Abstract
The plating industry is a high-pollutant sector because it consumes a significant amount of chemical compounds and produces a large volume of hazardous waste via the disposal of spent plating baths. Thus, the development of regeneration/purification routes to extend the lifetime of the plating baths may be a good opportunity to reduce both the environmental impact and the production costs of the plating industry. In this context, an innovative and in situ purification process, that uses magnetic nanoparticles (MNPs) to capture and remove contaminants and undesirable chemicals from the plating baths, extending their lifetime, was studied within the scope of the PureNano project. To support the process that has been developed, this work aimed to assess the sustainability of this recovery route and highlight its potential benefits. A comparative analysis was conducted between this novel route and conventional alternatives (i.e., underground disposal and incineration). To do so, the life cycle assessment (LCA) and life cycle costing (LCC) methodologies were used to evaluate the environmental impact and production costs, and an ecoefficiency analysis was performed to understand the trade-offs of each scenario. The results showed that MNPs were the main hotspot for the environmental impact and production costs. Overall, the purified plating baths may lead to lower environmental impacts (−98%) and processing costs (up to −95%) than other conventional alternatives. Regarding the ecoefficiency analysis, Scenario A (recovery route without MNPs recycling) and A-R (recovery route with MNPs valorisation) have a better economic/environmental impact relation than the conventional scenarios, i.e., incineration, and deposition in a landfill. However, Scenario A was the most ecoefficient scenario. In addition to this, further research is needed, namely, to search for other materials that may replace the most expensive and burdensome ones, and to investigate the use of renewable energy sources in MNPs production to improve their environmental and economic performances.
1. Introduction
Metal surface-finishing, often referred to as plating, is a key process in various industrial sectors such as automotive, aerospace, printing, consumer goods, and jewellery [1]. Plating involves the production of a metal coating, i.e., a thin metal layer, on the surface of a metallic or even plastic item or component. The application of a metal coating can prolong the lifetime of the plated substrate (e.g., by protecting it against corrosion and mechanical wear), improve its functionality (e.g., by reducing friction or increasing conductivity), or provide an aesthetically pleasing appearance [2,3,4,5,6,7]. The plating process requires the use of large-volume solutions, termed plating baths, in which various organic compounds and metal ions are dissolved in concentration as high as 300 g/L. Therefore, the plating industry has a significant environmental impact due to the consumption of large amounts of chemical compounds and the generation of substantial quantities of metal-containing wastewater [8]. Specifically, the plating industry produces approximately 300,000 tons of hazardous waste per year, which translates into an average of 16 tons per facility in the European Union (EU). This presents an important health and safety concern [9].
The composition of a plating bath is optimized for continuous operation over weeks or even months. However, this leads to the gradual accumulation of certain chemical species that hinder the plating process when their concentration surpasses a specific threshold. At this point, the efficiency of the plating process declines rapidly, and the quality of the final coatings is below par. As a result, the plating bath cannot be used anymore, and it is characterized as “spent”. The spent baths must be replaced with new ones and transferred to recycling centres for their appropriate treatment and disposal. In addition, the plating industry must address the problem of replacing traditional and intensely applied hard chromium (HC) metal coatings, because their production requires the use of highly toxic and carcinogenic Cr(VI) solutions which are now being restricted by Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH) regulations. Nickel-phosphorous (Ni-P) alloy coatings are promising alternatives to HC due to their good mechanical and tribological properties, which improve the wear-resistance of the plated objects. Currently, Ni-P alloy coatings are applied (i) as protective and decorative coatings in the automotive and aerospace industry, (ii) as catalytic coatings in water electrolysis to produce hydrogen, and (iii) as thin film coatings in microelectronics and sensors [10,11].
Notably, the desired properties of the Ni-P coatings can be enhanced via the optimisation of the relative phosphorous content, via the thermal post-treatment of the Ni-P coatings, or via the incorporation of nanoparticles in the Ni-P matrix. For example, the incorporation of “hard” silicon carbide nanoparticles (SiC NPs) in the Ni-P matrix as a means of reinforcement leads to Ni-P/SiC nanocomposite coatings showing a similar microhardness compared with HC [12,13].
A typical composition of a Ni-P/SiC plating bath contains dissolved nickel salts that provide Ni(II) cations to the cathode where they are reduced to their metallic Ni(0) state via the application of electric current and deposited on the substrate to be coated. Simultaneously, a pure nickel metal anode is oxidized to form Ni(II) ions to maintain a steady concentration of Ni(II) ions in the bath. However, these two reactions do not take place with the same efficiency, mainly due to the reduction of acidic protons to molecular hydrogen that takes place in parallel at the cathode. As a result, Ni(II) cations accumulate in the bath, which in turn disturbs the bath’s chemical and electrostatic fine balance. Once the concentration of Ni(II) cations surpasses a specific threshold, the outcome of the process does not meet the targeted specifications [11]. At this point, the bath is spent; it cannot be used anymore and must be replaced. This in turn means that large volumes of acidic solutions containing a high concentration of nickel and phosphorous species must be disposed of according to regulations. Considering these issues, actions should be taken to increase the sustainability of this high-polluting industry.
Intending to reduce global pollution from manufacturing industries, and, consequently, improving their environmental performance, the European Commission (EC) developed several reference documents that established different Best Available Techniques (BAT) to be implemented in different sectors [14]. In particular, in 2006, the EC made available a reference document on BAT for the “Surface Treatment of Metals and Plastics” [15]. In this report, the surface treatment of metals and plastics using different electrolytic or chemical processes were studied. About two hundred techniques were pointed out, and some of them relied on thematic areas such as drag-out reduction and control, electrode techniques, and process metals recovery. Furthermore, other materials may also be recovered (e.g., some acids) when applying other methods. This recovering approach is also supported by the pollution-prevention view which argues that metals existing in the spent baths must be recovered/recycled, and the pollutants extracted to increase the baths’ life cycle [16]. In this context, some efforts are being made, namely, to extract common metals from plating baths’ wastewater. For example, Wu et al. [17] studied the possibility of extracting Ni and Cu compounds with ethylenediamine tetra-acetic acid (EDTA) by electrolysis. Some studies investigated the potential of recovering Ni through the electrolytic processes, as in the case of Idhayachander and Palanivelu [18], who developed an electrolytic procedure to recover Ni from an electroless Ni solution. Moreover, Benvenuti et al. [19] aimed to find out the operational parameters that are necessary to scale up the electrodialysis process and to close the loop in the Ni electroplating industry. Also, other techniques for Ni recovery were found in the literature. For instance, Tanaka et al. [20] developed a solvent-extraction processing route that considered the recovery of Ni from an electroless spent bath along with its continuous extraction and stripping.
Indeed, wastewater treatment is the most investigated technique when searching for more environmentally friendly processing practices within the electroplating industry [21]. However, Laforest et al. [22] developed a multi-criteria decision tool to support industries from the metal-finishing sector in choosing the BAT and helping them to adopt cleaner and safer procedures in their companies. Afterwards, the authors applied the framework in a metal-finishing company to validate it and draw some conclusions. This Small and Midsize Enterprise (SME) aimed to remove metals to respect the legislation and reuse the treated effluent. The results showed that ion exchange resins were more advantageous than the evaporation alternative. Nevertheless, the latter was not comparable with reverse osmosis. Although the framework allowed the most appropriate practices to be chosen, Laforest et al. [22] stated that results should be complemented with a technical feasibility study.
While there have been efforts to reduce the environmental impact of the metal-finishing industry, further research is still essential to evaluate the effects of alternative practices and potential trade-offs. The study of different techniques from the sustainability point of view is of paramount importance to obtain feedback on potential sustainability hotspots and, thereby, support decisions, determining the most suitable course of action and the best practices to be pursued in the sector. In this context, Takuma et al. [8] assessed the environmental impacts of two different electroplating baths: (a) a conventional Ni bath (Watts bath); and (b) a citric acid-based bath to avoid boron emissions from the Watts baths. The functional unit (FU) considered was “the plating per 1 kg part”, and the following steps were studied: pre-treatment, plating, bath recovering, and the washing process to eliminate chemical contaminants. Despite the potential benefits of the Ni citrate bath, results showed that the overall environmental performance of the Watts bath was better than the former. To decrease the environmental burdens of both baths, the authors also evaluated a different wastewater treatment which made use of flocculants. Considering this scenario, a meaningful reduction of the plating effluent impacts was observed, but the overall result was not significantly affected. This study proved to be an important first step in assessing the potential environmental impacts of electroplating baths. Nevertheless, there were some limitations, namely: (i) the use of a life cycle impact-assessment method that mostly represents the Japanese reality; (ii) the utilisation of theoretical data for the raw materials based on stoichiometric relations; and (iii) the disregard of energy consumption and losses during feedstock production. Therefore, comparisons with this case study are difficult to make, and further research is needed in this field.
Even though some studies have explored the potential of new electroplating baths, such as citrate baths, to avoid harmful emissions of boric acid [8], a significant research gap can be identified: there is still limited knowledge, and a scarcity of studies focusing on assessing the environmental impacts of electroplating baths.
As aforementioned, the development of methods that (by removing the surplus of Ni species) regenerate the “spent” Ni-based baths may reduce both the production costs and the environmental impact of the surface-finishing industry and increase the bath’s lifetime. Consequently, these strategies may avoid hazardous emissions being emitted into the environment and reduce the use of raw materials to produce those products. In this context, a novel and in situ purification/regeneration process that removes contaminants and undesirable chemicals from the plating bath, allowing it to be reused, was developed in the framework of the PureNano project [23] to support waste reduction and process improvement in the plating industry. This recovery route makes use of functionalised magnetic nanoparticles (MNPs) to capture the contaminants of nickel-based plating baths. Overall, as the process allows for an extension of the bath’s lifetime, it is expected to yield environmental and economic benefits. However, as it constitutes an innovative process, literature documenting the associated environmental data is not available, and since studies assessing the sustainability of spent baths are scarce, new knowledge is required to support decisions and assess the sustainability of the spent bath valorisation strategies developed.
In general, this work aimed to assess the sustainability of a novel recovery route that extends the lifetime of a nickel-based plating bath using magnetic nanoparticles (MNPs). To do so, a comparative analysis between this valorisation route and conventional scenarios (i.e., underground disposal and incineration) was performed to evaluate the environmental impacts and economic performance of the different alternatives using LCA and LCC methodologies. Finally, an ecoefficiency analysis was carried out to understand the potential trade-offs of both environmental and economic performance.
