Abstract
The research employs both the literature and experimental data in order to develop reasonable strategies for melon fly control. The objects of research were sierozem soils of the Zhanakorgan region (Kyzylorda region), bentonite clays of the Sauran region (Turkestan region), and vermicompost obtained at the production site of the Research Institute “Ecology” at the International Kazakh-Turkish University, named after Khoja Ahmed Yasawi. The competitive agent ‘Vermiserbent’ was developed by combining sulphur-perlite-containing waste (SPCW), vermicompost, and natural bentonite clay. When incorporated into the soil, it serves as both an insecticide and a fertiliser recovery agent. The disinfection and enrichment of barren Sierozem soils in southern Kazakhstan could provide an eco-friendly approach to protect cucurbits (melon, watermelon, and pumpkin) against the melon fly. The average yield of watermelon treated with Vermiserbent increased by 2.3 t/ha compared to the control, melon by 4.6 t/ha, and pumpkin by 5.6 t/ha. The marketability of gourds as watermelons and melons after treatment with fertiliser increased by 1.2 times, and pumpkin by 1.1 times. The findings of studies conducted in agricultural fields in the Turkestan and Kyzylorda regions have shown that it is possible to produce environmentally sound gourds using a mixture of vermicompost, bentonite, and SPCW.
Keywords:
cucurbits; melon fly; sierozem soil; agent “Vermiserbent”; insecticide; disinfection; soil fertility 1. Introduction
One of the most essential state tasks in all countries is to solve the challenges of environmental protection, as well as the integrated rational use of natural resources. Every year, it is particularly important to solve the tasks of limiting anthropogenic influences on terrestrial ecosystems, as well as to develop scientific measures and strategies for managing them to prevent undesirable changes and assure environmental safety [1,2,3,4]. Special attention is paid to “industrial symbiosis”, which is based on using waste from one industry as a raw material for another.
Economic importance: The melon fly is a major pest of cucurbit crops, causing significant economic losses to farmers. A control strategy that can effectively manage this pest could help reduce these losses and increase the profitability of these crops. Environmental impact: The use of chemical pesticides to control melon fly populations can have negative environmental impacts, such as the pollution of soil and water resources. An effective control strategy that relies on non-chemical methods like disinfection and soil productivity management could help reduce these impacts. Food security is a significant concern, and cucurbit crops play a vital role in providing sustenance to many individuals, especially in the areas where they are cultivated. Implementing successful melon fly control measures could play a pivotal role in safeguarding a consistent and reliable supply of these crops, thereby making a valuable contribution to food security. Innovation: Developing effective non-chemical pest control strategies requires innovation and scientific research. The topic of an effective melon fly control strategy based on disinfection and soil productivity management represents an important area of ongoing research and innovation in the field of pest management. Overall, the topic of an effective melon fly control strategy based on disinfection and soil productivity management is relevant because it addresses important issues related to economic, environmental, and food security concerns, while also requiring innovation and scientific research to develop effective solutions.
Interest in the integrated use of natural resources and various wastes to produce new commercial goods has grown in both near and far countries such as the United States, England, Japan, China, Brazil, Europe, Russia, and Kazakhstan [5,6,7,8]. Numerous studies are being conducted in this direction to develop fertilisers, ameliorants, insecticides, and other agricultural agents [9].
Mineral fertilisers used in agriculture harm the soil, ecosystem, and agricultural products. Uncontrolled and unscientific use of chemical fertilisers lead to the deterioration of soil ecosystems and the increase of soil acidification and heavy metal ions [10]. The use of chemical fertilisers have adverse effects on crop quality and leads to ecological harm, including issues like water contamination, the release of greenhouse gases, and nitrogen leaching [11]. However, many modern methods used in environmental protection are not economically efficient and adversely affect the change of the soil layer. Enhancing soil health and elevating food quality (thus improving nutritional content) requires a shift towards cost-effective, environmentally friendly approaches. Various eco-friendly methods, such as biofertilisers, green manure, bacterialization, algal biofertilisers, and vermicomposting, play a pivotal role in promoting sustainable agricultural development. Additionally, agricultural residues represent a valuable resource for composting, serving as a crucial method for waste utilisation.
Hence, improper fertiliser application techniques can potentially harm soil health and disrupt soil-related ecosystem functions. The unbalanced use of chemical fertilisers can lead to shifts in soil pH, heightened vulnerability to pests, acidification, and the formation of soil crust, all of which contribute to diminished soil organic carbon levels and a decrease in beneficial soil organisms. This can hinder plant growth and reduce yields, and even result in the release of greenhouse gases [12].
Conversely, organic fertilisers have the capacity to enhance the physical and chemical attributes of the soil, including its structure, moisture retention, nutrient content, and cation exchange capacity. They also foster positive biological properties in the soil, leading to increased crop yields. Vermicompost, for instance, provides plant growth hormones like gibberellins, auxins, and cytokinins [13].
Fruit flies represent a significant group of pests that target cucurbit vegetables. In Kazakhstan, Cucurbitaceae crops cover a substantial area exceeding 80 thousand hectares, with around 70% of this cultivation concentrated in the southern region of the country [14]. Among these crops, the melon fly Zeugodacus cucurbitae (Diptera, Tephritidae) is a prominent global agricultural nuisance [15]. Z. cucurbitae has the capacity to infest over 130 host plants, including various vegetables and fruits. However, it primarily targets gourd and nightshade plants, such as cucumber, pumpkin, melon, watermelon, bitter melon, tomato, and eggplant. Adult insects are yellow flies with black spots 5–7 mm long. Mass flight of adults to feeding places begins in spring, when the soil warms up to 20 °C. During this period, the process of formation of the first fruits already begins in plants. After 6–7 days, insects start laying eggs in immature fruits to a depth of 2 mm. After 2–7 days, the eggs ripen, larvae hatch from them, which live in fruits and feed on their tissues. The larval phase lasts 8–13 days, and even longer in the last generation. The larva of the melon fly has a yellowish-white cylindrical body 8–10 mm long with two processes on the sides. Usually it gnaws through the fruit, comes out and, in the soil, forms a chrysalis at a depth of 12–13 cm. But often this process occurs in the fruits. In summer, the pupa develops within 13–20 days. Winters in soil at a depth of up to 18 cm.
