Adsorption of Lead from Aqueous Solution Using Activated Carbon Derived from Rice Husk Modified with Lemon Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Rice Husk Derived from Activated Carbon
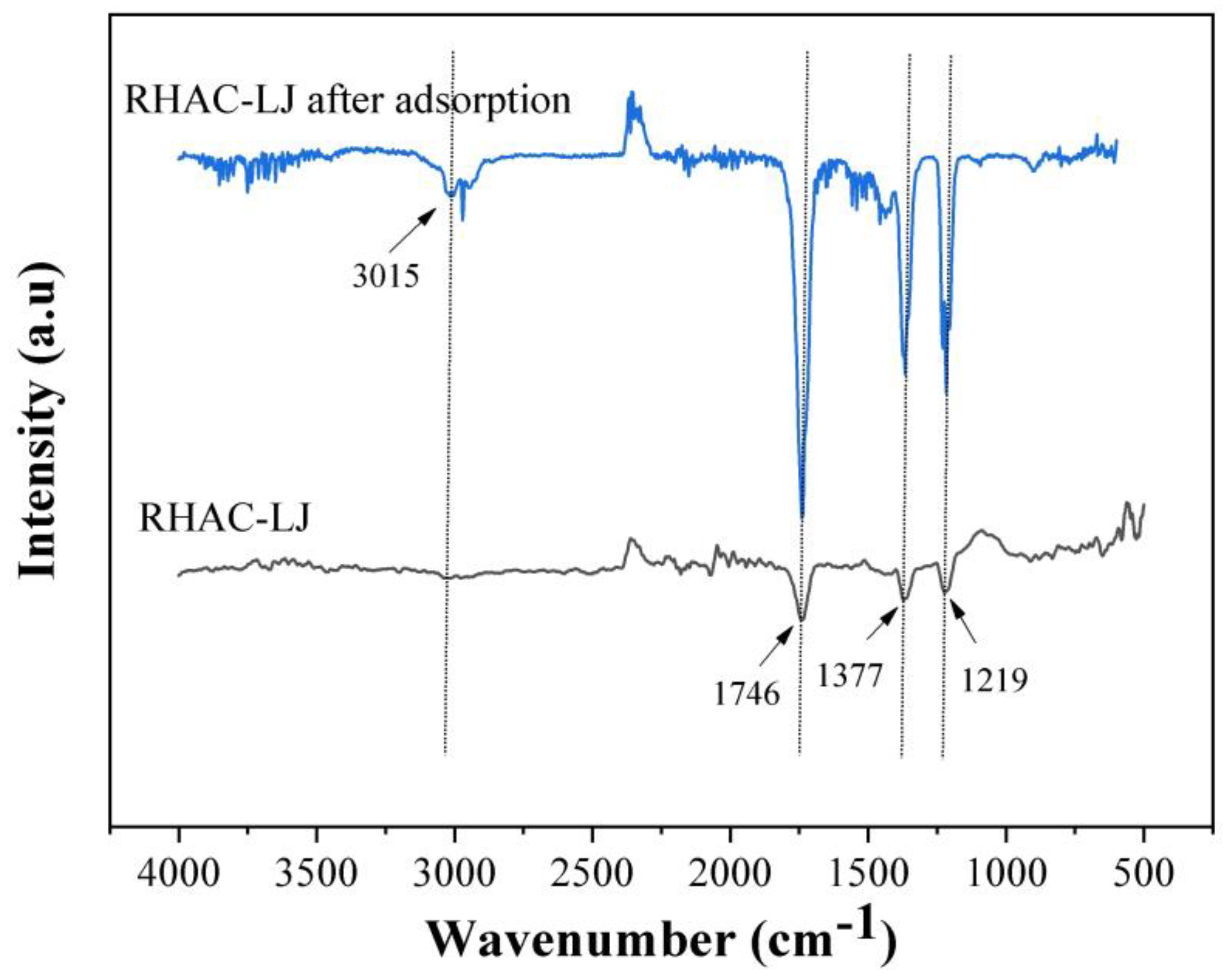
2.3. Characterization Analysis
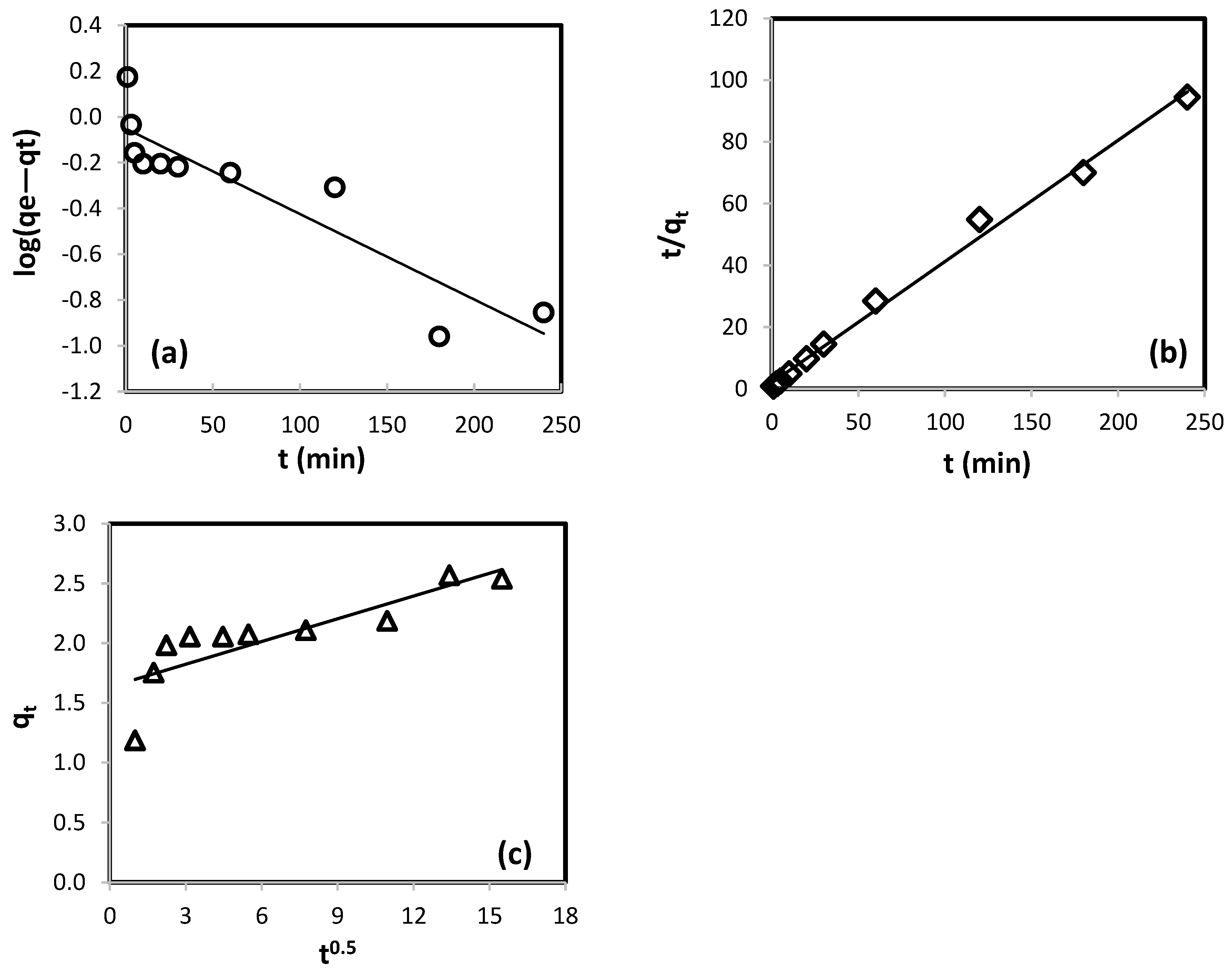
2.4. Kinetic Study
2.5. Experimental Design
3. Results and Discussion
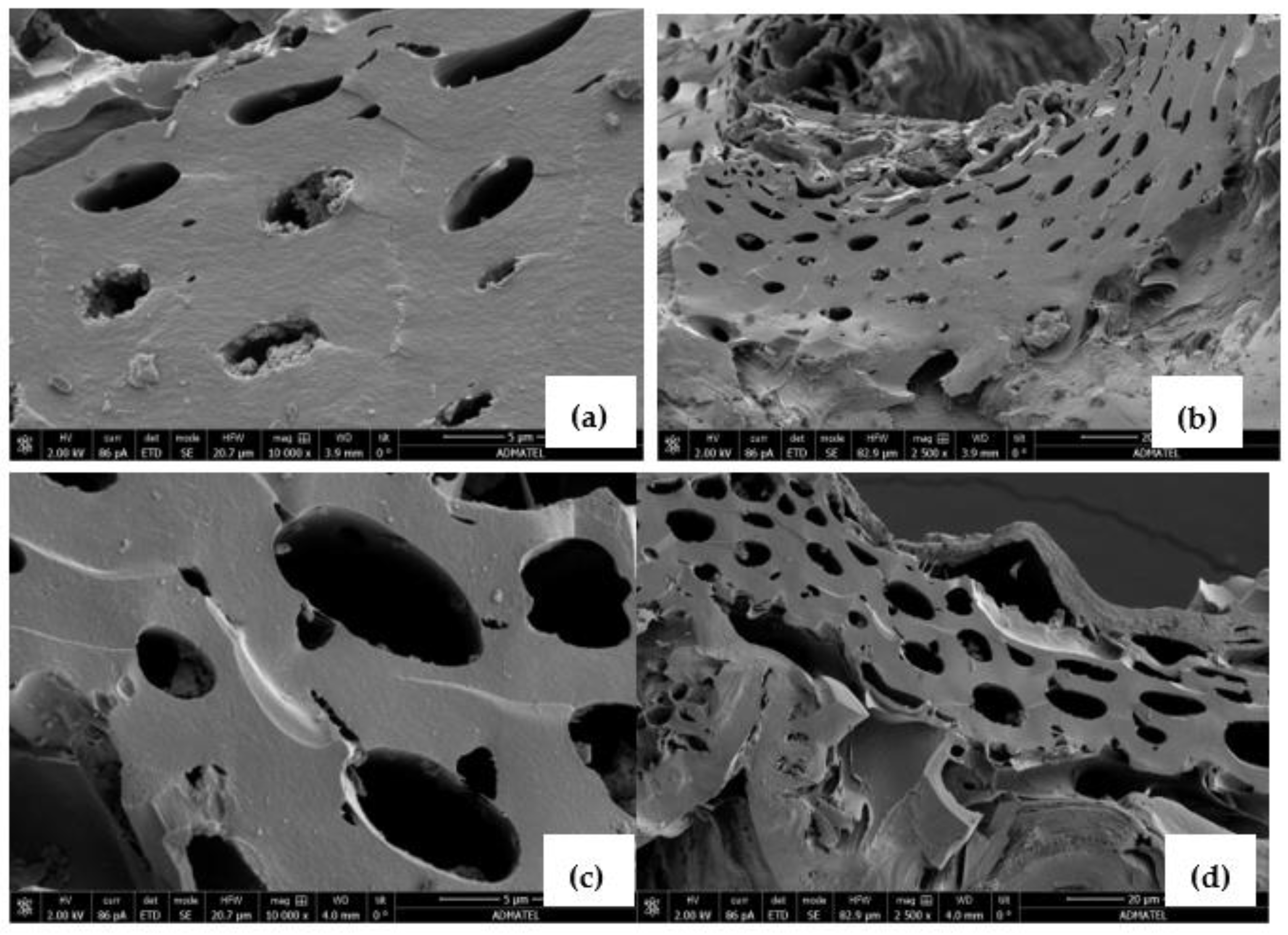
3.1. Characterization of Materials
3.2. Effect of Contact Time
3.3. Kinetic Study
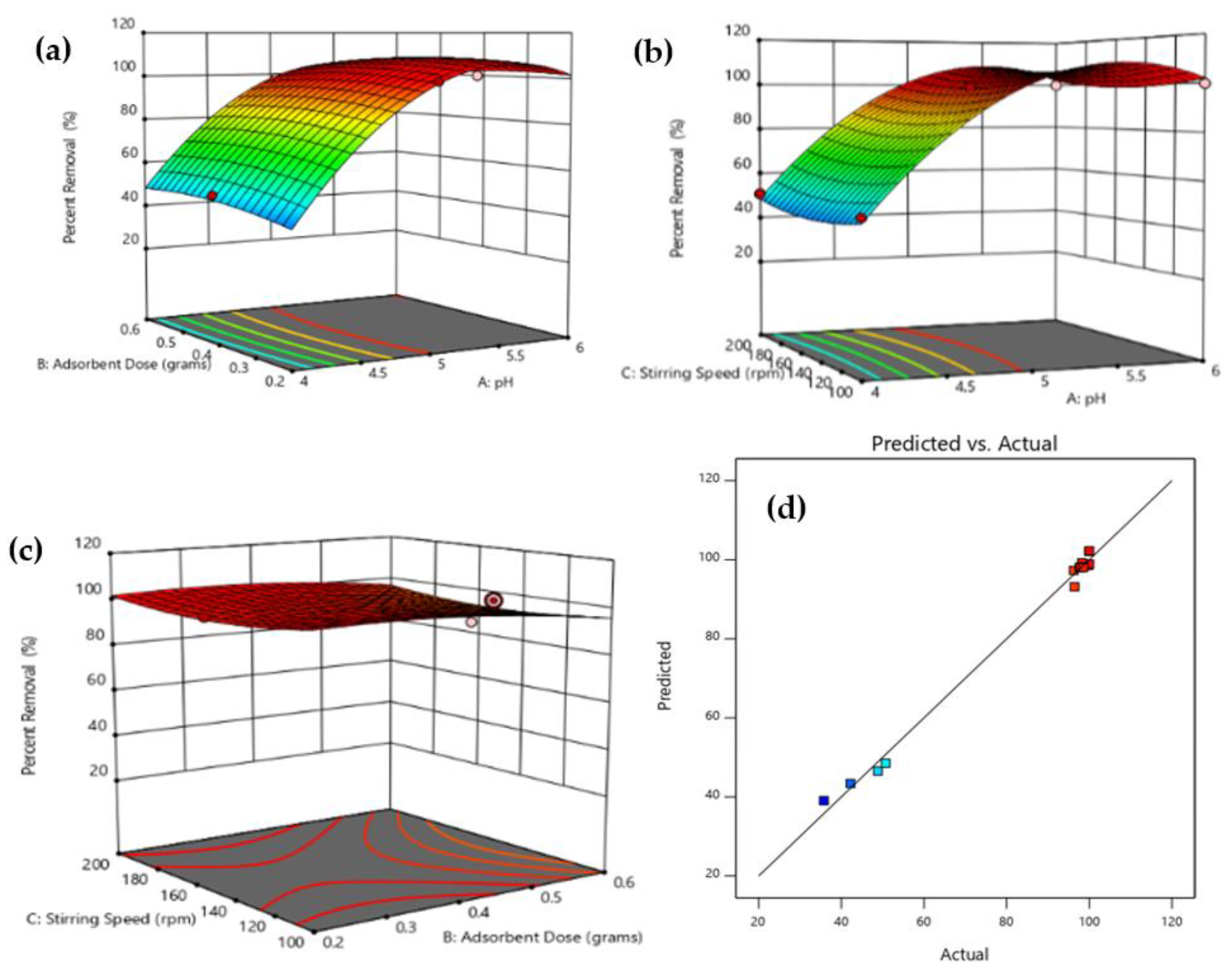
3.4. Optimization Using RSM Modelling
3.4.1. Establishment of the Mathematical Regression Model
3.4.2. Statistical Analysis and Interaction of Variables
3.4.3. Optimization and Validation of the Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azevedo, A.; Oliveira, H.A.; Rubio, J. Treatment and Water Reuse of Lead-Zinc Sulphide Ore Mill Wastewaters by High Rate Dissolved Air Flotation. Miner. Eng. 2018, 127, 114–121. [Google Scholar] [CrossRef]
- Acharya, J.; Sahu, J.N.; Mohanty, C.R.; Meikap, B.C. Removal of Lead(II) from Wastewater by Activated Carbon Developed from Tamarind Wood by Zinc Chloride Activation. Chem. Eng. J. 2009, 149, 249–262. [Google Scholar] [CrossRef]
- Yahya, M.D.; Obayomi, K.S.; Abdulkadir, M.B.; Iyaka, Y.A.; Olugbenga, A.G. Characterization of Cobalt Ferrite-Supported Activated Carbon for Removal of Chromium and Lead Ions from Tannery Wastewater via Adsorption Equilibrium. Water Sci. Eng. 2020, 13, 202–213. [Google Scholar] [CrossRef]
- Priya, A.K.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of Mechanism of Heavy Metals (Cr6+, Pb2+ & Zn2+) Adsorption from Aqueous Medium Using Rice Husk Ash: Kinetic and Thermodynamic Approach. Chemosphere 2022, 286, 131796. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Kumar, V.; Mohan, S.; Bano, D.; Hasan, S.H. Breakthrough Curve Modeling of Graphene Oxide Aerogel Packed Fixed Bed Column for the Removal of Cr(VI) from Water. J. Water Process Eng. 2017, 18, 150–158. [Google Scholar] [CrossRef]
- Badawy, N.M.; Naguib, D.M. Nano Metallothionein for Lead Removal from Battery Industry Waste Water. Biocatal. Agric. Biotechnol. 2021, 38, 102201. [Google Scholar] [CrossRef]
- Kalagbor, I. Adsorption of Cu2+ and Fe2+ from Single Metal Ion Solution Using Unmodified and Formaldehyde Modified Kola-Nut (Cola nitida) Testa. OSR J. Appl. Chem. 2017, 10, 12–18. [Google Scholar]
