Assessment of Benefits and Risk of Genetically Modified Plants and Products: Current Controversies and Perspective
Abstract
1. Introduction
2. Literature Search Method
3. Plant Genetic Transformation Methods
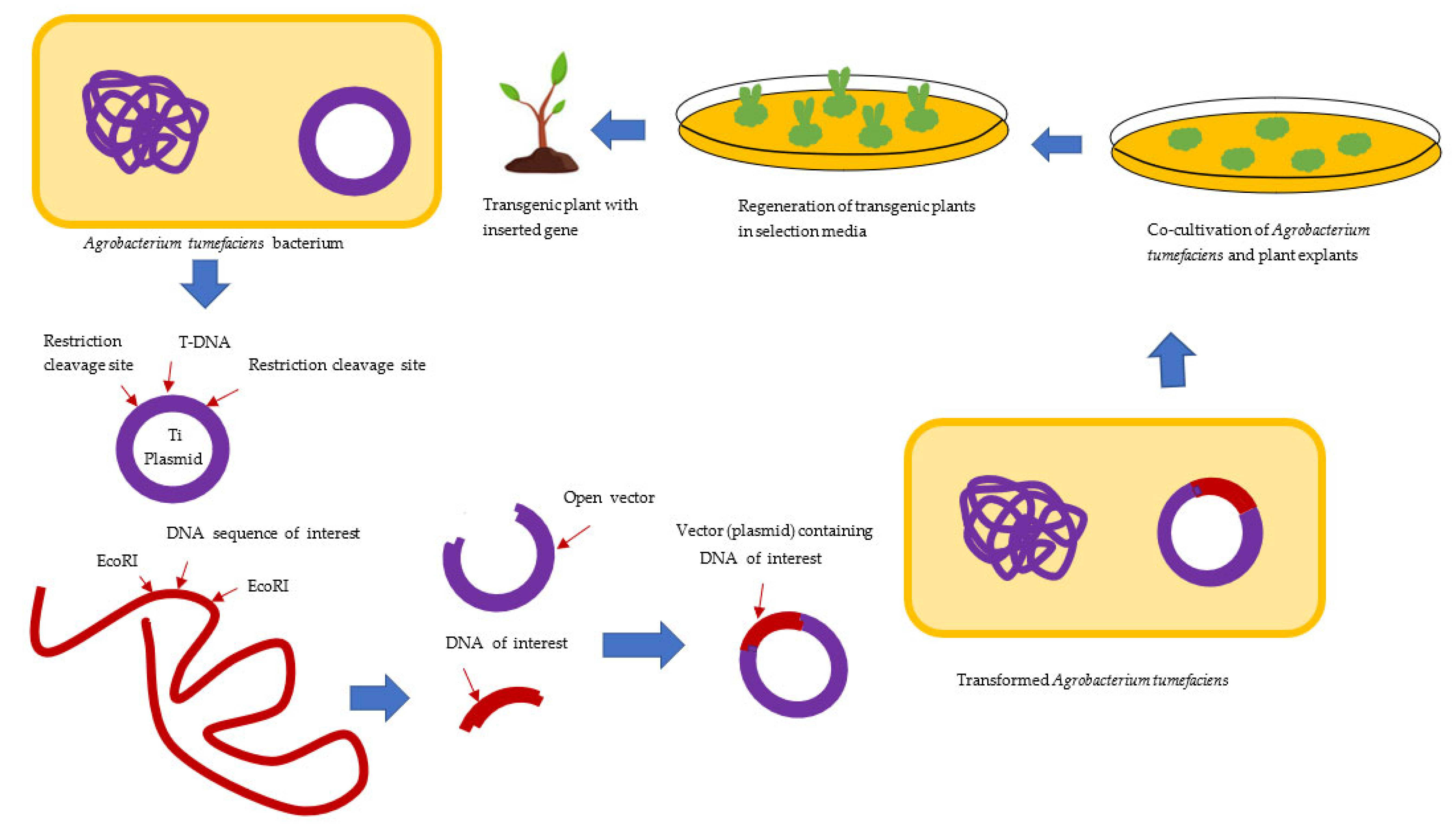
3.1. Agrobacterium-Mediated Transformation of the Plant
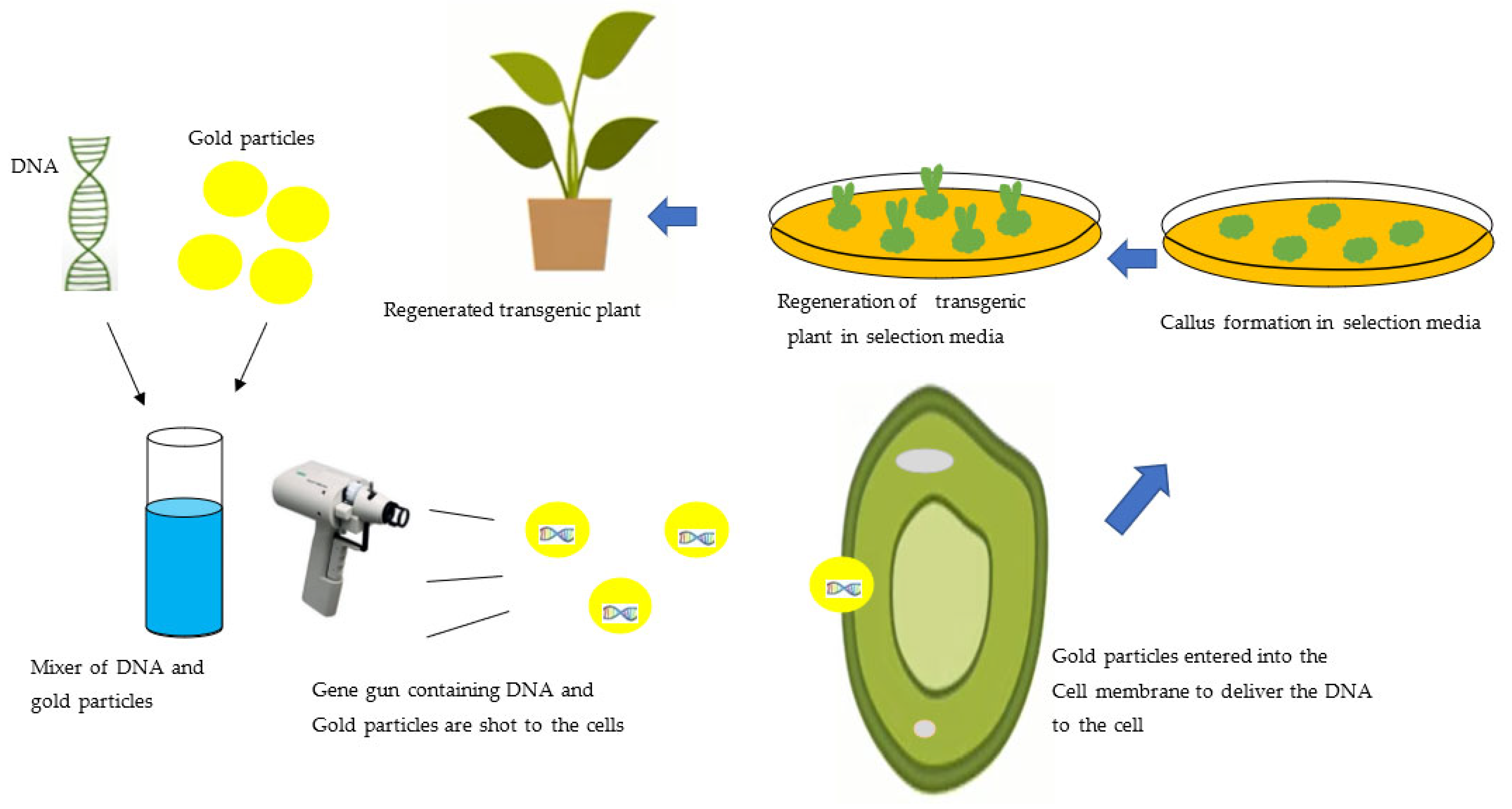
3.2. Biolistics Method of Genetic Transformation of the Plant
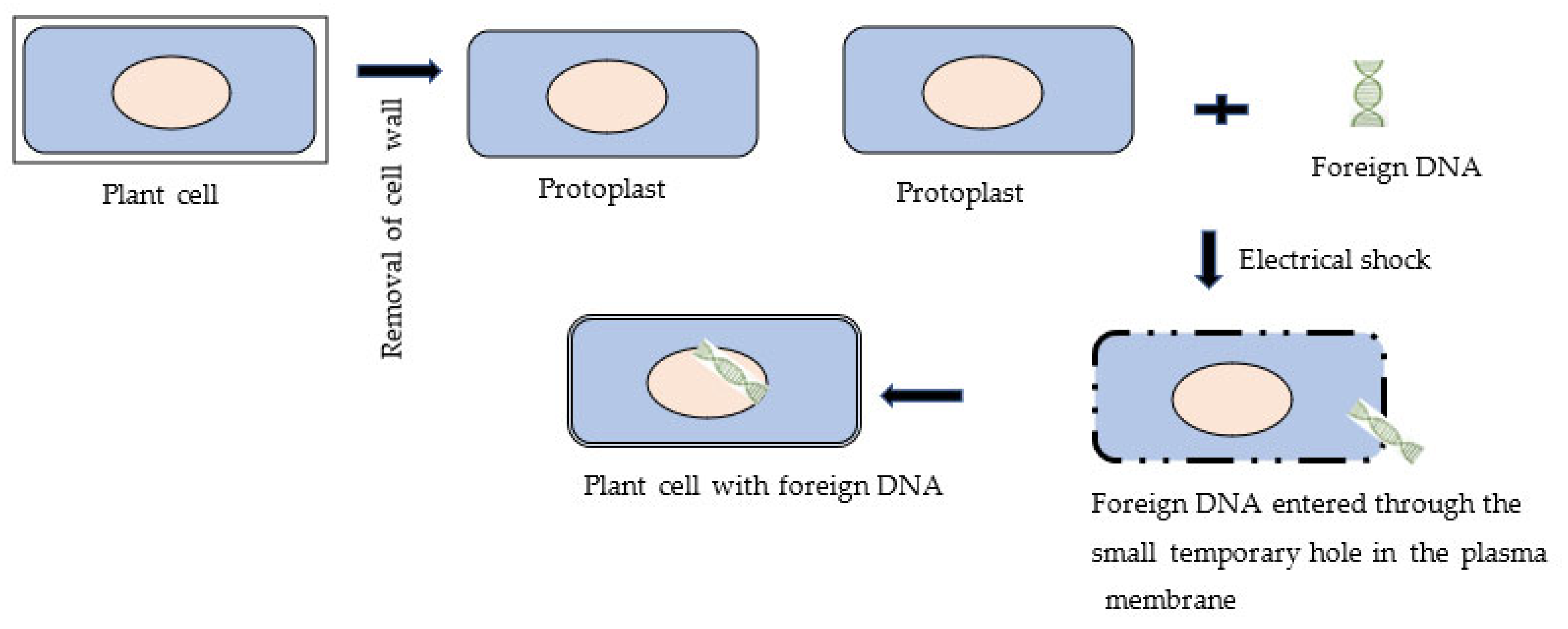
3.3. Electroporation Method of Genetic Transformation of the Plant
4. Benefits of Genetically Modified Plants and Products
4.1. Biofortification
4.2. Transgenic Approaches for Improving Phytochemicals and Biological Activities in Plants
| Scientific Name | Agrobacterium Strains/Vector | Gene | Phytochemicals | Biological Activity | References |
|---|---|---|---|---|---|
| Codonopsis lanceolata | LBA4404/pYBI121, | γ-tmt | Phenolic compounds and tocopherol | Antioxidant and antimicrobial activity | Ghimire et al. [25] |
| Perilla frutescens | LBA4404/pYBI130 | γ-tmt | Phenolic compounds and tocopherol | Antioxidant and antimicrobial activity | Ghimire et al. [57] |
| Lycopersicon esculentum L. | pBI101 | stilbene synthase (StSy) | Resveratrol | Antioxidant activity | D’Introno et al. [59] |
| Cucumis melo | MAFF 03–01724 (pRi1724) | rolC gene | Aroma essential oils (Z)-3-hexenol, (E)-2-hexenal, 1-nonanol, and (Z)-6-nonenol | Antimicrobial activity | Matsuda et al. [43] |
| Wheat | pMDC32 | Nicotianamine synthase 2 (OsNAS2) | Higher concentration of grain iron and zinc | Beasley et al. [60] | |
| Cassava | LBA4404/p8023 | FER1 and IRT1 | Higher concentration of iron and zinc | Narayanan et al. [61] | |
| Rice | pMDC32 | 35S-OsGGP | Increase concentrations of ascorbate | Broad et al. [62] | |
| Soybean | EHA105/pATPS1 | Overexpression of adenosine 5′-phosphosulfate sulfurylase 1 | Higher aamounts of sulfate, cysteine, and secondary metabolites in seeds | Kim et al. [63] | |
| Gynostemma pentaphyllum | ATCC 15834 | TL-DNA rolB | Triterpene saponins | Antitumor, immunopotentiating, antioxidant, antidiabetic | Chang et al. [64] |
| Momordica charantia | ATCC 15834 | rolC gene | Charantin | Antioxidant, antibacterial, antifungal | Thiruvengadam et al. [65] |
| Momordica dioica | KCTC 2703 | rolC gene | Phenolic compounds | Antioxidant, antibacterial. | Thiruvengadam et al. [66] |
| Cucumis anguria | KCTC 2703 | rolC gene | Phenolic compounds | antioxidant, antibacterial | Yoon et al. [67] |
| Lycopersicon esculentum Mill. | pBBC200/pBBC3 | LC and C1. | Flavonoids | Antioxidant activity | Le Gall et al. [68] |
| Rehmannia glutinosa | LBA4404/pMG-AhRS3 | Resveratrol Synthase Gene (RS3). | Phenolic compounds and Resveratrol | Antioxidant activity | Lim et al. [58] |
| Ipomoea batatas [L.] Lam. | pCAMBIA1300 | IbCAD1 | lignin contents, monolignol levels, and syringyl (S)/guaiacyl (G) | Stress tolerance | Lee et al. [69] |
| Miscanthus sinensis | LBA4404/pMBP1 | antisense COMT gene. | Lignin content | Lignin biosynthesis | Yoo et al. [70] |
| Cucumis melo | MAFF 03-01724 | rolCgene | Volatile compounds | Antimicrobial activity | Matsuda et al. [43] |
| Trigonella foenum-graecum L. | ARqua1 and LBA9402, nary vectorp35S::eGFP, | Green fluorescent protein gene [eGFP S65T variant | triterpene and steroidal saponins, phenolics, and galactomana | Heterologous expression | Garagounis et al. [45] |
| Sphagneticola calendulacea (L.) Pruski | LBA1334, pCAM:2 × 35S:g | rolA,rolB, rolC and gusA | Phenolics acid and flavonoids | Anti-hepatotoxic activity | Kundua et al. [46] |
| Morus notabilis | GV3101/pLGNL | MnMET1 | Flavonoid content | Inhibitory effect on Botrytis cinerea | Xin et al. [47] |
| Arabidopsis thaliana (L.) | pCAMBIA1301-AtMyB12 | AtMYB12 | Phenolic compounds | Increase in the flavonoid contents | Wang et al. [71] |
| Gynostemma pentaphyllum | ATCC 15834 | TL-DNA rolB | Triterpene saponins (gypenosides) | Antitumor, cholesterol lowering, immunopotentiating, antioxidant, hypoglycemic, antidiabetic activity | Chang et al. [64] |
| Aspergillus niger | ANIp7-laeA | LaeA | flaviolin, orlandin and kotan | Biosynthetic model for flaviolin | Wang et al. [72] |
| Nicotiana tabacum | pCAMBIA1301- | LlCCR | Phenolic compounds, | Wood properties | Prashant et al. [73] |
| Brassica rapa ssp. rapa | KCTC 2703 | rolC and virD2 | Phenolic compounds | Antioxidant activity, antimicrobial activity | Chung et al. [74] |
| Hypericum perforatum L. | Ri plasmid | rolB | Phenolic compounds, hypericin, and pseudohypericin | Antioxidant activity | Tusevski et al. [75] |
| Nicotiana tabacum L. | pGANE7/pBAK61 | AK-6b | Phenolic compounds | Auxin and cytokinin | Galis et al. [76] |
| Solanum tuberosum | LB4404/pBinKan-TX | TyrDC2 | Phenolic compounds, tyrosol glucoside | Increased resistance against pathogens | Landtag et al. [77] |
| Salvia miltiorrhiza Bunge | GV3101/pHB-GFP | RAS and CYP98A1 | Phenolic compounds | Antibacterial; Antioxidant activity; | Fu et al. [78] |
| Nicotiana tabacum L. | LB4404 | ipt-gene | Phenolic compounds | Peroxidase activity | Schnablová et al. [79] |
| Artemisia carvifolia Buch | GV3101 c/pPCV002 | rol Genes | Artemisinin | Increased production of artemisinin | Dilshad et al. [80] |
| Cucumis anguria L. | BA9402, A4, 15834, 13333, R1200, R1000 | rol A and rol B | Phenolic compounds | Antioxidant and antimicrobial activity | Sahayarayan et al. [81] |
| Medicago sativa | LBA4404 /pUC18-PAL | COMT and CCoAOMT | Phenolic compounds | Lignin biosynthesis | Guo et al. [82] |
| Nannochloropsis sp. | BA4404/pCAMBIA130404 | gus–mgfp5 | Phenolic compounds | Transient GUS expression in | Cha et al. [83] |
| Linum usitatissimum | C58C1:pGV2260 | Chalcone synthase (CHS), chalcone isomerase (CHI), and dihydroflavonol reductase (DFR) | Phenolic compounds, monounsaturated fatty acids, and lignans content | Antioxidant properties | Lorenc-Kukuła et al. [84] |
4.3. Transgenic Approaches for Environmental Protection
4.4. Transgenic Approaches for Removing Allergens
4.5. Transgenic Approaches for Phytoremediation
4.6. Transgenic Approaches for Vaccine Production
4.7. Transgenic Approach for Increased Biofuel Capacity in Plants
4.8. Increased Stress Resistance Capacity in Plants
5. Disadvantages of Genetically Modified Plants and Products
5.1. Human Health Hazards
5.2. Environmental Risks
5.3. Gene Flow
5.4. Increased Antibiotic Resistance
5.5. GMO Products Can Trigger Immune Reactions and Allergies
6. Biosafety Regulatory of GMO Foods and Products
7. Controversies of GM Foods and Products
8. Final Considerations and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- UN News. Over 820 Million People Suffering from Hunger; New UN Report Reveals Stubborn Realities of ‘Immense’ Global Challenge. UN News 2019. [Google Scholar]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Alexandratos, N.B.J. World Agriculture Towards 2030/2050. The 2012 Revision. Food and Agriculture Organization of the United Nations. ESA Working Paper no. 12-03. 2012. Available online: https://www.fao.org/3/ap106ee.pdf (accessed on 11 January 2023).
