Abstract
Sodium salinity negatively affects and reduces yields in international agricultural systems. This stress decreases crop growth and development, causing tissue death, flowering abortion, and senescence of the fertilized embryo, and negatively affects enzymatic activity, protein synthesis, among other processes. Rice is a cereal of great international demand for its nutritional properties and its productivity is affected by the presence of salts in agricultural surfaces. The objective of this article is to review the main effects of sodium salinity on morpho-physiological characteristics in rice cultivation. For the design and strategy of the information search, a methodology was followed to compile and summarize the existing studies on the effects of sodium salinity on this crop. The results of this search showed that sodium salts cause poor root growth, chlorosis, leaf curling and leaf scorching in this cereal; it also induces stomatal closure, inhibits photosynthesis, alters cell metabolism, causes oxidative stress in the crop, influences spikelet sterility and grain yield, among other effects.
1. Introduction
Salinity is one of the main abiotic factors; it is caused by different factors, including planting agricultural crops near coastal areas. It has a direct impact on the quality of agricultural soils and significantly affects the agricultural potential of crops [1,2]. Approximately 6% of the world’s arable land is affected by salts, representing more than 800 million hectares, with monetary losses amounting to 12 billion dollars in agricultural production [3,4,5]. Agricultural land is classified as saline when the electrical conductivity (EC) is 4 dS m−1 (approximately 40 mM NaCl) or more [6].
There are two types of salinization: natural (primary salinization) and anthropogenic (secondary salinization). The first is closely related to the water table with marine origins and the effects of sea intrusions in coastal areas, the primary minerals that form the rocks, the deposition of salts transported by the wind, seepage, upward capillary flow due to evapotranspiration [7,8,9]. On the other hand, secondary irrigation is due to poor management and use of poorly adapted soils, with drainage problems and unsuitable for irrigation, incorrect use of irrigation depths and their irregular distribution due to poor irrigation management, as well as excessive and intensive use of amendments or fertilizers and the use of industrial wastes or the use of wastewater for agricultural irrigation [10,11,12].
In addition, the intensive exploitation of groundwater resources, with special emphasis on coastal aquifers, induces saline intrusion through the developed artificial canals, the densely meshed system of rivers and/or natural reaches, resulting in the loss of water quality used for irrigation [13,14].
Under conditions of salt stress, plants absorb a large amount of salts, which are transferred from the soil solution to the outer cells of the root system, to the xylem vessels located in the radicle, and in turn, is transported from the roots to the shoots; then to the transpiratory flow through the leaves, which finally inhibits the absorption of nutrients by the plant [15,16,17]. Other effects caused by the presence of these salts on agricultural surfaces are a reduction in plant expansion, root vigor, inhibition and the retardation of growth and development, accelerated wilting, inhibition of the photosynthetic process, loss of turgor, cellular pH instability, accumulation of reactive oxygen species (ROS), membrane damage, ionic toxicity, osmotic imbalance, water imbalance, among others [18,19,20].
The main cause of salinization is sodium chloride (NaCl), which is abundant in most agricultural soils and is highly soluble; it limits the productivity and quality of areas devoted to agricultural cultivation internationally. Excessive concentrations of these salts and the deficit of water resources are factors that cause the conversion of fertile fields into marginal ones. The Food and Agriculture Organization of the United Nations (FAO) estimates that the impact of salinity on agricultural land amounts to more than 33% [21,22].
Various physiological, biochemical and molecular processes, water relations, transpiration, photosynthesis, cellular homeostasis, hormonal and enzymatic activities and gene expression patterns in plants are negatively affected by sodium salt stress. An accumulation of sodium salt causes an increase in soil pH and alkalinity, which in turn leads to osmotic stress and nutrient deficiency in plants due to its interference with the uptake of nutrients such as phosphorus, manganese, zinc, iron and copper [23,24].
Besides the osmotic and ionic stress induced by NaCl salt stress, this in turn causes other secondary stresses, for example, nutritional imbalances and oxidative stress cause the creation of reactive oxygen species (ROS) in plant radicles, such as hydrogen peroxide (H2O2), superoxide (O2) and hydroxide (OH) [25]. In addition, several biological processes are modified by the influence of high salt concentrations, such as germination, seed vigor, vegetative growth, flowering and fruit development [26].
Rice (Oryza sativa L.) is currently the main source of food for millions of people as the second most cultivated cereal in the world. Unfortunately, the poor management of soil resources, an increase in the presence of pathogens and the accumulation of phytotoxic substances affect the productivity of this crop, which still does not meet the existing demand [27,28].
Sodium salt stress is one of the factors that cause the greatest damage to crop growth, development and yield. Especially in the rice crop, this factor represents the main limiting factor in its productivity; the vegetative, reproductive and grain-filling stages are the most prone to this stress. Among the main symptoms caused by NaCl on O. sativa are the white tips of affected leaves, a decrease, retardation and irregular growth of seedlings, a reduction in tillering and, in severe cases, the death of this crop. This stress causes a significant reduction in the number of stems per plant, the number of spikelets per panicle, fertility, length and the number of panicles [29,30].
The complex mechanism of salt tolerance in soils involves responses at both the cellular and molecular levels. Therefore, it is necessary to urgently develop and investigate different methods and strategies for the elimination of the toxic effect of this stress [31].
Cuba has an agricultural area of 8709.3 million hectares and nearly 1 million of these have salinization problems, which represents 9.1% of the country’s surface area [32].
Therefore, it is necessary to review the main morphophysiological effects of salinity and specifically of sodium salinity on agricultural crops and especially on rice cultivation; as well as some alternatives which are currently being investigated in the mitigation of the negative effects of salt stress. This article aims to review the effects of sodium chloride on morphophysiological characteristics in rice cultivation.
2. Materials and Methods
The objective of this work is to review the main effects of sodium salinity on O. sativa. For the design and strategy of the information search, a methodology for literature review and information management of scientific topics was used, through its structuring and systematization, to compile and summarize the existing studies on the effects of sodium salinity on rice cultivation [33]. Using the keywords “sodium salinity” “rice” “sodium stress” “sodium chloride stress” “NaCl salinity” “NaCl stress” “NaCl-induced salinity” “NaCl salinity stress” “NaCl salinity stress” “sodium chloride-induced changes” in databases recognized by the scientific community, such as Springer (Spr), ResearchGate (RG), Sciencedirect (SD), Oxford Academic (OA), Google Scholar (GS) and Scielo (Sc). Publications of scientific articles in the international, regional and national contexts were analyzed. The bibliographic references of the selected articles were researched to recover other studies that could potentially be included in the review, which yielded a volume of information of 500 documents (Figure 1).
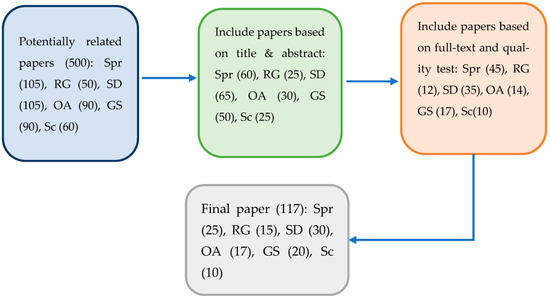
Figure 1.
The final work selection process. The arrows indicate the process of selection and reduction of articles at each stage from the beginning to the final selection of articles. The numbers in parentheses represent the articles analyzed in each database or scientific journal used at each stage of the review.
Subsequently, the inclusion criteria made it possible to determine the most relevant articles for this research. By focusing on articles detailing the effects caused mainly by sodium salinity on some biochemical, physiological and morphological processes, such as cell death, flowering abortion, senescence of the fertilized embryo, decrease in root, shoot and stem growth; in structural and anatomical changes in the crop, in the effects on enzyme activity, cell elongation and division, protein synthesis, DNA, RNA, chlorophyll and carotene content, crop yield, cell homeostasis, water, ionic and hormonal balance, nutrient transport and availability, carbohydrate synthesis, seed germination inhibition, photosynthetic rate decrease and stomatal closure in agricultural crops, specifically in rice; it was possible to narrow the search to 133 papers. Then, attention was focused on the influence of sodium salt stress on Morphological changes (poor root growth, leaf rolling, leaf tillering, chlorosis, leaf burning, and stunted plant growth), Physiological changes (inhibition of photosynthesis, stomatal closure, decreased water content, the higher concentration of osmolytes and lower osmotic potential), Biomass and grain yield (salt stress, spikelet sterility, fewer florets per panicle, less grain weight, less grain yield, and low harvest index), Biochemical changes (oxidative stress, altered metabolism, high Na+ transport to shoot, lower K uptake, and lower Zn and P uptake); leaving 117 papers of interest (Figure 1). The results of the literature review are presented below.
