Innovative Transformation and Valorisation of Red Mill Scale Waste into Ferroalloys: Carbothermic Reduction in the Presence of Alumina
Abstract
1. Introduction
1.1. Background
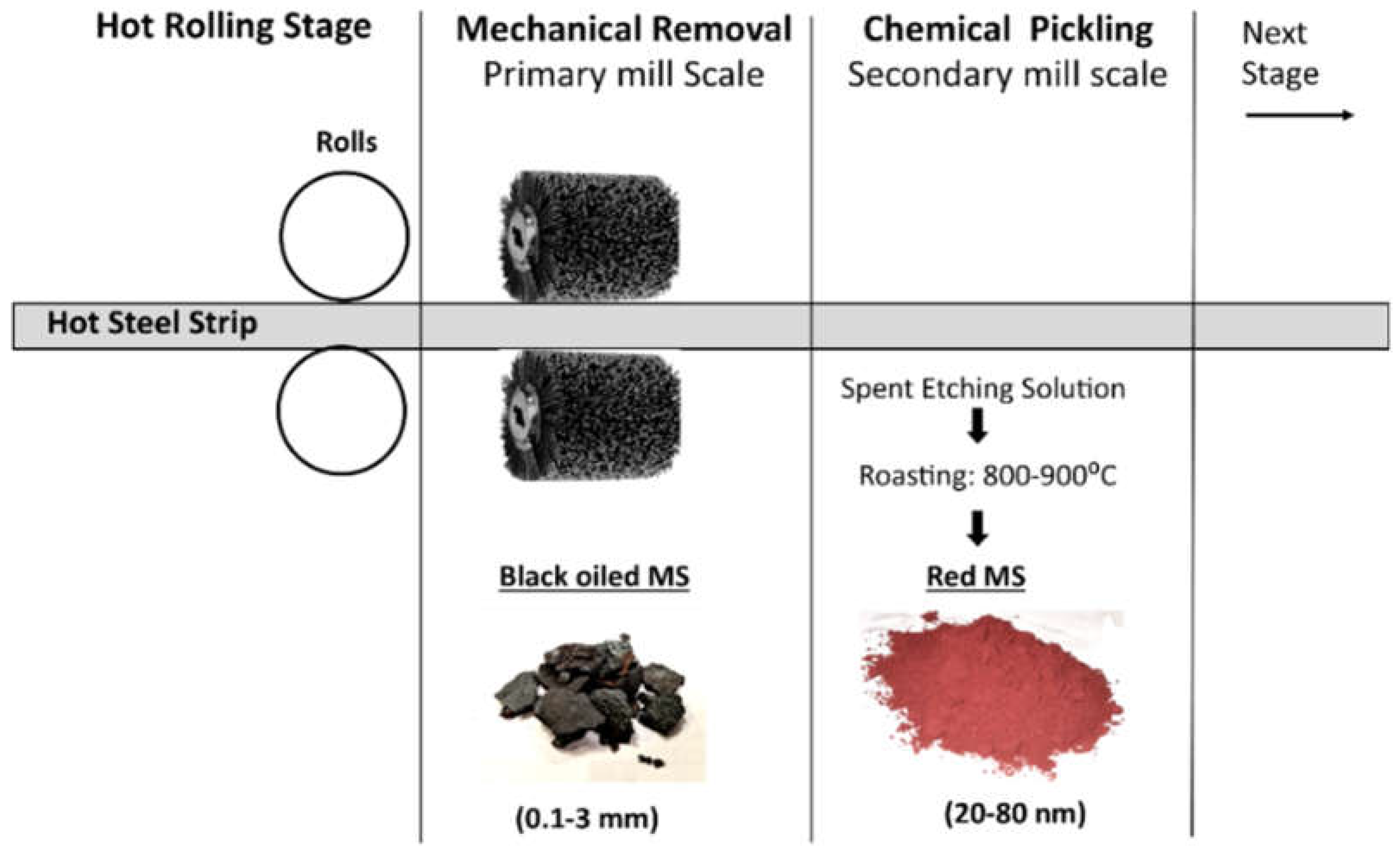
1.1.1. Generation of Mill Scales
1.1.2. Recycling of Mill Scale Waste
1.1.3. Low-Temperature Carbothermic Reduction of Alumina
1.2. Aim of This Investigation
2. Materials and Methods
2.1. Red MS Characterisation
2.2. Experimental
3. Results
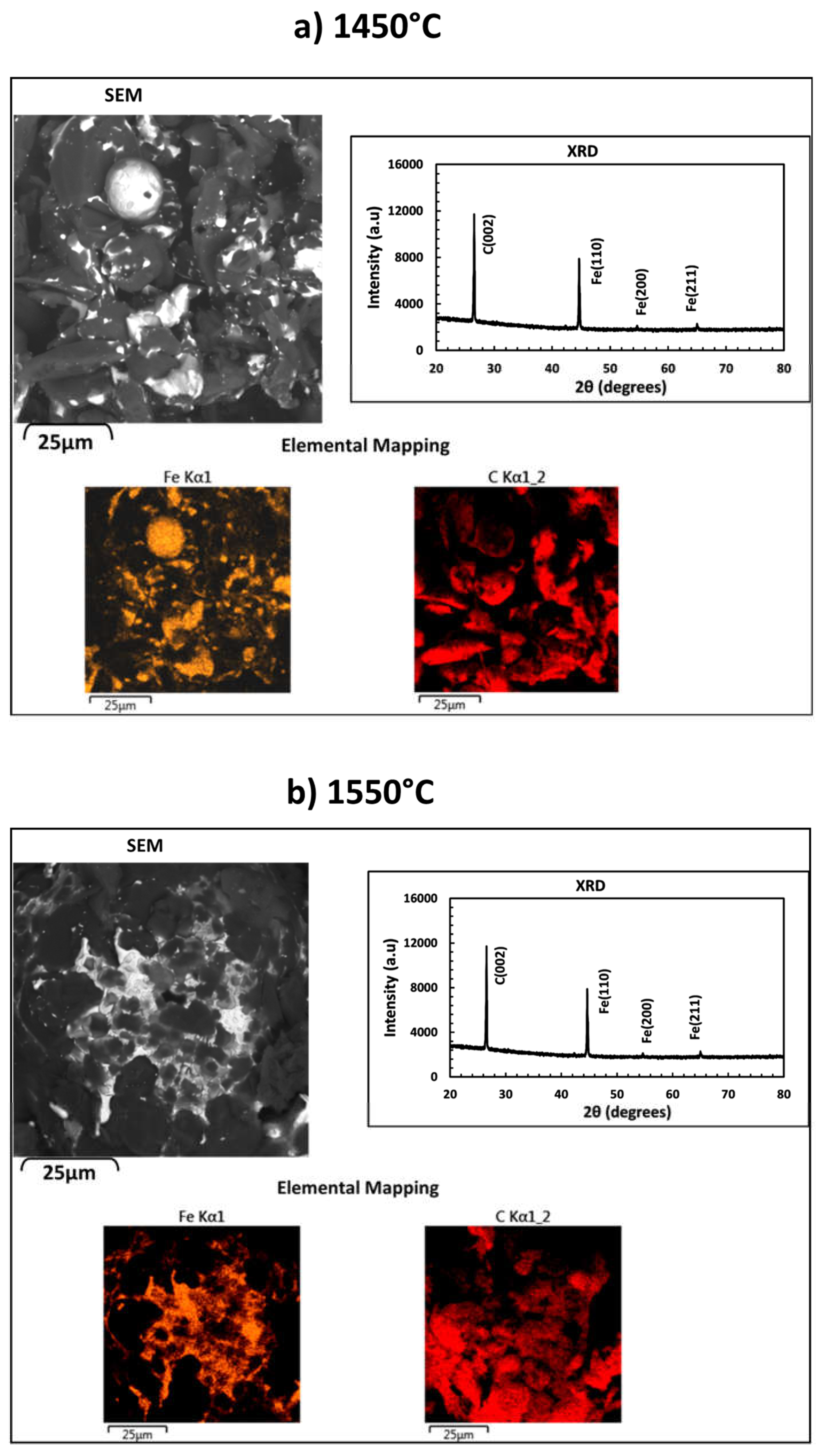
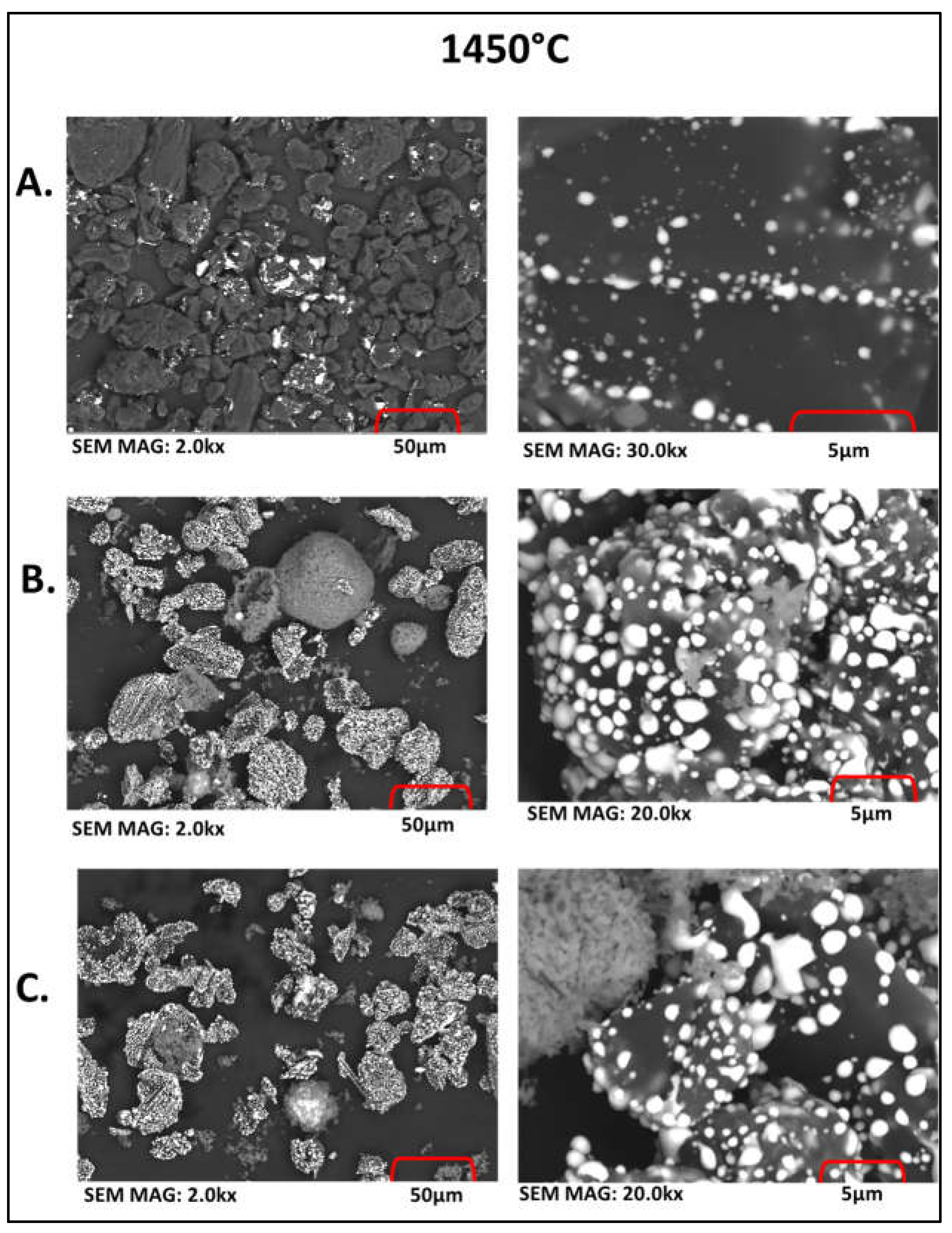
3.1. Carbothermic Reduction of Red MS
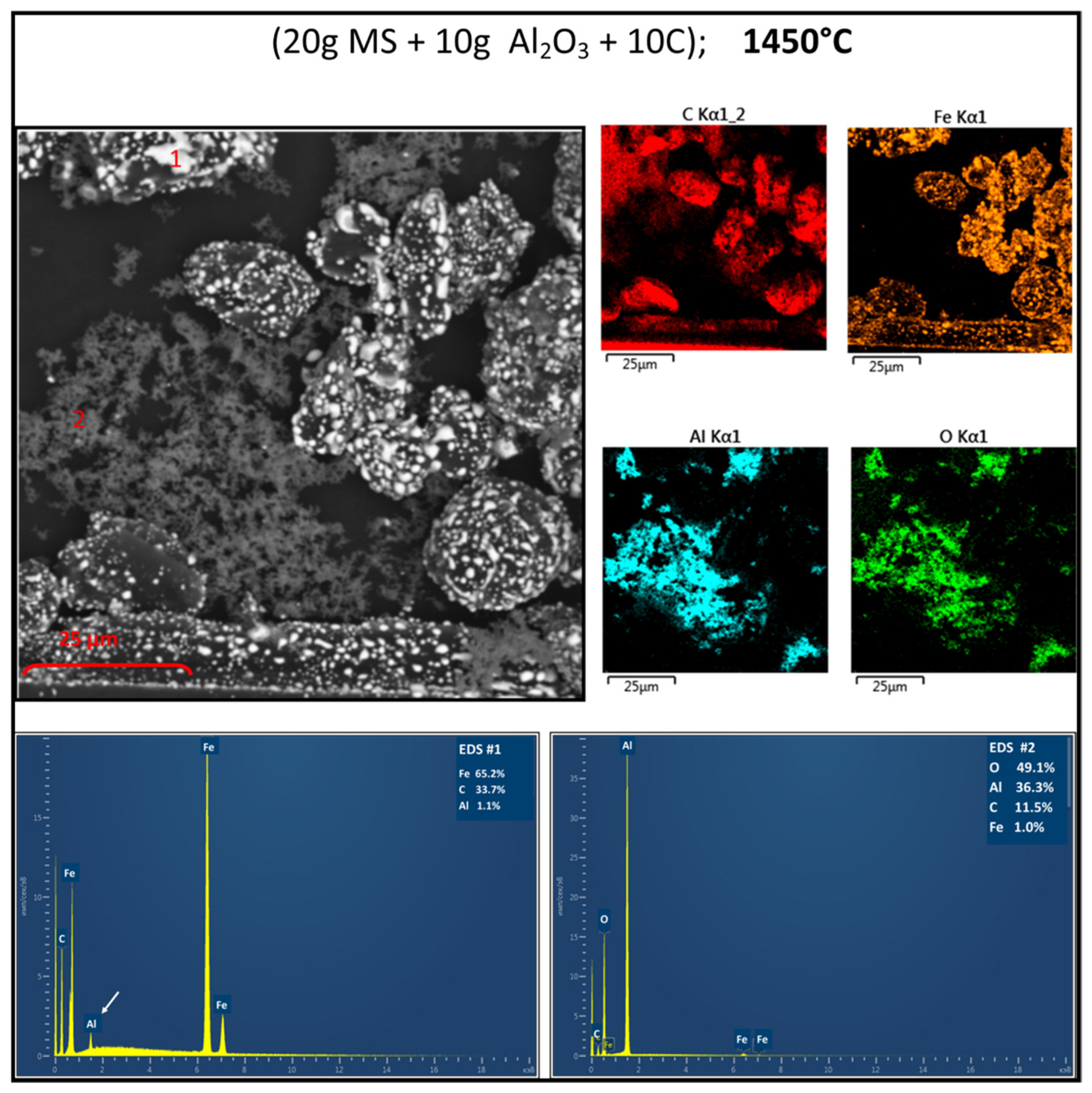