- Oliver, M.J. Why we need GMO crops in agriculture. MO Med. 2014, 111, 492–507. [Google Scholar]
- Moeller, L.; Wang, K. Engineering with Precision: Tools for the New Generation of Transgenic Crops. BioScience 2008, 58, 391. [Google Scholar] [CrossRef]
- Chandler, S.; Tanaka, Y. Genetic modifi cation in floriculture. Crit. Rev. Plant Sci. 2007, 26, 169–197. [Google Scholar] [CrossRef]
- Paiva, M.J.M.; Damasceno, I.A.M. O uso de dos alimentos geneticamente modificados: Principais desafios. Rev. Multidebates 2020, 4, 90–96. [Google Scholar]
- Torres, A.C.; Ferreira, A.T.; Sa, F.G.; Buso, J.A.; Caldas, L.S.; Nascimento, A.S.; Brigido, M.M.; Romano, E. Glossário De Biotecnologia Vegetal; Em Brapa-CNPH: Brasília, Brazil, 2000; p. 128. [Google Scholar]
- Welch, R.M. Biotechnology, biofortification and global health. Food Nutr Bull. 2005, 26, 4. [Google Scholar] [CrossRef]
- Nanjundan, J.; Singh, K.H.; Parmar, N.; Kumar, P.; Chauhan, D.K.; Khan, Y.J.; Thaku, A.K.; Sharma, D.; Singh, L. Genetic Engineering Strategies for Biotic and Abiotic Stress Tolerance and Quality Enhancement in Horticultural Crops: A Comprehensive Review. 3 Biotech 2017, 7, 1–35. [Google Scholar]
- Fischer, R.; Emans, N. Molecular farming of pharmaceutical proteins. Transgenic Res. 2000, 9, 279–299. [Google Scholar] [CrossRef]
- Lee, T.H.; Ho, H.K.; Leung, T.F. Genetically modified foods and allergy. Hong Kong Med. J. 2017, 23, 5–291. [Google Scholar] [CrossRef] [PubMed]
- Clive, J. Global Status of Commercialized Biotech/GM Crops; ISAAA Briefs 43; International Service for the Acquisition of Agri-Biotech Applications: Ithaca, NY, USA, 2011. [Google Scholar]
- Brookes, G.; Barfoot, P. Farm income and production impacts of using GM crop technology 1996–2016. GM. Crops Food 2018, 9, 59–89. [Google Scholar] [CrossRef] [PubMed]
- Gatew, H.; Mengistu, K. Genetically modified foods (GMOs); a review of genetic engineering. J. Life Sci. Biomed. 2019, 9, 157–163. [Google Scholar] [CrossRef]
- Ventura, M.V.A.; Batista, H.R.F.; Bessa, M.M.; Pereira, L.S.; Costa, E.M.; de Oliveira, M.H.R. Comparison of conventional and transgenic soybean production costs in different regions in Brazil. Res. Soc. Dev. 2020, 9, e154973977. [Google Scholar] [CrossRef]
- Kuiper, H.A.; Davies, H.V. The safe foods risk analysis framework suitable for GMOs? A case study. Food Control. 2010, 21, 1662–1676. [Google Scholar] [CrossRef]
- Twyman, R.M.; Kohli, A.; Stoger, E.; Christou, P. Foreign DNA: Integration and Expression in Transgenic Plants. In Genetic Engineering: Principles and Methods; Setlow, J.K., Ed.; Springer: Boston, MA, USA, 2002; Volume 24, pp. 107–136. [Google Scholar]
- Che1, P.; Chang, S.; Simon, M.K.; Zhang, Z.; Shaharyar, A.; Ourada, J.; O’Neill, D.; Torres-Mendoza, M.; Guo, Y.; Marasigan, K.M.; et al. Developing a rapid and highly efficient cowpea regeneration, transformation and genome editing system using embryonicaxis explants. Plant J. 2021, 106, 817–830. [Google Scholar] [CrossRef]
- Okpe, A.O.; Nka, F.A. Comparative Review of Plant Transformation Techniques. J. Adv. Biol. Biotechnol. 2021, 24, 1–18. [Google Scholar] [CrossRef]
- Matveeva, T.V.; Lutova, L.A. Horizontal gene transfer from Agrobacteriumto plants. Front. Plant Sci. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Xia, P.; Hu, W.; Liang, T.; Yang, D.; Liang, Z. An attempt toestablish an Agrobacterium-mediated transient expression system inmedicinal plants. Protoplasma 2020, 257, 1497–1505. [Google Scholar] [CrossRef]
- Ziemienowicz, A. Agrobacterium-mediated plant transformation: Factors, applications and recent advances. Bio Catal. Agric. Biotechnol. 2014, 3, 95–102. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Seong, E.S.; Lim, J.D.; Heo, K.; Kim, M.J.; Chung, I.M.; Juvik, J.A.; Yu, C.Y. Agrobacterium-mediated transformation of Codonopsis lanceolata using the c-TMT gene. Plant Cell. Tiss. Organ. Cult. 2008, 95, 265–274. [Google Scholar] [CrossRef]
- Niazian, M.; Sadat Noori, S.A.; Galuszka, P.; Mortazavian, S.M.M. Tissue culture-based Agrobacterium-mediated and in planta transformation methods. Czech J. Genet. Plant Breed. 2017, 53, 133–143. [Google Scholar] [CrossRef]
- Christou, P. Genetic transformation of crops using microprojectile bombardment. Plant J. Oxf. 1992, 2, 275–281. [Google Scholar] [CrossRef]
- Lacroix, B.; Citovsky, V. Biolistic Approach for Transient Gene Expression Studies in Plants. Methods Mol. Biol. 2020, 2124, 125–139. [Google Scholar]
- Hernandez-Garcia, C.; Bouchard, R.; Rushton, P.; Jones, M.; Chen, X.; Timko, M.; Finer, J. High level transgenic expression of soybean (Glycine max) GmERF and Gmubi gene promoters isolated by a novel promoter analysis pipeline. BMC Plant Biol. 2010, 10, 237. [Google Scholar] [CrossRef]
- Taylor, N.J.; Fauquet, C.M. Microparticle bombardment as a tool in plant science and agricultural biotechnology. DNA Cell Biol. 2002, 21, 963–977. [Google Scholar] [CrossRef]
- Al-Dosari, M.S.; Gao, X. Non-viral Gene Delivery: Principle, limitations, and recent progress. AAPS J. 2009, 11, 671–681. [Google Scholar] [CrossRef]
- Rubinsky, B. Irreversible electroporation in medicine. Technol. Cancer Res. Treat. 2007, 6, 255–260. [Google Scholar] [CrossRef]
- United Naations. United Naations. United Nations System Standing Committee on Nutrition (SCN). In 5th Report on the World Nutrition Situation Nutrition for Improved Development Outcomes; SCN: Geneva, Switzerland, 2004. [Google Scholar]
- Szenkovics, D.; Tonk, M.; Balog, A. Can genetically modified (GM) crops act as possible alternatives to mitigate world political conflicts for food? Food Energy Secur. 2021, 10, e268. [Google Scholar] [CrossRef]
- Kamthan, A.; Chaudhuri, A.; Kamthan, M.; Datta, A. Genetically modified (GM) crops: Milestones and new advances in crop improvement. Theor. Appl. Genet. 2016, 129, 1639–1655. [Google Scholar] [CrossRef]
- Singh, O.V.; Ghai, S.; Paul, D.; Jain, R.K. Genetically modified crops: Success, safety assessment, and public concern. Appl. Microbiol. Biotechnol. 2006, 71, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Goodman, R.E.; Hefle, S.L. The development of safety assessment for genetically modified foods. Asia Pac. Biotech. News 2006, 10, 614–616. [Google Scholar] [CrossRef]
- American Medical Association. Report 2 of the Council on Science and Public Health: Labeling of Bioengineered Foods; American Medical Association: Washington, DC, USA, 2012. [Google Scholar]
- Gilbert, P.R. Biotechnology Industries and Entrepreneurs; Darya Ganj: New Delhi, India, 2008; pp. 25–180. [Google Scholar]