- Ghosh, A.; Paul, S.; Bhattacharya, S.; Sasikumar, P.; Biswas, K.; Ghosh, U.C. Calcium Ion Incorporated Hydrous Iron(III) Oxide: Synthesis, Characterization, and Property Exploitation towards Water Remediation from Arsenite and Fluoride. Environ. Sci. Pollut. Res. 2019, 26, 4618–4632. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Chowdhury, S.; Omar, A.A.; Siyal, A.A.; Sufian, S. Response Surface Methodology and Artificial Neural Network for Remediation of Acid Orange 7 Using TiO2-P25: Optimization and Modeling Approach. Environ. Sci. Pollut. Res. 2020, 27, 34018–34036. [Google Scholar] [CrossRef]
- Babazad, Z.; Kaveh, F.; Ebadi, M.; Mehrabian, R.Z.; Juibari, M.H. Efficient Removal of Lead and Arsenic Using Macromolecule-Carbonized Rice Husks. Heliyon 2021, 7, e06631. [Google Scholar] [CrossRef]
- Bowden, G.D.; Pichler, B.J.; Maurer, A. A Design of Experiments (DoE) Approach Accelerates the Optimization of Copper-Mediated 18F-Fluorination Reactions of Arylstannanes. Sci. Rep. 2019, 9, 11370. [Google Scholar] [CrossRef] [PubMed]
- Guezzen, B.; Medjahed, B.; Benhelima, A.; Guendouzi, A.; Didi, M.A.; Zidelmal, S.; Abdelkrim Boudia, R.; Adjdir, M. Improved Pollutant Management by Kinetic and Box-Behnken Design Analysis of HDTMA-Modified Bentonite’s Adsorption of Indigo Carmine Dye. J. Ind. Eng. Chem. 2023, 125, 242–258. [Google Scholar] [CrossRef]
- Hong, S.; Cannon, F.S.; Hou, P.; Byrne, T.; Nieto-Delgado, C. Adsorptive Removal of Sulfate from Acid Mine Drainage by Polypyrrole Modified Activated Carbons: Effects of Polypyrrole Deposition Protocols and Activated Carbon Source. Chemosphere 2017, 184, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Arami, S.M.; Takallo, H.; Hosseini, M.; Radfard, M.; Soleimani, H.; Mohammadi, A.A. Modification of Pumice with HCl and NaOH Enhancing Its Fluoride Adsorption Capacity: Kinetic and Isotherm Studies. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1508–1520. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, S.; Belabbas, M.-A. Mid-Air Motion Planning of Floating Robot Using Heat Flow Method. IFAC-PapersOnLine 2019, 52, 19–24. [Google Scholar] [CrossRef]