Adult flies feed on fruit juice by piercing them with their ovipositor. The larvae consume the seeds and tissues, making the fruit completely unusable. Their damage is evidenced by small tubercles at the sites of puncture by the ovipositor and holes in the exit zone.
Utilizing agricultural waste as a secondary resource for the creation of diverse valuable commercial goods holds economic and ecological significance in agriculture. The importance lies in improving the environmental situation, lowering expenses, and boosting the environmental safety of the products.
The current research aimed to discover how the properties of sierozem soil alters when a mixture of vermicompost, bentonite, and sulphur-perlite-containing waste (SPCW) is applied, and what possibilities there are to produce environmentally safe cucurbits [14].
We have established for the first time that the use of only the above components allows us to obtain a new simultaneous effect—an increase in insecticidal action against melon flies and the production of environmentally friendly gourds with high yields. Insecticide-fertiliser in a solid form retains its physicochemical and biological properties over a wide temperature range and does not require special conditions for transportation and storage.
Below, Figure 1 shows the schematic way of obtaining insecticidal fertiliser ameliorant.
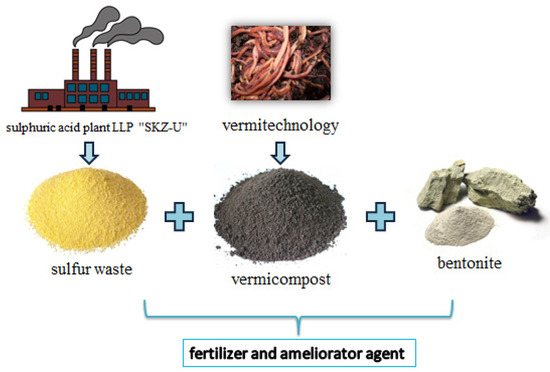
Figure 1.
Waste-based fertiliser ameliorant.
A complex insecticidal-fertiliser product for protecting watermelons and melons from the melon fly, which includes sulphur-perlite-containing waste from sulfuric acid production and bentonite as the main active ingredients; additionally, vermicompost is included in the composition. Figure 1 presents a possible way to obtain ameliorant fertiliser, based on the relationship between science and production. Thanks to the implementation of work that expands the “industrial symbiosis”, the following cluster relationship is successfully implemented by industries: sulphur-containing waste→sulfuric acid plant and with the help of vertitechnology→vermicompost and the addition of natural bentonite. With this cluster approach, thanks to the cycle, waste is used as efficiently as possible with virtually no loss of resources. Based on the appropriate use of agricultural waste, sulphuric acid production of SKZ-U LLP, and natural minerals, a complex ameliorant fertiliser with insecticidal properties has been developed.
Metagenomic soil analysis is a powerful tool for understanding the microbial communities present in soil. It involves analysing the genetic material (DNA or RNA) of all microorganisms present in a soil sample, without the need for culturing or isolating them. The importance of metagenomic soil analysis lies in its ability to provide a comprehensive view of the microbial diversity and function in soil, which is critical for various fields, including agriculture, environmental science, and biotechnology [16].
Here are some specific ways in which metagenomic soil analysis is important:
- Comprehending soil well-being: Soil constitutes an intricate ecosystem teeming with a wide range of microorganisms, and these microorganisms play a vital role in processes like nutrient cycling, plant development, and soil vitality. Metagenomic soil analysis can provide a detailed understanding of the microbial community composition and their functions in soil. This information can help identify beneficial microorganisms, potential pathogens, and other factors that affect soil health.
- Identifying novel microorganisms and functions: Metagenomic soil analysis can uncover new microorganisms that have not been previously described or cultured. These microorganisms may have unique metabolic pathways or functions that could be harnessed for biotechnological applications, such as the production of biofuels or bioremediation of contaminated soils.
- Monitoring soil changes: Metagenomic soil analysis can be used to monitor changes in soil microbial communities over time or in response to environmental stressors. For example, it can be used to assess the impact of agricultural practices, such as fertilisation or tillage, on soil microbial diversity and function.
- Developing sustainable agricultural practices: Metagenomic soil analysis can help identify microorganisms that contribute to plant growth promotion, disease suppression, and nutrient cycling. This information can be used to develop sustainable agricultural practices that reduce the need for synthetic fertilisers and pesticides, leading to improved soil health and reduced environmental impact.
In summary, metagenomic soil analysis is a powerful tool for understanding soil microbial diversity and function. Its applications in agriculture, environmental science, and biotechnology make it an important area of research for understanding and managing soil ecosystems.
Molecular techniques show great potential in identifying and researching a significant portion of non-cultivated microorganisms, enabling the examination of microbiome characteristics in their natural habitat without the need to isolate cultures in a pure form. Metagenomics involves the study of the complete genetic material obtained from an entire biological system. Within metagenomic approaches, the utmost emphasis is placed on analysing the 16S rRNA gene, upon which the contemporary phylogenetic classification of bacteria relies.
The research aims to address the following problems:
- –
- Crop Protection: Developing effective methods to control the melon fly will help prevent its detrimental impact on vegetable crops, especially on cucurbit crops like pumpkin, melon, and watermelon.
- –
- Increased Productivity: Soil productivity management contributes to higher crop yields and better crop quality, thereby ensuring food security.
- –
- Improved Environmental Sustainability: Implementing methods that are not reliant on chemical pesticides, such as disinfection and soil productivity management, can reduce the negative impact on ecosystems and enhance agricultural sustainability.
- –
- Efficient Resource Management: Responsible use of resources such as water and fertilisers can be part of a strategy for soil productivity management.
- –
- In essence, the research topic is geared towards enhancing crop yields, protecting agricultural crops from harmful pests, and concurrently addressing ecological sustainability and efficient resource management concerns.
Implementing a combination of soil disinfection and proactive soil productivity management practices will significantly reduce melon fly infestations, leading to improved crop yields and quality, while also promoting environmental sustainability by minimising the use of chemical pesticides.