3. Results
3.1. Morphological Effects of Sodium Salinity
The main factors that trigger the decrease in growth under salt stress are adverse changes in morphological structures, which undergo physiological changes due to salinity.
3.1.1. Effect of Sodium Salinity on Plant Height and Root Length
Plant height is a fundamental morphological parameter that under any abiotic and/or biotic stress condition undergoes modifications, which indicate changes in growth and development in the crop. The stomatal closure caused by salinity stress leads to an increase in temperature and a reduction in leaf elongation [34,35].
When the rice crop is exposed to sodium salinity concentrations, cell elongation and cell division are affected, which induces a significant reduction in the growth and productivity of roots and leaves [36,37].
Such stress induces a high uptake and accumulation of sodium (Na+) in the root zones of rice and, in turn, a low uptake, translocation and antagonistic accumulation of potassium (K+), which reduces the ability of the plant to perform osmotic adjustment and maintenance of turgor by suppressing plant growth or inhibiting metabolic activities. Direct competition between K+ and Na+ in the plasmalemma, impairment of the K+ transport process in xylem tissues due to Na+ and/or root leakage of K+ induced Na+, are some of the main causes of decreased tissue K+ concentrations [38].
Apoplastic leakage is the most important pathway in the cultivation of O. sativa because, being an aquatic species, it has limited control over water (H2O) loss from cell to cell. Large gaps develop in the cortical parenchyma of the root zone of this cereal to ensure oxygen transfer H2O from cell to cell. In the cortical parenchyma of the root zone of this cereal, large lacunae develop that ensure the transfer of oxygen. The entry of Na+ through the roots and the movement of Na+ to the leaves causes competition in the crop for K+ uptake and thus induces K+ deficiency in the plant, which activates the transport system for ions that have high affinity for K+ and low affinity for Na+ [39].
3.1.2. Effect of Sodium Salinity on Seedling Growth
Plant cells under the influence of sodium salt stress induce a reduction in shoot development and elongation due to dehydration and shrinkage. These modifications result in the development of symptoms in the form of visual lesions, especially in salt stress-sensitive rice genotypes. With general crop growth over a period of weeks and months, lesions and reduced lateral shoot development become clearly visible in those plants under the influence of sodium stress compared to those under non-saline conditions [40].
The seedling stage in the growth cycle of the crop in question is the most sensitive to the effects of salinity; there are several reports in which it is expressed that in this stage there is a significant reduction in the growth of shoots and roots. A prolonged saline exposure of rice cultivars induces a more rapid senescence of the leaves; the visible symptoms of this process begin 3–4 days after the crop is exposed to the effect of salts as yellowing and necrotic lesions on the tips of the oldest leaves [41].
The most critical stage in seedling establishment is seed germination, as it determines successful crop production. At these stages, it is particularly important to understand plant responses, in order to elucidate the mechanisms of plant resistance or sensitivity to salinity and its super-survival. There are reports of salt-sensitive cultivars of O. sativa, in which concentrations of 100 mMol NaCl or higher affect the germination of this cereal [42].
3.1.3. Effect of Sodium Salinity on Rice Leaf Growth and Mortality
The anatomical characteristics of leaves, such as the thickness of the leaf and mesophyll tissue, undergo significant changes when exposed to different concentrations of salt. The histological characteristics of the bundle, such as length, width, the thickness of phloem tissue and the diameter of the metaxylem vessel also undergo modifications due to the influence of this stress [43,44].
Salt stress changes in plant structural components, including leaf structure, are closely related to the physiological and biochemical activities of the leaves. Decreased photosynthetic rate, ultrastructural and metabolic damage and sequential leaf death are closely correlated with salt accumulation in expanding leaves. In rice plants subjected to sodium salt stress, leaf cells can also be damaged by transpiration and thus lead to growth inhibition in the crop [45,46].
There are reports that a greater increase in salt stress in the early seedling stage increases leaf mortality in rice. Leaf mortality at this stage varies from 0 to 100% when exposed to sodium salts for more than one week, causing in a short time, a reduction in the growth and development of the crop [47].
NaCl stress may be the reason for low leaf number by inhibiting leaf primordium formation. In the tillering stages of some rice varieties, leaf area indices and leaf area are also inhibited due to the effects of sodium salinity [48].
Several studies report on growth damage and leaf mortality due to the inhibitory effect of salinity, where a reduction in the growth and longevity of seedling leaves is appreciable after exposure to concentrations of 50 mMol NaCl [49].
3.1.4. Effect of Sodium Salinity on Vegetative and Reproductive Phases
In the morphophysiological parameters of the rice crop, the main effects of salinity stress are the inhibition of seed germination, a delay and reduction in root and shoot growth, a reduction in the number of stems per plant, in the number of grains per panicle and pollen viability, delays in seed establishment and the appearance of sterile spikelets, a reduction in total dry matter accumulation, poor leaf area development and direct effects on the establishment of the crop surface [50].
The most sensitive stages to NaCl stress in rice correspond to the early seedling growth and reproductive stages. Severe stress (NaCl > 100 mM) causes plants to die before reaching maturity. While in less severe conditions (NaCl < 50 mM), the delay in panicle initiation and flowering is appreciable, which causes a reduction in pollen viability and thus poor seed set. The presence of different Na+ concentrations in the panicle negatively affects O. sativa yield parameters such as tillering, number of spikelets, sterility and grain weight [51,52,53,54].
An increase in salt stress of 5 to 7.5 dS m−1 decreases the growth and fresh weight of rice seedlings. Several physiological parameters such as photosynthesis and plant growth are affected within a few weeks, depending on the salinity concentrations to which they are exposed. The main causes are changes in the osmotic and ionic state of the cell, a considerable increase in plant growth regulators and organic osmolytes such as abscisic acid (ABA), a loss of membrane permeability, a decrease in the partial pressure of intercellular carbon dioxide (CO2), a reduction in the turgor and stomatal conductance of the protective cells, direct effect on the efficiency of the photosynthetic process, and a reduction in the photosynthetic process [55,56,57,58,59].
If the tolerance mechanisms are not high enough to exclude salt from the transpiration flow, those leaves with longer transpiration time accumulate high levels of salt to toxic levels, triggering the death of the plant. In rice cultivation, the growth of new leaves is supported by the export of carbon dioxide from mature leaves; a correct balance between the rate of death of mature leaves and the production of young leaves is what sustains the life of the plant [47,60].
The root disposition of O. sativa is damaged by the presence of Cl− and Na+ salts. The damage of these salts in this cereal is easily recognizable since the damage generated by Cl− is recognizable by the wide-cut edge of the leaf that indicates burning; Na+ causes mottling and curling of the leaf [61].
3.2. Effects of Biomass and Grain Yield under Sodium Salt Stress
3.2.1. Effects of Sodium Salt Stress on Shoot Length
The growth and development of shoots are usually more affected than that of roots under salinity conditions. Reduced cytokinin concentrations in the root and an induced basipetal transport of auxins from the shoot to the root are some of the crop physiological mechanisms involved in the change of photosynthetic partitioning under the effect of NaCl. Thus, a reduction in productivity and yields occurs from the reduction in photosynthetic rate and growth [62,63].
In rice cultivars, sodium salts are capable of causing an ionic imbalance in shoot and root tissues. K+ is an essential nutrient in the plant as it is able to activate key enzymatic reactions, regulate cell membrane polarization and participate in osmoregulation. Therefore, a direct decrease in shoot length in O. sativa cultivars occurs as a result of K+ release or uptake. Moreover, this element has the same positive charge as Na+, but both have different functions in the cell. The presence in the roots of a low concentration of Na+ positively stimulates plant growth, maintains osmotic balance and, to a certain extent, replaces the function of K+ when it is deficient in the crop. It is widely known that the presence of high levels of Na+ in the cytosol is toxic [51,64].
3.2.2. Effects of Sodium Salt Stress on Seedling Biomass Production
Morphological and anatomical characteristics and physiological responses are affected by the influence of high concentrations of sodium chloride and sodium sulfate on agricultural surfaces. In the presence of these salts in the soil, water stress is the main effect, reducing plant growth and cell elongation as a result of the loss of crop turgor [21,65]. Branching in several herbaceous species is an important parameter to determine the total dry biomass capable of accumulating the crop, but it is easily modified by the effect of salt stress [66].