2. Materials and Methods
To reach the ultimate goal of this research work, the system under study, as well as the frameworks and the main assumptions, are pointed out in this section. Overall, the plating bath and its recovery route are characterised in Section 2.1 and Section 2.2. The LCA, LCC, and ecoefficiency analysis are described in Section 2.3, Section 2.4 and Section 2.5, accordingly.
2.1. Plating Bath
There are three major types of Ni-P baths, categorised based on the phosphorous (P) content, namely low P (1–5% w/w), medium P (6–9% w/w), and high P (10–13% w/w). The different P content can improve a specific property of the resulting coating, for example, a higher P content bath can produce Ni-P coatings with enhanced resistance to corrosion but reduced resistance to wear. Moreover, the degree of crystallinity of the Ni metal matrix is reduced with increasing P content. As a result, Ni-P coatings with a low P content are highly crystalline, whereas Ni-P coatings with a high P content can be completely amorphous. Herein, a medium-P bath was used which resulted in Ni-P coatings showing micro or nano-crystalline structures with enhanced mechanical properties [10].
For the sake of comparison, two types of medium-P baths were formulated, with and without SiC NPs. Both baths contained a high concentration of nickel salts, namely nickel sulphate (NiSO4) and nickel chloride (NiCl2). NiSO4 serves as the main source of Ni(II) cations which are reduced to their metallic Ni(0) state during electrodeposition. Nickel chloride is coadded in the bath to promote the oxidation of the sacrificial Ni metal anode and increase ion conductivity, which in turn lowers the required voltage. The Ni-P baths also contain dissolved phosphonic (H3PO3) and phosphoric (H3PO4) acids serving as pH buffering agents and as the phosphorous source. In addition, the electroplating baths have a small amount of an organic additive that acts as a brightener for the final coating, namely saccharin (≤2 g L−1). In the formulation of the Ni-P/SiC bath, SiC NPs and sodium dodecyl sulphate (SDS) were also added. Being very robust, the SiC NPs are co-deposited in the Ni-P metal matrix to enhance the mechanical properties (i.e., hardness and wear resistance) of the resulting nanocomposite coatings. SDS is a well-known surfactant which stabilises the dispersion of the SiC NPs in a highly acidic (pH ≤ 2) aqueous environment.
Regarding the electroplating conditions, direct or pulse current (10 Hz, 70% duty cycle) was applied with a low current density of 1 A dm−2 for 3 or 6 h, respectively, to achieve a deposit thickness of 30 μm, approximately. These parameters lead to nanocomposite coatings with a higher number of nanoparticles incorporated in the metal matrix [12,13,24,25].
2.2. Spent bath Valorisation
To extend the life cycle of this spent bath, a valorisation procedure developed during the PureNano project was studied. This technique employs functionalised magnetic nanoparticles (MNPs) tailor-made to selectively capture on their functionalised surface the excess nickel species in the spent baths that inhibit the Ni-P/SiC electroplating process. Afterwards, the [MNPs-(excess Ni2+)] aggregates may be removed via magnetic filtering, restoring the original chemical composition of the bath.
The MNPs have an iron-oxide ferromagnetic core and an external negatively charged polymer coating that can capture positively charged species. Importantly, the functionalised MNPs are stable at pH = 2–3, which is the operational pH window of a Ni-P/SiC electroplating bath. This posed a significant challenge since most iron-oxide nanoparticles are not stable at pH < 4. Moreover, the [MNPs-(excess Ni2+)] aggregates formed after the regeneration process can be removed via magnetic filtering, which is a more sustainable and safer method. Magnetic filtering also does not affect the concentration level of the SiC NPs in the bath, in contrast to simple filtration, often used when non-magnetic adsorbents are used for the regeneration of baths. Thus, this recovering route may have potential advantages when compared with other recovery methods.
2.3. Life Cycle Assessment
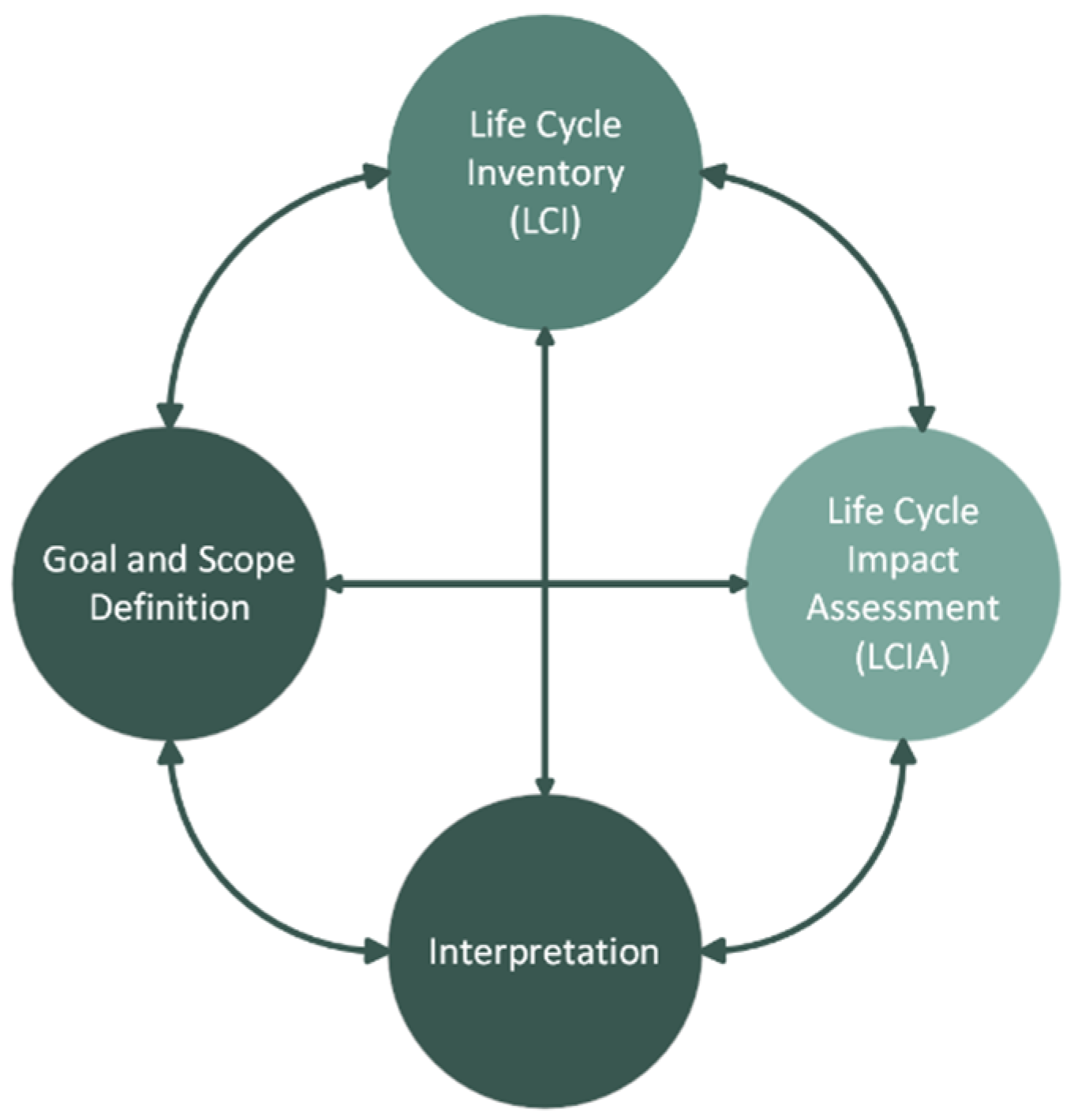
The Life Cycle Assessment (LCA) methodology is a useful framework to quantify the potential environmental impact throughout all stages of a product (or service) life cycle, or part of it. It is defined by the international standards ISO 14040:2006/Amd 1:2020 [26] and ISO 14044:2006/Amd 2:2020 [27]; it aims to identify the key environmental hotspots of a product system and thus inform stakeholders and possibly support decisions towards the improvement of the overall environmental performance of the system. LCA methodology can also be used to compare the environmental performance of alternative systems performing the same function (e.g., compare a new system with a conventional one). Consequently, it allows tracking of the main environmental impacts and benefits among alternatives and supports critical decisions regarding overall process improvement throughout a system’s life cycle (e.g., pollution prevention, and resource-consumption reduction). This methodology is divided into four stages that are interconnected (Figure 1) and will be implemented in the following sub-sections. The LCA studies are fundamental since they may be used to support the decision-making process of the different stakeholders, allowing them to choose the alternative with the best environmental performance [28].
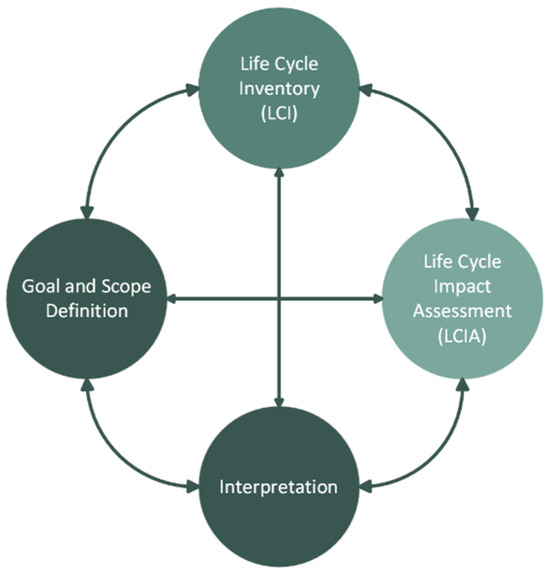
Figure 1.
LCA phases.