This hypothesis suggests that a holistic approach that combines soil disinfection with soil productivity management techniques will result in better melon fly control, increased agricultural productivity, and reduced environmental impact. The study would aim to test and validate this hypothesis through empirical research and data analysis.
2. Materials and Methods
The studies carried out in 2020–2022 covered the sierozem soils of Zhanakorgan (Kyzylorda region), bentonite clays of Sauran (Turkestan region), vermicompost obtained at the production site of the research institute “Ecology”, Khoja Ahmed Yasawi Kazakh-Turkish International University, and silicon-containing waste from the sulphuric acid plant LLP “SAP-U” (Kyzylorda region).
2.1. Experimental Design
The total area of the (Zhanakorgansky district, Otrarsky district, Savransky district) of each experimental field was 250 m2, each plot was 40 m2, and the settlement area was 20 m2. The plot options were laid out randomly in 4-fold replication. The experimental setup proceeded as outlined below: (1) The initial soil was used without the introduction of external elements (acting as the control). (2) For the treated soil, a combination of 20 metric tons per hectare of vermicompost, 2 metric tons per hectare of bentonite, and 5 metric tons per hectare of SPCW was integrated.
A 15–20 t/ha−1 (or 3–5 kg/m−2) of “Vermiserbent” (ratio 10:1:2.5) was applied to the soil surface of the experimental field previously infested with melon fly in autumn. Atmospheric precipitation during the autumn application partially dissolved the components, allowing the larvae in the surface layers to be destroyed. In early spring, the top layer of soil was then loosened (up to 20–30 cm), which allows the destruction of the remaining larvae.
To sterilise the seeds before sowing, they were immersed for up to 10 h in an extract obtained by boiling the waste under study with water (at a ratio of 1:5). The extract was diluted 10 times with water for prophylactic purposes and the plants in the experimental plots were sprayed. The plant application was conducted on two occasions, each time using a dosage of 2 L per hectare. The extract decomposes in the air to form hydrogen sulphide, the odour of which repels melon flies and other pests. The first treatment was performed during the first leaf formation, while the second treatment was performed during the formation of loops. In the control plot, the field was treated with a solution of the insecticide “Rapira” (2 L ha−1).
2.2. Agrochemical Analysis
The agrochemical characteristics of the soil were assessed employing the following techniques: humus content was determined using the Tyurin method, modified as per Nikitin [17,18]; soil pH was measured in accordance with GOST 26483-85 [19]; exchangeable cations were determined following the Gedroits method with trilonometric titration; total nitrogen (N) was analysed using the Kjeldahl method [20]; hydrolytic acidity was assessed following GOST 26212-91 [21]; mobile phosphorus (P) and exchangeable potassium (K) were determined in compliance with GOST 26207-91 [22].
The water extraction method was used to extract easily soluble salts from the soil. In a 750 cm3 dry flask, 100 g of air-dry soil was passed through a 1–2 mm diameter sieve, and 500 cm3 of distilled water (dH2O) without CO2 was added, sealed with a rubber stopper, and shaken for 5 min. The aqueous extract was then filtered with a tightly pleated philtre.
The average sugar content was determined according to GOST 8756.13-87 [23]. Sampling was carried out according to GOST 26671-2014 [24], while sample preparation is carried out according to GOST 26313-2014 [25].
The mass fraction of protein in cucurbits was determined using the biuret method modified by Jennings [26]. Around 5.0 g of the specimen were precisely weighed with an accuracy of ±0.001 g and then introduced into a dry Erlenmeyer flask of 250 cm3 in volume, sealed with a stopper. The sample was measured with a 0.1 cm3 graduated cylinder under a draft of 2 cm3 carbon tetrachloride to extract the fat, and then 100 cm3 Biuret reagent was added with a pipette. The sealed flask was shaken on a mechanical shaker for 60 min. Then the extract was centrifuged at 4500 rpm for 10 min. A transparent centrifugate was added to the cuvette of a photoelectric colourimeter with a solution layer thickness of 5 mm. The optical density was measured at 550 nm. The protein content in the sample (mg) was determined using a calibration curve.
2.3. Metagenomic Analysis
The investigation of the microbiome’s phylogenetic composition within the soil specimens was conducted using an Illumina HiSeq instrument (from the USA) at the University of Inner Mongolia (China), following the established protocol [27]. A comprehensive overview of the Illumina platform can be found on the official company website at https://www.illumina.com (accessed on 1 May 2023). The overall metagenomic analysis process involves several stages (illustrated in Figure 2), with each stage executed in accordance with the manufacturer’s prescribed standards [28].
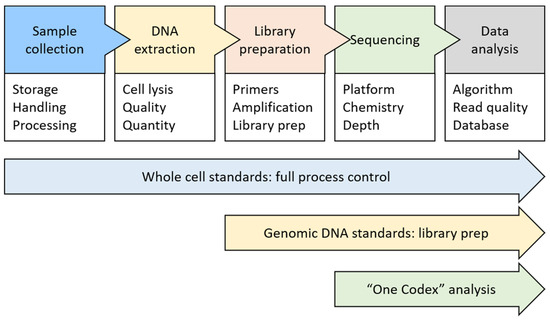
Figure 2.
The procedure for performing a metagenomic analysis on a soil specimen [29].
DNA extraction and PCR amplification were carried out as follows: Microbial DNA was extracted from coal samples using the E.Z.N.A.® soil DNA kit (Omega Bio-tek, Norcross, GA, USA), following the manufacturer’s instructions. The final concentration and purity of DNA were determined using a NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Waltham, MA, USA), and DNA quality was verified through electrophoresis in a 1% agarose gel.