It is known that salinity directly influences the capacity to accumulate and produce biomass in rice. This is due, firstly, to the osmotic effect caused by high salt concentrations in soils, which increases the water retention capacity of the soil and, in turn, increases the accumulation of specific ions in the plant and, secondly, to the effect of NaCl on the photosynthetic rate by reducing carboxylation in the chloroplast and thus directly affecting the plant’s ability to generate the reducing group, electron transport and the synthesis of adenosine triphosphate (ATP) in the thylakoid of the plant [21,46].
The fresh and dry biomass of rice is significantly reduced under sodium salt stress. In rice plants, growth is reduced when exposed to sodium salt stress [67]. This is due to the decrease in water potential in the cells, which induces stomatal closure and thus limits the assimilation of carbon dioxide (CO2). There are reports of scientific investigations in which a decrease in biomass is appreciable in the first 21 days up to the stage of development of 6 leaves in tolerant and resistant rice varieties grown under the influence of concentrations of 0 and 100 mM NaCl. At the same time, decreases in total rice biomass were reported under sodium salts, due to the inability of the plant to absorb water and the influence of osmotic stress [68,69].
3.2.3. Effects of NaCl Stress on Rice Grain Development
Salinity in the root zone seriously affects the final components of rice grain yield. In O. sativa grains, starch is the main reserve substance, representing 50–90% of the grain’s dry weight, while protein concentration is 5–12%. There are reports in which the total protein content of this cereal grain increased with sodium salt stress. The response of this crop to salt stress directly influences the significant increase in protein content, since this type of stress induces the synthesis of more than 40 polypeptides that are influenced by NaCl concentrations in rice [70].
In the developing grain, starch synthesis activity (1-4-glucan glucosyl transferase) is inhibited by salinity, and this abiotic stress in turn induces a decrease in the average weight of 1000 kernels. Amylose content, grain elongation and grain filling are usually reduced and inhibited at moderate to high salt concentrations. Grain filling in this cereal is influenced by the carbohydrate supply of the organs of origin, and those photosynthetically active leaves that are severely affected by NaCl [71,72].
A number of minerals such as calcium, magnesium and phosphorus, and trace elements such as iron, copper, zinc and manganese are the main nutritional constituents of rice grain. The vitamins thiamine, riboflavin and niacin, which are also affected by salt stress, are also found in this grain. The acquisition and absorption of macro and micronutrients are directly affected by this abiotic stress, according to several reports [73].
3.2.4. Effects of NaCl Stress on Sterility of Spikelets and Spikelets per Panicle
Under salinity conditions, there is an important problem regarding rice grain yield, which is the sterility of the panicle, and it is in the pollination and fertilization stages where this problem is most frequently observed, as a result of some genetic mechanisms and nutrient deficiencies under NaCl stress [74].
Several authors have postulated that Cl is a crucial component of the pollen tube. Therefore, the viability of the pollen grain can potentially be impaired by a sudden change in Cl upon exposure [75].
Several studies report a drastic decrease in the number of spikelets per plant in rice plants subjected to hydroponic conditions with different concentrations of sodium salts. This is detrimental to the grain yield of this cereal by reducing the development of the spikelets, especially the lower ones [73,76].
3.2.5. Effects of NaCl Stress on Grain Yield and Harvest Index
The null transformation of carbohydrates in the vegetative growth stage and spikelet development due to sodium salt stress currently represents the main cause of the decrease in grain yield of O. sativa [77]. According to some reports, different NaCl concentrations cause negative effects on several of the main yield components in this cereal, such as the number of spikelets per panicle, the number of tillers per plant and the percentage of sterile flowers. In turn, the translocation of soluble sugar content to the upper and lower spikelets and the inhibition of starch synthesis during grain development are other effects on the rice grain under sodium stress [78].
Salinity by NaCl significantly reduces the morphological variables of rice plantations, such as grain weight per plant, the total number of spikelets per plant, spikelet fertility, the average weight of 1000 grains, plant height, panicle length, tillering and number of panicles per plant. For each unit increase (dS/m−1) in soil electrical conductivity in the root zone greater than 3 dS/m−1, the yield of this cereal decreases by more than 10% [76,79].
3.3. Effects of Sodium Salts on Physiological Processes
3.3.1. Effects of Sodium Stress on Water and Nutrient Uptake
Osmotic stress, nutritional imbalance and ionic toxicity are the three main effects of sodium salt stress on plants [8]. The stomatal closure caused by this stress directly influences the loss of turgor in plant cells. In turn, this closure modifies the ability of leaves to fix carbon and facilitates the production of ROS, among which superoxides, hydrogen peroxide and singlet oxygen stand out. Scavenger enzymes are responsible for preventing the oxidation of membrane proteins, lipids or deoxyribonucleic acid (DNA) by ROS, including catalase, superoxide dismutase and ascorbate peroxidase. Photorespiration caused by high salt concentrations causes more than 70% of H2O2 production in the rice crop [21,80].
This abiotic stress, being in direct contact with the root, affects root growth and development. The symplastic, apolastic and transcellular pathways are responsible for the entry of H2O and solutes into the roots; however, in rice cultivation, the transport of water and solutes through transpiration is extremely important [81].
Different authors report that in rice cultivars subjected to different types or concentrations of salts, the exchangeable Na+ and water-soluble K+ contents are not affected. An increase in sodium levels in soils increases the contents of water-soluble Ca2+ and Mg2+ elements [70].
3.3.2. Effects of Osmotic Stress
In O. sativa cultivars, the influence of salt stress in early stages causes osmotic stress and decreased leaf development, while the plants of this cereal subjected to such abiotic stress over a long period of time, can experience ionic stress (Na+ and Cl−) and the early senescence of their older leaves [53].
The continuous exposure of rice plants to osmotic stress not only slows plant growth but also induces an accelerated increase in ABA, which is correlated with an increase in soil and leaf water potential. Therefore, stomatal closure in plants is closely influenced by increased ABA concentrations, which in turn, acts as a signal in mediating plant physiological responses to salt stress [82].
Stretch-activated channels, cytoskeleton-related mechanosensors, stretch-dependent ion (calcium) channels, redox-mediated systems and transmembrane protein kinases are the main detectors of osmotic stress as reported by several authors [65].
3.3.3. Effects of Ionic Toxicity by NaCl
Excessive accumulation of Na+ and Cl− in plant tissues and soil represent the most damaging effects of NaCl stress. The ionic imbalance in the plant and soil caused by the entry of these ions into the plant cell and the excessive absorption of Na+ and Cl− by the silver causes various alterations in the different physiological parameters of the plants [83].
The decrease in water absorption capacity in the root zone (phytosiological drought) is an effect entirely related to high salt concentrations in the soil profile [50], which in turn alters cellular metabolic functions due to salt toxicity and reduces the osmotic potential of the plant [53,84].
3.3.4. Effects of Sodium Stress on Water Relations
Due to the modifications in the water balance by the influence of different salinity concentrations, the opening and closing of the stomata and the water content in the plant are directly affected [43]. At the same time, as the availability of water in the soil decreases for the plants due to the effects of salts, the effort they must make to extract water from the environment increases. In order to maintain turgor in their cells, crops require an internal water potential close to 0. Among the metabolic adjustments that plants make to alleviate water stress conditions are the accumulation of various compatible osmolytes/solutes, such as sugars, amino acids, polyamines, betaines, polyhydric alcohols and quaternary ammonium compounds, as well as dehydrins, which are some of the water stress proteins [85].
3.3.5. Effects of Sodium Salinity on Nutrient Transport and Nutrient Availability
When Na+ concentrations are excessively high, it causes inhibition in the absorption and transport of calcium (Ca2+), which induces a calcium deficiency in plants by modifying the Na+/Ca2+ balance [78]. The presence of salts in the arable surfaces of rice plantations causes a nutrient imbalance in this crop. As the diffusion and mass flow of nitrates (NO3−), sulfates (SO42−), Ca, magnesium (Mg), and silicon (Si) decrease due to the effects of salt stress, the transport and availability of nutrients are affected. The uptake of nutrients, mainly K and Ca by plants is affected by the presence of sodium ions in the root zone. This ion negatively modifies cell enzymatic activity, as Na inhibits the absorption of K. The latter plays several important roles within the plant, such as the production of plant enzymes, the maintenance of cell turgor, the improvement of photosynthesis and the enablement of sugar and starch transport through the phloem, making it an essential element for the growth and development of plants [86].
High salinity produces a reduction in nitrogen (N) uptake in plants. There are reports showing the antagonistic effect of Na on phosphorus (P), K+, zinc (Zn), iron (Fe), Ca2+ and manganese (Mn), but in rice cultivation, it shows a synergistic effect on N and Mg [87].