- Houdebine, L.M. Impacts of genetically modified animals on the ecosystem and human activities. Glob. Bioeth. 2014, 25, 3–18. [Google Scholar] [CrossRef]
- Schubert, D. A different perspective on GM food. Nat. Biotechnol. 2002, 20, 969. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Hawthorne, W.J.; Manolios, N. Gene therapy in diabetes. Self Nonself Pharm. 2010, 1, 165–175. [Google Scholar] [CrossRef]
- Matsuda, Y.; Toyoda, H.; Sawabe, A.; Maeda, K.; Shimizu, N.; Fujita, N.; Fujita, T.; Nonomura, T.; Ouchi, S. A hairy root culture of melon produces aroma compounds. J. Agric. Food Chem. 2000, 48, 1417–1420. [Google Scholar] [CrossRef]
- Oller, A.L.W.; Agostini, E.; Talano, M.A.; Capozucca, C.; Milrad, S.R.; Tigier, H.A.; Medina, M.I. Overexpression of a basic peroxidase in transgenic tomato (Lycopersicon esculentum Mill. cv. Pera) hairy roots increases phytoremediation of phenol. Plant Sci. 2005, 169, 1102–1111. [Google Scholar] [CrossRef]
- Garagounis, C.; Beritza, K.; Georgopoulou, M.E.; Sonawane, P.; Haralampidis, K.; Goossens, A.; Aharoni, A.; Papadopoulou, K.K. A hairy-root transformation protocol for Trigonella foenum-graecum L. as a tool for metabolic engineering and specialised metabolite pathway elucidation. Plant Physiol. Biochem. 2020, 154, 451–462. [Google Scholar] [CrossRef]
- Kundua, S.; Salma, U.; Ali, M.N.; Hazra, A.K.; Mandal, N. Development of transgenic hairy roots and augmentation of secondary metabolites by precursor feeding in Sphagneticola calendulacea (L.) Pruski. Ind. Crops Prod. 2018, 121, 206–215. [Google Scholar] [CrossRef]
- Xin, Y.; Ma, B.; Zeng, Q.; He, W.; Qin, M.; He, N. Dynamic changes in transposable element and gene methylation in mulberry (Morus notabilis) in response to Botrytis cinerea. Hortic. Res. 2021, 8, 154. [Google Scholar] [CrossRef]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L.; et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, Y.; Li, M.; Cheng, T.; Li, S.W.; Zhang, J.; Xia, N.S. Oral immunization of animals with transgenic cherry tomatillo expressing HbsAg. World J. Gastroenterol. 2003, 9, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Van Eenennaam, A.L.; Lincoln, K.; Durrett, T.P.; Valentin, H.E.; Shewmaker, C.K.; Thorne, G.M. Engineering vitamin E content: From Arabidopsis mutant to soil oil. Plant Cell. 2003, 5, 3007–3019. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Shin, K.; Lönnerdal, B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry 2001, 40, 15771–15779. [Google Scholar] [CrossRef]
- Xiaoyan, S.; Yan, Z.; Shubin, W. Improvement Fe content of wheat (Triticum aestivum) grain by soybean ferritin expression cassette without vector backbone sequence. J. Agric. Food Biochem. 2012, 20, 766–773. [Google Scholar]
- Drakakaki, G.; Marcel, S.; Glahn, R.P.; Lund, E.K.; Pariagh, S.; Fischer, R.; Christou, P.; Stoger, E. Endosperm-specific co-expression of recombinant soybean ferritin and Aspergillus phytase in maize results in significant increases in the levels of bioavailable iron. Plant Mol. Biol. 2005, 59, 869–880. [Google Scholar] [CrossRef]
- Goto, F.; Yoshihara, T. Improvement of micronutrient contents by genetic engineering development of high iron content crops. Plant Biotechnol. 2001, 18, 7–15. [Google Scholar] [CrossRef]
- Masuda, H.; Usuda, K.; Kobayashi, T.; Ishimaru, Y.; Kakei, Y.; Takahashi, M.; Higuchi, K.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Overexpression of the barley nicotianamine synthase gene HvNAS1 increases iron and zinc concentrations in rice grains. Rice 2009, 2, 155–166. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Seong, E.S.; Lee, C.O.; Lim, J.D.; Lee, J.G.; Yoo, J.H.; Chung, I.M.; Kim, N.Y.; Yu, C.Y. Enhancement of α-tocopherol content in transgenic Perilla frutescens containing the γ-TMT gene. Afr. J. Biotechnol. 2011, 10, 2430–2439. [Google Scholar]
- Long, M.; Millar, D.J.; Kimura, Y.; Donovan, G.; Rees, J.; Fraser, P.D.; Bramley, P.M.; Bolwell, G.P. Metabolite profiling of carotenoid and phenolic pathways in mutant and transgenic lines of tomato: Identification of a high antioxidant fruit line. Phytochemistry 2006, 67, 1750–1757. [Google Scholar] [CrossRef]
- Lim, J.D.; Yang, D.C.; Yun, S.J.; Chung, I.M.; Sung, E.S.; Kim, M.J.; Heo, K.; Yu, C.Y. Isolation and Biological Activity of Resveratrol−3−O−β−D−GlucosideResveratrol−3−O−β−D−Glucoside in Transgenic Rehmannia glutinosa L. Transformed by Peanut Resveratrol Synthase Gene (RS3). Korean J. Med. Crop Sci. 2004, 12, 406–414. [Google Scholar]
- D’Introno, A.; Paradiso, A.; Scoditti, E.; D’Amico, L.; De Paolis, A.; Carluccio, M.A.; Nicoletti, I.; De Gara, L.; Santino, A.; Giovinazzo, G. Antioxidant and anti-inflammatory properties of tomato fruits synthesizing different amounts of stilbenes. Plant Biotechnol. J. 2000, 7, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.T.; Bonneau, J.P.; Sánchez-Palacios, J.T.; Moreno-Moyano, L.T.; Callahan, D.L.; Tako, E.; Glahn, R.P.; Lombi, E.; Johnson, A.A.T. Metabolic engineering of bread wheat improves grain iron concentration and bioavailability. Plant Biotechnol. J. 2019, 17, 1514–1526. [Google Scholar] [CrossRef]
- Narayanan, N.; Beyene, G.; Chauhan, R.D.; Gaitán-Solís, E.; Gehan, J.; Butts, P.; Siritunga, D.; Okwuonu, I.; Woll, A.; Jiménez- Aguilar, D.M.; et al. Biofortification of field-grown cassava by engineering expression of an iron transporter and ferritin. Nat. Biotechnol. 2019, 37, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Broad, R.C.; Bonneau, J.P.; Beasley, J.T.; Roden, S.; Sadowski, P.; Jewell, N.; Brien, C.; Berger, B.; Tako, E.; Glahn, R.P.; et al. Effect of Rice GDP-L-Galactose Phosphorylase Constitutive Overexpression on Ascorbate Concentration, Stress Tolerance, and Iron Bioavailability in Rice. Front. Plant Sci. 2020, 11, 595439. [Google Scholar] [CrossRef]
- Kim, W.-S.; Sun-Hyung, J.; Oehrle, N.W.; Jez, J.M.; Krishnan, H.B. Overexpression of ATP sulfurylase improves the sulfur amino acid content, enhances the accumulation of Bowman-Birk protease inhibitor and suppresses the accumulation of the β-subunit of β-conglycinin in soybean seeds. Sci. Rep. 2020, 10, 14989. [Google Scholar] [CrossRef]
- Chang, C.K.; Chang, K.S.; Lin, Y.C.; Liu, S.Y.; Chen, C.Y. Hairy root cultures of Gynostemma pentaphyllum (Thunb.) Makino: A promising approach for the production of gypenosides as an alternative of ginseng saponins. Biotechnol Lett. 2005, 27, 1165–1169. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Praveen, N.; Kim, S.H.; Chung, I.M. Establishment of Momordica charantia hairy root cultures for the production of phenolic compounds and the determination of their biological activities. Plant Cell Tissue Organ Cult. 2014, 118, 545–557. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Rekha, K.; Chung, I.M. Induction of hairy roots by Agrobacterium rhizogenes-mediated transformation of spine gourd (Momordica dioica Roxb. ex. willd) for the assessment of phenolic compounds and biological activities. Sci. Hortic. 2016, 198, 132–141. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Chung, I.M.; Thiruvengadam, M. Evaluation of phenolic compounds, antioxidant and antimicrobial activities from transgenic hairy root cultures of gherkin (Cucumis anguria L.). S. Afr. J. Bot. 2015, 100, 80–86. [Google Scholar] [CrossRef]