To target the hypervariable regions V3–V4 of the bacterial 16S rRNA gene, the primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were used for PCR amplification. The PCR reactions were carried out using a thermal cycler PCR system (GeneAmp 9700, Waltham, MA, USA) with the following program: 3 min of denaturation at 95 °C, 27 cycles of 30 s at 95 °C, 30 s of annealing at 55 °C, 45 s of elongation at 72 °C, and a final extension at 72 °C for 10 min. PCR reactions were performed in triplicate in a 20 µL reaction mixture consisting of 4 µL 5× FastPfu Buffer, 2 µL 2.5 mM dNTPs, 0.8 µL of each primer (5 µM), 0.4 µL FastPfu polymerase, and 10 ng template DNA. The resulting PCR products were then extracted from a 2% agarose gel, further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA), and quantified using QuantiFluor™-ST (Promega, Madison, WI, USA) according to the manufacturer’s protocol.
For Illumina MiSeq sequencing, purified amplicons were pooled in equimolar ratios and subjected to paired-end sequencing (2 × 300) on the Illumina MiSeq platform (Illumina, San Diego, CA, USA), following standard protocols established by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Sequencing data processing involved several steps: Raw fastq files were demultiplexed and subjected to quality filtering using Trimmomatic, and they were merged using FLASH. The filtering criteria included: (I) Trimming reads at sites with an average quality score of <20 within a 50 bps sliding window. (II) Precise alignment of primers with allowances for two nucleotide mismatches, with the removal of reads containing ambiguous bases. (III) Merging of sequences that overlapped for more than 10 bp based on their overlapping sequences. Operational taxonomic units (OTUs) were clustered at a 97% similarity cutoff using UPARSE (version 7.1). Chimeric sequences were identified and eliminated using the UCHIME algorithm. The taxonomic classification of each 16S rRNA gene sequence was performed through the RDP classifier algorithm against the Silva 16S rRNA database (SSU123), with a confidence threshold of 70%. All analyses of bacterial communities were conducted using the free online platform Majorbio I-Sanger Cloud Platform (www.i-sanger.com, accessed on 1 May 2023).
2.4. Soil Horizon Method
The soil of the experimental field was light sierozem soil. They have a profile with fuzzy transitions between horizons and are well permeable to water. The parent rock is loam. Humus reserves in the soil profile were found to be mostly concentrated in the upper layer of 0–20 cm (0.95–1.23%, 26.5–27.4 t ha−1), while it dropped moving downwards in the profile. Despite the low percentage, it can be found up to half a metre. These soils can be suitable for growing cucurbits and other crops after fertilisation and regular irrigation.
A soil excavation involves creating a trench with uniform, straight-edged sides, initially reaching a depth of 0.6 m. During the excavation, soil is only disposed of alongside the trench’s long sides. The process entails the systematic removal of soil in layers, revealing different genetic horizons (or sub-horizons) as the excavation progresses.
After reaching a depth of 0.6 m, a step-ledge is made with a height of about 0.4 m, then the pit is deepened by another 0.4 m and another step is made, etc. Usually, three steps are made in a complete soil section. Their width depends on the granulometric composition of the studied soil: in easily shedding soils (sandy-loamy sandy) they are wider (about 0.4–0.5 m), in more stable (clay-loamy) they are narrower (0.3 m).
At the end of the deepening of the soil cut, the front (front) and side walls are cleaned with a shovel, while the bayonet of the shovel turns in the opposite direction so that the handle mounted on it does not interfere.
A measuring (for example, tailor’s) tape is attached to the upper edge of the cleaned front wall with a pin or needle, which is stretched down the centre to determine the thickness of individual horizons (subhorizons) of the soil.
The left side of the front wall (to the left of the measuring tape) remains unaffected by the work on describing the soil profile, the right (working) one is intended for the removal of soil samples.
The fresh section is carefully examined and genetic horizons and subhorizons are preliminarily distinguished. It is recommended that the final selection of soil horizons (subhorizons) be carried out as the final stage in the description of the section after each of the studied features (colour, moisture content, granulometric composition, structure, etc.) has been described. The preliminary identification of genetic horizons (subhorizons) is carried out on the basis of a change in colour in the soil stratum from top to bottom.
Horizons (subhorizons) are described in order from top to bottom.
In the process of describing the soil profile, samples are taken as material for laboratory work of the necessary analyses. Samples are wrapped in paper or placed in special bags along with labels indicating the location of the pit and the number of the description point.
2.5. Method for Determination of Nitrogen
A 0.200 g sample of soil is taken on a laboratory balance and placed in a heat-resistant test tube with a capacity of 50 mL. Then, 2 mL of a solution with a mass fraction of hydrogen peroxide of 30% is poured into the test tube along the wall, wetting the entire sample of soil with it. After 2 min, 3 mL of concentrated sulfuric acid containing selenium is added with a dispenser. The contents of the test tubes are stirred in a circular motion, placed in a device for heating test tubes, placed in a fume hood and the test tubes are gradually heated to 400 °C. Ashing is carried out at this temperature until the solution is completely discoloured. Then the solution is left to cool at room temperature and topped up with distilled water to the mark on the test tube. If heat-resistant tubes or a heating device are not available, 50 mL Kjeldahl flasks may be used. In this case, after the organic matter has been ashed, the solution is quantitatively transferred into volumetric flasks with a capacity of 50 mL and topped up with distilled water to the mark.
Following this, 1 mL of a clear solution obtained by decomposition of the soil is transferred by a dispenser into a dry flat-bottomed or conical flask with a capacity of 100 mL. Then, 45 mL of the working staining reagent is added to the solution with a dispenser, 2.5 mL of the working solution of hypochlorite is added. After adding each reagent, the solution is stirred, the flask with the solution is left for 1 h for the formation of a stable colour. The optical density of the coloured solution is measured relative to the zero solution in a cuvette with an absorbing layer thickness of 1 cm at a wavelength of 655 nm.
3. Results
The agrochemical characteristics of the Sierozem soils examined in the research exhibited the following properties: the pH (measured in KCl) ranged from 5.90 to 6.50; hydrolytic acidity fell within the range of 2.38 to 2.76 milliequivalents per 100 g of soil; the mobile phosphorus (P) content varied from 25.0 to 31.0 milligrams per kilogram; total nitrogen (N) content was in the range of 0.095% to 0.117%; exchangeable potassium (K) content ranged from 120 to 130 milligrams per kilogram; the total absorbed bases ranged from 13.6 to 14.0 milliequivalents per 100 g of soil. Calcium (Ca) cations were the dominant presence across all soil layers, comprising approximately 7.5 milliequivalents or around 54.3% in the 0–40 cm layer.