2.3.1. Goal and Scope Definition
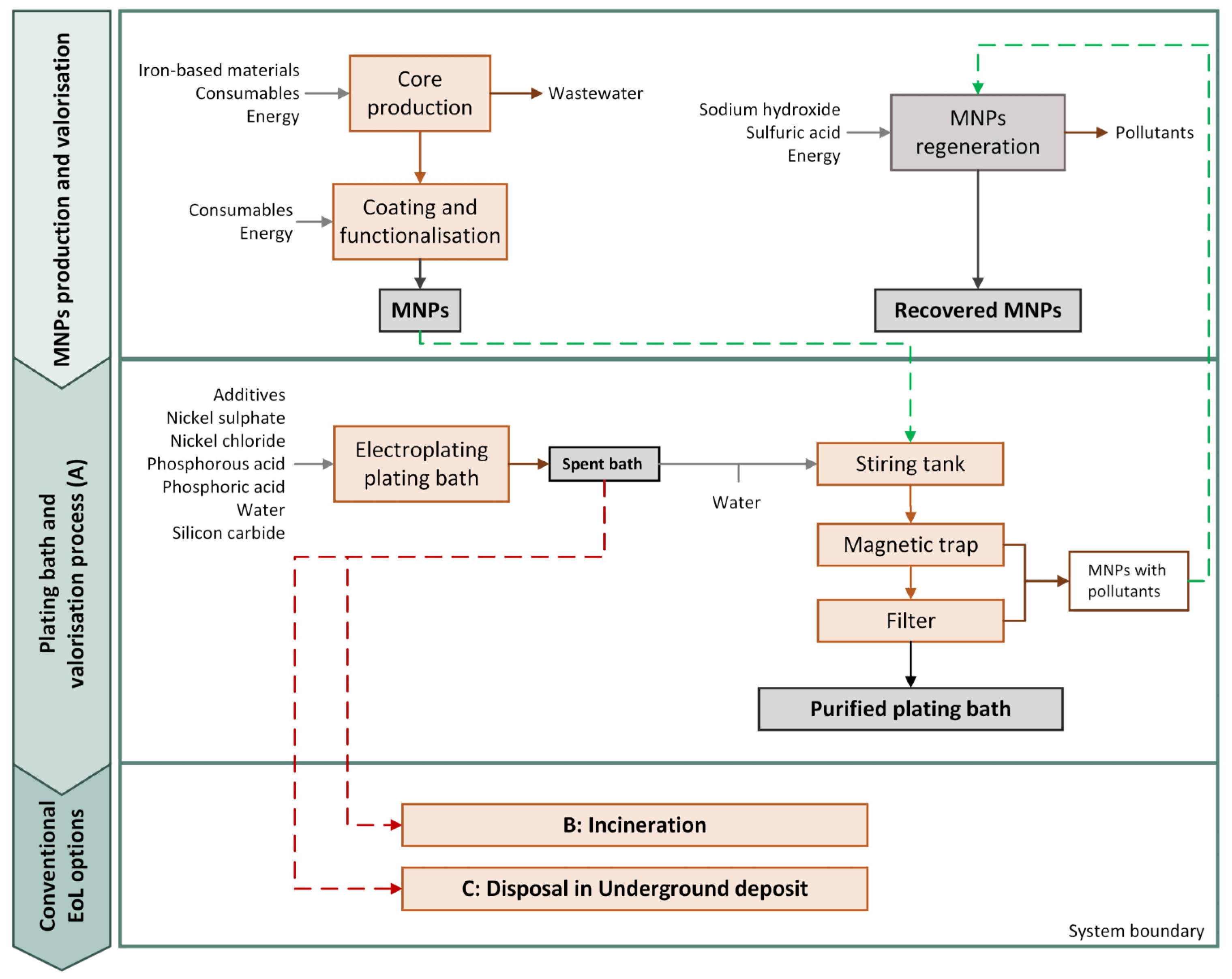
The LCA methodology was applied to perform a comparative analysis between the purification process of a spent bath and the business-as-usual (BAU) scenarios, i.e., the spent bath’s incineration or its disposal in an underground deposit. Using a functional unit (FU) of 1 kg of spent bath to be treated, the environmental impacts were assessed from the raw material production and acquisition, until the spent bath end-of-life (EoL), i.e., cradle-to-grave. Figure 2 shows the system under study. Overall, four different scenarios were assessed:
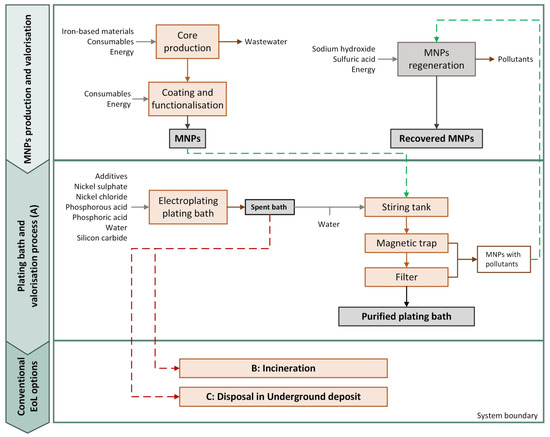
Figure 2.
Flowchart of the system under study.
- Scenario A: which considers the production of the MNPs and their use in the spent bath purification process. In this scenario, the MNPs with pollutants that are obtained after the valorisation process were deposed in an underground deposit;
- Scenario A-R: which considers the production of the MNPs and their use in the spent bath purification process. Nevertheless, herein the MNPs with pollutants were processed to obtain recycled MNPs that may be used in a subsequent purification process;
- Scenario B: Considers the BAU alternative of incineration of the spent bath after water evaporation;
- Scenario C: Contemplates the BAU alternative of disposal of the spent bath in an underground deposit.
2.3.2. Life Cycle Inventory (LCI)
After identifying the approach, the system boundaries, and process steps, the LCI was gathered considering primary data from partners. This information was completed with secondary data from the available literature and the EcoInvent database (v3.8). To sum up, the complete inventory is depicted in Table 1 for all scenarios under study.

Table 1.
Life cycle inventory for the system under study. FU: 1 kg of spent bath to be treated.
In this study, the following assumptions were made. The cathode and anode were considered out of scope because their requirements are the same in all scenarios, which do not influence the comparison between scenarios. Considering the MNPs production, some materials used were not available in the EcoInvent database. Therefore, to model their environmental impacts, proxies available in the literature [29] were used. Specifically, the LCI for the valorisation of the MNPs was gathered based on data available in the literature [30].
Regarding scenarios A and A-R, it is important to note that only the materials that were added to the previous (purified) bath (2nd iteration of the process) were considered as inputs. In this context, the elements already existing in the solution had no impact (i.e., cut-off allocation was assumed). The same allocation principle was used for the MNPs recycling, where its potential environmental impacts were only associated with the valorisation process. On the other hand, in a scenario where the MNPs were not recovered (A), it was considered that the MNPs with the metal ions (pollutants) were disposed in a landfill.
Finally, since the main objective of this study is to support decision-makers to understand and choose the best alternative from an environmental point of view, the transport of materials was not considered.
2.3.3. Life Cycle Impact Assessment (LCIA)
Afterwards, the LCIA was performed using SimaPro (v9.3) software (PRé Sustainability, Amersfoort, The Netherlands). More specifically, the environmental burdens were obtained applying the ReCiPe 2016 (v1.1) method [31] and considering the Hierarchist (H) approach. Therefore, the impacts were calculated for 18 midpoints. Since the ecotoxicity categories (i.e., terrestrial, marine, and freshwater ecotoxicity) are known to have more uncertainty than the others, the environmental impacts were analysed for the midpoints depicted in Table 2.

Table 2.
Midpoints under the study.
2.4. Life Cycle Costing
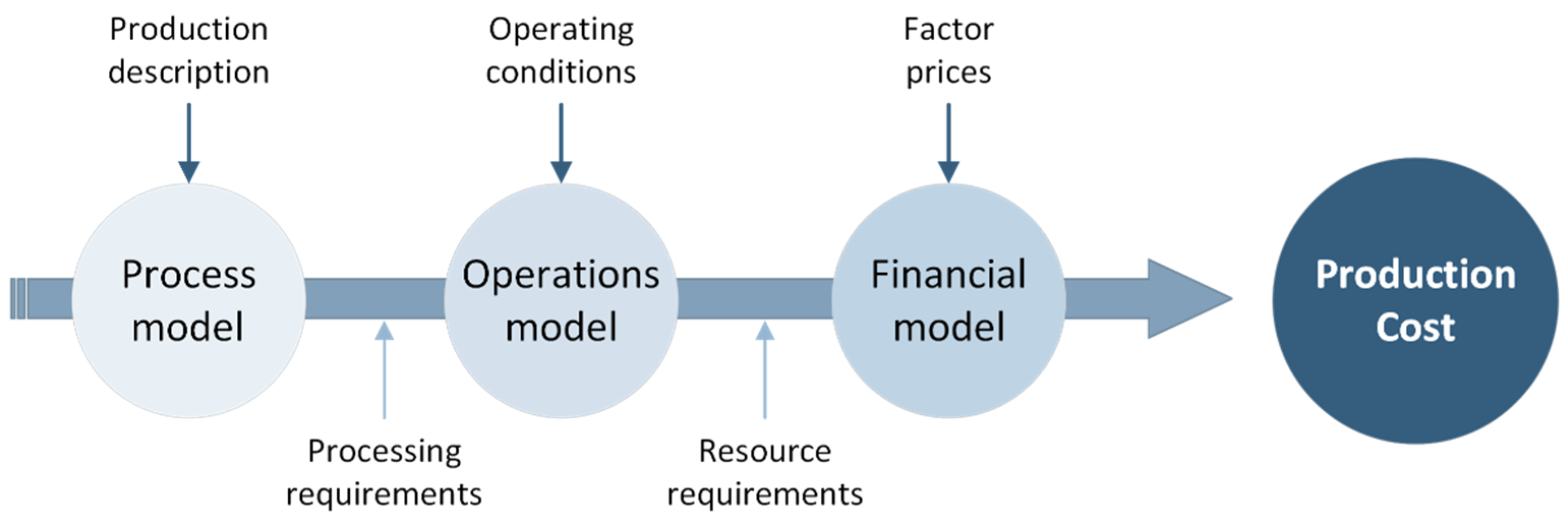
Life Cycle Costing (LCC) aims to estimate the costs of the technical solutions through the life cycle of a product or/and service, with the purpose of supporting decisions towards the most viable processes and products for further development (e.g., process scale up). This framework considers all costs related to a product or a process across its life cycle, and it is often used to calculate and assess the best option from an economic point of view [32]. More specifically, the process-based cost model (PBCM) is a methodology widely used to account for the main cost drivers of a system (i.e., material cost, energy cost, labour cost, equipment cost). These costs are also analysed regarding the influence of the production volume, i.e., they are divided in fixed and variable costs [33]. The PBCM is divided into three sub-models (Figure 3) [34]. First, the process model contains the general inputs of the process and relates the final product description and the process conditions required to produce it, such as part-production cycle time. Second, the operations model covers the operating conditions of the process, which includes the number of shifts, working time and production volume. This model calculates the necessary resources, namely, equipment, labour, and energy, among others, to produce the desired production volume, i.e., the required inventory. Third, the financial model comprises the cost data regarding the inputs used in the operations model (e.g., cost per kg, cost per kWh, etc.).