3.3.6. Effects of NaCl Salinity on Stomatal Closure
The initial response of the plant to sodium stress is stomatal closure. The closure of stomata and the decrease in photosynthetic rate under stress conditions are due to the low capacity of plants to assimilate carbon dioxide. Additionally, increasing NaCl concentrations decrease transpiration, root water potential and ABA transport from the root zone to the stem, which induces stomatal closure, which is of vital importance in crop survival under salt stress, in order to avoid dehydration of leaf tissues [88].
3.3.7. Effects of Sodium Salts on Photosynthesis
The level of toxicity of adult leaves in rice crops increases when exposed to a high concentration of salt, which induces premature senescence in the leaves and thus decreases the photosynthetic leaf area available in this cereal [89].
Several authors report that the influence of salt stress directly affects photosynthetic capacity and chlorophyll synthesis in crops, which causes a reduction in the activity of the photosystem II (PS-II) reaction center, in turn blocking electron transfer, limiting carbon assimilation and even the per-oxidation or dissociation of the thylakoid membrane [48].
Concentrations of photosynthetic pigments in the leaves of salt-stressed plants decrease as a result of the reduction in water potential in the crop. The absorption of N, a vital element in the composition of chlorophyll, is hindered by the presence of NaCl on the cultivable surfaces, which in turn prevents the absorption of another important element in chlorophyll biosynthesis, Mg; it also causes a reduction in the production of photosynthetic pigments by modifying the specific activities of the enzymes responsible for this process and, by subjecting the precursors of chlorophyll, hinders the biosynthesis of chlorophyll in the plant. Under salt stress the uptake of CO2 is directly affected; this stress primarily affects the growth process, and thus decreases the expansion capacity of the leaves and this in turn hinders the ability to capture photosynthetically active radiation [90,91].
The process of carbon reduction by the Calvin cycle is decreased by the limited uptake of CO2 due to the occurrence of salt stress, and this in turn decreases the oxidation of nicotinamide adenine dinucleotide phosphate (NADP+) which serves as an electron acceptor in the photosynthetic machinery. During the photosynthetic electron transfer process, an excessive reduction of ferrodoxin enables the transfer of electrons from PS-I to oxygen, which triggers the Mehler reaction by forming superoxide radiochannels, and this in turn triggers a large number of chain reactions that end up generating more aggressive active oxygen species (AOS). Oxidative stress is considered to be the slightest imbalance in cellular redox homeostasis, resulting in the production of AOS from the reduction of univalent oxygen. The rates of AOS production, such as superoxide radical, hydrogen peroxide, hydroxyl radical, alkoxyl radical (AR) and singlet oxygen formation through electron leakage to oxygen are intensified under the influence of salt stress. Cytotoxic AOS generated from various metabolic processes in mitochondria and peroxisomes through oxidative damage of lipids, proteins and nucleic acids destroys the proper functioning of cellular metabolism [92].
Several studies have reported the ability of plants to create a complex defense system against AOS damage. This antioxidant system consists of carotenoids, ascorbate, glutathione, a-tocopherols and various enzymes such as superoxide dismutase, catalase, glutathione peroxidase, peroxidases and others that are closely linked to the ascorbate-glutathione cycle, ascorbate peroxides, dehydroascorbate reductase, monodehydroacorbate reductase and glutathione reductase [30].
The decrease in photosynthetic rate in O. sativa crop due to the influence of salt stress is mainly due to stomatal closure, reduction of rubisco efficiency, and the dislocation of essential cations in the multiple membrane structures of the leaf; all this leads to modifications in permeability, swelling and inefficiency of the granae [93].
There is research that reports on the photosynthetic capacity exhibited by the panicle in the rice crop, which contributes to grain filling and, in particular, the spikelets, which, such as the flag leaf, have a high photosynthetic capacity [72].
3.3.8. Effects of NaCl Stress on Hormones
Nowadays, several phytohormones play a decisive role in the daily interactions between the plant and the surrounding environment, in this case, the influence of salt stress. These plant hormones are the main coordinators between the ability of the crop to respond to stress and plant growth [74,94].
- (a)
- Abscisic Acid (ABA)
Cellular factors and environmental stimuli are the main regulators of plant growth and development [95]. One of the most important regulators is ABA. Various physiological processes, among which are, semilayer development, bud dormancy, seed germination, vegetative growth, water scarcity, osmotic regulation, xylem fiber differentiation, and environmental stress responses, among others, are directly influenced by this phytohormone. Under drought and saline stress conditions, it is released in the root zone and transported to the leaves. Therefore, it plays a key role in responses to these stresses. Plants can tolerate these stresses by decreasing the availability of H2O by closing the stomata and accumulating osmoprotectants and proteins for osmotic adjustment when high concentrations of this phytohormone are present. It is closely linked to reactive oxygen species, such as H2O2, which, by increasing the concentration of ABA in guard cells, induces stomatal closure [96].
In rice crops under sodium salt stress, ABA levels increase considerably in the root zones, which causes a decrease in the concentrations of zataine and zeatin ribosides in leaves and root exudates. Grain filling in the crop in question is directly influenced by this hormone, which is why sucrose transport to the grains and starch synthesis capacity in intact grains is negatively affected by high concentrations of this hormone [97].
- (b)
- Auxins
It is now known that the ratio between stimulant and inhibitory substances decreases drastically under stress conditions, a variation in plant growth regulating substances directly affects the growth phases. Auxins are phytohormones that act mainly on cell division mechanisms and gibberellic auxins on differentiation or elongation. This growth hormone is also responsible for regulating gravitropic responses in plants. In roots closer to the gravitropic stimulus, a greater lateral accumulation of auxin occurs, resulting in directional growth toward the gravity vector. The opposite occurs in halotropism, where the greatest lateral auxin concentrations are higher in the root zone farthest from the influence of salt stress, leading to root growth away from the areas with the highest NaCl stress concentrations [95].
- (c)
- Cytokinins
The main hormone directly involved and playing a key role in shoot and root growth, apical dominance, as well as in responses to different biotic and abiotic stresses is cytokinin. In multiple agricultural crops, yields are greatly improved by overexpression of the IPT gene (encoding isopentenyl transferase), which is directly involved in cytokinin biosynthesis, and down-regulation of the CKX gene (encoding cytokinin oxidase), which is responsible for cytokinin degradation. In the cultivation of O. sativa, the presence of this phytohormone is essential throughout its vegetative cycle; it is responsible for various processes such as root development, the meristematic activity of the shoots, vegetative and reproductive branching, the number of spikelets per panicle and in the final grain yield [98].
- (d)
- Gibberellins (GA)
Seed germination, cell and shoot elongation, leaf expansion, transition to the flowering stage, flower and fruit growth and development are regulated by GA. Being an antagonist of ABA, it enables seed germination under stress conditions by employing various mechanisms, such as the induction of enzyme synthesis and stimulation through the tonoplast of the plasma membrane protons pump (H+-ATPase). Under salt stress conditions it is possible to increase the growth and development of cultures by treatment with this phytohormone. The ability to decrease ribonuclease activity, increase reducing sugars, the antioxidant activity of enzymes and protein synthesis, are some of the many positive effects GA has on rice and other crops [99,100].
- (e)
- Ethylene
The hormonal imbalance triggered by salt stress induces a high ethylene biosynthesis, which negatively affects crop growth and development. The hormonal sensitivity of this phytohormone to salinity depends largely on the agricultural crop, the growth stage of the crop, the concentration of ethylene in its tissues and the duration of exposure. The growth and development of spikelets, mainly the lower spikelets in rice plants, are affected by high ethylene production. The decrease in growth and development of O. sativa plants under stress conditions is due to the high production of 1-aminocyclopropane-1-carboxylic acid, which is induced by the accumulation of ethylene [101].
Excessive ethylene production in plants significantly decreases plant growth and development, leading to the premature death of the crop. Therefore, in order for rice plantations to be able to survive under salinity stress, it is of utmost importance to maintain ethylene homeostasis within the rice crop. Currently, the use of ethylene inhibitors is very popular, as well as the chemical control of ethylene biosynthesis in crops subjected to different levels of salinity. Some allow crops to maintain good grain yields, such as the inhibitors aminoethoxyvinylglycine (AVG) and 1-methylcyclopropene (1-MCP) [102].
Several authors report a positive effect on starch content in upper and lower spikelets, spikelet fertility, grain yield and harvest index of rice cultivars with the application of 1-MCP [76].