- Le, A.H.Q.; Hoang, H.Y.; Le Van, T.; Hoang Nguyen, T.; Uyen Dao, M. Adsorptive Removal of Benzene and Toluene from Aqueous Solutions by Oxygen-Functionalized Multi-Walled Carbon Nanotubes Derived from Rice Husk Waste: A Comparative Study. Chemosphere 2023, 336, 139265. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low Cost Adsorbents for the Removal of Organic Pollutants from Wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Adeyanju, C.A.; Iwuozor, K.O.; Odeyemi, S.O.; Emenike, E.C.; Ogunniyi, S.; Te-Erebe, D.K. Retort Carbonization of Bamboo (Bambusa vulgaris) Waste for Thermal Energy Recovery. Clean Technol. Environ. Policy 2023, 25, 937–947. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Iwuchukwu, F.U.; Eyankware, O.E.; Iwuozor, K.O.; Olotu, K.; Bright, O.C.; Igwegbe, C.A. Flash Pyrolysis of Biomass: A Review of Recent Advances. Clean Technol. Environ. Policy 2022, 24, 2349–2363. [Google Scholar] [CrossRef]
- Kaushik, A.; Basu, S.; Singh, K.; Batra, V.S.; Balakrishnan, M. Activated Carbon from Sugarcane Bagasse Ash for Melanoidins Recovery. J. Environ. Manag. 2017, 200, 29–34. [Google Scholar] [CrossRef]
- Iriarte-Velasco, U.; Alvarez-Uriarte, J.; Chimeno-Alanis, N.; González-Velasco, J. Natural Organic Matter Adsorption onto Granular Activated Carbons: Implications in the Molecular Weight and Disinfection Byproducts Formation. Ind. Eng. Chem. Res. 2008, 47, 7868–7876. [Google Scholar] [CrossRef]
- Hashemian, S.; Salari, K.; Yazdi, Z.A. Preparation of Activated Carbon from Agricultural Wastes (Almond Shell and Orange Peel) for Adsorption of 2-Pic from Aqueous Solution. J. Ind. Eng. Chem. 2014, 20, 1892–1900. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Flores, E.D.; Genuino, D.A.D.; Futalan, C.M.; Wan, M.-W. Adsorption of Eriochrome Black T (EBT) Dye Using Activated Carbon Prepared from Waste Rice Hulls—Optimization, Isotherm and Kinetic Studies. J. Taiwan Inst. Chem. Eng. 2013, 44, 646–653. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Utilization of Rice Husks as a Feedstock for Preparation of Activated Carbon by Microwave Induced KOH and K2CO3 Activation. Bioresour. Technol. 2011, 102, 9814–9817. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, Y.; Wang, Z.; Li, Y.; Wang, L.; Ding, L.; Gao, X.; Ma, Y.; Guo, Y. Application Studies of Activated Carbon Derived from Rice Husks Produced by Chemical-Thermal Process—A Review. Adv. Colloid Interface Sci. 2011, 163, 39–52. [Google Scholar] [CrossRef]
- Shao, S.; Ma, L.; Li, X.; Zhang, H.; Xiao, R. Preparation of Activated Carbon with Heavy Fraction of Bio-Oil from Rape Straw Pyrolysis as Carbon Source and Its Performance in the Aldol Condensation for Aviation Fuel as Carrier. Ind. Crops Prod. 2023, 192, 115912. [Google Scholar] [CrossRef]