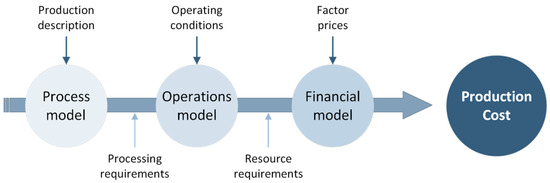
Figure 3.
The PBCM structure.
In this study, the PBCM methodology was used to calculate the life-cycle costs of the different scenarios presented in Section 2.3. This cost analysis aimed to compare the economic performance of the valorisation route of the spent bath with conventional (BAU) alternatives of disposing of spent baths to support the decision-making process. To do so, the boundaries for this analysis remain the same as considered for the LCA, i.e., cradle-to-grave). Moreover, the same unit of measurement (1 kg of spent bath to be treated) was also considered. According to the methodological approach described previously, the cost data were divided into four categories and gathered in Table 3.

Table 3.
Cost data used for the LCC analysis.
Overall, it was assumed that there was an average of 20 working days per month at 8 h a day, leading to a total of 250 working days per year for all scenarios. Moreover, the disposal and incineration costs considered were 36.5 EUR/ton and 32.5 EUR/ton, respectively. The capital cost of equipment was considered based on the best knowledge of the partners, and the corresponding life-time usage for the production line (ratio between “time used in the process under study” and “equipment lifetime”).
2.5. Ecoefficiency Evaluation
Finally, an ecoefficiency analysis was performed following the international standard ISO 14045:2012 [35]. According to this document, the ecoefficiency analysis is seen as a management tool that evaluates the environmental impacts and the life cycle costs of a system, jointly. Thus, the ecoefficiency considers environmental impacts assessment through the Life Cycle Assessment (LCA) methodology standardised by ISO 14040 [26] and ISO 14044 [27]. The main aims of ISO 14045 are to establish a common structure and terminology to assess ecoefficiency, give a clear orientation for the interpretation of results, and encourage a robust, accurate and useful communication of results.
In this methodology, both LCA and LCC studies’ results may be integrated, which is valuable to jointly analyse the potential environmental and economic benefits of alternative improvement strategies [32], as applied in different sectors.
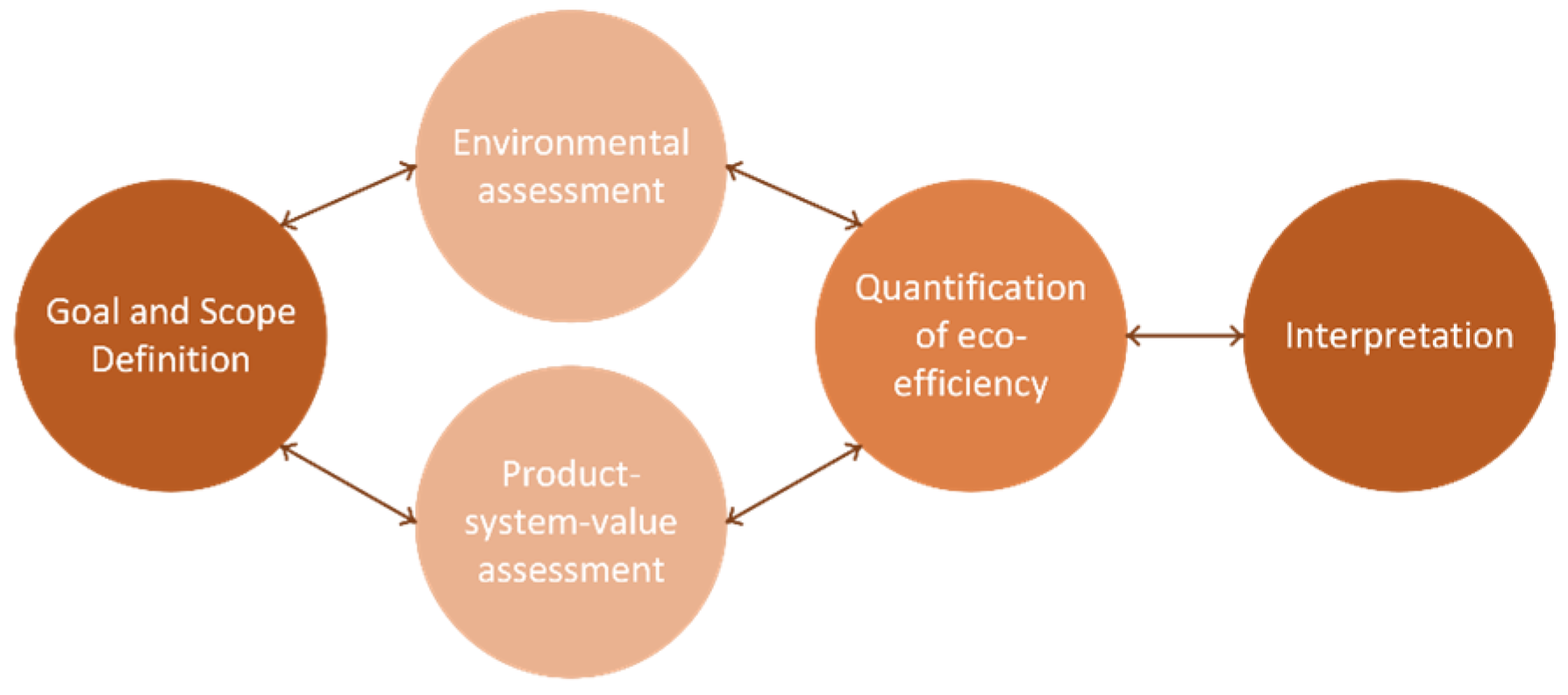
The ecoefficiency analysis has five different stages, all interactive among each other (Figure 4). This methodology has several applications, namely, in product development and improvement, strategic planning, policy making, and sustainability assessment. In the next sub-sections, each step of this framework will be applied and described.
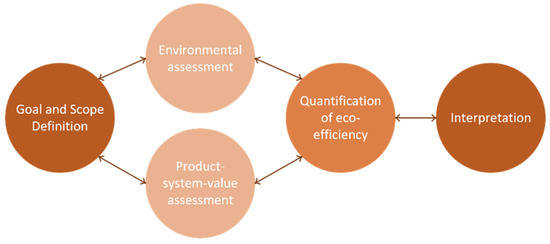
Figure 4.
Ecoefficiency assessment approach.
2.5.1. Goal and Scope Definition
Aiming to compare the environmental and economic performances of the different scenarios herein studied, an ecoefficiency analysis was performed using the scope described for the LCA and LCC assessments. Thus, the system boundaries remained the same, i.e., cradle-to-grave, as well as the FU (1 kg of spent bath to be treated).
2.5.2. Environmental Assessment
The environmental assessment was carried out according to the steps described in Section 2.3. To calculate the ecoefficiency indicator associated with the scenarios under study, only the global warming potential (GW) category (measured in kg of CO2-eq) was selected since it is a well-known environmental indicator.
2.5.3. Product System Value Assessment
In this work, the product system value was estimated using Equation (1). In general, the life-cycle costs of the technical solutions evaluated were obtained using the PBCM methodology (Section 2.4). On the other hand, since it was not possible to identify the product value, the product system value indicator was defined to be the inverse of the costs of the technologies.
2.5.4. Quantification of Ecoefficiency
Finally, the ecoefficiency was calculated through Equation (2) and using the results obtained in the last two steps. In other words, the ecoefficiency may be understood as the ratio between the product system value and the environmental performance of each scenario. In this context, scenarios with higher indicators have better economic and/or environmental performances. Therefore, to improve ecoefficiency, it is essential to lower both the costs (increasing the system’s value) and/or the environmental impacts associated with the system.
3. Results and Discussion
Since the interpretation of results is the final step of each methodology carried out, the results of the three frameworks, i.e., LCA, LCC, and the ecoefficiency analysis, are depicted and analysed in detail in Section 3.1, Section 3.2 and Section 3.3, respectively.
3.1. Environmental Analysis
In this sub-section, the environmental performance of all scenarios is analysed in detail. Firstly, a comparative analysis was carried out to compare the valorisation routes with different BAU scenarios, i.e., B—incineration, and C—Disposal in an underground deposit, and see the potential trade-offs of each scenario. Secondly, both baths (used in scenario A, and virgin) were studied to see their potential hotspots.
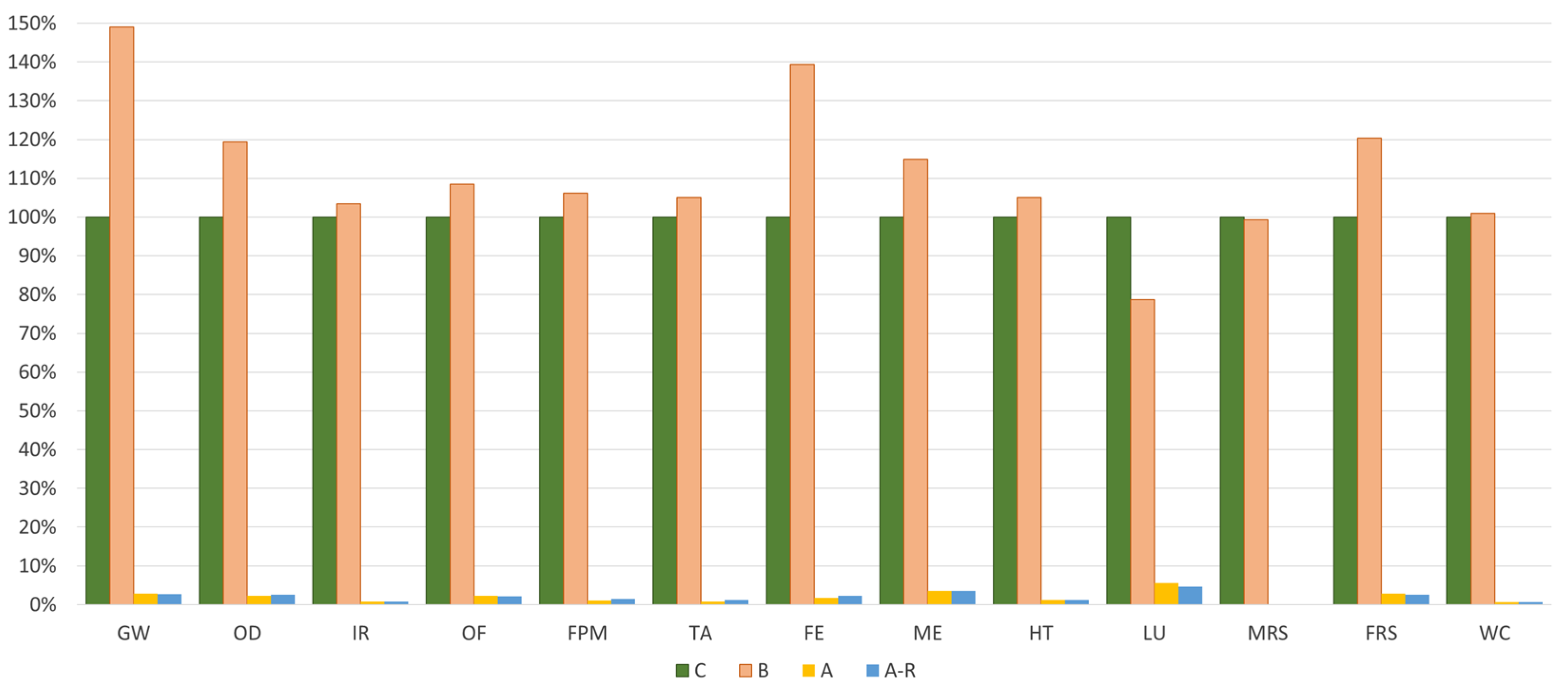
Considering the comparative analysis, Table 4 depicts the total environmental impacts of all scenarios under study for each midpoint analysed. Figure 5 shows the relative environmental impacts of the four different scenarios, assuming the disposal scenario as the reference one (100%). It means that all the impacts were normalised considering this reference scenario.