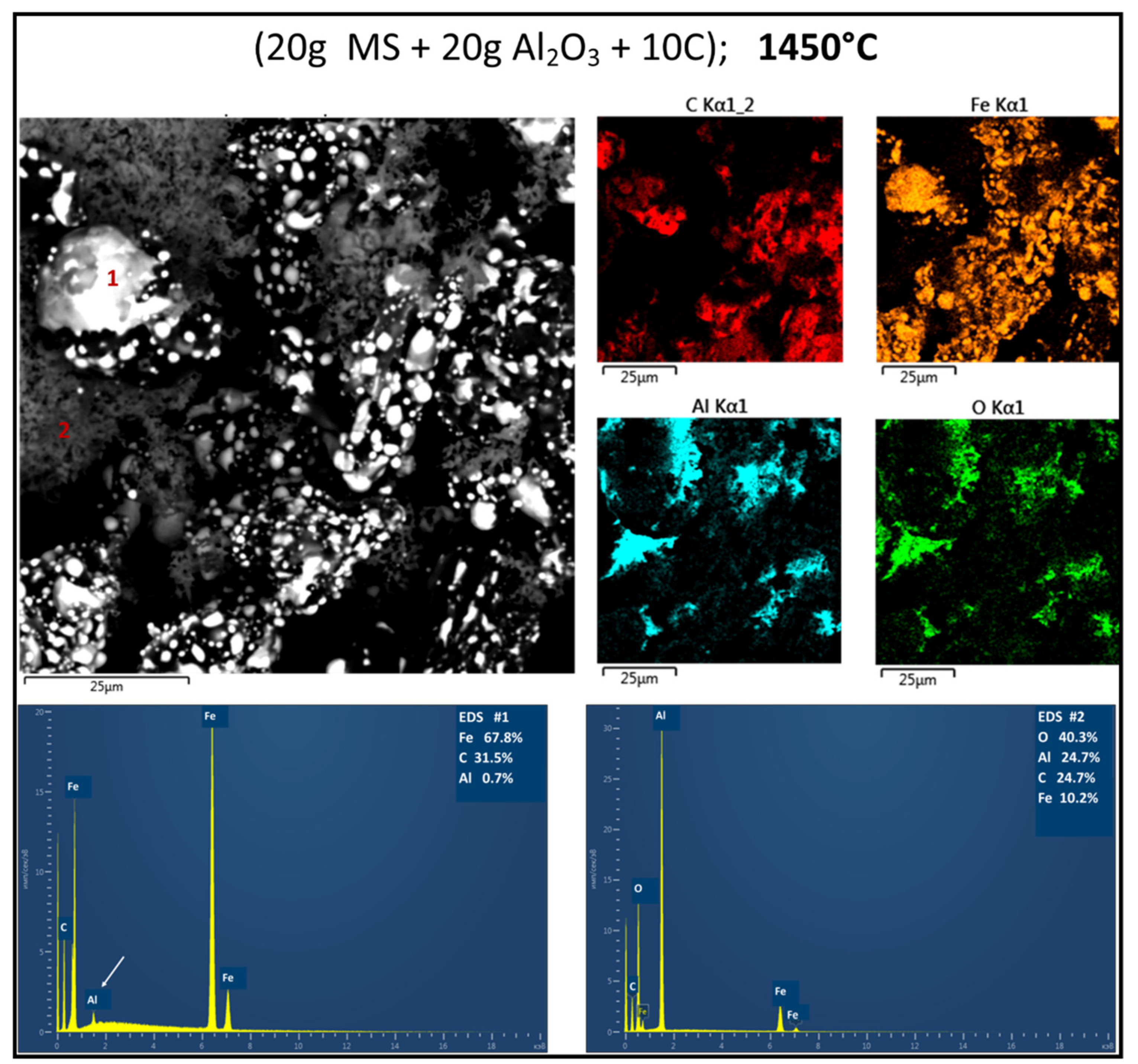
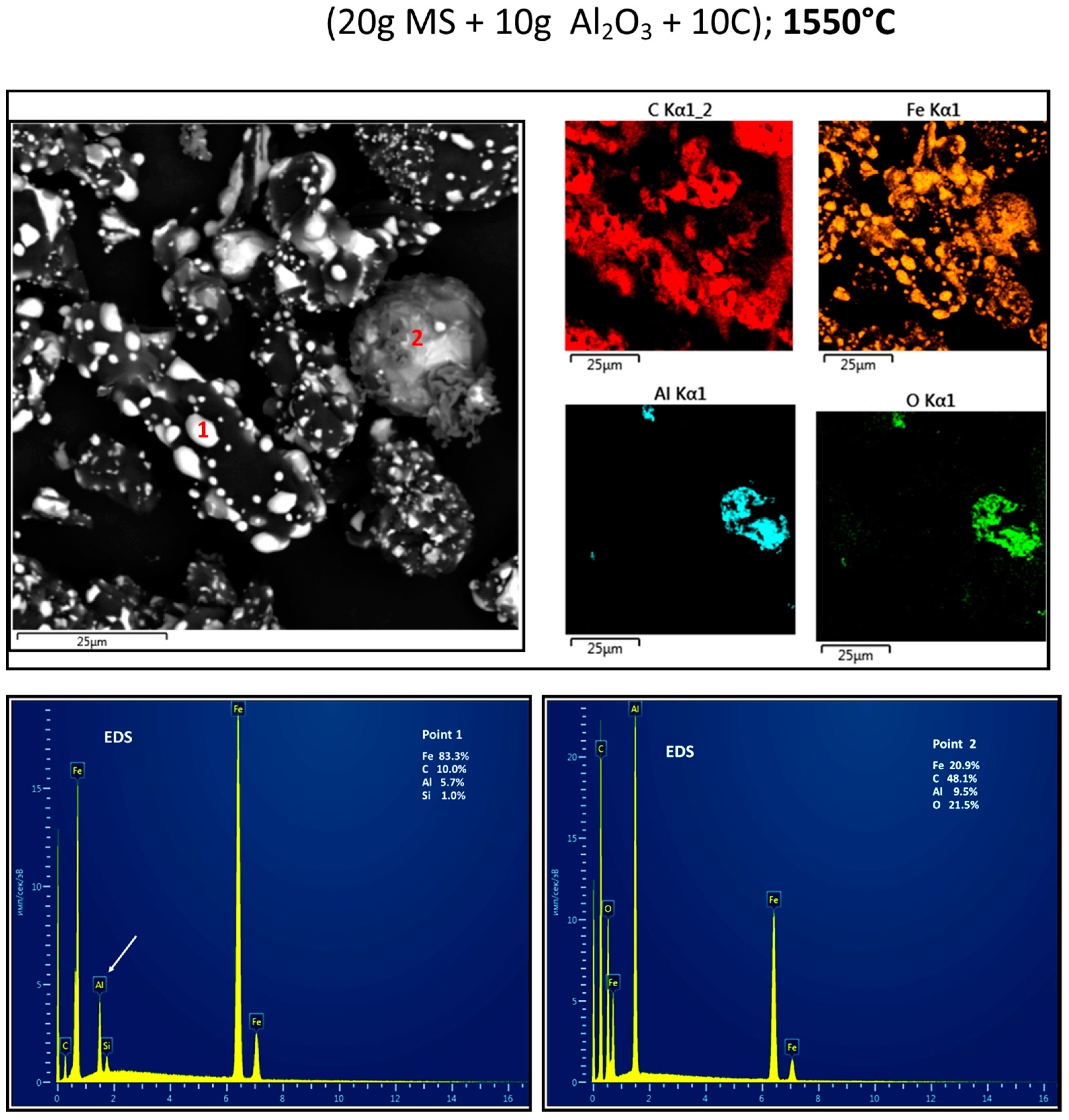
3.2. Reduction Behaviour of Red MS-Al2O3-C Blends
3.3. Structure Determination
4. Discussion
4.1. Impact on Reactivity
4.2. Lower Temperature for Alumina Reduction
- (1)
- The reduction of iron oxide and its subsequent carburisation in the molten state.
- (2)
- The disintegration of alumina into sub-oxide gases AlO and Al2O (Al2O3 + C = 2AlO + CO, Al2O3 + 2C = Al2O + 2CO); this reaction is known to have a slow kinetics [45].
- (3)
- AlO and Al2O gases are captured by the carburised molten iron.
- (4)