- Björklund, K.; Li, L.Y. Adsorption of Organic Stormwater Pollutants onto Activated Carbon from Sewage Sludge. J. Environ. Manag. 2017, 197, 490–497. [Google Scholar] [CrossRef]
- Lim, J.S.; Abdul Manan, Z.; Wan Alwi, S.R.; Hashim, H. A Review on Utilisation of Biomass from Rice Industry as a Source of Renewable Energy. Renew. Sustain. Energy Rev. 2012, 16, 3084–3094. [Google Scholar] [CrossRef]
- Menya, E.; Olupot, P.W.; Storz, H.; Lubwama, M.; Kiros, Y. Production and Performance of Activated Carbon from Rice Husks for Removal of Natural Organic Matter from Water: A Review. Chem. Eng. Res. Des. 2018, 129, 271–296. [Google Scholar] [CrossRef]
- Chang, S.H. Rice Husk and Its Pretreatments for Bio-Oil Production via Fast Pyrolysis: A Review. BioEnergy Res. 2020, 13, 23–42. [Google Scholar] [CrossRef]
- Go, A.W.; Conag, A.T.; Igdon, R.M.B.; Toledo, A.S.; Malila, J.S. Potentials of Agricultural and Agro-Industrial Crop Residues for the Displacement of Fossil Fuels: A Philippine Context. Energy Strategy Rev. 2019, 23, 100–113. [Google Scholar] [CrossRef]
- Dadebo, D.; Ibrahim, M.G.; Fujii, M.; Nasr, M. Sequential Treatment of Surfactant-Laden Wastewater Using Low-Cost Rice Husk Ash Coagulant and Activated Carbon: Modeling, Optimization, Characterization, and Techno-Economic Analysis. Bioresour. Technol. Rep. 2023, 22, 101464. [Google Scholar] [CrossRef]
- Wang, L.-H.; Lin, C.-I. Adsorption of Lead(II) Ion from Aqueous Solution Using Rice Hull Ash. Ind. Eng. Chem. Res. 2008, 47, 4891–4897. [Google Scholar] [CrossRef]
- Naiya, T.K.; Bhattacharya, A.K.; Mandal, S.; Das, S.K. The Sorption of Lead(II) Ions on Rice Husk Ash. J. Hazard. Mater. 2009, 163, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L. Comparison of Rice Husk- and Dairy Manure-Derived Biochars for Simultaneously Removing Heavy Metals from Aqueous Solutions: Role of Mineral Components in Biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Shi, J.; Fan, X.; Tsang, D.C.W.; Wang, F.; Shen, Z.; Hou, D.; Alessi, D.S. Removal of Lead by Rice Husk Biochars Produced at Different Temperatures and Implications for Their Environmental Utilizations. Chemosphere 2019, 235, 825–831. [Google Scholar] [CrossRef]
- Deiana, C.; Granados, D.; Venturini, R.; Amaya, A.; Sergio, M.; Tancredi, N. Activated Carbons Obtained from Rice Husk: Influence of Leaching on Textural Parameters. Ind. Eng. Chem. Res. 2008, 47, 4754–4757. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in Porous and Nanoscale Catalysts for Viable Biomass Conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef]