- Le Gall, G.; DuPont, M.S.; Mellon, F.A.; Davis, A.L.; Collins, G.J.; Verhoeyen, M.E.; Colquhoun, I.J. Characterization and Content of Flavonoid Glycosides in Genetically Modified Tomato (Lycopersicon esculentum) Fruits. J. Agric. Food Chem. 2003, 51, 2438–2446. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Kim, S.E.; Park, S.U.; Lim, Y.H.; Choi, H.Y.; Kim, W.G.; Ji, C.Y.; Kim, H.S.; Kwak, S.S. Tuberous roots of transgenic sweet potato overexpressing IbCAD1 have enhanced low-temperature storage phenotypes. Plant Physiol. Biochem. 2021, 166, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Seong, E.S.; Ghimire, B.K.; Heo, K.; Jin, X.; Yamada, T.; Clark, L.V.; Sacks, E.J.; Yu, C.Y. Establishment of Miscanthus sinensis with decreased lignin biosynthesis by Agrobacterium–mediated transformation using antisense COMT gene. Plant Cell Tissue Organ Cult. 2018, 133, 359–369. [Google Scholar] [CrossRef]
- Wang, F.; Kong, W.; Wong, G.; Fu, L.; Peng, R.; Li, Z.; Yao, Q. AtMYB12 regulates flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis thaliana. Mol. Gen. Genom. 2016, 291, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, X.; Tabudravu, J.; Wang, S.; Deng, H.; Pan, L. The chemical profile of activated secondary metabolites by overexpressing LaeA in Aspergillus niger. Microbiol. Res. 2021, 248, 126735. [Google Scholar] [CrossRef] [PubMed]
- Prashant, S.; Srilakshmi Sunita, M.; Pramod, S.; Gupta, R.K.; Anil Kumar, S.; Karumanchi, S.R.; Rawal, S.K.; Kavi Kishor, P.B. Down-regulation of Leucaena leucocephala cinnamoyl CoA reductase (LlCCR) gene induces significant changes in phenotype, soluble phenolic pools and lignin in transgenic tobacco. Plant Cell Rep. 2011, 30, 2215–2231. [Google Scholar] [CrossRef]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Production of glucosinolates, phenolic compounds and associated gene expression profiles of hairy root cultures in turnip (Brassica rapa ssp. rapa). 3 Biotech 2016, 6, 175. [Google Scholar] [CrossRef]
- Tusevski, O.; Petreska Stanoeva, J.; Stefova, M.; Pavokovic, D.; Gadzovska Simic, S. Identification and quantification of phenolic compounds in Hypericum perforatum L. transgenic shoots. Acta Physiol. Plant 2014, 36, 2555–2569. [Google Scholar] [CrossRef]
- Galis, I.; Kakiuchi, Y.; Simek, P.; Wabiko, H. Agrobacterium tumefaciens AK-6b gene modulates phenolic compound metabolism in tobacco. Phytochemistry 2004, 65, 169–179. [Google Scholar] [CrossRef]
- Landtag, J.; Baumert, A.; Degenkolb, T.; Schmidt, J.; Wray, V.; Scheel, D.; Strack, D.; Rosahl, S. Accumulation of tyrosol glucoside in transgenic potato plants expressing a parsley tyrosine decarboxylase. Phytochemistry 2002, 60, 683–689. [Google Scholar] [CrossRef]
- Fu, R.; Shi, M.; Deng, C.; Zhang, Y.; Zhang, X.; Wang, Y.; Kai, G. Improved phenolic acid content and bioactivities of Salvia miltiorrhiza hairy roots by genetic manipulation of RAS and CYP98A. Food Chem. 2020, 331, 1273652. [Google Scholar] [CrossRef] [PubMed]
- Schnablova, T.; Synkova, H.; Vicankova, A.; Burketova, L.; Eder, J.; Cvikrova, M. Transgenic ipt tobacco over producing cytokinins over accumulates phenolic compounds during in vitro growth. Physiol. Bio Chem. 2006, 44, 526–534. [Google Scholar]
- Dilshad, E.; Cusido, R.M.; Estrada, K.R.; Bonfill, M.; Mirza, B. Genetic Transformation of Artemisia carvifolia Buch with rol Genes Enhances Artemisinin Accumulation. PLoS ONE 2015, 10, e0140266. [Google Scholar] [CrossRef] [PubMed]
- Sahayarayan, J.; Udayakumar, R.; Arun, M.; Ganapathi, A.; Alwahibi, M.S.; Aldosari, N.S.; Morgan, A.M.A. Effect of different Agrobacterium rhizogenes strains for in-vitro hairy root induction, total phenolic, flavonoids contents, antibacterial and antioxidant activity of (Cucumis anguria L.). Saudi J. Biol. Sci. 2020, 27, 2972–2979. [Google Scholar] [CrossRef]
- Guo, D.; Chen, F.; Dixon, R.A. Monolignol biosynthesis in microsomal preparations from lignifying stems of alfalfa (Medicago sativa L.). Phytochemistry 2002, 61, 657–667. [Google Scholar] [CrossRef]
- Cha, T.S.; Chen, C.F.; Yee, W.; Aziz, A.; Loh, S.H. Cinnamic acid, coumarin and vanillin: Alternative phenolic compounds for efficient Agrobacterium-mediated transformation of the unicellular green alga, Nannochloropsis sp. J. Microbiol. Methods 2011, 84, 430–434. [Google Scholar] [CrossRef]
- Lorenc-Kukuła, K.; Amarowicz, R.; Oszmiański, J.; Doer_mann, P.; Starzycki, M.; Skała, J.; Zuk, M.; Kulma, A.; Szopa, J. Pleiotropic Effect of Phenolic Compounds Content Increases in Transgenic Flax Plant. J. Agric. Food Chem. 2005, 53, 3685–3692. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal volatiles from bacillus atrophaeus gbsc56 promote growth and stimulate induced systemic resistance in tomato against meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Peng, D.; Wan, D.; Cheng, C.; Ye, X.; Sun, M. Nematode-specific cadherin CDH-8 acts as a receptor for Cry5B toxin in Caenorhabditis elegans. Appl. Microbiol. Biotechnol. 2018, 102, 3663–3673. [Google Scholar] [CrossRef]
- Stewart, C.N., Jr.; Adang, M.J.; All, J.N.; Raymer, P.L.; Ramachandran, S.; Parrott, W.A. Insect control and dosage effects in transgenic canola containing a synthetic Bacillus thuringiensis cryIAc gene. Plant Physiol. 1996, 112, 115–120. [Google Scholar] [CrossRef][Green Version]
- Singh, S.; Kumar, N.R.; Maniraj, R.; Lakshmikanth, R.; Rao, K.Y.S.; Muralimohan, N.; Arulprakash, T.; Karthik, K.; Shashibhushan, N.B.; Vinutha, T. Expression of Cry2Aa, a Bacillus thuringiensis insecticidal protein in transgenic pigeon pea confers resistance to gram pod borer, Helicoverpa armigera. Sci. Rep. 2018, 8, 8820. [Google Scholar] [CrossRef] [PubMed]
- Bríza, J.; Pavingerová, D.; Vlasák, J.; Niedermeierová, H. Norway spruce (Picea abies) genetic transformation with modified Cry3A gene of Bacillus thuringiensis. Acta Biochim. Pol. 2013, 60, 395–400. [Google Scholar] [CrossRef]
- Heimlich, R.E.; Fernandez-Cornejo, J.F.; McBride, W.; Klotz-Ingram, C.; Jans, S.; Brooks, N. Adoption of Genetically Engineered Seed in US Agriculture: Implication for Pesticide Use; sld001; USDA Publication: Washington, DC, USA, 2000. [Google Scholar]
- United States Department of Agriculture. Genetically Engineered Crops: Has Adoption Reduced Pesticide Use? Agricultural Outlook August. 2000. Available online: www.ers.usda.gov/publications/agoutlook/aug2000/ao273f.pdf (accessed on 31 August 2000).