- The subsequent reduction of these gases to Al by the solute carbon.
- (5)
- The dissolution of reduced Al into molten iron due to its high affinity resulted in the formation of ferro-aluminium alloys.
5. Conclusions
- A novel processing route was developed for extracting iron from red mill scales and its conversion to ferro-aluminium alloys. The key innovation of this study lies in blending red MS with Al2O3 prior to the carbothermic reduction. The yield of iron and ferrous alloys from red MS was significantly enhanced when mixed with alumina in a range of concentrations.
- Most studies on extracting iron from iron-bearing wastes do not use blends; instead, the waste is treated alone as a low-grade iron resource [49]. The interactions between alumina and red MS led to significant increases in reduction reactivity. This breakthrough result means that higher quantities of metal could be extracted using red MS-Al2O3 blends than MS alone, leading to higher yields and significant economic benefits during waste processing.
- This study showed that red MS could be used to prepare iron aluminides as well as Fe-Al alloys in a range of compositions. These alloys are known for their low density and good mechanical strength and can be up to 30% lighter than commercial stainless steel and other structural materials [50,51,52]. Iron aluminides find applications in power generation systems due to their excellent high-temperature corrosion resistance in oxidizing and sulfidizing environments [53].
- This study showed that secondary red mill scales are a valuable material source for preparing high-value ferrous alloys. This study has the potential to open new research areas in industrial waste management wherein the use of nanosized reactants could offer significantly higher process efficiencies and productivity in a range of applications.