- Muniandy, L.; Adam, F.; Mohamed, A.R.; Ng, E.-P. The Synthesis and Characterization of High Purity Mixed Microporous/Mesoporous Activated Carbon from Rice Husk Using Chemical Activation with NaOH and KOH. Microporous Mesoporous Mater. 2014, 197, 316–323. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Xu, X.; Meng, X.; Qu, J.; Wang, Z.; Liu, C.; Qu, B. Preparation, Characterization and Application of Activated Carbon from Corn Cob by KOH Activation for Removal of Hg(II) from Aqueous Solution. Bioresour. Technol. 2020, 306, 123154. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, H.; Dai, P.; Shen, X.; Zhang, J.; Zhao, T.; Zhu, Z. Microwave-Assisted Preparation and Characterization of Mesoporous Activated Carbon from Mushroom Roots by Phytic Acid (C6H18O24P6) Activation. J. Taiwan Inst. Chem. Eng. 2016, 67, 532–537. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Gao, W.; Wang, Z.; Ma, Y.; Wang, Z. Simultaneous Preparation of Silica and Activated Carbon from Rice Husk Ash. J. Clean. Prod. 2012, 32, 204–209. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, J.; Zhang, H.; Yang, S.; Qi, J.; Wang, Z.; Xu, H. Use of Rice Husk-Based Porous Carbon for Adsorption of Rhodamine B from Aqueous Solutions. Dye. Pigment. 2005, 66, 123–128. [Google Scholar] [CrossRef]
- Youssef, A.M.; Ahmed, A.I.; El-Bana, U.A. Adsorption of Cationic Dye (MB) and Anionic Dye (AG 25) by Physically and Chemically Activated Carbons Developed from Rice Husk. Carbon Lett. 2012, 13, 61–72. [Google Scholar] [CrossRef]
- Somasundaram, S.; Sekar, K.; Gupta, V.K.; Ganesan, S. Synthesis and Characterization of Mesoporous Activated Carbon from Rice Husk for Adsorption of Glycine from Alcohol-Aqueous Mixture. J. Mol. Liq. 2013, 177, 416–425. [Google Scholar] [CrossRef]
- Ali, I.H.; Al Mesfer, M.K.; Khan, M.I.; Danish, M.; Alghamdi, M.M. Exploring Adsorption Process of Lead (II) and Chromium (VI) Ions from Aqueous Solutions on Acid Activated Carbon Prepared from Juniperus Procera Leaves. Processes 2019, 7, 217. [Google Scholar] [CrossRef]
