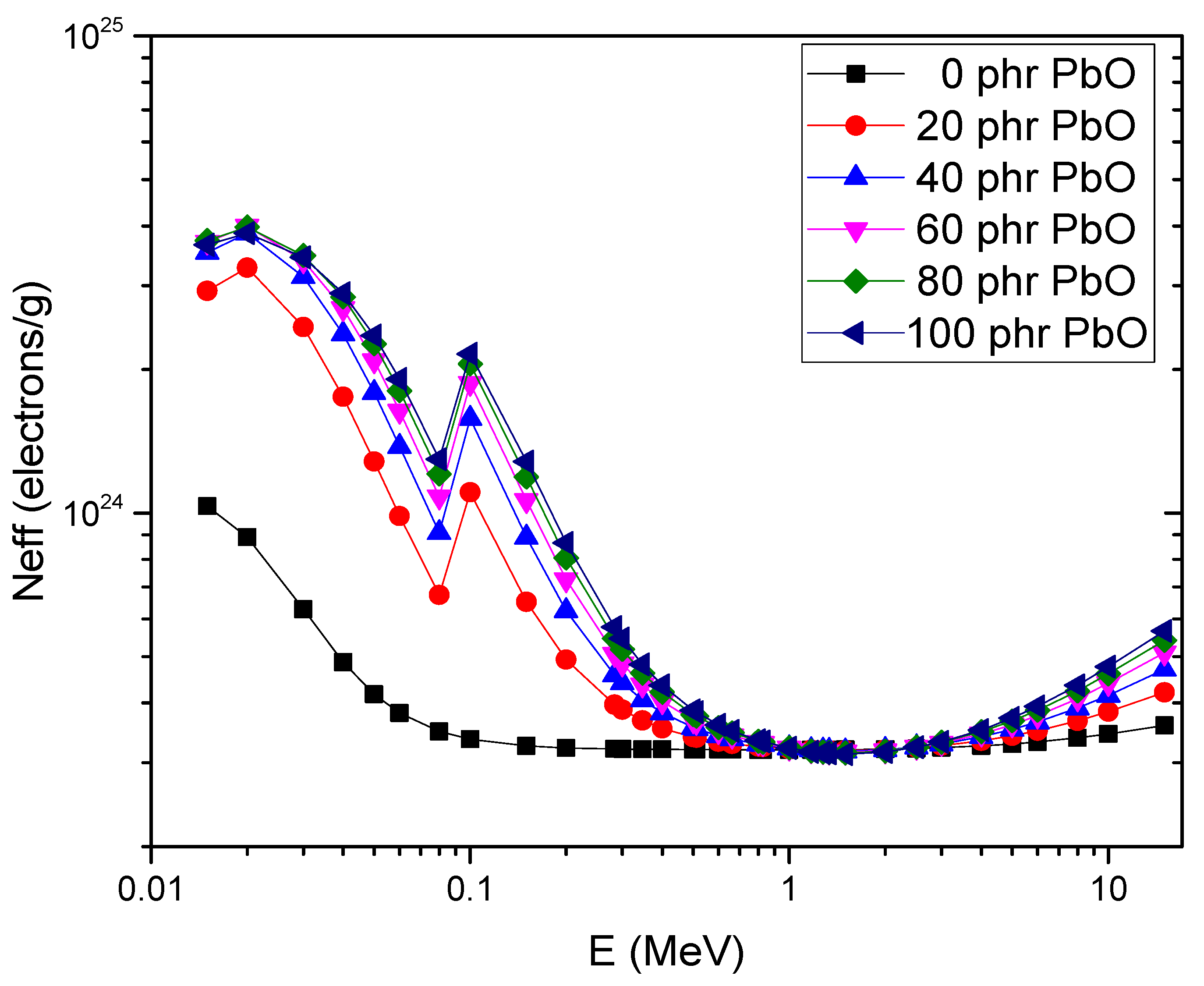
4.5. Gamma-Ray Shielding Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nambiar, S.; Yeow, J.T.W. Polymer-composite materials for radiation protection. ACS Appl. Mater. Interfaces 2012, 4, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Facio, A.; Benavides, R.; Martínez-Pardo, M. Thermal stability of PVC formulations gamma irradiated at different dose rates. Radiat. Phys. Chem. 2014, 97, 75–80. [Google Scholar] [CrossRef]
- Canel, A.; Korkut, H.; Korkut, T. Improving neutron and gamma flexible shielding by adding medium-heavy metal powder to epoxy based composite materials. Radiat. Phys. Chem. 2019, 158, 13–16. [Google Scholar] [CrossRef]
- Sayyed, M. Investigation of shielding parameters for smart polymers. Chin. J. Phys. 2016, 54, 408–415. [Google Scholar] [CrossRef]
- Mann, K.S.; Rani, A.; Heer, M.S. Shielding behaviors of some polymer and plastic materials for gamma-rays. Radiat. Phys. Chem. 2015, 106, 247–254. [Google Scholar] [CrossRef]
- Mirji, R.; Lobo, B. Computation of the mass attenuation coefficient of polymeric materials at specific gamma photon energies. Radiat. Phys. Chem. 2017, 135, 32–44. [Google Scholar] [CrossRef]
- Martin, J.E. Physics for Radiation Protection, 3rd ed.; Wiley-VCH: Hoboken, NJ, USA, 2013. [Google Scholar]
- Harish, V.; Nagaiah, N.; Prabhu, T.N.; Varughese, K.T. Preparation and characterization of lead monoxide filled unsaturated polyester based polymer composites for gamma radiation shielding applications. J. Appl. Polym. Sci. 2009, 112, 1503–1508. [Google Scholar] [CrossRef]
- Drobny, J.G. Ionizing Radiation and Polymers: Principles, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Harish, V.; Nagaiah, N. Thermal degradation of lead monoxide filled polymer composite radiation shields. AIP Conf. Proc. 2011, 89–90. [Google Scholar] [CrossRef]
- Mandal, S.; Alam, S. Studies on gamma radiation resistance polyethersulphone films reinforced by lead oxide. Mater. Res. Innov. 2013, 17, 373–376. [Google Scholar] [CrossRef]
- Mheemeed, A.K.; Hasan, H.I.; Al-Jomaily, F.M. Gamma-ray absorption using rubber—Lead mixtures as radiation protection shields. J. Radioanal. Nucl. Chem. 2011, 291, 653–659. [Google Scholar] [CrossRef]