- Tohidfar, M.; Zare, N.; Jouzani, G.S.; Eftekhari, S.M. Agrobacterium mediated transformation of alfalfa (Medicago sativa) using a synthetic cry3a gene to enhance resistance against alfalfa weevil. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 113, 227–235. [Google Scholar] [CrossRef]
- Alfonso-Rubí, J.; Ortego, F.; Castañera, P.; Carbonero, P.; Díaz, I. Transgenic expression of trypsin inhibitor CMe from barley in Indica and Japonica rice, confers resistance to the rice weevil Sitophilus oryzae. Transgenic Res. 2003, 12, 2331. [Google Scholar] [CrossRef] [PubMed]
- Tougou, M.; Furutani, N.; Yamagishi, N.; Shizukawa, Y.; Takahata, Y.; Hidaka, S. Development of resistant transgenic soybeans with inverted repeat-coat protein genes of soybean dwarf virus. Plant Cell Rep. 2006, 25, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.C.; Gomes, K.A.; de Carvalho Filho, M.M.; Ricardo Harakava, R.; Carels, N.; Siqueira, W.J.; Latado, R.R.; de Argollo Marques, D. Agrobacterium-mediated transformation of Jatropha curcas leaf explants with a fungal chitinase gene. Afr. J. Biotechnol. 2016, 15, 2006–2016. [Google Scholar]
- Macrae, T.C.; Baur, M.E.; Boethel, D.J.; Fitzpatrick, B.J.; Gao, A.G.; Gamundi, J.C.; Harrison, L.A.; Kabuye, V.T.; Mcpherson, R.M.; Miklos, J.A.; et al. Laboratory and field evaluations of transgenic soybean exhibiting highdose expression of a synthetic Bacillus thuringiensis cry1A gene for control of Lepidoptera. J. Econ. Entomol. 2005, 98, 577–587. [Google Scholar] [CrossRef]
- Tohidfar, M.; Ghareyazie, B.; Mosavi, M.; Yazdani, S.; Golabchian, R. Agrobacterium-mediated transformation of cotton (Gossypium hirsutum) using a synthetic cry1Ab gene for enhanced resistance against Heliothis armigera. Iran. J. Biotechnol. 2008, 6, 164–173. [Google Scholar]
- Papolu, P.K.; Dutta, T.K.; Tyagi, N.; Urwin, P.E.; Lilley, C.J.; Rao, U. Expression of a cystatin transgene in eggplant provides resistance to root-knot nematode, Meloidogyne incognita. Front Plant. Sci. 2016, 7, 1122. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Liu, H.M.; Liu, X.Z. Production of transgenic kiwifruitplants harboring the SbtCry1Ac gene. Genet. Mol. Res. 2015, 14, 8483–8489. [Google Scholar] [CrossRef]
- Heydarian, Z.; Yu, M.; Gruber, M.; Glick, B.R.; Zhou, R.; Hegedus, D.D. Inoculation of soil with plant growth promoting bacteria producing 1-aminocyclopropane-1-carboxylate deaminase or expression of the corresponding acdS gene in transgenic plants increases salinity tolerance in Camelina sativa. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, J.; Qin, X.; Shi, X.; Luo, L.; Zhang, G.; Yu, H.; Li, C.; Hu, M.; Liu, Q.; et al. JcCBF2 gene from Jatropha curcas improves freezing tolerance of Arabidopsis thaliana during the early stage of stress. Mol. Biol. Rep. 2015, 42, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Feng, Y.; Ahn, S.J. Alteration of leaf shape, improved metal tolerance, and productivity of seed by overexpression of CsHMA3 in Camelina sativa. Biotechnol. Biofuels 2014, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Losada, O.A.; Fonseca, C.A.G. Alimentos transgenicosy alergenicidad. Rev. Fac. Med. 2007, 55, 251–269. [Google Scholar]
- Dodo, H.W.; Konan, N.K.; Chen, F.C.; Egnin, M.; Viquez, O.M. Alleviating peanut allergy using genetic engineering: The silencing of the immunodominant allergen Ara h 2 leads to its significant reduction and a decrease in peanut allergenicity. Plant Biotechnol. J. 2008, 6, 135–145. [Google Scholar] [CrossRef]
- Le, L.Q.; Mahler, V.; Lorenz, Y.; Scheurer, S.; Biemelt, S.; Vieths, S.; Sonnewald, U. Reduced allergenicity of tomato fruits harvested from Lyc e 1-silenced transgenic tomato plants. J. Allergy Clin. Immunol. 2006, 118, 1176–1183. [Google Scholar] [CrossRef]
- Gilissen, L.J.; Bolhaar, S.T.; Matos, C.I.; Rouwendal, G.J.; Boone, M.J.; Krens, F.A.; Zuidmeer, L.; Van Leeuwen, A.; Akkerdaas, J.; Hoffmann-Sommergruber, K.; et al. Silencing the major apple allergen Mal d 1 by using the RNA interference approach. J. Allergy Clin. Immunol. 2005, 115, 364–369. [Google Scholar] [CrossRef]
- Herman, E.M.; Helm, R.M.; Jung, R.; Kinney, A.J. Genetic modification removes an immunodominant allergen from soybean. Plant Physiol. 2003, 132, 36–43. [Google Scholar] [CrossRef]
- Bhalla, P.L.; Swoboda, I.; Singh, M.B. Reduction in allergenicity of grass pollen by genetic engineering. Int. Arch. Allergy Immunol. 2001, 124, 51–54. [Google Scholar] [CrossRef]
- Garbisu, C.; Hernandez, A.J.; Barrutia, O.; Alkorta, I.; Becerril, J.M. Phytoremediation: A technology using green plants to remove contaminants from polluted areas. Rev. Environ. Health 2002, 17, 173–188. [Google Scholar] [CrossRef]
- Sun, L.; Ma, Y.; Wang, H.; Huang, W.; Wang, X.; Han, L.; Sun, W.; Han, E.; Wang, B. Overexpression of PtABCC1 contributes to mercury tolerance and accumulation in Arabidopsis and poplar. Biochem. Biophys. Res. Commun. 2018, 497, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, C.; Ros, R.; Haro, A.D.; Walker, D.J.; Bernal, M.P.; Serrano, R.; Avino, J.N. A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Biochem. Biophys. Res. Commun. 2003, 303, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dhankher, O.P.; Carreira, L.; Lee, D.; Chen, A.; Schroeder, J.I.; Balish, R.S.; Meagher, R.B. Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol. 2004, 45, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.N.; Anderson, C.W.; Stewart, R.B.; Robinson, B.H. Mercury volatilisation and phytoextraction from base-metal mine tailings. Environ. Pollut. 2005, 136, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Bizily, S.P.; Rugh, C.L.; Meagher, R.B. Phytodetoxification of hazardous organomercurials by genetically engineered plants. Nat. Biotechnol. 2000, 18, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Van Huysen, T.; Abdel-Ghany, S.; Hale, K.L.; LeDuc, D.; Terry, N.; Pilon-Smits, E.A. Overexpression of cystathionine-gamma- synthase enhances selenium volatilization in Brassica juncea. Planta 2003, 218, 71–78. [Google Scholar] [CrossRef]
- Wolfe, N.L.; Hoehamer, C.F. Enzymes used by plants and microorganisms to detoxify organic compounds. In Phytore-Mediation: Transformation and Control of Contaminants; McCutcheon, S.C., Schnoor, J.L., Eds.; New Wiley: New York, NY, USA, 2003; pp. 159–187. [Google Scholar]
- Collins, C.; Laturnus, F.; Nepovim, A. Remediation of BTEX and trichloroethene. Current knowledge with special emphasis on phytoremediation. Environ. Sci. Pollut. Res. Int. 2002, 9, 86–94. [Google Scholar] [CrossRef]
- Gullner, G.; Komives, T.; Rennenberg, H. Enhanced tolerance of transgenic poplar plants overexpressing g-glutamylcysteine synthetase towards chloroacetanilide herbicides. J. Exp. Bot. 2001, 52, 971–979. [Google Scholar] [CrossRef]
- Flocco, C.G.; Lindblom, S.D.; Smits, E.A. Overexpression of enzymes involved in glutathione synthesis enhances tolerance to organic pollutants in Brassica juncea. Int. J. Phytochem. 2004, 6, 289–304. [Google Scholar] [CrossRef]
- Migocka, M.; Papierniak, A.; Kosieradzka, A.; Posyniak, E.; Maciaszczyk-Dziubinska, E.; Biskup, R.; Garbiec, A.; Marchewka, T. Cucumber metal tolerance protein CsMTP9 is a plasma membrane H1-coupled antiporter involved in the Mn21 and Cd21 efflux from root cells. Plant J. 2015, 84, 10451058. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Bhattacharya, S.; Maiti, M.K. Enhanced cadmium accumulation and tolerance in transgenic tobacco overexpressing rice metal tolerance protein gene OsMTP1 is promising for phytoremediation. Plant. Physiol. Biochem. 2016, 105, 297309. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Wu, S.; Cui, Y.; Zhang, L.; Dong, H. Overexpression of the Tamarix hispida ThMT3 gene increases copper tolerance and adventitious root induction in Salix matsudana Koidz. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 121, 469479. [Google Scholar] [CrossRef]
- Zanella, L.; Fattorini, L.; Brunetti, P.; Roccotiello, E.; Cornara, L.; D’Angeli, S.; Della Rovere, F.; Cardarelli, M.; Barbieri, M.; Sanità di Toppi, L. Overexpression of AtPCS1 in tobacco increases arsenic and arsenic plus cadmium accumulation and detoxification. Planta 2016, 243, 605622. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.N.; Naing, A.H.; Yun, B.W.; Kim, C.K. Overexpression of RsMYB1 enhances heavy metal stress tolerance in transgenic petunia by elevating the transcript levels of stress tolerant and antioxidant genes. bioRxiv 2018, 3028, 286849. [Google Scholar]
- Chen, J.; Yang, L.; Yan, X.; Liu, Y.; Wang, R.; Fan, T.; Ren, Y.; Tang, X.; Xiao, F.; Liu, Y.; et al. Zinc-finger transcription factor ZAT6 positively regulates cadmium tolerance through glutathionedependent pathway in Arabidopsis. Plant Physiol. 2016, 171, 01882.2015. [Google Scholar] [CrossRef]
- Liu, D.; An, Z.; Mao, Z.; Ma, L.; Lu, Z. Enhanced heavy metal tolerance and accumulation by transgenic sugar beets expressing Streptococcus thermophilus StGCSGS in the presence of Cd, Zn and Cu alone or in combination. PLoS ONE 2015, 10, e0128824. [Google Scholar]
- Wang, J.W.; Li, Y.; Zhang, Y.X.; Chai, T.Y. Molecular cloning and characterization of a Brassica juncea yellow stripe-like gene, BjYSL7, whose overexpression increases heavy metal tolerance of tobacco. Plant Cell Rep. 2013, 32, 651662. [Google Scholar] [CrossRef]
- Brunetti, P.; Zanella, L.; Paolis, A.D.; Litta, D.D.; Cecchetti, V.; Falasca, G.; Barbieri, M.; Altamura, M.M.; Costantino, P.; Cardarelli, M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 38153829. [Google Scholar] [CrossRef]
- Meng, J.G.; Zhang, X.D.; Tan, S.K.; Yang, Z.M. Genome-wide identification of Cd-responsive NRAMP transporter genes and analyzing expression of NRAMP 1 mediated by miR167 in Brassica napus. Biometals 2017, 30, 917931. [Google Scholar] [CrossRef]
- Banuelos, G.; Terry, N.; LeDuc, D.L.; Pilon-Smits, E.A.; Mackey, B. Field trial of transgenic Indian mustard plants shows enhanced phytoremediation of selenium-contaminated sediment. Environ. Sci. Technol. 2005, 39, 1771. [Google Scholar] [CrossRef]