- Rashid, U.S.; Bezbaruah, A.N. Citric Acid Modified Granular Activated Carbon for Enhanced Defluoridation. Chemosphere 2020, 252, 126639. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Liu, S.; Tan, X.; Zeng, G.; Zeng, W.; Ding, Y.; Cao, W.; Zheng, B. Enhanced Adsorption of Methylene Blue by Citric Acid Modification of Biochar Derived from Water Hyacinth (Eichornia crassipes). Environ. Sci. Pollut. Res. 2016, 23, 23606–23618. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, X.; Zhang, R.; Lu, J. Removal of Aniline from Aqueous Solution Using Pine Sawdust Modified with Citric Acid and β-Cyclodextrin. Ind. Eng. Chem. Res. 2014, 53, 887–894. [Google Scholar] [CrossRef]
- Isoda, N.; Rodrigues, R.; Silva, A.; Gonçalves, M.; Mandelli, D.; Figueiredo, F.C.A.; Carvalho, W.A. Optimization of Preparation Conditions of Activated Carbon from Agriculture Waste Utilizing Factorial Design. Powder Technol. 2014, 256, 175–181. [Google Scholar] [CrossRef]
- Kalderis, D.; Bethanis, S.; Paraskeva, P.; Diamadopoulos, E. Production of Activated Carbon from Bagasse and Rice Husk by a Single-Stage Chemical Activation Method at Low Retention Times. Bioresour. Technol. 2008, 99, 6809–6816. [Google Scholar] [CrossRef] [PubMed]
- Liou, T.-H. Development of Mesoporous Structure and High Adsorption Capacity of Biomass-Based Activated Carbon by Phosphoric Acid and Zinc Chloride Activation. Chem. Eng. J. 2010, 158, 129–142. [Google Scholar] [CrossRef]
- Yapo, B.M. Lemon Juice Improves the Extractability and Quality Characteristics of Pectin from Yellow Passion Fruit By-Product as Compared with Commercial Citric Acid Extractant. Bioresour. Technol. 2009, 100, 3147–3151. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Feng, J.; Dai, Y.; Chang, S. Mechanisms of Strontium’s Adsorption by Saccharomyces cerevisiae: Contribution of Surface and Intracellular Uptakes. Chemosphere 2019, 215, 15–24. [Google Scholar] [CrossRef]
- Rady, M.M.; Alharby, H.F.; Desoky, E.-S.M.; Maray, A.R.M.; Mohamed, I.A.A.; Howladar, S.M.; Elmohsen, Y.H.A.; Faraz, A.; Ali, S.; AbdelKhalik, A. Citrate-Containing Lemon Juice, as an Organic Substitute for Chemical Citric Acid, Proactively Improves Photosynthesis, Antioxidant Capacity, and Enzyme Gene Expression in Cadmium-Exposed Phaseolus Vulgaris. S. Afr. J. Bot. 2023, 160, 88–101. [Google Scholar] [CrossRef]
- Deshmukh, M.B.; Patil, S.S.; Jadhav, S.D.; Pawar, P.B. Green Approach for Knoevenagel Condensation of Aromatic Aldehydes with Active Methylene Group. Synth. Commun. 2012, 42, 1177–1183. [Google Scholar] [CrossRef]
- Chen, J.P.; Wu, S.; Chong, K.-H. Surface Modification of a Granular Activated Carbon by Citric Acid for Enhancement of Copper Adsorption. Carbon 2003, 41, 1979–1986. [Google Scholar] [CrossRef]