Table 4.
Total quantitative environmental impacts for all scenarios under study. FU: 1 kg of spent bath to be treated.
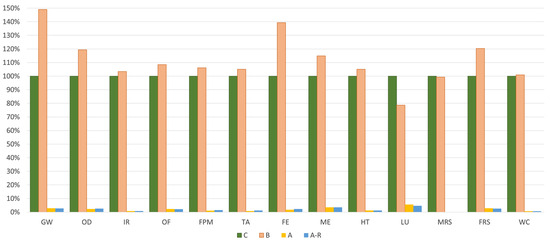
Figure 5.
Scenarios comparison for all scenarios under study. UF: 1 kg of spent bath to be treated.
Regarding the spent bath purification route, Figure 5 demonstrates that this technique results in a substantial reduction of environmental impacts across all the analysed midpoint categories. Both the A and the A-R (purification system with MNPs valorisation) reduce the environmental burdens to about 98%. Nevertheless, when considering the incineration route (B), this scenario has 12% more impacts than the base case (disposal).
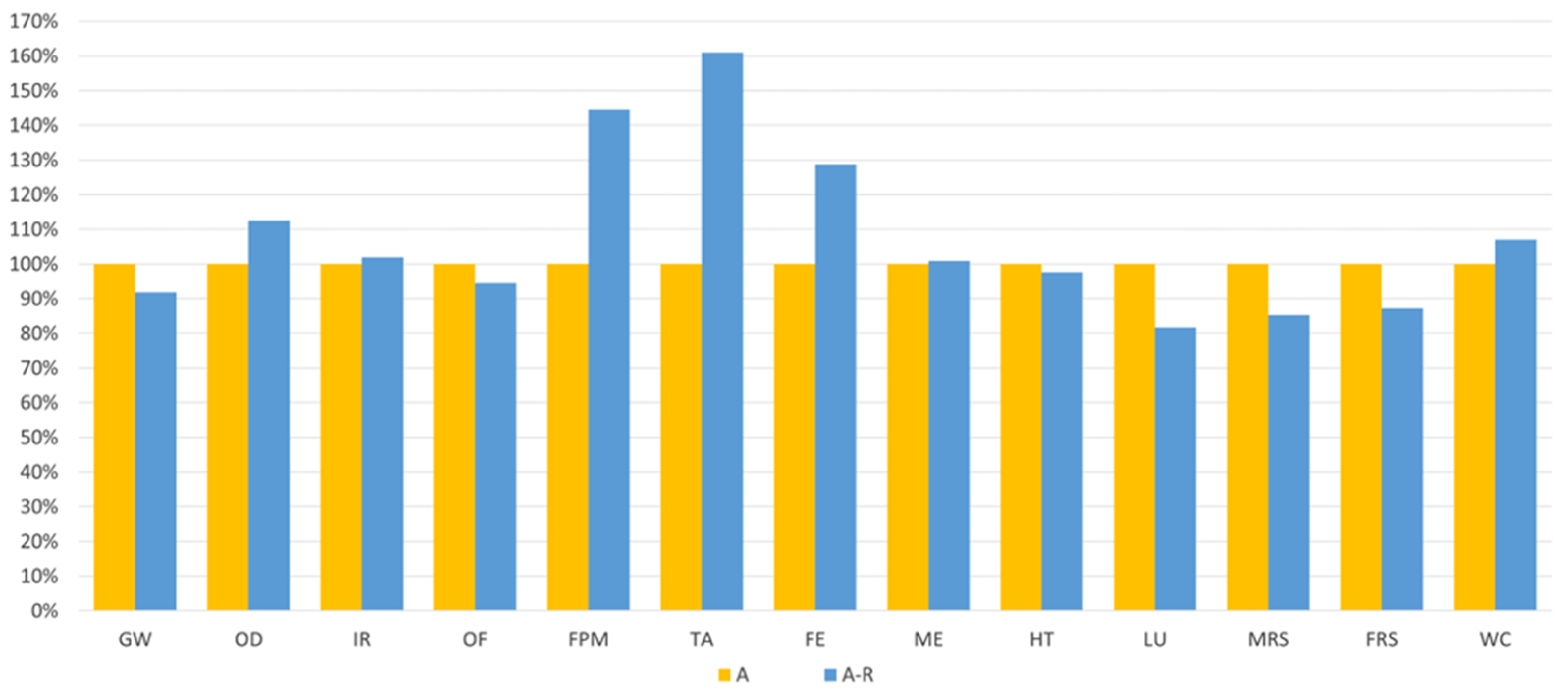
Comparing the valorisation process of the spent bath with (A-R) and without (A) MNPs recycling, Figure 6 shows that the impacts of the purification could be reduced through MNPs recycling (A-R) in some categories, namely in GW (−8%), OF (−6%), HT (−2%), LU (−18%), MRS (−15%), and FRS (−13%). However, when considering the average global impacts, this scenario (A-R) exhibits over 7% higher impact than Scenario A.
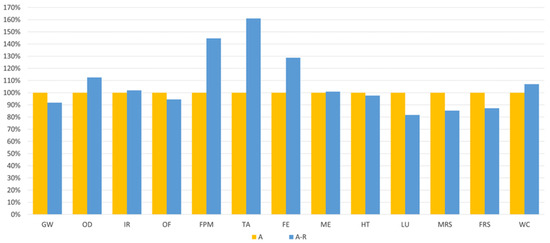
Figure 6.
Relative comparison between the purification route with virgin MNPs (A) and with recycled MNPs (A-R).
These results highlight the importance of analysing various impact categories in an LCA study, because focusing solely on a single impact indicator, such as GW (carbon emissions), may lead to an incomplete evaluation and not fully capture the existing trade-offs associated with a process or product. Considering multiple impact categories ensures a comprehensive understanding of environmental impacts and allows for a more robust assessment.
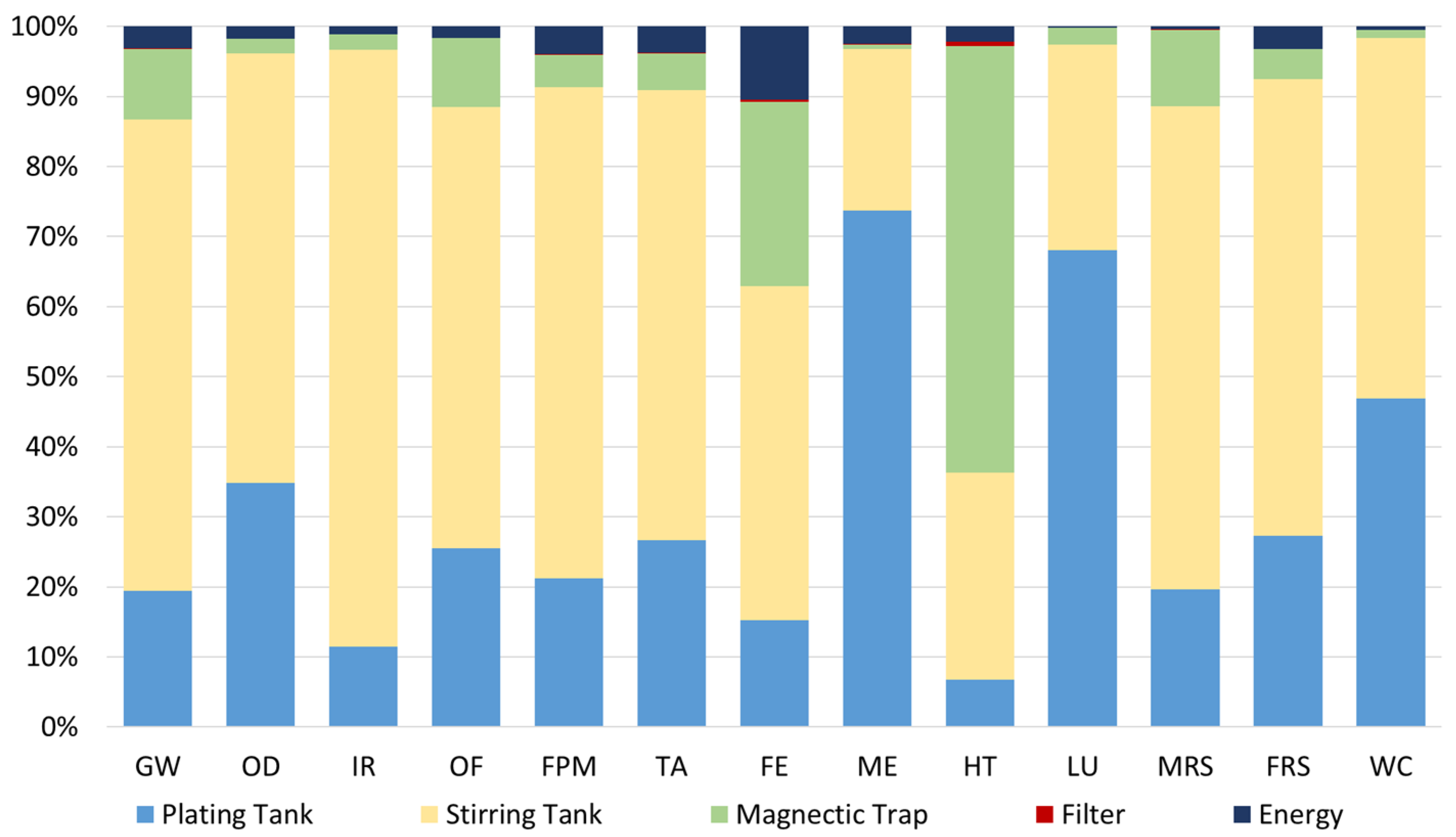
Looking in more detail for the results of the spent bath of the valorisation process without MNPs recovery (Scenario A), Figure 7 depicts the relative environmental impact for each category under analysis. As already referred to, these results are associated with the second iteration of the process, which means that only the purification process and the materials that need to be added to the purified bath (to achieve the required properties) were considered. The initial spent bath was assumed to have no impacts (cut-off).