- Belgin, E.E.; Aycik, G.A. Preparation and radiation attenuation performances of metal oxide filled polyethylene based composites for ionizing electromagnetic radiation shielding applications. J. Radioanal. Nucl. Chem. 2015, 306, 107–117. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Khatib, A.M.; Badawi, M.S.; Rashad, A.R.; El-Sharkawy, R.M.; Thabet, A.A. Recycled high-density polyethylene plastics added with lead oxide nanoparticles as sustainable radiation shielding materials. J. Clean. Prod. 2018, 176, 276–287. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Khatib, A.M.; Badawi, M.S.; Rashad, A.R.; El-Sharkawy, R.M.; Thabet, A.A. Fabrication, characterization and gamma rays shielding properties of nano and micro lead oxide-dispersed-high density polyethylene composites. Radiat. Phys. Chem. 2018, 145, 160–173. [Google Scholar] [CrossRef]
- Özdemir, T.; Güngör, A.; Akbay, I.; Uzun, H.; Babucçuoglu, Y. Nano lead oxide and epdm composite for development of polymer based radiation shielding material: Gamma irradiation and attenuation tests. Radiat. Phys. Chem. 2018, 144, 248–255. [Google Scholar] [CrossRef]
- Majeed, A.H.; Hamza, M.S.; Kareem, H.R. Effect of Ading Nanocarbon Black on the Mechanical Properties of Epoxy. Diyala J. Eng. Sci. 2014, 7, 94–108. [Google Scholar] [CrossRef]
- Fu, J.-F.; Yu, W.-Q.; Dong, X.; Chen, L.-Y.; Jia, H.-S.; Shi, L.-Y.; Zhong, Q.-D.; Deng, W. Mechanical and tribological properties of natural rubber reinforced with carbon blacks and Al2O3 nanoparticles. Mater. Des. 2013, 49, 336–346. [Google Scholar] [CrossRef]
- Shahamatifard, F.; Rodrigue, D.; Park, K.W.; Frikha, S.; Mighri, F. Natural rubber nanocomposites: Effect of carbon black/multi-walled carbon nanotubes hybrid fillers on the mechanical properties and thermal conductivity. Polym. Technol. Mater. 2021, 60, 1686–1696. [Google Scholar] [CrossRef]
- Maiti, M.; Sadhu, S.; Bhowmick, A.K. Effect of carbon black on properties of rubber nanocomposites. J. Appl. Polym. Sci. 2005, 96, 443–451. [Google Scholar] [CrossRef]
- Abdelsalam, A.A.; Araby, S.; El-Sabbagh, S.H.; Abdelmoneim, A.; Hassan, A.M. Effect of carbon black loading on mechanical and rheological properties of natural rubber/styrene-butadiene rubber/nitrile butadiene rubber blends. J. Thermoplast. Compos. Mater. 2019, 34, 490–507. [Google Scholar] [CrossRef]