3.4. Salinity Tolerance Mechanisms
The salt shock that occurs due to the action of salts modifies gene expression. In sudden exposures of 150 mmolL−1 NaCl concentrations in rice plants, the genes present in the roots after 15 min under salt stress differs from those present after one week [103].
There are cellular costs that various cultures must incur to achieve survival under sodium stress conditions; these are the cost of salt exclusion, intracellular compartmentalization and salt excretion through salt glands. Relative to the cost required to perform osmotic adjustment, the above cost is relatively small. For the plant to be able to use one mole of NaCl as osmotic, the number of moles of ATP needed is 4 in root cells and 7 in leaf cells, while the number of moles needed to synthesize an organic compound is an order of magnitude higher. The tolerance mechanisms adopted by plants to cope with sodium stress are mainly of two types: those that minimize the entry of salts into the plant and those that reduce salt concentrations in the cytoplasm [104,105].
The mechanisms by which salt is excluded from leaves are the following (Table 1):

Table 1.
The mechanisms by which salt is excluded from leaves.
3.4.1. Osmotic Adjustment
The limit of tolerance that the rice crop can withstand to deal with the different concentrations of toxic ions is determined by the osmotic adjustment. One of the best strategies used by the crop to cope with Na salt stress is the reduction of NaCl accumulation in the cell cytoplasm and/or the compartmentalization of these ions in the vacuoles, as well as the accumulation in the cell cytoplasm of compatible compounds such as organic solutes, free sugar, glycinabetaine and proline. In rice, carbohydrates or trehalose are more essential than proline under osmotic stress conditions [106].
The activation of mechanisms that have a direct impact on cellular dehydration, protein denaturation (including those of PS-II) and destabilization of cellular structures is due to the presence of high Na+ concentrations in the apoplastic solution. In order to adjust the osmotic potential between the cytosol and the apoplastic solution, compatible solutes (non-toxic metabolites) are stored in the cell cytoplasm. In plants, various sugars (fructose, glucose and sucrose), complex sugars (trehalose, raffinose and fructans) and amino acids and their derivatives (proline, glycine-betaine and proline-betaine) are responsible for this indispensable function. Proline is the most compatible solute with O. sativa and has an essential role as a protector against hyperosmotic stress, increasing its concentrations in rice subjected to NaCl stress, especially in tolerant genotypes. The equilibrium of the osmotic pressure of the ions inside the vacuoles (where Na+ and Cl− are also located) is made possible by the accumulation of these compatible solutes in the cytoplasm [107,108].
3.4.2. Mechanisms for Alleviating and Strengthening Stress Tolerance in Crops
Currently, there are several alternatives that seek to mitigate and strengthen the mechanisms against stress in crops, among which the following stand out:
- (a)
- Salt stress relief using microorganisms
In order to reduce the toxic effects of salinity on plant growth and development, several mechanisms and strategies are currently available, such as genetic engineering and the application of plant growth-promoting bacteria (PGPB). Several studies report how a highly specialized group of PGPB, specifically plant growth-promoting rhizobacteria (PGPR), facilitates plant nitrogen acquisition and fixation, phytohormone production, siderophore synthesis and phosphorus solubilization by directly stimulating plant growth and development processes. To improve the conditions of plants under abiotic stress it is advisable to apply PGPR; this promoter causes physical and chemical changes in plants as a result of the systemic tolerance induced by it [109,110].
There are several studies that report the improvement of rice productivity under saline conditions with the application of rhizospheric soil microbes, such as Bacillus pumilus and Pseudomonas pseudoalcaligenes [111,112].
The effect of salt stress on various agricultural crops is currently being investigated. For example, there are reports on the application of PGPB, specifically the bacterial strains FD48 and RABA6 in the cultivation of O. sativa under sodium salt stress conditions, where there was a significant increase in the dry biomass of shoots and roots [113]. In turn, other authors reported significant benefits in rice growth parameters, chemical properties and soil biological activity with the application of Pseudomonas multiresinivorans, Microbacterium steraromaticum and Bacillus subtilis individually and/or in combination with manure [110].
On the other hand, the application of arbuscular mycorrhizae (Claroideoglomus etunicatum) at different salinity concentrations in the rice crop reported an increase in aerial dry biomass. This increased the colonization of arbuscular mycorrhizae by 36–43% and reduced the negative impact of NaCl concentrations of 75 and 150 mM to which the crop was subjected [111].
Other authors investigated the capacity of tolerance to Na salt stress of this cereal through the application to rice seeds of 24-epibrasinolide and two analogs of spirostannic brassinosteroids; where Biobras-16 in concentrations lower than 0.01 μmol·L−1 was the most effective in stimulating the growth of rice seedlings in a saline medium [112].
- (b)
- Application of silicon to reduce salt stress
Although the ability of silicon to activate an induced reduction mechanism in Na uptake in rice is still inconclusive, the main action of silicon is its ability to reduce leaf transpiration rates and thus reduce Na uptake by the plant. A large part of rice species is sensitive to salinity due to the accumulation and translocation of Na and Cl that occur during transpiration, which in turn involves the loading of the xylem through the symplast. Therefore, it is necessary to promote and/or devise any strategy aimed at reducing the incidence of the negative effects of Na and Cl inside the plants. The expression of silicon transporters in rice for radial transport and xylem loading is able to accumulate silicon in more than 10% of dry matter and, in turn, shoots accumulate twice as much as roots. Therefore, the translocation of Na and Cl from the roots to the shoot is able to be limited by silicon application, and silicon in turn strengthens the cells, prevents root oxygen loss, maintains membrane integrity and maintains leaf water status [113,114].
- (c)
- Tolerance to manganese-induced salt stress
Plants require this essential and important micronutrient for the various metabolic functions in which it plays a determining role, such as the processes linked to photosynthesis and respiration, the synthesis of ATP, fatty acids, amino acids, lipids, proteins, flavonoids and hormone activation. There are reports where this micronutrient rejuvenates the accumulation and translocation of Na ions in the growth and development stage of O. sativa under the influence of NaCl stress [114].
The 133 articles consulted, study the effects of salinity on growth and physiological changes, which represents a solid, proven and consistent source of information on the main effects of sodium salinity on the O. sativa crop. This review allows summarizing in a single document the physiological and morphological changes in the metabolism of the rice crop, which determine the subsistence, as well as the productivity of rice under the influence of this abiotic stress; due to which plants and specifically this cereal developed tolerance mechanisms. There is a field in this review that is not studied in all its depth, such as those related to biochemical processes, since these generate physiological and morphological modifications in plants subjected to stress by sodium salts.
From the bibliographic review carried out in the preparation of this article, it is possible to take different paths with the objective of reducing the incidence of this abiotic factor in this crop. This review serves as a window to deepen future research related to this topic and the processes and mechanisms affected by NaCl, such as biochemical, hormonal and cellular changes.
4. Conclusions and Future Prospects
Salt stress negatively affects crop growth and development by causing tissue death, flowering abortion and fertilized embryo senescence. It also affects enzyme activity, protein synthesis, DNA, RNA and mitosis in plants. The factors affecting salinity in recent years have focused on the biochemical and physiological mechanisms of plants and on obtaining more genetically tolerant varieties, without taking into account the importance of the rhizosphere-associated microbiota of rice plants, linked to their nutrition and induction of tolerance to salinity stress. Future studies should also focus on PGPR, and their influence on stress-relieving mechanisms, including host root architecture alternations, biosynthesis and metabolism of phytohormones, osmoregulation, production of extracellular polysaccharides (EPS) that can serve as humectants, production of antioxidants for reactive oxygen species (ROS), enhancement of nodulation, soil fertility and regulation of salt stress-responsive genes.
A clearer understanding of the mechanism that causes PGPR to interact with the plant will provide an essential insight into the practical use of these bacteria through agronomic applications to the soil. Similarly, the study of the microflora existing in the rhizosphere of halophilic plants may constitute a valuable resource in the tolerance of rice crops to salinity.
Author Contributions
Conceptualization, L.I.R.C., M.T.G.G. and J.J.H.; methodology, Z.G.U., M.T.G.G. and L.I.R.C.; validation, Y.F.C. and M.T.G.G.; formal analysis, Z.G.U.; investigation, L.I.R.C., M.T.G.G., J.J.H. and Z.G.U.; writing—original draft preparation, L.I.R.C., Z.G.U. and M.M.R.J.; writing—review and editing, L.I.R.C., Z.G.U. and M.M.R.J.; visualization, Z.G.U.; supervision, M.T.G.G. and Y.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to anonymous editors and reviewers for providing valuable insights on the discussed topics.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant salt stress: Adaptive responses, tolerance mechanism and bioen-gineering for salt tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, J.; Zhang, Y.; Xu, X.; Wang, L.; Ouyang, Z. Evaluation of coastal farming under salinization and optimized fertilization strategies in China. Sci. Total Environ. 2021, 797, 149038. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Land and Plant Nutrition Management Service. 2008. Available online: https://www.fao.org/documents/card/en/c/cb9929en/ (accessed on 26 July 2022).