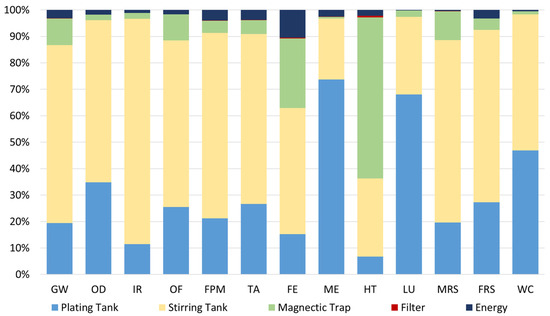
Figure 7.
Relative environmental impacts associated with the spent bath valorisation process (A). FU: 1 kg of spent bath to be treated.
Regarding the scenario without recycled MNPs, i.e., Scenario A, it is possible to conclude that most of the impacts (about 57%) are associated with the stirring tank due to the addition of MNPs in this stage. This material is responsible for 56% of the global environmental impacts of the process due to the materials used to functionalise and coat the nanoparticles, and the high energy demand that is required for the MNPs production. In this scenario (A), the additives used in the spent bath have also expressive potential impacts, i.e., 24% and 5%, because to keep the bath properties, the original amount of these additives, contained in the virgin bath, had to be added in the second iteration. Moreover, the MNPs with pollutants which were captured in the magnetic trap, and then, disposed of, are responsible for 11% of the impacts, on average, being more meaningful in the HT category (61%).
On the other hand, the energy consumed during the purification process is responsible for less than 3% of the impacts.
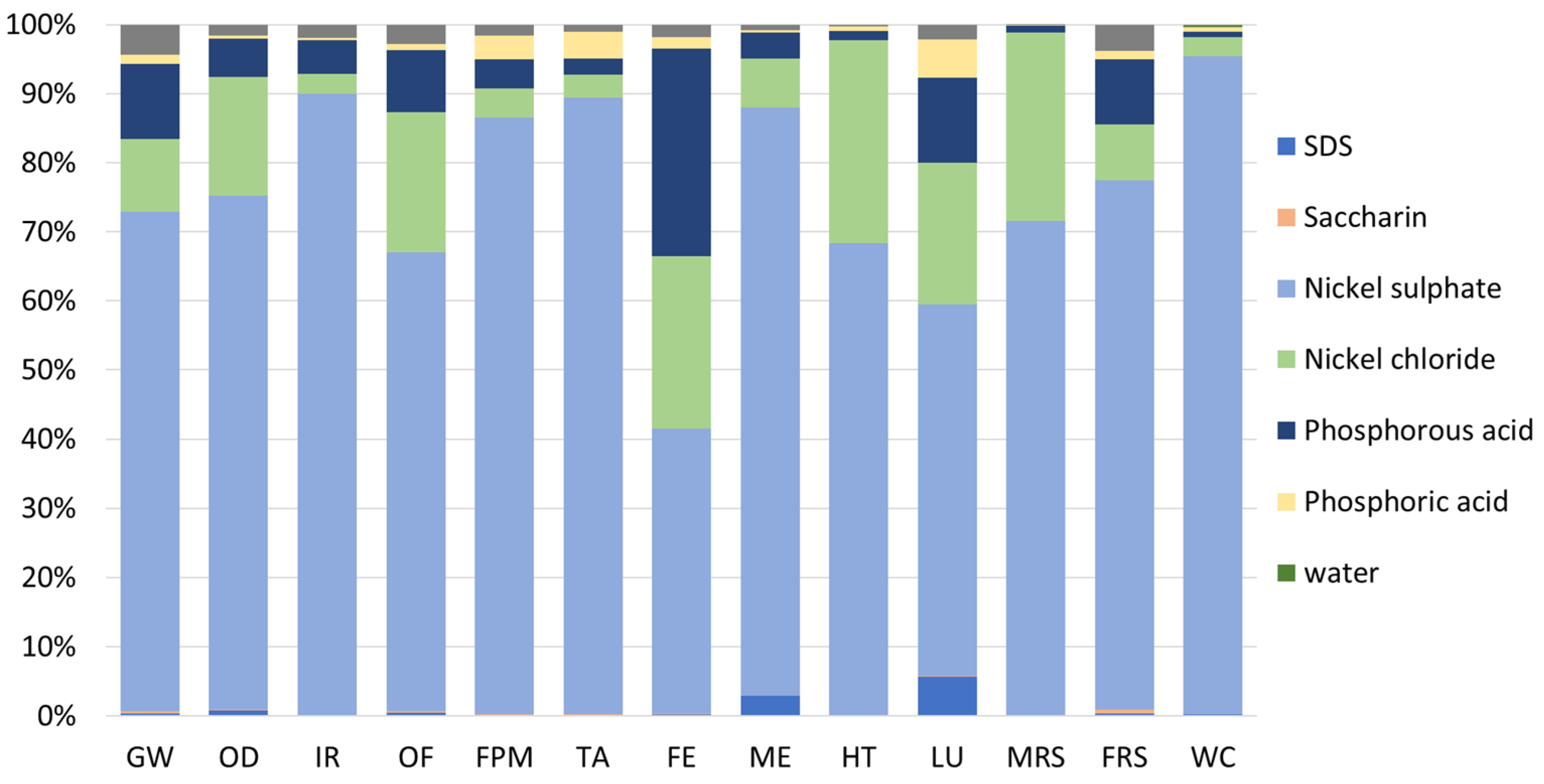
Finally, Figure 8 depicts the relative impacts of each material used in the virgin plating bath. Thus, it is possible to observe that most of the impacts are related to the nickel-based materials, i.e., nickel sulphate, and nickel chloride, which represent 75% and 14% of the impacts, accordingly. Moreover, the phosphorous acid is also responsible for considerable impacts (about 7%). In contrast, other materials, such as additives (i.e., SDS and saccharin) and water, have negligible contributions to the overall environmental impacts, each accounting for less than 1%.
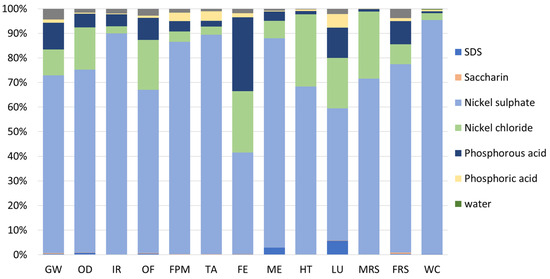
Figure 8.
Relative environmental impacts associated with the virgin plating bath. FU: 1 kg of spent bath to be treated.
3.2. Cost Analysis
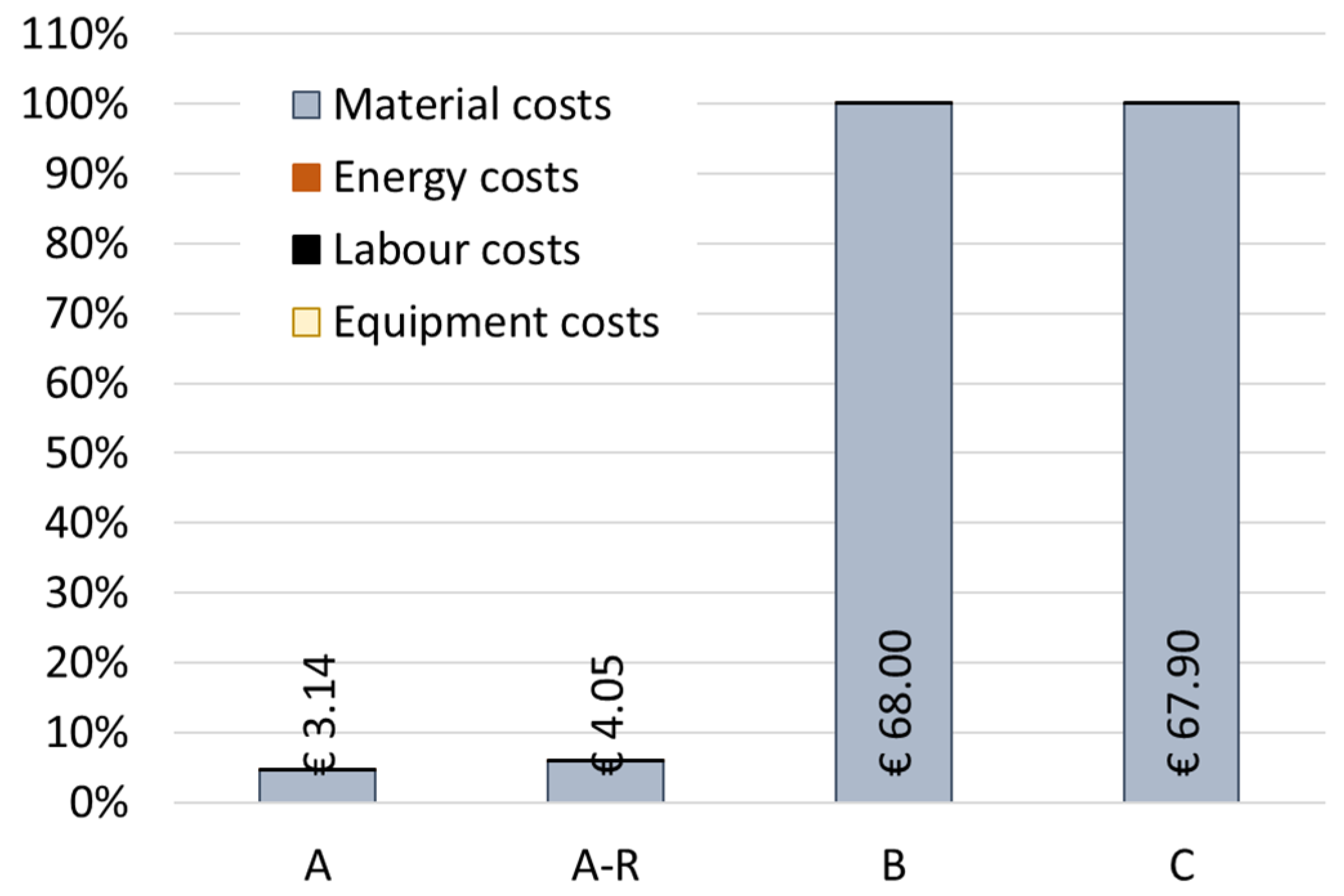
The economic performance of the scenarios was also evaluated. Figure 9 presents a relative comparison of the calculated life cycle costs for the four scenarios, with the BAU scenario as the baseline (C = 100%). Among the scenarios, the purification route without recycling MNPs (Scenario A) presents the lowest processing costs at 3.14EUR/kg of spent bath. When compared with scenario C (underground deposit), both purification alternatives (A and A-R) allow BAU costs to be reduced by more than 90%. This fact happens due to the recovery of material inputs that are very expensive, namely nickel sulphate and SDS. Thus, fewer quantities of materials (resources) are needed in the second iteration of the process. Nevertheless, the recycling process A-R proves to be 29% more expensive than Scenario A.