- Abdel-Baki, M.; Salem, A.; Abdel-Wahab, F.; El-Diasty, F. Bond character, optical properties and ionic conductivity of Li2O/B2O3/SiO2/Al2O3 glass: Effect of structural substitution of Li2O for LiCl. J. Non-Crystalline Solids 2008, 354, 4527–4533. [Google Scholar] [CrossRef]
- Singh, V.P.; Badiger, N.; Kaewkhao, J. Radiation shielding competence of silicate and borate heavy metal oxide glasses: Comparative study. J. Non-Crystalline Solids 2014, 404, 167–173. [Google Scholar] [CrossRef]
- Berger, M.J. Photon Cross Sections Database. NIST Standard Reference Database 8 (XGAM). 1998. Available online: http//physics.nist.gov/PhysRefData/Xcom/Text/XCOM.html (accessed on 10 October 2022).
- Tijani, S.; Kamal, S.M.; Al-Hadeethi, Y.; Arib, M.; Hussein, M.; Wageh, S.; Dim, L. Radiation shielding properties of transparent erbium zinc tellurite glass system determined at medical diagnostic energies. J. Alloy. Compd. 2018, 741, 293–299. [Google Scholar] [CrossRef]
- Taylor, M.L.; Smith, R.L.; Dossing, F.; Franich, R.D. Robust calculation of effective atomic numbers: The Auto-Zeffsoftware. Med. Phys. 2012, 39, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Tonguc, B.T.; Arslan, H.; Al-Buriahi, M.S. Studies on mass attenuation coefficients, effective atomic numbers and electron densities for some biomolecules. Radiat. Phys. Chem. 2018, 153, 86–91. [Google Scholar] [CrossRef]
- Arulmozhi, K.T.; Mythili, N. Studies on the chemical synthesis and characterization of lead oxide nanoparticles with different organic capping agents. AIP Adv. 2013, 3, 122122. [Google Scholar] [CrossRef]
- Atef, S.; El-Nashar, D.E.; Ashour, A.H.; El-Fiki, S.; El-Kameesy, S.U.; Medhat, M. Effect of gamma irradiation and lead content on the physical and shielding properties of PVC/NBR polymer blends. Polym. Bull. 2019, 77, 5423–5438. [Google Scholar] [CrossRef]
- Intom, S.; Kalkornsurapranee, E.; Johns, J.; Kaewjaeng, S.; Kothan, S.; Hongtong, W.; Chaiphaksa, W.; Kaewkhao, J. Mechanical and radiation shielding properties of flexible material based on natural rubber/ Bi2O3 composites. Radiat. Phys. Chem. 2020, 172, 108772. [Google Scholar] [CrossRef]
- Bel, T.; Arslan, C.; Baydogan, N. Radiation shielding properties of poly (methyl methacrylate) / colemanite composite for the use in mixed irradiation fields of neutrons and gamma rays. Mater. Chem. Phys. 2019, 221, 58–67. [Google Scholar] [CrossRef]