- Instead of dumping industrial waste into landfills or trying to just get rid of it in any way possible, there is an urgent need to identify the true potential of the waste and the materials within it. In addition to utilising red MS waste for producing new alloys, new research directions have opened on the role of nanosized reactants in reduction reactions. It is expected that these energy-efficient waste recycling options will lead to waste valorisation and economic as well as environmentally sustainable resource utilisation and management.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worldsteel Association. Environment and Climate Change. 2021. Available online: https://worldsteel.org/steel-topics/environment-and-climate-change/ (accessed on 16 August 2023).
- World Steel Organization. Buildings-and-Infrastructure. 2023. Available online: https://worldsteel.org/steel-topics/steel-markets/buildings-and-infrastructure/ (accessed on 16 August 2023).
- Basabe, V.V.; Szpunar, J.A. Growth rate and phase composition of oxide scales during hot rolling of low carbon steels. ISIJ Int. 2004, 44, 1554–1559. [Google Scholar] [CrossRef]
- Iluţiu-Varvara, D.A.; Aciu, C.; Pică Sava, E.M. Research on the chemical characterization of the oily mill scale for natural resources conservation. Procedia Eng. 2017, 18, 439–443. [Google Scholar] [CrossRef]
- Vlaicu, G.; Bancuta, I.; Stihi, C.; Gheboianu, A.I. The study of scale formation on hot rolled ingots and billets. J. Sci. Arts 2010, 12, 161–164. [Google Scholar]
- Khaerudini, D.S.; Chanif, I.; Insiyanda, D.R.; Destyorini, F.; Alva, S.; Pramono, A. Preparation and characterization of mill scale industrial waste reduced by biomass-based carbon. J. Sustain. Metall. 2019, 5, 510–518. [Google Scholar] [CrossRef]
- Rieger, J.; Schenk, J. Residual processing in the European steel industry: A technological overview. J. Sustain. Metall. 2019, 5, 295–309. [Google Scholar] [CrossRef]
- Paswan, D.; Malathi, M.; Minj, R.K.; Bandopadhyay, D. Mill scale: A potential raw material for iron and steel making. Steel World 2015, 21, 54–56. [Google Scholar]
- Umadevi, T.; Brahmacharyulu, A.; Karthik, P.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Recycling of steel plant mill scale via iron ore sintering plant. Ironmak. Steelmak. 2012, 39, 222–227. [Google Scholar] [CrossRef]
- Li, L.F.; Caenen, P.; Daerden, M.; Vaes, D.; Meers, G.; Dhondt, C.; Celis, J.-P. Mechanism of single and multiple step pickling of 304 stainless steel in acid electrolytes. Corros. Sci. 2005, 47, 1307–1324. [Google Scholar] [CrossRef]
- Lindell, D.; Pettersson, R. Pickling of process-oxidized austenitic stainless steels in HNO3-HF mixed acid. Steel Res. Int. 2010, 81, 542–551. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Rawat, J. Influence of iodide ions on inhibitive performance of Tetraphenyl-dithia-octaaza-cyclotetradeca-hexaene (PTAT) during pickling of mild steel in hot sulfuric acid. Mater. Chem. Phys. 2001, 70, 95–99. [Google Scholar] [CrossRef]
- Pickling of Hot Rolled Strip of Carbon Steel in Pickling Lines. 2013. Available online: https://www.ispatguru.com/pickling-of-scale-formed-on-hot-rolled-strip-of-carbon-steel/ (accessed on 15 August 2023).
- Singh Raman, R.K. Characterisation of ‘rolled-in’, ‘fragmented’ and ‘red’ scale formation during secondary processing of steels. Eng. Fail. Anal. 2006, 13, 1044–1050. [Google Scholar] [CrossRef]
- Eissa, M.; Ahmed, A.; El-Fawkhry, M. Conversion of Mill Scale Waste into Valuable Products via Carbothermic Reduction. J. Metall. 2015, 2015, 926028. [Google Scholar] [CrossRef]
- Kumar, K.S.; Sah, R.; Sekhar, V.R.; Vishwanath, S.C. Development and use of mill scale briquettes in BOF. Ironmak. Steelmak. 2017, 44, 134–139. [Google Scholar] [CrossRef]
- Martın, M.I.; López, F.A.; Torralba, J.M. Production of sponge iron powder by reduction of rolling mill scale. Ironmak. Steelmak. 2012, 39, 155–162. [Google Scholar] [CrossRef]
- Sen, R.; Pandel, U. Closed crucible reduction of lump powdered mill scale or iron ore by coal: The sequential methodology and mechanism for optimization of process parameters. Adv. Powder Technol. 2020, 31, 3760–3773. [Google Scholar] [CrossRef]
- Sen, R.; Maan, A.; Goyal, U.; Birdhaniya, A.; Pandel, U. In crucible reduction of mill scale by lean grade coal: Study of time, temperature and arrangement for optimum reduction conditions. Mater. Today Proc. 2018, 5, 7256–7263. [Google Scholar] [CrossRef]