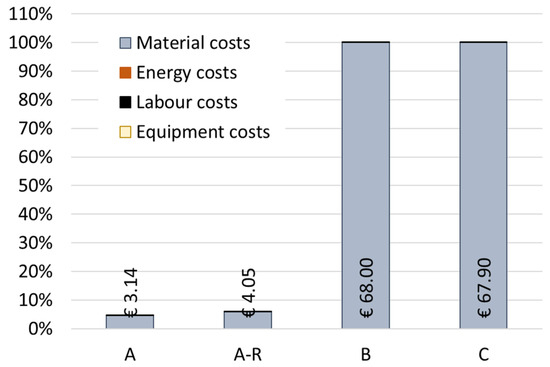
Figure 9.
Scenarios comparison for the total production costs. UF: 1 kg of spent bath to be treated.
Overall, the expensive materials, such as SDS, and others that are used in a high amount (i.e., nickel sulphate), were the main hotspots in Scenario A and Scenarios B and C. For the A-R scenario, the recycling process of the nanoparticles proved to be most expensive, making recycled MNPs the main potential hotspots of this scenario (56% of the total costs). On the other hand, the energy, labour, and equipment costs proved to be negligible in all spent bath purification scenarios.
3.3. Ecoefficiency Analysis
Finally, the ecoefficiency of each scenario must also be studied. To do so, the costs associated with each scenario and their specific product value—calculated according to Equation (1)—are presented in Table 5. The ecoefficiency indicators of the scenarios are depicted in Table 6. In general, the results showed that Scenario A was the most sustainable scenario with the best ecoefficiency performance.

Table 5.
Total costs and product value of all scenarios analysed.

Table 6.
Ecoefficiency indicator for all scenarios under study.
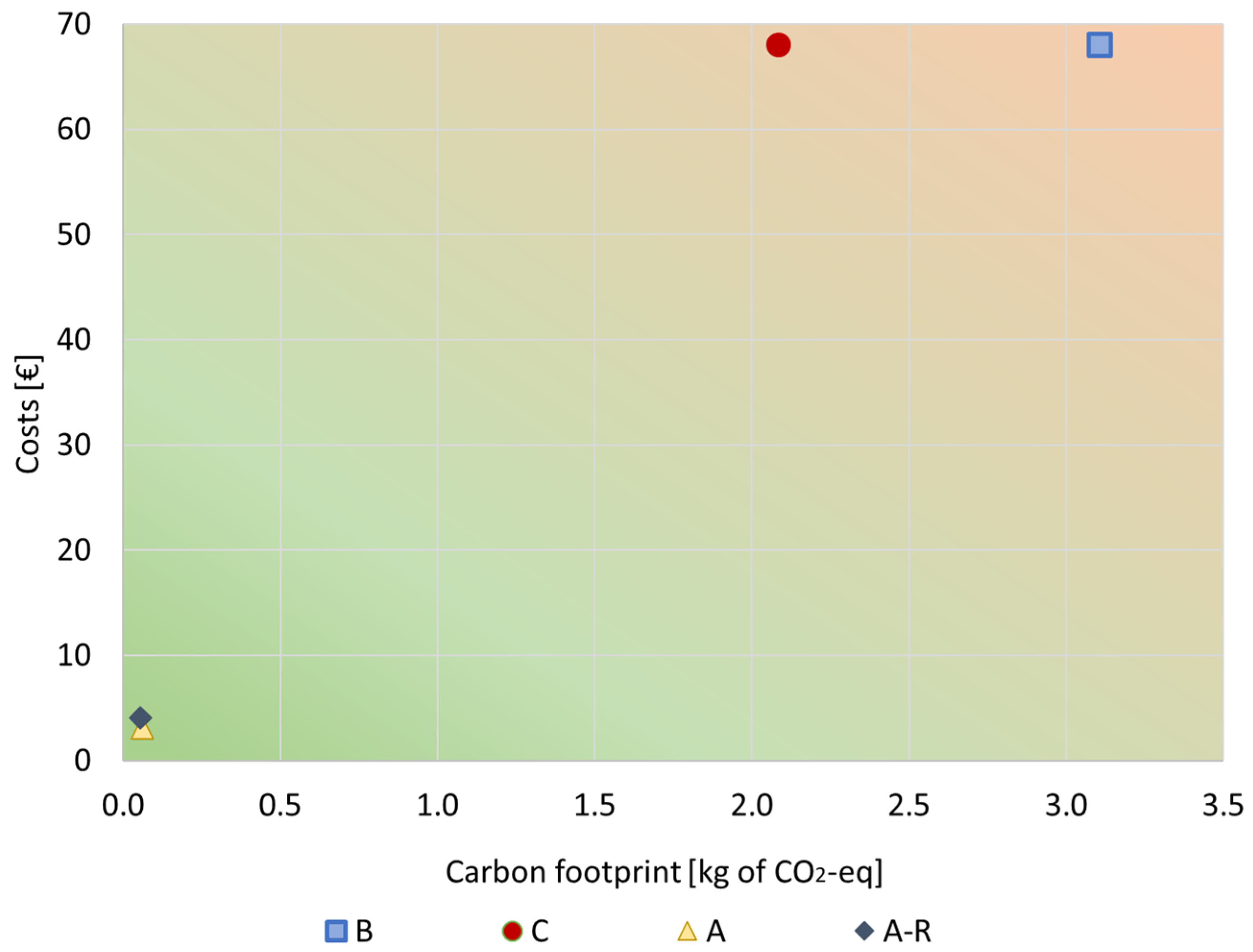
To better understand the results and draw some conclusions, the economic and environmental analyses are represented in a graph (Figure 10) where the relation between costs and carbon emissions may be observed. Overall, Figure 10 shows that the purification processes (A and A-R) had better economic vs. environmental performance than the BAU alternatives (B and C). This is due to the recovery of expensive and burdensome materials included in the original bath (e.g., nickel-based materials and other additives such as SDS), which, thanks to the purification route, can be preserved in the bath to be reused instead of wasted. Therefore, in the purified bath, a smaller amount of materials is required when compared to a virgin plating bath.
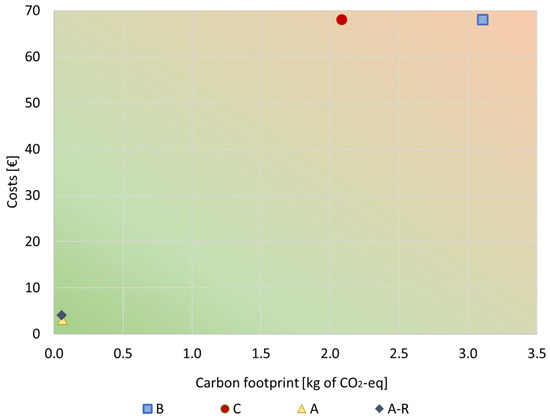
Figure 10.
Total costs vs. environmental impacts relation for all scenarios under study. FU: 1 kg of spent bath to be treated.
Comparing the A and A-R scenarios, their carbon emission is not much different. Scenario A-R has slightly slower impact per spent bath (5.56 × 10−2 kg of CO2-eq/kg) than A (6.06 × 10−2 kg of CO2-eq/kg of spent bath) because of the lower amount of MNPs added to the plating bath. Nonetheless, since the MNPs’ recycling process was more expensive than producing virgin MNPs, the A-R scenario had higher costs (4.05 EUR/kg of spent bath) than the A scenario (3.14 EUR/kg of spent bath). Thus, further work is still needed to improve the efficiency of the recycling process and reduce its costs.
On the other hand, incineration (B) was the scenario with the worst relation of costs and carbon footprint, presenting the highest costs (68 EUR/kg of spent bath) and highest environmental impacts (3.1 kg of CO2-eq/kg of spent bath).
In general, since MNPs’ production and recycling were considered a potential hotspot, further research is needed to improve their sustainability, reducing costs and environmental impacts. For example, considering the MNPs’ production, the materials used to coat the MNPs and make them functional were considered the main environmental and economic hotspot. Thus, more research to evaluate other options and estimate their environmental and economic implications are needed. Moreover, since electricity also has considerable burdens, the use of renewable sources (e.g., wind power and photovoltaics) may reduce the environmental impacts associated with the MNPs’ production and increase the ecoefficiency. Furthermore, additional research is recommended since the MNPs’ recycling was modelled according to the literature, and the impacts of the process may decrease with the when upscaling and/or optimizing the process.
4. Conclusions and Further Research
The main objectives of the present study were to assess the sustainability of a valorisation/purification process to extend the life of nickel-based plating baths as well as to compare its environmental and economic performance with conventional alternatives (i.e., underground disposal and incineration).
Overall, the LCA and LCC results showed that the purified plating baths lead to lower environmental impacts (less 98%) and processing costs (about −95%) than other conventional alternatives. The use of nanoparticles to purify the spent baths were considered an important hotspot for the environmental impacts and life cycle costs due to the materials and energy used during their production and recycling process. Nevertheless, this valorisation alternative can lead to lower environmental burdens and costs than other conventional alternatives. The amount of MNPs used in the purification was shown to be a critical factor; thus, studies on the optimal MNPs amount are advisable to purify other baths.