- Aggarwal, S.L. Rubber Technology, 3rd ed.; Morton, M., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1987; p. 638. [Google Scholar] [CrossRef]
- Huang, W.; Yang, W.; Ma, Q.; Wu, J.; Fan, J.; Zhang, K. Preparation and characterization of γ-ray radiation shielding PbWO4/EPDM composite. J. Radioanal. Nucl. Chem. 2016, 309, 1097–1103. [Google Scholar] [CrossRef]
- Hubbell, J.H. Review of photon interaction cross section data in the medical and biological context. Phys. Med. Biol. 1999, 44, R1–R22. [Google Scholar] [CrossRef]
- Hubbell, J. Photon mass attenuation and energy-absorption coefficients. Int. J. Appl. Radiat. Isot. 1982, 33, 1269–1290. [Google Scholar] [CrossRef]


| Ingredients | Amounts (phr) * |
|---|---|
| Acrylonitrile butadiene rubber (NBR) | 50 |
| Styrene-butadiene rubber (SBR) | 50 |
| Butadiene rubber (BR) | 20 |
| Stearic acid | 2 |
| ZnO | 5 |
| Processing oil | 10 |
| Dioctyl phthalate (DOP) | 10 |
| N330 (nanocarbon black) | 40 |
| Mercapto benzothiazole sulfenamide (MBTS) rubber accelerator | 2 |
| Trimethyl-quinoline (TMQ) | 1 |
| Sulfur (S) | 2 |
| PbO | 20–100 |
| Element | PbO Concentration (phr) | |||||
|---|---|---|---|---|---|---|
| 0 | 20 | 40 | 60 | 80 | 100 | |
| C | 77.60 | 72.91 | 75.98 | 74.98 | 69.57 | 66.56 |
| N | 09.10 | 11.92 | 6.65 | 8.17 | 7.95 | 4.17 |
| O | 07.00 | 6.23 | 5.87 | 5.27 | 5.22 | 4.39 |
| S | 04.41 | 2.62 | 2.79 | 2.72 | 4.16 | 4.21 |
| Ca | 0.87 | 0.39 | 0.36 | 0.26 | 0.50 | 0.45 |
| Zn | 1.02 | 0.45 | 0.5 | 0.48 | 0.59 | 0.58 |
| Pb | 0 | 5.47 | 7.93 | 8.10 | 11.71 | 19.64 |
| Energy (MeV) | PbO (phr) | µm (cm2/g) | HVL (cm) | Zeff (Electrons/Atom) | Neff (Electrons/g) × 1023 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Theoretical | Experimental | Theoretical | Experimental | Theoretical | Experimental | Theoretical | Experimental | ||
| 0.662 | 0 | 0.082 | 0.082 | 2.468 | 2.454 | 3.798 | 3.800 | 3.193 | 3.190 |
| 20 | 0.084 | 0.084 | 2.338 | 2.362 | 4.278 | 4.280 | 3.285 | 3.280 | |
| 40 | 0.087 | 0.086 | 2.247 | 2.254 | 4.748 | 4.695 | 3.360 | 3.321 | |
| 60 | 0.088 | 0.088 | 2.188 | 2.194 | 5.212 | 5.210 | 3.414 | 3.410 | |
| 80 | 0.208 | 0.208 | 0.922 | 0.922 | 5.656 | 5.660 | 3.462 | 3.460 | |
| 100 | 0.091 | 0.091 | 2.098 | 2.097 | 6.117 | 6.120 | 3.493 | 3.490 | |
| 1.173 | 0 | 0.062 | 0.062 | 3.241 | 3.241 | 3.796 | 3.800 | 3.192 | 3.190 |
| 20 | 0.062 | 0.062 | 3.166 | 3.176 | 4.154 | 4.150 | 3.190 | 3.190 | |