- Hussain, O.A.; Hathout, A.S.; Abdel-Mobdy, Y.E.; Rashed, M.M.; Abdel Rahim, E.A.; Fouzy, A.S.M. Preparation and Characterization of Activated Carbon from Agricultural Wastes and Their Ability to Remove Chlorpyrifos from Water. Toxicol. Rep. 2023, 10, 146–154. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of Pyrolysis Temperature on Soybean Stover- and Peanut Shell-Derived Biochar Properties and TCE Adsorption in Water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Théorie Der Sogenannten Adsorption Gelöster Stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Teymur, Y.A.; Güzel, F. Ultra-Efficient Removal of Methylene Blue, Oxytetracycline, and Lead(II) by Activated Carbon Derived from Black Cumin (Nigella sativa) Processing Solid Waste. J. Water Process Eng. 2023, 54, 103940. [Google Scholar] [CrossRef]
- Boudrahem, F.; Aissani-Benissad, F.; Aït-Amar, H. Batch Sorption Dynamics and Equilibrium for the Removal of Lead Ions from Aqueous Phase Using Activated Carbon Developed from Coffee Residue Activated with Zinc Chloride. J. Environ. Manag. 2009, 90, 3031–3039. [Google Scholar] [CrossRef]

| Operating Factor | Unit | Range and Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| pH | 4 | 5 | 6 | |
| Adsorbent dosage | g | 0.2 | 0.4 | 0.6 |
| Stirring speed | rpm | 100 | 150 | 200 |
| Kinetic Model | Parameters | |
|---|---|---|
| Pseudo-first order | qe,theoretical (mg/g) | 0.9499 |
| k1 (min−1) | 0.000852 | |
| R2 | 0.8292 | |
| Pseudo-second order | qe,theoretical (mg/g) | 2.5439 |
| k2 (g/mg·min) | 3.3697 | |
| R2 | 0.9941 | |
| Intraparticle diffusion | C | 1.6330 |
| kid (g/mg·min0.5) | 0.0635 | |
| R2 | 0.6980 |
| Adsorbent | Activating Agent | Surface Area (m2/g) | Pore Diameter (nm) | Kinetic Rate Constant (g/mg·min) | Reference |
|---|---|---|---|---|---|
| Rice husk ash | Not applicable | 57.50 | not available | 0.267 | [34] |
| Activated carbon from black cumin waste | K2CO3 | 2211 | 2.80 | 0.00151 | [63] |
| Activated carbon from coffee residue | ZnCl2 | 890 | not available | 0.123 | [64] |
| Biochar from rice husk | Not applicable | 27.8 | not available | not available | [35] |
| Rice husk biochar | Not applicable | 193.15 | 6.80 | 0.0179 | [36] |
| RHAC-LJ | Lemon juice | 112.87 | 3.95 | 3.369 | Present work |
| Run | Independent Variables | |||
|---|---|---|---|---|
| pH | Adsorbent Dosage (g) | Stirring Speed (rpm) | Removal Efficiency (%) | |
| 1 | 5 | 0.2 | 200 | 99.99 |
| 2 | 5 | 0.4 | 150 | 98.32 |