- Sista, K.S.; Dwarapudi, S.; Nerune, V.P. Direct reduction recycling of mill scale through iron powder synthesis. ISIJ Int. 2019, 59, 787–794. [Google Scholar] [CrossRef]
- Bagatini, M.C.; Fernandes, T.; Silva, R.; Galvão, D.F.; Flores, I.V. Mill scale and flue dust briquettes as alternative burden to low height blast furnaces. J. Clean. Prod. 2020, 276, 124332. [Google Scholar] [CrossRef]
- Jikar, P.C.; Dhokey, N.B. Influence of process parameters on counter current reactor reduction of oxidized mill scale waste and its co-relationship with mathematical model. J. Sustain. Metall. Mater. Sci. 2020, 6, 622–630. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, B.B.; Xiong, L.; Li, G.H.; Jiang, T. Recycling of carbonaceous iron-bearing dusts from iron & steel plants by composite agglomeration process (CAP). Ironmak. Steelmak. 2017, 44, 532–543. [Google Scholar]
- Su, P.; Zhang, J.; Li, Y. Chemical fixation of toxic metals in stainless steel pickling residue by Na2S∙xH2O, FeSO4∙6H2O and phosphoric acid for beneficial uses. J. Environ. Sci. 2020, 90, 364–374. [Google Scholar] [CrossRef]
- Stocks, C.; Wood, J.; Guy, S. Minimization and recycling of spent acid wastes from galvanizing plants. Resour. Conserv. Recycl. 2005, 44, 153–166. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, T.; Sun, W.; He, D.; Lu, C.; Fu, X. Acid recovering and iron recycling from pickling waste acid by extraction and spray pyrolysis techniques. J. Clean. Prod. 2021, 312, 127747. [Google Scholar] [CrossRef]
- Dahlgren, L. Treatment of Spent Pickling Acid from Stainless Steel Production: A Review of Regeneration Technologies with Focus on the Neutralization Process for Implementation in Chinese Industry. Master’s Thesis, KTH, Stockholm, Sweden, 2010. [Google Scholar]
- Machado, R.M.; Gameiro, M.L.F.; Rodrigues, J.M.A.; Ismael, M.R.; Reis, M.T.; Carvalho, J.M. Recovery of hydrochloric acid from galvanizing industrial effluents. Separ. Sci. Technol. 2017, 52, 1333–1340. [Google Scholar] [CrossRef]
- Leonzio, G. Recovery of metal sulphates and hydrochloric acid from spent pickling liquors. J. Clean. Prod. 2016, 129, 417–426. [Google Scholar] [CrossRef]
- Xu, J.; Lu, S.; Fu, D. Recovery of hydrochloric acid from the waste acid solution by diffusion dialysis. J. Hazard Mater. 2009, 165, 832–837. [Google Scholar] [CrossRef]
- Chang, Y.; Zhai, X.; Li, B.; Fu, Y. Removal of iron from acidic leach liquor of lateritic nickel ore by goethite precipitate. Hydrometallurgy 2010, 101, 84–87. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Yin, S.; Long, H.; Li, S. Research on extraction of zinc from spent pickling solution using Aliquat 336. Hydrometallurgy 2020, 193, 105322. [Google Scholar] [CrossRef]
- Mishra, R.K.; Rout, P.C.; Sarangi, K.; Nath Sarma, K.C. Solvent extraction of Fe(III) from the chloride leach liquor of low-grade iron ore tailings using Aliquat 336. Hydrometallurgy 2011, 108, 93–99. [Google Scholar] [CrossRef]
- Calandra, A.J.; Castellano, C.E.; Ferro, C.M. The electrochemical behavior of different graphite/cryolite alumina melt interfaces under potentio-dynamic perturbations. Electrochim. Acta 1979, 24, 425–437. [Google Scholar] [CrossRef]
- European Commission. IPCC Reference Document on Best Available Techniques in the Non-ferrous Metals Industries. 2001. Available online: http://cartuja.jrc.es/pub/english.cgi/d733223 (accessed on 16 August 2023).
- Russell, A.S. Pitfalls and pleasures in new Aluminum process development. Metall. Trans. B 1981, 12, 203–215. [Google Scholar] [CrossRef]
- Khanna, R.; Ikram-ul-haq, M.; Said, S.F.; Sahajwalla, V.; Mukherjee, P.S.; Seetharaman, S. Reduction reactions in Al2O3-C-Fe and Al2O3-Fe2O3-C systems at 1823K. ISIJ Int. 2014, 54, 1485–1490. [Google Scholar] [CrossRef]
- Khanna, R.; Ikram-ul-haq, M.; Sahajwalla, V. Carbothermic reduction of alumina: On the role of molten iron and reaction mechanisms. ISIJ Int. 2016, 56, 1300–1302. [Google Scholar] [CrossRef]
- Schiemann, M.; Wirtz, S.; Scherer, V.; Bärhold, F. Spray roasting of iron chloride FeCl2: Laboratory scale experiments and a model for numerical simulation. Powder Technol. 2012, 228, 301–308. [Google Scholar] [CrossRef]
- Khanna, R.; Konyukhov, Y.V.; Ikram-ul-haq, M.; Burmistrov, I.; Cayumil, R.; Belov, V.A.; Rogachev, S.O.; Leybo, D.V.; Mukherjee, P.S. An innovative route for valorising iron and aluminium oxide rich industrial wastes: Recovery of multiple metals. J. Environ. Manag. 2021, 295, 113035. [Google Scholar] [CrossRef]