| 40 | 0.062 | 0.062 | 3.119 | 3.125 | 4.506 | 4.510 | 3.189 | 3.190 | |
| 60 | 0.062 | 0.062 | 3.099 | 3.089 | 4.853 | 4.850 | 1.179 | 1.180 | |
| 80 | 0.062 | 0.062 | 3.084 | 3.088 | 5.185 | 5.190 | 3.173 | 3.170 | |
| 100 | 0.062 | 0.062 | 3.068 | 3.062 | 5.534 | 5.530 | 3.161 | 3.160 | |
| 1.332 | 0 | 0.058 | 0.058 | 3.461 | 3.462 | 3.796 | 3.800 | 3.192 | 3.190 |
| 20 | 0.058 | 0.058 | 3.389 | 3.385 | 4.145 | 4.140 | 3.182 | 3.180 | |
| 40 | 0.058 | 0.057 | 3.346 | 3.393 | 4.487 | 4.413 | 3.175 | 3.125 | |
| 60 | 0.058 | 0.058 | 3.331 | 3.326 | 4.824 | 4.820 | 3.160 | 3.160 | |
| 80 | 0.058 | 0.057 | 3.320 | 3.311 | 5.147 | 5.061 | 3.150 | 3.096 | |
| 100 | 0.058 | 0.060 | 3.307 | 3.164 | 5.487 | 5.679 | 3.134 | 3.238 | |
| 2.51 | 0 | 0.042 | 0.042 | 4.847 | 4.853 | 3.818 | 3.820 | 3.210 | 3.210 |
| 20 | 0.042 | 0.042 | 4.712 | 4.783 | 4.196 | 4.200 | 3.223 | 3.220 | |
| 40 | 0.042 | 0.042 | 4.624 | 4.614 | 4.568 | 4.570 | 3.233 | 3.230 | |
| 60 | 0.042 | 0.041 | 4.580 | 4.583 | 4.935 | 4.813 | 3.232 | 3.153 | |
| 80 | 0.042 | 0.042 | 4.546 | 4.562 | 5.285 | 5.290 | 3.234 | 3.230 | |
| 100 | 0.042 | 0.042 | 4.510 | 4.500 | 5.653 | 5.650 | 3.238 | 3.230 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamal, R.; Salama, E.; Elshimy, H.; El-Nashar, D.E.; Bakry, A.; Ehab, M. Gamma Attenuation and Mechanical Characteristics of a Lead/NBR/SBR Rubber Composite with Black Nanocarbon Reinforcement. Sustainability 2023, 15, 2165. https://doi.org/10.3390/su15032165
Gamal R, Salama E, Elshimy H, El-Nashar DE, Bakry A, Ehab M. Gamma Attenuation and Mechanical Characteristics of a Lead/NBR/SBR Rubber Composite with Black Nanocarbon Reinforcement. Sustainability. 2023; 15(3):2165. https://doi.org/10.3390/su15032165
Chicago/Turabian StyleGamal, Romisaa, Elsayed Salama, Hassan Elshimy, Doaa E. El-Nashar, Assem Bakry, and Mohamed Ehab. 2023. "Gamma Attenuation and Mechanical Characteristics of a Lead/NBR/SBR Rubber Composite with Black Nanocarbon Reinforcement" Sustainability 15, no. 3: 2165. https://doi.org/10.3390/su15032165
APA StyleGamal, R., Salama, E., Elshimy, H., El-Nashar, D. E., Bakry, A., & Ehab, M. (2023). Gamma Attenuation and Mechanical Characteristics of a Lead/NBR/SBR Rubber Composite with Black Nanocarbon Reinforcement. Sustainability, 15(3), 2165. https://doi.org/10.3390/su15032165