- Dominguez-Solis, J.R.; Lopez-Martin, M.C.; Ager, F.J.; Ynsa, M.D.; Romero, L.C.; Gotor, C. Increased cysteine availability is essential for cadmium tolerance and accumulation in Arabidopsis thaliana. Plant Biotechnol. J. 2004, 2, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, X.; Wang, R.; Wang, J.; Le, S.; Zhao, Y. Overexpression of Three Duplicated BnPCS Genes Enhanced Cd Accumulation and Translocation in Arabidopsis thaliana Mutant cad1. Bull. Environ. Contam. Toxicol. 2019, 102, 146–152. [Google Scholar] [CrossRef]
- Nehnevajova, E.; Mireddy, E.; Stolz, A.; Gerdemann-Knörck, M.; Novák, O.; Strnad, M.; Schmülling, T. Root enhancement in cytokinin-deficient oilseed rape causes leaf mineral enrichment, increases the chlorophyll concentration under nutrient limitation and enhances the phytoremediation capacity. BMC Plant Biol. 2019, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kimura, E.; Koike, S.; Nojima, J.; Futai, E.; Sasagawa, N.; Watanabe, Y.; Ishiura, S. Transgenic rice expressing amyloid βpeptide for oral immunization. Int. J. Biol. Sci. 2011, 7, 301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rybicki, E.P. Plant-made vaccines for humans and animals. Plant Biotechnol. J. 2010, 8, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ge, X.; Dolan, M.C. Towards high-yield production of pharmaceutical proteins with plant cell suspension cultures. Biotechnol. Adv. 2011, 29, 278–299. [Google Scholar] [CrossRef]
- Sala, F.; Rigano, M.M.; Barbante, A.; Basso, B.; Walmsley, A.M.; Castiglione, S. Vaccine antigen production in transgenic plants: Strategies, gene constructs and perspectives. Vaccine 2003, 21, 803–808. [Google Scholar] [CrossRef]
- Daniell, H.; Kumar, S.; Dufourmantel, N. Breakthrough in chloroplast genetic engineering of agronomically important crops. Trend. Biotechnol. 2005, 23, 238–245. [Google Scholar] [CrossRef]
- Scotti, N.; Rigano, M.M.; Cardi, T. Production of foreign proteins using plastid transformation. Biotechnol. Adv. 2012, 30, 387–397. [Google Scholar] [CrossRef]
- Marsian, J.; Fox, H.; Bahar, M.W.; Kotecha, A.; Fry, E.E.; Stuart, D.I.; Macadam, A.J.; Rowlands, D.J.; Lomonossoff, G.P. Plant-made polio type 3 stabilized VLPs—A candidate synthetic polio vaccine. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Richter, L.J.; Thanavala, Y.; Arntzen, C.J.; Mason, H.S. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat. Biotechnol. 2000, 18, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Yusibov, V.; Hooper, D.C.; Spitsin, S.V.; Fleyish, N.; Kean, R.B.; Mikheeva, T.; Deka, D.; Karasev, A.; Cox, S.; Randall, J.; et al. Expression in plants and immunogenecity of plant virus based experimental rabies vaccine. Vaccine 2002, 20, 3155–3164. [Google Scholar] [CrossRef]
- Tacket, C.O. Plant-based vaccines against diarrheal diseases. Trans. Am. Clin. Climatol. Assoc. 2007, 118, 79–87. [Google Scholar]
- Mason, H.S.; Warzecha, H.; Tsafir, M.S.; Arntzen, C.J. Edible plant vaccines: Applications for prophylactic and therapeutic molecular medicine. Trends Mol. Med. 2002, 8, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Makarkov, A.I.; Chierzi, S.; Pillet, S.; Murai, K.K.; Landry, N.; Ward, B.J. Plant-made virus-like particles bearing influenza hemagglutinin (HA) recapitulate early interactions of native influenza virions with human monocytes/macrophages. Vaccine 2017, 35, 4629–4636. [Google Scholar] [CrossRef] [PubMed]
- Abha, K.; Shaila, M.S.; Lakshmi, S.G. Transgenic plants as a source of “edible vaccines” for two morililiviral animal diseases. In Proceedings of the Abstracts of 10th IAPTC&B Congress 72-A, Orlando, FL, USA, 23–28 June 2002. [Google Scholar]
- Dennis, S.J.; O’Kennedy, M.M.; Rutkowska, D.; Tsekoa, T.; Lourens, C.W.; Hitzeroth, I.I.; Meyers, A.E.; Rybicki, E.P. Safety and immunogenicity of plant-produced African horse sickness virus-like particles in horses. Vet. Res. 2018, 49, 1–6. [Google Scholar] [CrossRef]
- Yusibov, V.; Streatfield, S.J.; Kushnir, N. Clinical development of plant-produced recombinant pharmaceuticals: Vaccines, antibodies and beyond. Hum. Vaccin. 2011, 7, 313–321. [Google Scholar] [CrossRef]
- Watson, J.; Koya, V.; Leppla, S.H.; Daniell, H. Expression of Bacillus anthracis protective antigen in transgenic chloroplasts of tobacco, a non-food/feed crop. Vaccine 2004, 22, 4374–4384. [Google Scholar] [CrossRef]
- Lamphear, B.J.; Jilka, J.M.; Kesl, L.; Welter, M.; Howard, J.A.; Streatfield, S.J. A corn-based delivery system for animal vaccines: An oral transmissible gastroenteritis virus vaccine boosts lactogenic immunity in swine. Vaccine 2004, 22, 2420–2424. [Google Scholar] [CrossRef]
- Kim, T.G.; Gruber, A.; Langridge, W.H.R. HIV-1 gp120 V3 cholera toxin B subunit fusion gene expression in transgenic potato. Protein Expres. Purif. 2004, 37, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Maloney, B.J.; Takeda, N.; Suzaki, Y.; Ami, Y.; Li, T.C.; Miyamura, T.; Arntzen, C.J.; Mason, H.S. Challenges in creating a vaccine to prevent hepatitis E. Vaccine 2005, 23, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Gressel, J. Transgenics are imperative for biofuel crops. Plant Sci. 2008, 174, 246–263. [Google Scholar] [CrossRef]
- Smith, A.M. Prospects for increasing starch and sucrose yields for bioethanol production. Plant J. 2008, 54, 546–558. [Google Scholar] [CrossRef]
- Vanden Wymelenberg, A.; Sabat, G.; Mozuch, M.; Kersten, P.J.; Cullen, D.; Blanchette, R.A. Structure, organization, and transcriptional regulation of a family of copper radical oxidase genes in the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl. Environ. Microbiol. 2006, 72, 4871–4877. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Cheng, X.; Sun, J.; Marita, J.; Ralph, J.; Chiang, V. Combinatorial modification of multiple lignin traits in trees through multigene co-transformation. Proc. Natl. Acad. Sci. USA 2003, 100, 4939–4944. [Google Scholar] [CrossRef]
- Myers, A.M.; Morell, M.K.; James, M.G.; Ball, S.G. Recent progress toward understanding the biosynthesis of the amylopectin crystal. Plant Physiol. 2000, 122, 989–998. [Google Scholar] [CrossRef]
- Ralph, J.; Akiyama, T.; Kim, H.; Lu, F.; Schatz, P.F.; Marita, J.M.; Ralph, S.A.; Reddy, M.S.S.; Chen, F.; Dixon, R.A. Effects of coumarate 3-hydroxylase down-regulation on lignin structure. J. Biol. Chem. 2006, 281, 8843–8853. [Google Scholar] [CrossRef]
- Chabannes, M.; Barakate, A.; Capierre, C.; Marita, J.M.; Ralph, J.; Peem, M.; Danoun, S.; Halpin, C.; Grima-Pettenati, J.; Boudet, A.M. Strong decrease in lignin content without significant alteration of plant development is induced by simultaneous down-regulation of cinnamoyl CoA reductase (CCR) and cinnamyl alcohol dehydrogenase (CAD) in tobacco plants. Plant J. 2001, 28, 257–270. [Google Scholar] [CrossRef]
- Blaschke, L.; Legrand, M.; Mai, C.; Polle, A. Lignification and structural biomass production in tobacco with suppressed caffeic/5-hydroxy ferulic acid-O-methyl transferase activity under ambient and elevated CO2 concentrations. Physiol. Plant 2004, 121, 75–83. [Google Scholar] [CrossRef]
- Lardizabal, K.; Effertz, R.; Levering, C.; Mai, J.; Pedroso, M.; Jury, T.; Aasen, E.; Gruys, K.; Bennett, K. Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiol. 2008, 148, 9–96. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, E.; Edwards, P.; Byrne, J.; Stymne, S.; Dehesh, K. The distribution of caprylate, caprate and laurate in lipids from developing and mature seeds of transgenic Brassica napus L. Planta 2000, 212, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Van Camp, W. Yield enhancement genes: Seeds for growth. Curr. Opin. Biotechnol. 2005, 16, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.P.; Gallardo, F.; Pascual, M.B.; Sampalo, R.; Romero, J.; Torres de Navarra, A.; Ca´novas, F.M. Improved growth in a field trial of transgenic hybrid poplar overexpressing glutamine synthetase. New Phytol. 2004, 164, 137–145. [Google Scholar] [CrossRef]
- Hammond, B.; Dudek, R.; Lemen, L.; Nemeth, M. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol. 2004, 42, 1003–1014. [Google Scholar] [CrossRef]
- Schroder, M.; Poulsen, M.; Wilcks, A.; Kroghsbo, S.; Miller, A.; Frenzel, T.; Danier, J.; Rychlik, M.; Emami, K.; Gatehouse, A. A 90-day safety study of genetically modified rice expressing Cry1Ab protein (Bacillus thuringiensis toxin) in Wistar rats. Food Chem. Toxicol. 2007, 45, 339–349. [Google Scholar] [CrossRef]
- Setamou, M.; Berna, J.S.; Legaspi, J.C.; Mirkov, T.E. Parasitism and location of sugarcane borer (Lepidoptera: Pyralidae) by Cotesia flavipes (Hymenoptera: Braconidae) on transgenic and conventional sugarcane. Environ. Entomol. 2002, 31, 1219–1225. [Google Scholar] [CrossRef]
- Moellenbeck, D.J.; Peters, M.L.; Bing, J.W.; Rouse, J.R.; Higgins, L.S.; Sims, L.; Nevshemal, T.; Marshall, L.; Ellis, R.T. Insecticidal proteins from Bacillus thuringiensis protect corn from corn rootworms. Nat. Biotechnol. 2001, 19, 668–672. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, K.; Liu, Y.; Yang, S.; Zhang, J.; Gong, X.; Ma, F. Overexpression of MdMIPS1 enhances salt tolerance by improving osmosis, ion balance, and antioxidant activity in transgenic apple. Plant Sci. 2020, 301, 110654. [Google Scholar] [CrossRef]
- Prakash, D.; Verma, S.; Bhatia, R.; Tiwary, B.N. Risks and Precautions of Genetically Modified Organisms. ISRN Ecol. 2011, 2011, 369573. [Google Scholar] [CrossRef]
- Deepa, A. Genetically Modified Foods: Benefits and Risks; Massachusetts Medical Society: Boston, MA, USA, 2015. [Google Scholar]
- Pelletier, D.L. Science, law, and politics in FDA’s genetically engineered foods policy: Scientific concerns and uncertainties. Nutr. Rev. 2005, 63, 210–223. [Google Scholar] [CrossRef] [PubMed]
- USDA. Biotechnology Frequently Asked Questions; USDA: Washington, DC, USA, 1998.