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, A.; Palma, P.; Alvarenga, P.; Gonçalves, M.C. Soil salinity risk in a climate change scenario and its effect on crop yield. In Climate Change and Soil Interactions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 351–396. [Google Scholar] [CrossRef]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Santamaría-César, J.; Figueroa-Viramontes, U.; Medina-Morales, M.C. Productividad de la alfalfa en condiciones de salinidad en el Distrito de Riego 017, Comarca Lagunera. Ídem 2004, 22, 343–349. [Google Scholar]
- García, M.; Jáuregui, D.; Medina, E. Adaptaciones anatómicas foliares en especies de angiospermas que crecen en la zona costera del estado falcón (Venezuela). Acta Botánica Venez. 2008, 31, 291–306. [Google Scholar]
- Terrazas, J.M. Aprovechamiento Del Suelo Salino: Agricultura Salina Y recuperación De Suelos. Apthapi 2019, 5, 1539–1563. [Google Scholar]
- Martínez-Villavicencio, N.; López-Alonzo, C.V.; Pérez-Leal, R.; Basurto-Sotelo, M. Efectos Por Salinidad En El Desarrollo Vegetativo: Effects of Salinity on Vegetative Growth. Tecnociencia Chih. 2020, 5, 156–161. [Google Scholar]
- Loc, H.H.; Lixian, M.; Park, E.; Dung, T.D.; Shrestha, S.; Yoon, Y.J. How the saline water intrusion has reshaped the agricultural landscape of the Vietnamese Mekong Delta, a review. Sci. Total Environ. 2021, 794, 148651. [Google Scholar] [CrossRef]
- Bhatt, T.; Sharma, A.; Puri, S.; Minhas, A.P. Salt tolerance mechanisms and approaches: Future scope of halotolerant genes and rice landraces. Rice Sci. 2020, 27, 368–383. [Google Scholar] [CrossRef]
- Ganapati, R.K.; Naveed, S.A.; Zafar, S.; Wang, W.; Xu, J. Saline-Alkali Tolerance in Rice: Physiological Response, Molecular Mechanism, and QTL Identification and Application to Breeding. Rice Sci. 2022, 29, 412–434. [Google Scholar] [CrossRef]
- Wang, Y.X.; Wang, J.; Zhao, X.Q.; Yang, S.; Huang, L.Y.; Du, F.P.; Li, Z.K.; Zhao, X.Q.; Fu, B.Y.; Wang, W.S. Overexpression of the transcription factor gene OsSTAP1 increases salt tolerance in rice. Rice 2020, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Wang, C.C.; Xie, J.P.; Zhang, F.; Lu, J.L.; Shi, X.R.; Shi, Y.Y.; Zhou, Y.L. Stress-activated protein kinase OsSAPK7 regulates salt-stress tolerance by modulating diverse stress-defensive responses in rice. Rice Sci. 2021, 28, 547–556. [Google Scholar] [CrossRef]
- Liu, X.W.; Feike, T.; Chen, S.Y.; Shao, L.W.; Sun, H.Y.; Zhang, X.Y. Effects of saline irrigation on soil salt accumulation and grain yield in the winter wheat-summer maize double cropping system in the low plain of North China. J. Integr. Agric. 2016, 15, 2886–2898. [Google Scholar] [CrossRef]
- Medina, L.R. La agricultura, la salinidad y los hongos micorrízicos arbusculares: Una necesidad, un problema y una alternativa. Cultiv. Trop. 2016, 37, 42–49. [Google Scholar]
- Khan, M.S.; Akther, T.; Ali, D.M.; Hemalatha, S. An investigation on the role of salicylic acid alleviate the saline stress in rice crop (Oryza sativa (L.)). Biocatal. Agric. Biotechnol. 2019, 18, 101027. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant plant growth–promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Singh, Y.P.; Arora, S.; Mishra, V.K.; Singh, A.K. Synergizing Microbial Enriched Municipal Solid Waste Compost and Mineral Gypsum for Optimizing Rice-Wheat Productivity in Sodic Soils. Sustainability 2022, 14, 7809. [Google Scholar] [CrossRef]
- Xu, Z.; Shao, T.; Lv, Z.; Yue, Y.; Liu, A.; Long, X.; Zhou, Z.; Gao, X.; Rengel, Z. The mechanisms of improving coastal saline soils by planting rice. Sci. Total Environ. 2019, 703, 135529. [Google Scholar] [CrossRef] [PubMed]
- Pathania, P.; Rajta, A.; Singh, P.C.; Bhatia, R. Role of plant growth-promoting bacteria in sustainable agriculture. Biocatal. Agric. Biotechnol. 2020, 30, 101842. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.H.; Gao, J.P.; Li, L.G.; Cai, X.L.; Huang, W.; Chao, D.; Zhu, M.; Wang, Z.; Luan, S.; Lin, H. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005, 37, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.; Rask, K.A.; Vestergård, M.; Johansen, J.L.; Priemé, A.; Frøslev, T.G.; Ekelund, F. Specialized microbiomes facilitate natural rhizosphere microbiome interactions counteracting high salinity stress in plants. Environ. Exp. Bot. 2021, 186, 104430. [Google Scholar] [CrossRef]
- Zhou, Z.; Fan, J.; Zhang, J.; Yang, Y.; Zhang, Y.; Zan, X.; Li, X.; Wan, J.; Gao, X.; Chen, R.; et al. OsMLP423 Is a Positive Regulator of Tolerance to Drought and Salt Stresses in Rice. Plants 2022, 11, 1653. [Google Scholar] [CrossRef]
- Rodríguez, N.D.; Torres, C.N.; Chaman, M.E.; Hidalgo, J.E.M. Efecto del estrés salino en el crecimiento y contenido relativo del agua en las variedades IR-43 y amazonas de Oryza sativa “arroz” (Poaceae). Arnaldoa 2019, 26, 931–942. [Google Scholar] [CrossRef]
- Razzaque, S.; Haque, T.; Elias, S.M.; Rahman, M.S.; Biswas, S.; Schwartz, S.; Ismail, A.M.; Walia, H.; Juenger, T.E.; Seraj, Z.I. Reproductive stage physiological and transcriptional responses to salinity stress in reciprocal populations derived from tolerant (Horkuch) and susceptible (IR29) rice. Sci. Rep. 2017, 7, 46138. [Google Scholar] [CrossRef]
- National office of Statistics and Information of the Republic of Cuba. Available online: http://www.onei.gob.cu/publicaciones-tipo/Anuario (accessed on 11 May 2022).