- Saffarzadeh, A.; Shimaoka, T. Occurrence and significance of secondary iron-rich products in landfilled MSWI bottom ash. Int. J. Waste Resour. 2014, 4, 150. [Google Scholar]
- Predescu, A.M.; Matei, E.; Berbecaru, A.C.; Râpă, M.; Sohaciu, M.G.; Predescu, C.; Vidu, R. An innovative method of converting ferrous mill scale wastes into superparamagnetic nano adsorbents for water decontamination. Materials 2021, 14, 2539. [Google Scholar] [CrossRef]
- Tang, J.; Pei, Y.; Hu, Q.; Pei, D.; Xu, J. The recycling of ferric salt in steel pickling liquors: Preparation of nano-sized iron oxide. Procedia Environ. Sci. 2016, 31, 778–784. [Google Scholar] [CrossRef]
- Lundgren, M.; Khanna, R.; Ökvist, L.S.; Sahajwalla, V.; Björkman, B. The evolution of structural order as a measure of thermal history of coke in the blast furnace. Matall. Mater. Trans. B 2014, 45, 603–616. [Google Scholar] [CrossRef]
- Frank, R.A.; Finn, C.W.; Elliott, J.F. Physical chemistry of the carbothermic reduction of alumina in the presence of a metallic solvent: Part II. Measurements of kinetics of reaction. Metall. Trans. B 1989, 20, 161–173. [Google Scholar] [CrossRef]
- Okomoto, H. The C-Fe (Carbon-Iron) System. J. Phase Equilib. 1992, 13, 543–565. [Google Scholar] [CrossRef]
- Murray, J.L. Fe–Al binary phase diagram. In Alloy Phase Diagrams; Baker, H., Ed.; ASM International: Materials Park, OH, USA, 1992; Volume 54. [Google Scholar]
- Eggersmann, M.; Mehrer, H. Diffusion in intermetallic phases of the Fe–Al system. Philos. Mag. A 2000, 80, 1219–1244. [Google Scholar] [CrossRef]
- Khanna, R.; Konyukhov, Y.V.; Zinoveev, D.; Jayasankar, K.; Burmistrov, I.; Kravchenko, M.; Mukherjee, P.S. Red mud as a secondary resource of low-grade iron: A global perspective. Sustainability 2022, 14, 1258. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K.; Liu, C.T. Processing, properties, and applications of nickel and iron aluminides. Prog. Mater Sci. 1997, 42, 177–192. [Google Scholar] [CrossRef]
- Liu, C.T.; George, E.P.; Maziasz, P.J.; Schneibel, J.H. Recent advances in B2 iron aluminide alloys: Deformation, fracture, and alloy design. Mater. Sci. Eng. A 1998, 258, 84–98. [Google Scholar] [CrossRef]
- Sina, H.; Corneliusson, J.; Turba, K.; Iyengar, S. A study on the formation of iron aluminide (FeAl) from elemental powders. J. Alloys Compd. 2015, 636, 261–269. [Google Scholar] [CrossRef]
- Tortorelli, P.F.; Natesan, K. Critical factors affecting the high-temperature corrosion performance of iron aluminides. Mater. Sci. Eng. A 1998, 258, 115–125. [Google Scholar] [CrossRef]
- Major Steel-Producing Countries 2021 and 2020 Million Tonnes, Crude Steel Production. Available online: https://worldsteel.org/steel-topics/statistics/world-steel-in-figures-2022/ (accessed on 25 September 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanna, R.; Konyukhov, Y.; Li, K.; Jayasankar, K.; Maslennikov, N.; Zinoveev, D.; Kargin, J.; Burmistrov, I.; Leybo, D.; Kravchenko, M.; et al. Innovative Transformation and Valorisation of Red Mill Scale Waste into Ferroalloys: Carbothermic Reduction in the Presence of Alumina. Sustainability 2023, 15, 16810. https://doi.org/10.3390/su152416810
Khanna R, Konyukhov Y, Li K, Jayasankar K, Maslennikov N, Zinoveev D, Kargin J, Burmistrov I, Leybo D, Kravchenko M, et al. Innovative Transformation and Valorisation of Red Mill Scale Waste into Ferroalloys: Carbothermic Reduction in the Presence of Alumina. Sustainability. 2023; 15(24):16810. https://doi.org/10.3390/su152416810
Chicago/Turabian StyleKhanna, Rita, Yuri Konyukhov, Kejiang Li, Kalidoss Jayasankar, Nikita Maslennikov, Dmitry Zinoveev, Jumat Kargin, Igor Burmistrov, Denis Leybo, Maksim Kravchenko, and et al. 2023. "Innovative Transformation and Valorisation of Red Mill Scale Waste into Ferroalloys: Carbothermic Reduction in the Presence of Alumina" Sustainability 15, no. 24: 16810. https://doi.org/10.3390/su152416810
APA StyleKhanna, R., Konyukhov, Y., Li, K., Jayasankar, K., Maslennikov, N., Zinoveev, D., Kargin, J., Burmistrov, I., Leybo, D., Kravchenko, M., & Mukherjee, P. S. (2023). Innovative Transformation and Valorisation of Red Mill Scale Waste into Ferroalloys: Carbothermic Reduction in the Presence of Alumina. Sustainability, 15(24), 16810. https://doi.org/10.3390/su152416810