The analysis of the aqueous extract from the soil aimed to provide insights into the composition and quantity of readily soluble salts, as well as the extent and nature of soil salinity. The most common salts responsible for inducing soil salinity and detrimental effects on plants are calcium (Ca), magnesium (Mg), and sodium (Na) carbonates, bicarbonates, chlorides, and sulphates. Adverse effects on crop quality and yield volume start to occur when salt concentrations reach 0.1% of the soil’s dry weight. The results of the chemical analysis of the aqueous extract obtained from completely dry soil prior to the application of the fertiliser-amendment treatment are presented in Table 1.

Table 1.
The content of components in the aqueous extract (average value, %).
Based on the information derived from the aqueous extract analysis, it was determined that the Sierozem soil under investigation did not exhibit salinity. In the topmost layer spanning from 0–100 cm, the salt content ranged from 0.104% to 0.112% (Table 1). The aqueous extract contained no carbonates. The chlorine ion concentration in the upper 0–100 cm (1 m) layer did not surpass the toxicity threshold, averaging 0.010%. At the same time, bicarbonate ions dominated, accounting for more than 41% of the total salts.
An analysis of the cationic composition of the aqueous extract revealed a drop in calcium (Ca) reserves and an increase in sodium (Na) as it passes from the upper to the lower horizon. The content of magnesium (Mg) cations in a metre (0–100 cm) layer was evenly distributed. The alkalinity of the soil can be explained by a relatively high content of sodium and calcium bicarbonate (Table 1).
As we move from the upper to lower soil layers, there is a notable reduction in the quantity of humic substances, a rise in the pH level of the environment, and a decline in the finer fractions within the soil’s particle composition. Table 2 shows initial the mechanical composition of the profile of the sierozem soil of the Otrar and Sauran districts of the Turkistan region and the Zhanakorgan district of the Kyzylorda region.

Table 2.
Granulometric and microaggregate composition of soils.
Sufficiently high soil alkalinity can be explained by a precipitation shortage in the region. The removal of weathering and soil formation products from soils and parent rock is limited in this regard. A high alkali level in the soil is well known to be detrimental to most crops, including melons. Plant metabolism is disrupted in an alkaline environment, and the solubility and availability of nutrients, as well as iron, copper, manganese, boron, and zinc, are reduced. In the case of an alkaline reaction, plant toxic chemicals, such as soda and sodium aluminate, develop in the soil solution [30,31,32,33].
When the pH rises sharply, the root hairs of the plants experience alkali burn, which has a negative effect on their further development and can lead to death. The strongly alkaline soils of the study area have pronounced negative agrophysical properties, which are accompanied by a strong peptisation of soil colloids and humic substances dissolution. These soils become textured (lose their structure), excessively sticky when wet and hard when dry, with poor filtration and unsatisfactory air conditions.
Only extensive recultivation measures with the inclusion of organomineral compositions can improve these alkaline soils. As a fertiliser and reclamation agent (FRA), a “Vermiserbent” mixture of vermicompost, bentonite, clay, and SPCW was chosen (Figure 3).
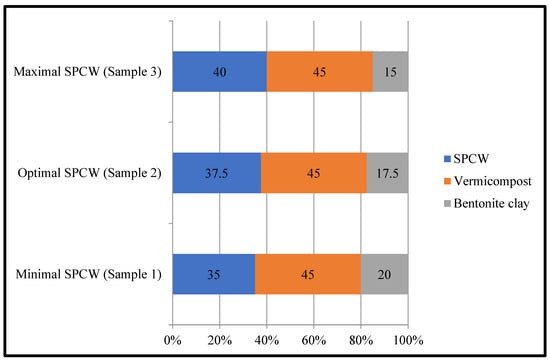
Figure 3.
Optimal and boundary contents of components in FRA “Vermiserbent” (wt.%).
The optimal ratios of the “Vermiserbent” components were determined based on laboratory, extended laboratory, and field trials on farm soils. The components of “Vermiserbent” are characterised by the properties described below.
All components of “Vermiserbent” contain calcium compounds. Calcium displaces the absorbed sodium, making the Solonetz horizon more structurally and water permeable, consequently allowing salts to be washed out of the lower horizons.
Vermicompost improves soil fertility and structure, has high agrochemical and growth-promoting properties, and delivers a consistent, high, ecologically safe crop yield with outstanding flavour. Vermicompost has great physical and chemical properties, including structural water resistance (95–97%) and complete moisture capacity (200–250%), allowing it to be used not only as a fertiliser but also as an effective ameliorant and soil conditioner.
Bentonite from the Urangai deposit (Turkestan region) contains 60–70% montmorillonite minerals. It is a veritable treasure trove of natural trace elements. Due to the presence of the main nutrients K (0.8–1.4%), N (0.8–1.0%), P (0.08–0.10%), a complex of trace elements, SiO2 (57.5%), and CaCO3 (13.5%), bentonite can be utilised as a fertiliser and an ameliorant.
Waste from sulphuric acid production contains in its composition 10 to 20% sulphur compounds (free sulphur, polysulphides, thiosulphate, sulphate, mercaptans), perlite, gypsum, and slaked lime [34]. When introduced into the soil, it not only acts as a fertiliser and ameliorating agent, but it also shows an insecticidal action that kills many pests.
The yield, marketability, and calorie content of melon, watermelons, and pumpkins from the control and trial plots were determined during the study (Table 3).

Table 3.
Production trial results on the “Vermiserbent” impact on cucurbit yield.
Following the application of “Vermiserbent,” the nutritional content of melon is determined based on the following standard parameters (expressed in grams per 100 g of melon): 88.5 g of water; 0.63 g of proteins; 0.25 g of fat; 0.77 g of carbohydrates; 0.02 g of ascorbic acid; 0.6 g of fibre; 0.4 g of pectin; and 0.5 g of ash.