- Gómez-Luna, E.; Fernando-Navas, D.; Aponte-Mayor, G.; Betancourt-Buitrago, L.A. Methodology for the bibliographic review and information management of scientific topics, through its structuring and systematization. Dyna 2014, 81, 158–163. [Google Scholar] [CrossRef]
- Rajendran, K.; Tester, M.; Roy, S.J. Quantifying the three main componenents of salinity tolerence in cereals. Plant Cell Environ. 2009, 23, 237–249. [Google Scholar] [CrossRef]
- Siraul, X.R.R.; Richard, A.J.; Robert, T.F. A new screening method for osmotic component of salinity tolerance in cereals using infrared thermography. Funct. Plant Biol. 2009, 36, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, B.; Sudiro, C.; Jabir, R.; Schiavo, F.L.; Hyder, M.Z.; Yasmin, T. Adaptive behaviour of roots under salt stress correlates with morpho-physiological changes and salinity tolerance in rice. Int. J. Agric. Biol. 2019, 21, 667–674. [Google Scholar] [CrossRef]
- Khan, M.H.; Panda, S.K. Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol. Plant. 2008, 30, 81–89. [Google Scholar] [CrossRef]
- Schachtman, D.P. Molecular insights into the structure and function of plant K+ transport mechanisms. Biochim. Et Biophys. Acta (BBA) Biomembr. 2000, 1465, 127–139. [Google Scholar] [CrossRef]
- La¨uchli, A.; Grattan, S.R. Plant growth and development under salinity stress. In Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Berlin, Germnay, 2009; pp. 1–32. ISBN 978-1-4020-5577-5. [Google Scholar]
- Chang, J.; Cheong, B.E.; Natera, S.; Roessner, U. Morphological and metabolic responses to salt stress of rice (Oryza sativa L.) cultivars which differ in salinity tolerance. Plant Physiol. Biochem. 2019, 144, 427–435. [Google Scholar] [CrossRef]
- Kumar, V.; Shriram, V.; Jawali, N.; Shitole, M.G. Differential response of indica rice genotypes to NaCl stress in relation to physiological and biochemical parameters. Arch. Agron. Soil Sci. 2007, 53, 581–592. [Google Scholar] [CrossRef]
- Nassar, R.M.A.; Kamel, H.A.; Ghoniem, A.E.; Alarcón, J.J.; Sekara, A.; Ulrichs, C.; Abdelhamid, M.T. Physiological and Anatomical Mechanisms in Wheat to Cope with Salt Stress Induced by Seawater. Plants 2020, 9, 237. [Google Scholar] [CrossRef]
- Rizwan, R.; Muhammad, A.A.; Muhammad, I.; Iqbal, H.; Ali, A.; Umar, F.; Mudassir, I.S. Major Constraints for Global Rice Production: Changing Climate, Abiotic and Biotic Stresses. In Rice Research for Quality Improvement: Genomics and Genetic Engineering; Roychoudhury, A., Ed.; Springer: Singapore, 2020; Chapter 2; pp. 15–45. [Google Scholar] [CrossRef]
- Alamgir, A.N.M.; Ali, M.Y. Effects of NaCl salinity on leaf characters and physiological growth attributes of different genotypes of rice (Oryza sativa L.). Bangladesh J. Bot. 2006, 35, 99–107. [Google Scholar]
- Munns, R.; James, R.A.; Lauchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Yeo, A.R.; Yeo, M.E.; Flowers, S.A.; Flowers, T.J. Screening of rice (Oryza sativa L.) genotypes for physiological characters contributing to salinity resistance, and their relationship to overall performance. Theor. Appl. Genet. 1991, 79, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Rafii, M.Y.; Yusoff, M.M.; Ali, N.S.; Yusuff, O.; Datta, D.R.; Anisuzzaman, M.; Ikbal, M.F. Advanced Breeding Strategies and Future Perspectives of Salinity Tolerance in Rice. Agronomy 2021, 11, 1631. [Google Scholar] [CrossRef]
- Abdullah, Z.; Khan, M.A.; Flowers, T. Causes of sterility in seed set of rice under salinity stress. J. Agron. Crop Sci. 2001, 187, 25–32. [Google Scholar] [CrossRef]
- Rao, P.S.; Mishra, B.; Gupta, S.R.; Rathore, A. Reproductive stage tolerance to salinity and alkalinity stresses in rice genotypes. Plant Breed. 2008, 127, 256–261. [Google Scholar] [CrossRef]
- Sultana, N.; Ikeda, T.; Itoh, R. Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ. Exp. Bot. 1999, 42, 211–220. [Google Scholar] [CrossRef]
- Grattan, R.; Zeng, L.; Shannon, M.; Roberts, S. Rice is more sensitive to salinity than previously thought. Calif. Agric. 2002, 56, 189–195. [Google Scholar] [CrossRef]
- Shereen, A.; Mumtaz, S.; Raza, S.; Khan, M.A.; Solangi, S. Salinity effects on seedling growth and yield componentsof different inbred rice lines. Pak. J. Bot. 2005, 37, 131–139. [Google Scholar]
- Hussain, S.; Cao, X.; Zhong, C.; Zhu, L.; Khaskheli, M.A.; Fiaz, S.; Jin, Q. Sodium chloride stress during early growth stages altered physiological and growth characteristics of rice. Chil. J. Agric. Res. 2018, 78, 183–197. [Google Scholar] [CrossRef]
- Muhammad, J.; Muhammad, A.; Noor, S.A.; Muhammad, Y.; Javaid, I.; Xiuling, L.; Depeng, W.; Shah, F. Modulation in growth, gas exchange, and antioxidant activities of salt-stressed rice (Oryza sativa L.) genotypes by zinc fertilization. Arab. J. Geosci. 2019, 12, 775. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 2000, 135, 1–9. [Google Scholar] [CrossRef]
- Chaves, M.; Flexas, V.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Akram, R. Plant Growth and Morphological Changes in Rice Under Abiotic Stress. Adv. Rice Res. Abiotic Stress Toler. 2019, 69–85. [Google Scholar] [CrossRef]
- Zheng, L.; Shannon, M.C.; Lesch, S.M. Timing of salinity stress affects rice growth and yield components. Agric. Water Manag. 2001, 48, 191–206. [Google Scholar] [CrossRef]
- Gay, F.; Maraval, B.; Roques, S.; Gunata, Z.; Boulanger, R.; Audeber, A.; Mestres, C. Effect of salinity on yield and 2-acetyl-1-pyrroline content in the grains of three fragrant rice cultivars (Oryza sativa L.) in Camargue (France). Field Crops Res. 2009, 117, 154–160. [Google Scholar] [CrossRef]
- Negrão, S.; Courtois, B.; Ahmadi, N.; Abreu, I.; Saibo, N.; Oliveira, M.M. Recent updates on salinity stress in rice: From physiological to molecular responses. Crit. Rev. Plant Sci. 2011, 30, 329–377. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, Z.; Liu, J.; Liu, X.; Liu, F. Growth and physiological responses of cotton plants to salt stress. J. Agron. Crop Sci. 2021, 207, 565–576. [Google Scholar] [CrossRef]
- Marcaida III, M.; Li, T.; Angeles, O.; Evangelista, G.K.; Fontanilla, M.A.; Xu, J.; Ali, J. Biomass accumulation and partitioning of newly developed Green Super Rice (GSR) cultivars under drought stress during the reproductive stage. Field Crops Res. 2014, 162, 30–38. [Google Scholar] [CrossRef]
- Kumar, V.; Shriram, V.; Nikam, T.D.; Jawali, N.; Shitole, M.G. Sodium chloride-induced changes in mineral nutrients and proline accumulation in indica rice cultivars differing in salt tolerance. J. Plant Nutr. 2008, 31, 1999–2017. [Google Scholar] [CrossRef]
- Riaz, M.; Arif, M.S.; Ashraf, M.A.; Mahmood, R.; Yasmeen, T.; Shakoor, M.B.; Fahad, S. A comprehensive review on rice responses and tolerance to salt stress. Adv. Rice Res. Abiotic Stress Toler. 2019, 133–158. [Google Scholar] [CrossRef]
- Irakoze, W.; Prodjinoto, H.; Nijimbere, S.; Bizimana, J.B.; Bigirimana, J.; Rufyikiri, G.; Lutts, S. NaCl-and Na2SO4-induced salinity differentially affect clay soil chemical properties and yield components of two rice cultivars (Oryza sativa L.) in Burundi. Agronomy 2021, 11, 571. [Google Scholar] [CrossRef]
- Fabre, D.; Siband, P.; Dingkuhn, M. Characterizing stress effects on rice grain development and filling using grain weight and size distribution. Field Crops Res. 2005, 92, 11–16. [Google Scholar] [CrossRef]
- Ndayiragije, A.; Lutts, S. Long term exogenous putrescine application improves grain yield of a salt-sensitive rice cultivar exposed to NaCl. Plant Soil 2007, 291, 225–238. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, A.; Safdar, L.B.; Zafar, M.M.; Rui, Y.; Shakeel, A.; Shaukat, A.; Ashraf, M.; Gong, W.; Yuan, Y. Salt stress induces physiochemical alterations in rice grain composition and quality. J. Food Sci. 2020, 85, 14–20. [Google Scholar] [CrossRef]
- Hasamuzzaman, M.; Masayuki, F.; Islamm, M.N.; Ahamed, K.U.; Kamrin, N. Performance of four irrigated rice varieties under different levels of salinity stress. Int. J. Integr. Biol. 2009, 6, 85–89. [Google Scholar]