| 3 | 5 | 0.6 | 200 | 98.22 |
| 4 | 5 | 0.6 | 100 | 96.24 |
| 5 | 4 | 0.4 | 200 | 50.73 |
| 6 | 6 | 0.4 | 100 | 99.93 |
| 7 | 5 | 0.2 | 100 | 99.74 |
| 8 | 4 | 0.2 | 150 | 35.78 |
| 9 | 6 | 0.6 | 150 | 96.43 |
| 10 | 6 | 0.2 | 150 | 99.83 |
| 11 | 4 | 0.6 | 150 | 42.21 |
| 12 | 5 | 0.4 | 150 | 97.66 |
| 13 | 4 | 0.4 | 100 | 48.83 |
| 14 | 5 | 0.4 | 150 | 97.75 |
| 15 | 6 | 0.4 | 200 | 99.99 |
| 16 | 5 | 0.4 | 150 | 97.94 |
| 17 | 5 | 0.4 | 150 | 98.60 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9070.63 | 9 | 1007.85 | 142.97 | <0.0001 |
| A | 5974.88 | 1 | 5974.88 | 847.57 | <0.0001 |
| B | 0.6272 | 1 | 0.6272 | 0.0890 | 0.7741 |
| C | 2.19 | 1 | 2.19 | 0.3113 | 0.5943 |
| AB | 24.16 | 1 | 24.16 | 3.43 | 0.1066 |
| AC | 0.8464 | 1 | 0.8464 | 0.1201 | 0.7391 |
| BC | 0.7482 | 1 | 0.7482 | 0.1061 | 0.7541 |
| A2 | 2975.73 | 1 | 2975.73 | 422.12 | <0.0001 |
| B2 | 35.58 | 1 | 35.58 | 5.05 | 0.0595 |
| C2 | 48.69 | 1 | 48.69 | 6.91 | 0.0340 |
| Residual | 49.35 | 7 | 7.05 | ||
| Lack of Fit | 48.72 | 3 | 16.24 | 103.18 | 0.0003 |
| Pure Error | 0.6295 | 4 | 0.1574 | ||
| Cor Total | 9119.98 | 16 |
| Removal Efficiency (%) | ||
|---|---|---|
| Predicted | 98.49% | |
| Observed | Run 1 | 97.05% |
| Run 2 | 96.32% | |
| Run 3 | 97.17% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Futalan, C.C.; Diana, E.; Edang, M.F.A.; Padilla, J.M.; Cenia, M.C.; Alfeche, D.M. Adsorption of Lead from Aqueous Solution Using Activated Carbon Derived from Rice Husk Modified with Lemon Juice. Sustainability 2023, 15, 15955. https://doi.org/10.3390/su152215955
Futalan CC, Diana E, Edang MFA, Padilla JM, Cenia MC, Alfeche DM. Adsorption of Lead from Aqueous Solution Using Activated Carbon Derived from Rice Husk Modified with Lemon Juice. Sustainability. 2023; 15(22):15955. https://doi.org/10.3390/su152215955
Chicago/Turabian StyleFutalan, Cybelle Concepcion, Emmanuel Diana, Ma. Florita Andrea Edang, Jelly May Padilla, Marie Chela Cenia, and Dale Mhar Alfeche. 2023. "Adsorption of Lead from Aqueous Solution Using Activated Carbon Derived from Rice Husk Modified with Lemon Juice" Sustainability 15, no. 22: 15955. https://doi.org/10.3390/su152215955
APA StyleFutalan, C. C., Diana, E., Edang, M. F. A., Padilla, J. M., Cenia, M. C., & Alfeche, D. M. (2023). Adsorption of Lead from Aqueous Solution Using Activated Carbon Derived from Rice Husk Modified with Lemon Juice. Sustainability, 15(22), 15955. https://doi.org/10.3390/su152215955