The sugar composition in watermelon was as follows: 1.82% glucose; 2.96% fructose; 2.87% sucrose. In the pulp of the fruit, the sugar is 12 g per 100 g. Fructose predominates in watermelon.
The pumpkin variety known as “Matilda” falls into the category of early-maturing varieties. In 100 g of the fruit, the primary components are present in the following proportions: 1.0 g of protein, 0.1 g of fat, 44.2 g of carbohydrates, and 89.3 g of water.
In terms of aesthetics, the produced melons, watermelons, and pumpkins are not inferior to the control samples. The nitrate content were below the permissible standard values. The quantitative composition of macro- and microelements in the melons and watermelons cultivated in both the control and experimental plots showed no significant variations. The melons and watermelons from the experimental plots, on the other hand, were juicier and sweeter than those from the control plot. The experimental plots produced more melons, watermelons, and pumpkins than the control plots, and the fruits were larger as well.
We studied the microbiota of substrates obtained using vermitechnology. Typically, alterations in bacterial composition as manure passes through the digestive system of worms are examined by comparing the microbial cell complexes in the manure ingested by the worms and the resulting coprolites. In order to comprehend the mechanisms behind microbiota changes in manure as it passes through worms, it is crucial to have knowledge of the bacterial composition. Consequently, the taxonomic makeup and population levels of bacterial species in the soil and coprolite were investigated (after being subjected to Vermiserbent treatment) as shown in Figure 4 and Figure 5. In the microbiological analysis of the soil before and after treatment are designated BIII and BIV.
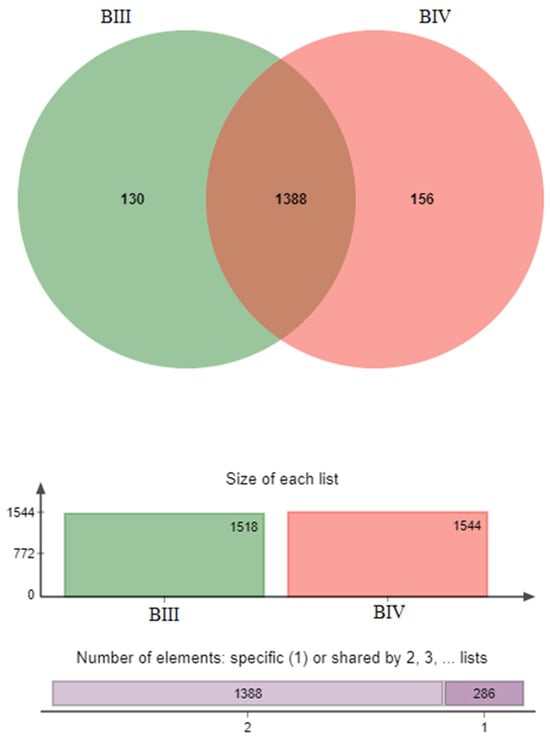
Figure 4.
General views in two groups of samples (BIII, BIV).
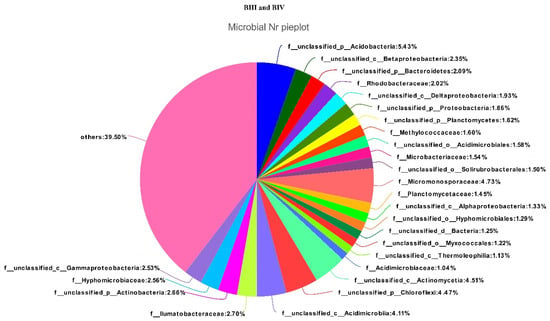
Figure 5.
Composition and ratio of individual species in two general samples (BIII, BIV).
The segment where the various sample groups intersect represents the shared species among them, while the section that does not overlap signifies the distinct species unique to each sample group, with the associated number denoting the quantity of these species. (Figure 4). This means that there are 1388 species in the two samples, and 130 species only in sample BIII, and 156 species in sample BIV.
If you look at the samples separately, then there will be the Figure 6 and Figure 7. In these pictures, we will see their diversity, that they are rich in species composition in the soil after the experiments.
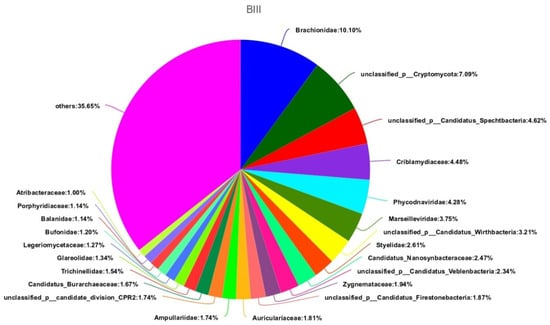
Figure 6.
Composition and ratio of individual species in sample BIII.
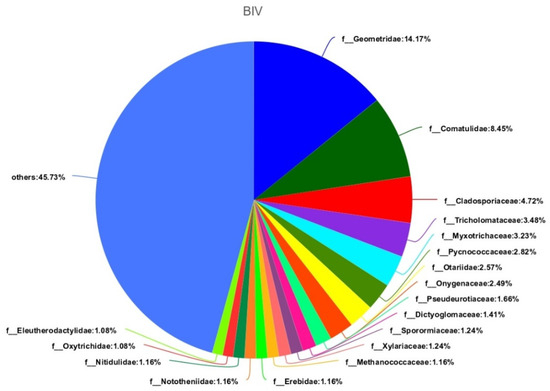
Figure 7.
Composition and ratio of individual species in sample BIV.
To illustrate the comparison of two samples in terms of percentages, we can represent them as depicted in Figure 8. The horizontal axis/vertical axis displays the specimen names, while the vertical axis/horizontal axis indicates the species proportion in the specimens. The bars, distinguished by various colours, correspond to distinct species, with the length of each bar symbolizing the respective species proportion.
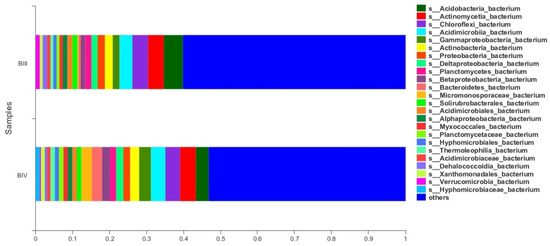
Figure 8.