- Irakoze, W.; Prodjinoto, H.; Nijimbere, S.; Rufyikiri, G.; Lutts, S. NaCl and Na2SO4 salinities have different impact on photosynthesis and yield-related parameters in rice (Oryza sativa L.). Agronomy 2020, 10, 864. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.J.; Zhu, L.F.; Yu, S.M.; Sanjoy, K.K.; Jin, Q.Y. Effects of 1-methylcyclopropene on function of flag leafand development of superior and inferior spikelets in rice cultivars differing in panicle types. Field Crops Res. 2015, 177, 64–74. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, J.H.; Zhong, C.; Zhu, L.F.; Cao, X.C.; Yu, S.M.; Jin, Q.Y. Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- Irakoze, W.; Vanpee, B.; Rufyikiri, G.; Dailly, H.; Nijimbere, S.; Lutts, S. Comparative effects of chloride and sulfate salinities on two contrasting rice cultivars (Oryza sativa L.) at the seedling stage. J. Plant Nutr. 2019, 42, 1001–1015. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, S.K.; Singh, M.K.; Singh, V.K.; Modi, A.; Singh, P.K.; Kumar, A. Plant growth–promoting rhizobacteria and their functional role in salinity stress management. Abat. Environ. Pollut. 2020, 151–160, 151–160. [Google Scholar] [CrossRef]
- Kronzucker, J.K.; Britto, T.D. Sodium transport in plants: A critical review. New Phytol. 2011, 189, 54–81. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- James, R.A.; Blake, C.; Byrt, C.S.; Munns, R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J. Exp. Bot. 2011, 62, 2939–2947. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Ali, S.; Khan, N. Delineation of mechanistic approaches employed by plant growth promoting microorganisms for improving drought stress tolerance in plants. Microbiol. Res. 2021, 249, 126771. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N. Beneficial plant-microbe interactions for agricultural sustainability. J. Appl. Biol. Biotechnol. 2021, 9, 1–4. [Google Scholar] [CrossRef]
- Garcia, M.J.; Lucena, C.; Romera, F.J.; Alcántara, E.; Pérez-Vicente, R. Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. J. Exp. Bot. 2010, 61, 3885–3899. [Google Scholar] [CrossRef]
- Amirjani, M.R. Effect of NaCl on some physiological parameters of rice. Eurpoean J. Biol. Sci. 2010, 3, 6–16. [Google Scholar]
- Okon, O.G. Effect of Salinity on Physiological Processes in Plants. Microorg. Saline Environ. Strateg. Funct. 2019, 56, 237–262. [Google Scholar] [CrossRef]
- Ghosh, B.; Ali, M.N.; Saikat, G. Response of rice under salinity stress: A review update. J. Rice Res. 2016, 4, 167. [Google Scholar] [CrossRef]
- Mandhania, S.; Madan, S.; Sawhney, V. Antioxidant defense mechanism under salt stress in wheat seedlings. Biol. Plant. 2006, 227, 227–231. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Effects of salinity on sodium content and photosynthetic responses of rice seedlings differing in salt tolerance. J. Plant Physiol. 2000, 157, 54–58. [Google Scholar] [CrossRef]
- Mokrani, S.; El-hafid, N. Microbes Associated with Crops: Functional Attributes for Crop Productivity. In Soil Microbiomes for Sustainable Agriculture. Sustainable Development and Biodiversity; Yadav, A.N., Ed.; Springer: Cham, Switzerland, 2021; Volume 27, pp. 31–54. [Google Scholar] [CrossRef]
- Blakeslee, J.J.; Spatola, T.; Kriechbaumer, V. Auxin biosynthesis: Spatial regulation and adaptation to stress. J. Exp. Bot. 2019, 70, 5041–5049. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Villagra, J.; Figueroa, C.; Luengo-Escobar, A.; Morales, M.; Inostroza-Blancheteau, C.; Reyes-Díaz, M. Abscisic Acid and Plant Response under Adverse Environmental Conditions. In Plant Performance under Environmental Stress; Husen, A., Ed.; Springer: Cham, Switzerland, 2021; pp. 17–47. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.H.; Liu, K.; Wang, Z.Q.; Liu, L.J. Abscisic acid and ethylene interact in rice spikelets in response to water stress during meiosis. J. Plant Growth Regul. 2007, 26, 318–328. [Google Scholar] [CrossRef]
- Nutan, K.K.; Rathore, R.S.; Tripathi, A.K.; Mishra, M.; Pareek, A.; Singla-Pareek, S.L. Integrating the dynamics of yield traits in rice in response to environmental changes. J. Exp. Bot. 2020, 71, 490–506. [Google Scholar] [CrossRef]
- Schulze, E.D.; Beck, E.; Buchmann, N.; Clemens, S.; Müller-Hohenstein, K.; Scherer-Lorenzen, M. Water deficiency (drougth). In Plant Ecology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Illouz-Eliaz, N.; Ramon, U.; Shohat, H.; Blum, S.; Livne, S.; Mendelson, D.; Weiss, D. Multiple gibberellin receptors contribute to phenotypic stability under changing environments. Plant Cell 2019, 31, 1506–1519. [Google Scholar] [CrossRef]
- Hussain, S.; Huang, J.; Zhu, C.; Zhu, L.; Cao, X.; Hussain, S.; Zhang, J. Pyridoxal 5′-phosphate enhances the growth and morpho-physiological characteristics of rice cultivars by mitigating the ethylene accumulation under salinity stress. Plant Physiol. Biochem. 2020, 154, 782–795. [Google Scholar] [CrossRef]
- Watkins, C.B. Postharvest ripening regulation and innovation in storage technology. Acta Hortic. 2008, 796, 51–58. [Google Scholar] [CrossRef]
- Kawasaki, S.; Borchert, C.; Deyholos, M.; Wang, H.; Brazille, S.; Kawai, K.; Bohnert, H.J. Gene expression profiles during the initial phase of salt stress in rice. Plant Cell 2001, 13, 889–905. [Google Scholar] [CrossRef]
- Céccoli, G. Analysis of Intraspecific Variability for Salinity Tolerance Mechanisms and the Response of Leaf Expansion to This Stress in Sunflower (Helianthus annuus L.). Ph.D. Thesis, Faculty of Agricultural Sciences, National University of Córdoba, Córdoba, Argentina, 2013. [Google Scholar]
- Hossen, M.S.; Karim, M.F.; Fujita, M.; Bhuyan, M.H.M.B.; Nahar, K.; Masud, A.A.C.; Mahmud, J.A.; Hasanuzzaman, M. Comparative Physiology of Indica and Japonica Rice under Salinity and Drought Stress: An Intrinsic Study on Osmotic Adjustment, Oxidative Stress, Antioxidant Defense and Methylglyoxal Detoxification. Stresses 2022, 2, 156–178. [Google Scholar] [CrossRef]
- Nounjan, N.; Theerakulpisut, P. Effect of exogenous proline and trehalose on physiological responses in rice seedlings during salt-stress and after recovery. Plant Soil Environ. 2012, 58, 308–315. [Google Scholar] [CrossRef]
- Demiral, T.; T¨urkan, I. Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ. Exp. Bot. 2006, 56, 72–79. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 197, 945–963. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Dimkpa, C.; Weinand, T.; Ash, F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Env. 2009, 32, 1682–1694. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.B.; Patel, S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol. Plant. 2011, 33, 797–802. [Google Scholar] [CrossRef]
- Gutierrez-Manero, F.J.; Ramos-Solano, B.; Probanza, A.; Mehouachi, J.; Tadeo, F.R.; Talon, M. The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol. Plant. 2001, 111, 206–211. [Google Scholar] [CrossRef]
- Shobana, N.; Thangappan, S.; Uthandi, S. Plant Growth-Promoting Bacillus sp. Cahoots Moisture Stress Alleviation in Rice Genotypes by Triggering Antioxidant Defense System. Microbiol. Res. 2020, 239, 126518. [Google Scholar] [CrossRef]
- Bhambure, A.B.; Mahajan, G.R.; Kerkar, S. Salt Tolerant Bacterial Inoculants as Promoters of Rice Growth and Microbial Activity in Coastal Saline Soil. Proceedings of the National Academy of Sciences. India Sect. B Biol. Sci. 2017, 88, 1531–1538. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Azcon, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef]
- Reyes, Y.; Núñez, M.; Mazorra, L.M.; Martínez, L.; Ravelo, E.; Dell’Amico, J.; Menéndez, J.L.; Pérez, G. Mechanisms of action of brassinosteroids and their analogues on plant responses to abiotic stresses. An. De La Acad. De Cienc. De Cuba. 2021, 11, e873. [Google Scholar]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The Role of silicon in higher plants under salinity and drought stress. Front. Plants Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Lekklar, C.; Chadchawan, S.; Boon-Long, P.; Pfeiffer, W.; Chaidee, A. Salt stress in rice: Multivariate analysis separates four components of beneficial silicon action. Protoplasma 2018, 256, 331–347. [Google Scholar] [CrossRef]
- Rahman, A.; Hossain, M.S.; Mahmud, J.A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganeseinduced salt stress tolerance in rice seedlings: Regulation of ion homeostasis, antioxidant defense and glyoxalase systems. Physiol. Mol. Biol. Plants 2016, 22, 291–306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).