- Yul, R.J.; Choi, J.Y.; Li, M.S.; Jin, B.R.; Je, Y.H. Bacillus thuringiensis as a Specific, Safe, and Effective Tool for Insect Pest Control. J. Microbiol. Biotechnol. 2007, 17, 547–559. [Google Scholar]
- Hall, J.; Matos, M.; Langford, C.H. Social exclusion and transgenic technology: The case of Brazilian agriculture. J. Bus. Ethics. 2008, 77, 45–63. [Google Scholar] [CrossRef]
- Hilbeck, A.; Baumgartner, M.; Fried, P.M.; Bigler, F. Effects of transgenic Bacillus thuringiensis corn-fed prey on mor- tality and development time of immature Chrysoperla carnea (Neuroptera: Chrysopidae). Environ. Entomol. 1998, 27, 480–487. [Google Scholar] [CrossRef]
- Arnaud, J.F.; Viard, F.; Delescluse, M.; Cuguen, J. Evidence for gene flow via seed dispersal from crop to wild relatives in Beta vulgaris (Chenopodiaceae): Consequences for the release of genetically modified crop species with weedy lineages. Proc. R. Soc. B Biol. Sci. 2003, 270, 1565–1575. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Scierenbeck, K.A. Hybridization as a stimulus for the evolution of invasiveness in plants? Proc. Natl. Acad. Sci. USA 2000, 97, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Gressel, J. Molecular biology of weed control. Transgenic Res. 2000, 9, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Ricroch, A.E.; Berge, J.E.; Kuntz, M. Evaluation of genetically engineered crops using transcriptomic, proteomic, and metabolomic profiling techniques. Plant Physiol. 2011, 155, 1752–1761. [Google Scholar] [CrossRef]
- Keese, P. Risks from GMOs due to Horizontal Gene Transfer. Environ. Biosaf. Res. 2008, 7, 123–149. [Google Scholar] [CrossRef]
- Dutton, A.; Klein, H.; Romeis, J.; Bigler, F. Uptake of Bt-toxin by herbivores feeding on transgenic maize and consequences for the predator Chrysoperia carnea. Ecol. Entomol. 2002, 27, 441–447. [Google Scholar] [CrossRef]
- Tudisco, R.; Lombardi, P.; Bovera, F.; Cutrignelli, M.I.; Mastellone, V.; Terzi, V.; Avallone, L.; Infascelli, F. Genetically Modified Soya Bean in Rabbit Feeding: Detection of DNA fragments and evaluation of metabolic effects by enzymatic analysis. J. Anim. Sci. 2006, 82, 193–199. [Google Scholar] [CrossRef]
- Romeis, J.; Dutton Bigler, F. Bacillus thuringiences toxin (Cry 1 Ab) has no direct on larvae of the green lacewing Chrysoperla carnea (stephens) (Neuroptera: Chrysopidae). J. Insect. Physiol. 2004, 50, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Bob, B.M.W.; Candolin, U. Behavioral responses to changing environments. Behav. Ecol. 2015, 26, 665–673. [Google Scholar]
- Chekol, C. The Health Effects of Genetically Modified Foods: A Brief Review. Int. J. Nutr. Sci. 2021, 6, 1047. [Google Scholar] [CrossRef]
- Paparini, A.; Romano-Spica, V. Public health issues related with the consumption of food obtained from genetically modified organisms. Biotechnol. Annu. Rev. 2004, 10, 85–122. [Google Scholar] [PubMed]
- Alexandrova, N.; Georgieva, K.; Atanassov, A. Biosafety regulations of GMOs: National and International aspects and regional cooperation. Biotechnol. Biotechnol. Equip. 2005, 19, 153–172. [Google Scholar] [CrossRef]
- de Vendômois, J.S.; Roullier, F.; Cellier, D.; Séralini, G.E. A Comparison of the effects of three GM corn varieties on mammalian health. Int. J. Biol. Sci. 2009, 5, 706–726. [Google Scholar] [CrossRef]
- Wozniak, E.; Tyczewska, A.; Twardowski, T. Bioeconomy development factors in the European Union and Poland. New Biotechnol. 2021, 60, 2–8. [Google Scholar] [CrossRef]
- Cribbs, A.P.; Perera, S.M. Focus: Genome editing: Science and bioethics of CRISPR-Cas9 gene editing: An analysis towards separating facts and fiction. Yale J. Biol. Med. 2017, 90, 625. [Google Scholar]
- Vibha Ahuja Regulation of emerging gene technologies in India. BMC Proc. 2018, 12, 14.
- MoEFCC; DBT. Guidelines, User’s Guide and Risk Analysis Framework. In Environmental Risk Assessment of GE Plants; MoEFCC: New Delhi, India, 2016. [Google Scholar]
- MoEFCC; BCIL. Frequently Asked Questions About GE Plants. In Phase II Capacity Building Project on Biosafety; MoEFCC: New Delhi, India, 2015. [Google Scholar]
- Ishii, T.; Araki, M. A future scenario of the global regulatory landscape regarding genome-edited crops. GM Crop. Food 2017, 8, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Rosado, A.; Craig, W. Biosafety regulatory systems overseeing the use of genetically modified organisms in the latin america and caribbean region. AgBioForum 2017, 20, 120–132. [Google Scholar]
- Global Agriculture. Venezuela passes new seed law banning genetically modified crops. 2016. Available online: https://www.globalagriculture.org/whatsnew/news/en/31519.html. (accessed on 11 January 2023).
- APBREBES. The New Seed Law of Venezuela; APBREBES: Geneva, Switzerland, 2016. [Google Scholar]
- Gatica-Arias, A. The regulatory current status of plant breeding technologies in some Latin American and the Caribbean countries. Plant Cell Tissue Organ Cult. 2020, 141, 229–242. [Google Scholar] [CrossRef]
- Smith, P.; Katovich, E. Are GMO policies “trade related”? Empirical analysis of Latin America. Appl. Econ. Perspect. Policy 2017, 39, 286–312. [Google Scholar] [CrossRef]
- ISAAA. Brief 55–2019. Executive Summary, Biotech Crops Drive Socio-Economic Development and Sustainable Development in the New Frontier; ISAAA: Nairobi, Kenya, 2019. [Google Scholar]
- Turnbull, C.; Lillemo, M.; Hvoslef-Eide, T. Global regulation of genetically modified crops amid the gene edited crop boom—A review. Front. Plant Sci. Plant Biotechnol. 2021, 12, 1–19. [Google Scholar] [CrossRef]
- Zarrilli, S. International trade in gmos and gm products: National and multilateral legal frameworks. In Policy Issues in International Trade and Commodities Study Series no. 29; United Nations: New York, NY, USA, 2005; pp. 1–16. [Google Scholar]
- Rosado, A.; Eriksson, D. Biosafety legislation and the regulatory status of the products of precision breeding in the Latin America and the Caribbean region. Plants People Planet 2021, 4, 214–231. [Google Scholar] [CrossRef]
- Akaakohol, M.A.; Aye, G.C. Diversification and farm household welfare in Makurdi, Benue state, Nigeria. Dev. Stud. Res. 2014, 1, 168–175. [Google Scholar] [CrossRef]
- ASSAf (Academies of Science of South Africa). GMOs for African agriculture: Challenges and opportunities. Workshop Proceedings Report. ASSAf: 2010. Available online: www.assaf.org.za (accessed on 11 January 2023).
- EFSA (European Food Safety Authority). Guidance on the environmental risk assessment of genetically modified plants. EFSA J. 2010, 8, 1879. [Google Scholar] [CrossRef]
- Bawa, A.S.; Anilakumar, K.R. Genetically modified foods: Safety, risks and public concerns-a review. J. Food Sci. Technol. 2013, 50, 1035–1046. [Google Scholar] [CrossRef]
- Tung, O.J.L. A comparative analysis of the South African and burkinabe experiences with genetically modified crop regulation. In Verfassung und Recht in Übersee/Law and Politics in Africa, Asia, Latin America; Nomos Verlagsgesellschaft: Baden-Baden, Germany, 2017; pp. 3–29. [Google Scholar]
- Anderson, K.; Nielsen, C. Golden Rice and The Looming Gmo Trade Debate: Implications for the Poor; CEPR. Discussion Papers 4195; CEPR: London, UK, 2004. [Google Scholar]
- Deffor, E.W. Consumer acceptance of genetically modified foods in the greater accra region of Ghana. J. Biosaf. Health Educ. 2014, 2, 116. [Google Scholar] [CrossRef]
- Newell-McGloughlin, M.; Burke, J. Biotechnology crop adoption: Potential and challenges of genetically improved crops. Encycl. Agric. Food Syst. 2014, 2014, 69–93. [Google Scholar]
- Akinbo, O.; Obukosia, S.; Ouedraogo, J.; Sinebo, W.; Savadogo, M.; Timpo, S.; Mbabazi, R.; Maredia, K.; Diran Makinde, D.; Ambali, A. Commercial release of genetically modified crops in Africa: Interface between biosafety regulatory systems and varietal release systems. Front. Plant Sci. 2021, 12, 605937. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.G.; Downie, R. African Perspectives on Genetically Modified Crops: Assessing the Debate in Zambia, Kenya and South Africa. A Report of the Csis Global Food Security Project; Centre for Strategic & International Studies: Washington, DC, USA, 2010. [Google Scholar]
- ISAAA. Global Status of Commercialised Biotech/GM Crops in ISAAA Brief Ithaca; ISAAA: New York, NY, USA, 2019. [Google Scholar]
- ABNE. African biosafety network of expertise (ABNE) african union development agency NEPAD (AUDA-NEPAD); ABNE: Midrand, South Africa, 2019. [Google Scholar]
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2018: Executive Brief; ISAAA: Ithaca, NY, USA, 2018. [Google Scholar]
- Kimenju, S.C.; De Groote, H.; Karugia, J.; Mbogoh, S.; Poland, D. Consumer awareness and attitudes toward GM foods in Kenya. Afr. J. Biotechnol. 2005, 4, 1–10. [Google Scholar]
- Nyinondi, P.S.; Dulle, F.W.; Nawe, J. Perception of agricultural biotechnology among farmers. Journalists and Scientists in Tanzania. Univ. Dares Salaam Libr. J. 2017, 12, 106–120. [Google Scholar]
- ISAAA. Global Status of Commercialised Biotech/Gm Crops in 2017: Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years; ISAAA Brief: New York, NY, USA, 2017; Volume 53, pp. 25–26. [Google Scholar]
- ISAAA. Global Status of Commercialised Biotech/GM Crops in ISAAA Brief no. 54; ISAAA: Ithaca, NY, USA, 2018. [Google Scholar]
- Aerni, P. Stakeholder attitudes towards the risks and benefits of genetically modified crops in South Africa. Environ. Sci. Policy 2005, 8, 464–476. [Google Scholar] [CrossRef]
- Aerni, P.; Bernauer, T. Stakeholder attitudes toward GMOs in the Philippines, Mexico, and South Africa: The issue of public trust. World Dev. 2006, 34, 557–575. [Google Scholar] [CrossRef]
- Marques, M.D.; Critchley, C.R.; Walshe, J. Attitudes to genetically modified food over time: How trust is, and the media cycle predict support. Public Underst. Sci. 2015, 24, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.; Schurman, R. The complex choreography of agricultural biotechnology in Africa. Afr. Affairs 2020, 119, 499–525. [Google Scholar] [CrossRef]
- MOA (Ministry of Agriculture). Measures of Labelling Administration of Agricultural GMO–Decree 10 (in Chinese), 2002. Available online: http://www.moa.gov.cn/fwllm/zxbs/xzxk/bszl/201405/t20140527_3917464.htm (accessed on 11 January 2023).
- MOA (Ministry of Agriculture of China). Guideline for Safety Inspection of Field Trials of GM Crops, 2012. Available online: http://www.moa.gov.cn/zwllm/zcfg/qtbmgz/200606/t20060612_627852.htm (accessed on 22 August 2015).