Composition and ratio of individual species in two samples of comparative nature (BIII, BIV).
The research conducted enables us to establish connections between the characteristics of microbial communities’ structure and diversity, as examined through 16S rRNA gene sequencing, and the attributes of soil properties and their origin.
The abundant presence of microorganisms within a soil sample may be indicative of soil health and fertility. Microorganisms are pivotal contributors to soil well-being and productivity as they facilitate the decomposition of organic matter, release essential nutrients, and uphold soil structure. Certain microorganisms like bacteria, fungi, and protozoa can also assist in managing plant diseases and pest issues. Moreover, soil microorganisms can aid in carbon sequestration, offering a means to mitigate climate change.
4. Conclusions
In conclusion, the analysis of the aqueous extract from the Sierozem soil samples has provided valuable insights into the soil’s properties and composition. The findings indicate that the studied Sierozem soil does not exhibit salinity issues, as the salt content in the topmost 0–100 cm layer ranged from 0.104% to 0.112%, and no carbonates were detected in the aqueous extract. Chlorine ion concentration in this layer remained below the toxicity threshold, averaging 0.010%. Notably, bicarbonate ions were the predominant salt component, constituting over 41% of the total salts.
Furthermore, the cationic composition analysis revealed some changes from the upper to lower horizons. There was a decrease in calcium (Ca) levels and an increase in sodium (Na) content as we moved down the soil profile. The content of magnesium (Mg) cations appeared to be evenly distributed within the 0–100 cm layer. The alkalinity of the soil was associated with the relatively high presence of sodium and calcium bicarbonate.
Additionally, the transition from upper to lower soil layers was marked by a reduction in humic substances, an elevation in soil pH levels, and a decrease in finer soil fractions. This mechanical composition change is depicted in Table 2, providing insight into the profile of Sierozem soil in different districts.
Overall, this analysis of the Sierozem soil’s aqueous extract has contributed to our understanding of its chemical and mechanical characteristics, highlighting its suitability for various agricultural practices and land management.
In summary, the significant soil alkalinity observed in the region can be attributed to the limited precipitation, which hinders the removal of weathering and soil formation products from the soils and parent rock. This high alkaline level poses a challenge to agricultural practices, particularly for melon cultivation. Alkaline environments disrupt plant metabolism, reduce the solubility and availability of essential nutrients such as iron, copper, manganese, boron, and zinc, and promote the formation of plant-toxic compounds like soda and sodium aluminate in the soil solution.
The adverse effects of high pH levels extend to the root system of plants, leading to alkali burns on root hairs and impeding their growth, potentially resulting in plant mortality. Moreover, the strongly alkaline soils in the study area exhibit unfavourable agrophysical properties, including excessive peptization of soil colloids and the dissolution of humic substances. These soils tend to lose their structure, becoming overly sticky when wet and hard when dry, with poor filtration and inadequate air conditions.
To address these challenges and enhance the quality of these alkaline soils, comprehensive recultivation measures are required. The application of “Vermiserbent,” a mixture composed of vermicompost, bentonite, clay, and SPCW, has been identified as a suitable fertilisation and reclamation agent (FRA). This blend offers the potential to mitigate the detrimental effects of alkalinity, restore soil structure, and create a more conducive environment for successful crop cultivation.
The new agent “Vermiserbent”, when applied to the soil, influences the formation of agrophysical (structure, bulk density, air permeability, water holding capacity, etc.), physicochemical, and biological properties of the soil. The intensification of physiological and biochemical processes by the agent “Vermiserbent” in cucurbits accelerates the passage of phenophase and shortens the duration of the vegetation period by an average of 7–10 days. The developed composition is a source of plant nutrients. Further research should be focused on determining the biological effect of insecticide ameliorant on melon flies. It would also be interesting to use insecticide–ameliorant on other agricultural pests.
Author Contributions
Conceptualization, G.S. and A.A.; methodology, Z.Y.; software, K.T.; validation, A.H., Z.Y. and K.T.; formal analysis, G.S.; investigation, A.A.; resources, K.T.; data curation, A.H.; writing—original draft preparation, Z.Y.; writing—review and editing, K.T.; visualization, G.S.; supervision, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khoroshavin, L.B.; Belyakov, V.A.; Svalov, E.A. Basic Technologies for Processing Industrial and Municipal Solid Waste; Ministry of Education and Science Ros. Federation, Ural Federal University, Noskov, A.S., Eds.; Ural Publishing House: Yekaterinburg, Russia, 2016; 220p. [Google Scholar]
- Chertow, M.; Park, J. Scholarship and Practice in Industrial Symbiosis: 1989–2014. Tak. Stock Ind. Ecol. 2016, 5, 87–116. [Google Scholar]
- Andersson, E.; Arfwidsson, O.; Bergstand, V.; Göransson, T.; Haag, L.; Sund, L.; Svedlund, S. Industrial Symbiosis in Stenungsund. 2013. Available online: http://www.industriellekologi.se/symbiosis/stenungsund.html (accessed on 1 February 2023).