When comparing the results obtained in the ecoefficiency analysis, the results showed that A and A-R had the best relation between costs and environmental impacts (carbon footprint). Since the recycling of MNPs had higher costs than their production, A (valorisation system with virgin MNPs) was considered the most ecoefficient scenario.
In conclusion, when compared with BAU alternatives, the technique evaluated seemed to have environmental and economic benefits; it allows metals to be recycled and pollutants to be removed from electroplating baths. This purification alternative may help to increase the sustainability of the plating baths and extends bath life cycles, specifically in cases where expensive and burdensome materials are used.
Since MNPs’ production and recycling were considered a hotspot, further research is needed to improve their sustainability. For instance, other alternative materials can be studied to replace the most expensive and burdensome ones. Moreover, the use of renewable energy sources (e.g., wind power and photovoltaics) may improve the environmental and economic performance of the MNPs’ production.
Author Contributions
Conceptualization, B.M. and H.M.; Formal analysis, B.M.; Writing—original draft, B.M., E.P. and H.M.; Writing—review & editing, B.M., A.G., A.Z.-K. and H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 821431, within the PureNano project. The authors would also like to acknowledge some of the PureNano partners for their fruitful collaboration, namely Captive, and RISE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wielage, B.; Lampke, T.; Zacher, M.; Dietrich, D. Electroplated Nickel Composites with Micron- to Nano-Sized Particles. Key Eng. Mater. 2008, 384, 283–309. [Google Scholar] [CrossRef]
- Rezayat, M.; Yazdi, M.S.; Zandi, M.D.; Azami, A. Tribological and Corrosion Performance of Electrodeposited Ni–Fe/Al2O3 Coating. Results Surf. Interfaces 2022, 9, 100083. [Google Scholar] [CrossRef]
- Darvishzadeh, A.; Nasouri, K. Manufacturing, Modeling, and Optimization of Nickel-Coated Carbon Fabric for Highly Efficient EMI Shielding. Surf. Coatings Technol. 2021, 409, 126957. [Google Scholar] [CrossRef]
- Salehikahrizsangi, P.; Raeissi, K.; Karimzadeh, F.; Calabrese, L.; Proverbio, E. Effects of Surface Morphology on Erosion–Corrosion and Corrosion Resistance of Highly Hydrophobic Nickel-Tungsten Electrodeposited Film. Coatings 2021, 11, 1084. [Google Scholar] [CrossRef]
- Bostani, B.; Arghavanian, R.; Parvini Ahmadi, N.; Yazdani, S. Fabrication and Properties Evaluation of Functionally Graded Ni-NCZ Composite Coating. Surf. Coatings Technol. 2017, 328, 276–282. [Google Scholar] [CrossRef]
- Ramazan, K.; Esma, S.; Belkıs, U. Statistical Optimisation of Organic Additives for Maximum Brightness and Brightener Analysis in a Nickel Electroplating Bath. Trans. IMF 2015, 93, 89–96. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Safyari, M.; Mori, G. Hydrogen Absorption Rate and Hydrogen Diffusion in a Ferritic Steel Coated with a Micro- or Nanostructured ZnNi Coating. Electrochem. Commun. 2022, 134, 107169. [Google Scholar] [CrossRef]
- Takuma, Y.; Sugimori, H.; Ando, E.; Mizumoto, K. Comparison of the Environmental Impact of the Conventional Nickel Electroplating and the New Nickel Electroplating. Int. J. Life Cycle Assess. 2018, 23, 1609–1623. [Google Scholar] [CrossRef]
- Reis, M.T.A.; Ismael, M.R.C. Electroplating Wastes. Phys. Sci. Rev. 2018, 3, 20180024. [Google Scholar] [CrossRef]
- Pillai, A.M.; Rajendra, A.; Sharma, A.K. Electrodeposited Nickel–Phosphorous (Ni–P) Alloy Coating: An in-Depth Study of Its Preparation, Properties, and Structural Transitions. J. Coatings Technol. Res. 2012, 9, 785–797. [Google Scholar] [CrossRef]
- Lelevic, A.; Walsh, F.C. Electrodeposition of Ni P Alloy Coatings: A Review. Surf. Coatings Technol. 2019, 369, 198–220. [Google Scholar] [CrossRef]
- Zoikis-Karathanasis, A.; Pavlatou, E.A.; Spyrellis, N. Pulse Electrodeposition of Ni–P Matrix Composite Coatings Reinforced by SiC Particles. J. Alloys Compd. 2010, 494, 396–403. [Google Scholar] [CrossRef]
- Ahmadkhaniha, D.; Zanella, C. The Effects of Additives, Particles Load and Current Density on Codeposition of SiC Particles in NiP Nanocomposite Coatings. Coatings 2019, 9, 554. [Google Scholar] [CrossRef]
- European Commission Sustainable Production: Best Available Techniques. Available online: https://joint-research-centre.ec.europa.eu/scientific-activities-z/sustainable-production-best-available-techniques_en (accessed on 3 April 2023).
- European IPPC Bureau. European Commission Surface Treatment of Metals and Plastics (STM). Available online: https://eippcb.jrc.ec.europa.eu/reference/surface-treatment-metals-and-plastics (accessed on 4 April 2023).
- Bless, D. A Review of Electroplating Nickel Bath Life Extension, Nickel Recovery & Copper Recovery from Nickel Baths. Plat. Surf. Finish. 2000, 87, 72–78. [Google Scholar]
- Wu, L.; Garg, S.; Xie, J.; Zhang, C.; Wang, Y.; Waite, T.D. Electrochemical Removal of Metal-Organic Complexes in Metal Plating Wastewater: A Comparative Study of Cu-EDTA and Ni-EDTA Removal Mechanisms. Environ. Sci. Technol. 2023, 57, 12476–12488. [Google Scholar] [CrossRef]
- Idhayachander, R.; Palanivelu, K. Electrolytic Recovery of Nickel from Spent Electroless Nickel Bath Solution. E-J. Chem. 2010, 7, 1412–1420. [Google Scholar] [CrossRef]
- Benvenuti, T.; Rodrigues, M.A.S.; Bernardes, A.M.; Zoppas-Ferreira, J. Closing the Loop in the Electroplating Industry by Electrodialysis. J. Clean. Prod. 2017, 155, 130–138. [Google Scholar] [CrossRef]
- Tanaka, M.; Huang, Y.; Yahagi, T.; Hossain, M.K.; Sato, Y.; Narita, H. Solvent Extraction Recovery of Nickel from Spent Electroless Nickel Plating Baths by a Mixer-Settler Extractor. Sep. Purif. Technol. 2007, 62, 97–102. [Google Scholar] [CrossRef]
- Scarazzato, T.; Panossian, Z.; Tenório, J.A.S.; Pérez-Herranz, V.; Espinosa, D.C.R. A Review of Cleaner Production in Electroplating Industries Using Electrodialysis. J. Clean. Prod. 2017, 168, 1590–1602. [Google Scholar] [CrossRef]
- Laforest, V.; Raymond, G.; Piatyszek, É. Choosing Cleaner and Safer Production Practices through a Multi-Criteria Approach. J. Clean. Prod. 2013, 47, 490–503. [Google Scholar] [CrossRef]
- PureNano H2020 PureNano. Available online: https://www.purenano-h2020.eu/ (accessed on 3 July 2023).
- Ahmadkhaniha, D.; Tsongas, K.; Tzetzis, D.; Zanella, C. Study of the Effect of Pulse Plating Parameters on the Electrodeposition of NiP and NiP/SiC Coatings and Their Microhardness Values. Trans. IMF 2021, 99, 29–37. [Google Scholar] [CrossRef]
- Cai, C.; Zhu, X.B.; Zheng, G.Q.; Yuan, Y.N.; Huang, X.Q.; Cao, F.H.; Yang, J.F.; Zhang, B. Electrodeposition and Characterization of Nano-Structured Ni-SiC Composite Films. Surf. Coatings Technol. 2011, 205, 3448–3454. [Google Scholar] [CrossRef]
- ISO 14040:2006/Amd 1:2020; Environmental Management-Life Cycle Assessment-Principles and Framework-Amendment 1. ISO: Geneva, Switzerland, 2020.
- ISO 14044:2006/Amd 2:2020; Environmental Management-Life Cycle Assessment-Requirements and Guidelines-Amendment 2. ISO: Geneva, Switzerland, 2020.
- Moura, B.; Monteiro, H.; Mata, T.M.; Iten, M.; Martins, A.A. Environmental Life Cycle Assessment of Early-Stage Development of Ergosterol Extraction from Mushroom Bio-Residues. J. Clean. Prod. 2022, 355, 131623. [Google Scholar] [CrossRef]
- Muñoz, I.; Rodríguez, C.; Gillet, D.M.; Moerschbacher, B. Life Cycle Assessment of Chitosan Production in India and Europe. Int. J. Life Cycle Assess. 2018, 23, 1151–1160. [Google Scholar] [CrossRef]
- Garcia Gonzalez, M.N.; Quiroga-Flores, R.; Börjesson, P. Life Cycle Assessment of a Nanomaterial-Based Adsorbent Developed on Lab Scale for Cadmium Removal: Comparison of the Impacts of Production, Use and Recycling. Clean. Environ. Syst. 2022, 4, 100071. [Google Scholar] [CrossRef]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollande3r, A.; van Zelm, R. ReCiPe 2016 v1.1 A Harmonized Life Cycle Impact Assessment Method at Midpoint and Endpoint Level Report I: Characterization RIVM Report 2016–0104a. Int. J. Life Cycle Assess. 2016, 22, 138–147. [Google Scholar] [CrossRef]
- Monteiro, H.; Cruz, P.L.; Moura, B. Integrated Environmental and Economic Life Cycle Assessment of Improvement Strategies for a Ceramic Industry. J. Clean. Prod. 2022, 345, 131173. [Google Scholar] [CrossRef]
- Nadeau, M.-C.; Kar, A.; Roth, R.; Kirchain, R. A Dynamic Process-Based Cost Modeling Approach to Understand Learning Effects in Manufacturing. Int. J. Prod. Econ. 2010, 128, 223–234. [Google Scholar] [CrossRef]
- Calado, E.A.; Leite, M.; Silva, A. Integrating Life Cycle Assessment (LCA) and Life Cycle Costing (LCC) in the Early Phases of Aircraft Structural Design: An Elevator Case Study. Int. J. Life Cycle Assess. 2019, 24, 2091–2110. [Google Scholar] [CrossRef]
- ISO 14045:2012; Environmental Management-Eco-Efficiency Assessment of Product Systems-Principles, Requirements and Guidelines. ISO: Geneva, Switzerland, 2012.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).