- Domenech, T.; Davies, M. Structure and Morphology of industrial symbiosis networks: The case of Kalundborg. Procedia Soc. Behav. Sci. 2011, 10, 79–89. [Google Scholar] [CrossRef]
- Mavropoulos, A.; Wilson, D.; Velis, S.; Cooper, J.; Eppelquist, B. Globalization and Waste Management. In Step 1: Concepts and Facts; International Solid Waste Association: Vienna, Austria, 2012. [Google Scholar]
- Ratnawati, B.; Yani, M.; Suprihatin, S.; Hardjomidjojo, H. Waste processing techniques at the landfill site using the material flow analysis method. Glob. J. Environ. Sci. Manag. 2023, 9, 73–86. [Google Scholar] [CrossRef]
- Rasskazova, A.V.; Alexandrova, T.N.; Lavrik, N.A. The increase of effectiveness of power utilization of brown coal of Russian Far East and prospects of valuable metals extraction. Eurasian Min. 2014, 1, 25–27. [Google Scholar]
- Vetoshkin, A.G. Processing of Industrial and Domestic Wastes (Technology and Equipment for the Protection of the LITHOSPHERE): Training Manual; Publishing house DIA: Moscow, Russia, 2015; 400p. [Google Scholar]
- Marchenko, L.A.; Artyushin, A.A.; Smirnov, I.G.; Mochkova, T.V.; Spiridonov, A.Y.; Kurbanov, R.K. Technology for the application of pesticides and fertilizers by unmanned aerial vehicles in digital agriculture. Agric. Mach. Technol. 2019, 13, 38–45. [Google Scholar] [CrossRef]
- Environmental Impacts of Pesticides. May 2016. Available online: https://www.slu.se/en/Collaborative-Centres-and-Projects/centre-for-chemical-pesticides-ckb1/information-about-pesticides-in-the-environment-/exposure-and-environmental-impact/ (accessed on 1 February 2017).
- Mezhevov, A.S. The use of biomeliorants to increase the productivity of low-humus soils. Sci. J. Russ. Res. Inst. Land Reclam. Probl. 2020, 4, 2020. [Google Scholar]
- Methods for Determining the Composition of Exchangeable Cations and Absorption Capacity of Soils. Available online: http://www.bibliotekar.ru/2-9-67-himiya-pochvy/34.htm (accessed on 1 February 2023).
- Sainova, G.A.; Kozhamberdiev, E.M.; Akbasova, A.D.; Ibraimov, U.K. Sulfur-containing waste from sulfuric acid production of CKZ-U LLP is a valuable commercial resource. Almaty Altyn. Baspa 2021, 216, 107–109. [Google Scholar]
- The Melon Fly Has Already Been Identified in Nine Regions of Southern Kazakhstan. Available online: https://otyrar.kz/2013/07/dynnaya-muxa-vyyavlena-uzhe-v-devyati-rajonax-yuzhnogo-kazaxstana/ (accessed on 12 July 2013).
- Shelly, T.E.; Kurashima, R.S. Capture of melon flies and Oriental fruit flies (Diptera: Tephritidae) in traps baited with torula yeast-borax or CeraTrap in Hawaii. Fla. Entomol. 2018, 101, 144–146. [Google Scholar] [CrossRef]
- Lawson, K.E.; Wu, S.; Bhattacharjee, A.S. Metabolic network analysis reveals microbial community interactions in anammox granules. Nat. Commun. 2017, 8, 15416. [Google Scholar] [CrossRef] [PubMed]
- GOST 26213-91; Determination of Organic Matter by the Tyurin Method in the Modification of TSINAO. Gosstandart of Russia: Moscow, Russia, 1991.
- GOST 62213-91; Determination of Organic Matter by the Tyurin Method in the Nikitin Modification. Gosstandart of Russia: Moscow, Russia, 1991.
- GOST 26483-85 Soil; Preparation of salt Extract and Determination of its pH by the TSINAO Method. Gosstandart of Russia: Moscow, Russia, 1985.
- GOST 26107-84 Soil; Methods for Determining Total Nitrogen by the Kjeldahl Method. Gosstandart of Russia: Moscow, Russia, 1984.
- GOST 26212-91; Determination of Hydrolytic Acidity by the Kappen Method in the Modification of TSINAO. Gosstandart of Russia: Moscow, Russia, 1991.
- GOST 26207-91; Determination of Mobile Compounds of Phosphorus and Potassium by the Kirsanov Method in the Modification of TSINAO. Gosstandart of Russia: Moscow, Russia, 1991.
- GOST 8756.13-87; Fruit and Vegetable Processing Products. Methods for the Determination of Sugars. Gosstandart of Russia: Moscow, Russia, 1987.
- GOST 26671-2014; Fruit and Vegetable Processing Products, Canned Meat and Meat-Growing. Preparation of Samples for Laboratory Analysis. Gosstandart of Russia: Moscow, Russia, 2014.
- GOST 26313-2014; Fruit and Vegetable Processing Products. Acceptance Rules and Sampling Methods. Gosstandart of Russia: Moscow, Russia, 2014.
- Handbook of Human Health. Available online: https://med7.net/page/205049000134225195162213217243208088054111214100/ (accessed on 1 February 2023).
- Delmont, T.O.; Robe, P.; Cecillon, S.; Clark, I.M.; Constancias, F. Accessing the soil metagenome for studies of microbial diversity. Appl Env. Microbiol. 2011, 77, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Oulas, A.; Pavloudi, C.; Polymenakou, P.; Pavlopoulos, G.A.; Papanikolaou, N.; Kotoulas, G. Metagenomics: Tools and insights for analyzing next-generation sequencing data derived from biodiversity studies. Bioinform. Biol. Insights 2015, 9, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Research Report. Available online: https://www.ncste.kz/assets/report_files/2020/AP05134797-OT-20/en_62208__1044122_1604062853.docx (accessed on 1 February 2023).
- Samofalova, I.A.; Samofalova, M. Chemical Composition of Soils and Soil-Forming Rocks [Text]: Textbook; Russian Federation, FGOU VPO “Perm State Agricultural Academy”; Publishing House of FGOU VPO “Perm State Agricultural Academy”: Perm, Russia, 2009; 132p. [Google Scholar]
- Klimenko, O.; Ivanova, A.; Klimenko, N. Influence of soil alkalinity on the mobility of plant nutrients. Bull. Nikitovsky Bot. Gard. 2007, 95, 46–50. [Google Scholar]
- Hale, B.; Evans, L.; Lambert, R. Effects of cement or lime on Cd, Co, Cu, Ni, Pb, Sb and Zn mobility in field-contaminated and aged soils. J. Hazard. Mater. 2012, 199, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, Z.G.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Cui, F.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, T.A.; Tolpeshta, I.I.; Trofimov, S.Y. Soil acidity. Acid-base buffer capacity of soils. In Aluminum Compounds in the Solid Phase of the Soil and in the Soil Solution, 2nd ed.; Grif & K: Tula, Russia, 2012; 124p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).