- Xiao, Z.; Kerr, W.A. Biotechnology in China–regulation, investment, and delayed commercialization. GM CROPS FOOD 2022, 13, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Loppacher, L.J.; Kerr, W.A. Biotechnology in China: Food policy and international trade issues. In Food Policy Control and Research; Riley, A.P., Ed.; Nova Science Publishers: New York, NY, USA, 2005; pp. 1–15. [Google Scholar]
- Kim, H.Y.; Kim, J.H.; Mi-Hwa Oh, M.H. Regulation and detection methods for genetically modified foods in Korea. Pure Appl. Chem. 2010, 82, 129–137. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- ILSI. Nutritional and Safety Assessments of Foods And Feeds Nutritionally Improved through Biotechnology: Case Studies. Available online: http://www.ilsi.org/foodbiotech/publications/10_ilsi2008_casestudies_crfsfs.pdf (accessed on 11 January 2023).
- Shahzadi, F.; Malik, M.F.; Ali, R. Genetically Modified Food Controversies: A Review. Int. J. Sci. Eng. Res. 2015, 6, 2072–2089. [Google Scholar]
- De Vos, C.J.; Swanenburg, M. Health effects of feeding genetically modified (GM) crops to livestock animals: A review. Food Chem. Toxicol. 2018, 117, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.H.; Ipharraguerre, I.R. Livestock performance: Feeding biotech crops. J. Dairy Sci. 2001, 1, E9–E18. [Google Scholar] [CrossRef]
- Rizzi, A.; Raddadi, N.; Sorlini, C.; Nordgrd, L.; Nielsen, K.M.; Daffonchio, D. The stability and degradation of dietary DNA in the gastrointestinal tract of mammals: Implications for horizontal gene transfer and the biosafety of GMOs. Crit. Rev. Food Sci. Nutr. 2012, 52, 142–161. [Google Scholar] [CrossRef]
- Nielsen, K.M.; Bones, A.M.; Smalla, K.; van Elsas, J.D. Horizontal gene transfer from transgenic plants to terrestrial bacteria − a rare event? FEMS Microbiol. Rev. 1998, 22, 79–103. [Google Scholar] [CrossRef]
- Breckling, B.; Reuter, H.; Middelhoff, U.; Glemnitz, M.; Wurbs, A.; Schmidt, G.; Windhorst, W. Risk indication of genetically modified organisms (GMO): Modelling environmental exposure and dispersal across different scales: Oilseed rape in Northern Germany as an integrated case study. Ecol. Indic. 2011, 11, 936–941. [Google Scholar] [CrossRef]
- Beckwith, M.; Hadlock, T.; Suffron, H. Public perceptions of plant biotechnology—A focus group study. New Genet Soc. 2003, 22, 93–109. [Google Scholar] [CrossRef]
- Lundquist, K.A. Unapproved genetically modified corn: It’s what’s for dinner. Iowa Law Rev. 2015, 100, 825–851. [Google Scholar]
- Mikkelsen, T.R.; Andersen, B.; Jorgensen, R.B. The risk of crop transgene spread. Nature 1996, 380, 31. [Google Scholar] [CrossRef]
- Snow, A.A. Illegal gene flow from transgenic creeping bentgrass: The saga continues. Mol. Ecol. 2012, 21, 4663–4664. [Google Scholar] [CrossRef] [PubMed]
- Pineyro-Nelson, A.; Van Heerwaarden, J.; Perales, H.; Serratos-Hernández, J.A.; Rangel, A.; Hufford, M.B.; Gepts, P.; Garay-Arroyo, A.; Rivera-Bustamante, R.; Alvarez-Buylla, E.R. Resolution of the Mexican transgene detection controversy: Error sources and scientific practice in commercial and ecological contexts. Mol. Ecol. 2009, 18, 4145–4150. [Google Scholar] [CrossRef]
- Wegier, A.; Pineyro-Nelson, A.; Alarcon, J.; Galvez-Mariscal, A.; Varez-Buylla, E.R.; Pinero, D. Recent long-distance transgene flow into wild populations conforms to historical patterns of gene flow in cotton (Gossypium hirsutum) at its centre of origin. Mol. Ecol. 2011, 20, 4182–4194. [Google Scholar] [CrossRef]
- Acevedo, F.; Huerta., E.; Burgeff, C.; Koleff, P.; Sarukhan, J. Is transgenic maize what Mexico really needs? Nat. Biotechnol. 2011, 29, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Samuels, J. Transgene flow from Bt brinjal: A real risk? Trends Biotechnol 2013, 31, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Qaim, M. Benefits of genetically modified crops for the poor: Household income, nutrition, and health. New Biotechnol. 2010, 27, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L. The terminator. Altern. J. 2006, 32, 24. [Google Scholar]
- Maghari, M.B.; Ardekani, M.A. Genetically Modified Foods and Social Concerns. Avicenna J. Med. Biotech. 2011, 3, 109–117. [Google Scholar]
- Tironi, M.; Salazar, M.; Valenzuela, D. Resisting and accepting: Farmers’ hybrid epistemologies in the GMO controversy in Chile. Technol. Soc. 2013, 35, 93–104. [Google Scholar] [CrossRef]
- Kangmennaanga, J.; Oseia, L.; Frederick, A.; Armah, F.A. Genetically modified organisms and the age of (Un) reason? A critical examination of the rhetoric in the GMO public policy debates in Ghana. Futures 2016, 83, 37–49. [Google Scholar] [CrossRef]
- Soleri, D.; Cleveland, D.; Cuevas, F. Transgenic crops and crop varietal diversity: The case of maize in Mexico. BioScience 2006, 56, 503–513. [Google Scholar] [CrossRef]
- Rzymski, P.; Królczyk, A. Attitudes toward genetically modified organisms in Poland: To GMO or not to GMO? Food Sec. 2016, 8, 689–697. [Google Scholar] [CrossRef]
- Liu, L.; Cao, C. Who owns the intellectual property rights to Chinese genetically modified rice? Evidence from patent portfolio analysis. Biotechnol. Law Rep. 2014, 33, 181–192. [Google Scholar] [CrossRef]
- Wong, A.Y.T.; Chan, A.W.K. Genetically modified foods in China and the United States: A primer of regulation and intellectual property protect. Food Sci. Hum. Wellness 2016, 5, 124–140. [Google Scholar] [CrossRef]
- Rodriguez-Ferrand, G. Restrictions on Genetically Modified Organisms, The Law Library of Congress, Global Legal Research Center. 2014. Available online: http://www.loc.gov/law/help/restrictions-on-gmos/ (accessed on 11 January 2023).
- Cui, K.; Shoemaker, S.P. Public perception of genetically-modified (GM) food: A Nationwide Chinese Consumer Study. Npj Sci. Food. 2018, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
| Scientific Name | Plant Parts | A. tumefaciens Strains/Vector | Gene | Biotic and Abiotic Resistance | References |
|---|---|---|---|---|---|
| Medicago sativa | Leaves and petiole | Agrobacterium. tumefaciens LBA4404/ AGL01/s GV101 | cry3a (Bt Toxin) | Insect resistance | Tohidfar et al. [92] |
| Oryza sativa L. | Seed | Particle bombardment | Itr1gene | Insect resistance | Alfanso-Rubi et al. [93] |
| Glycine max L. | Somatic embryo | Micro projectile bombardment | Viral coat protein | Soybean dwarf virus resistance | Tougou et al. [94] |
| Jatropha curcas L. | Leaves | Agrobacterium tumefaciens EHA 105 strain | Chitinase | Disease resistance | Franco et al. [95] |
| Glycine max L. | Leaves | Agrobacterium tumefaciens | cry1A gene (tic107) | Insect resistance | Macrae et al. [96] |
| Gossypium hirsutum var Coker | Seed | Agrobacterium tumefaciens (LBA 4404)/pBI121 | cry1Ab gene | Insect resistance | Tohidfar et al. [97] |
| Brinjal | Leaves | Agrobacterium tumefaciens LBA4404/pBI121 | cystatin gene | Higher rate of inhibition of root-knot nematode in transgenic plant | Papolu et al. [98] |
| Kiwi fruits | Leaves | Agrobacterium tumefaciens LBA4404/pBin513 | sbtCryIAcgene | Resistance against Oraesia excavate | Zhang et al. [99] |
| Camelina sativa L. | Floral parts | Agrobacterium rhizogenes (pB172)/plasmid pKYLX71.1 | acdS: ACC deaminase | Salinity tolerance | Heydarian et al. [100] |
| Arabidopsis thaliana L. | Seedlings | Agrobacterium tumefaciens GV3101/pBI121 expression vector | Transcription factor JcCBF2 | Freezing tolerance | Wang et al. [101] |
| Camelina sativa L. | Flower, stem, leaf, and root | Agrobacterium tumefaciens/pCB302-3 vectors | CsHMA3 | Heavy metals tolerance | Park et al. [102] |
| Plant | Gene | A. tumefaciens Strains/Vector | Product | Activity | References |
|---|---|---|---|---|---|
| Arabidopsis thaliana L. and Poplar | PtABCC1 | A. tumefaciens GV3101/pCX-SN | ABC transporter | Hg tolerance | Sun et al. [110] |
| Arabidopsis thaliana L. | TpNRAMP5 | pMD19-T, HBT95-GFP, pCAMBIA1305.1, | Numerous natural resistance-associated macrophage proteins | Increased accumulation of Cd, Co, and Mn | Peng et al. [86] |
| Arabidopsis thaliana L. | CsMTP9 | pENTR/D-TOPO vector into pMDC43 or pMDC83 | Metal transport protein 9 | Increased accumulation of Mn and Cd | Migocka et al. [121] |
| Tobacco | OsMTP1 | E. coli, DH10B (GIBCO BRLp/UC18) | Metal transport protein 1 | Cd hyperaccumulation | Das et al. [122] |
| Salix matsudana | ThMT3 | A. tumefaciens LBA4404/PROKII-ThMT3 | Metallothionein | Increased Cu tolerance and root growth | Yang et al. [123] |
| Tobacco | AtPCS1 | A. tumefaciens LBA4404/pBI121 and pCAMBIA | Phytochelatin synthase | Cd and As accumulation | Zanella et al. [124] |
| Petunia | RsMYB1 | A. tumefaciens C58C1/pB7WG2D | Transcription factor | Enhanced tolerant to Cd,, Cu, Zn | Ai et al. [125] |
| Arabidopsis thaliana L. | ZAT6 | A. tumefaciens GV3101/pXB93 | Zinc-finger transcription factor | Enhanced Cd tolerance | Chen et al. [126] |
| Beta vulgaris | St GCS-GS | A. tumefaciens EHA105/pGWB2 | StGCS-GS | Increased Cd, Zn, Cu tolerance | Liu et al. [127] |
| Rice | TaPCS1 | A. tumefaciens EHA105/pBI121 | Phytochelatin synthase, non-protein thiols | Cd hypersensitivity | Wang et al. [128] |
| Arabidopsis thaliana L. | AtABCC3 | A. tumefaciens GV3101/pER8 | Phytochelatin | Increased Cd tolerance | Brunetti et al. [129] |
| Brassica napus | BnNRAMP1b | ycf1 (Y04069), zrc1 (Y00829), smf1 (Y06272), BY4741/pYES2 | Transport functions | Enhanced uptake of Cd, Zn, Mn | Meng et al. [130] |
| Indian mustard | gshI, gshII and APS1 | pFF19 | γ-Glu-Cys synthetase, glutathione Synthetase, and ATP sulfurylase | Enhanced Se, | Banuelos et al. [131] |
| Arabidopsis thaliana L. | OASTd | A. tumefaciens CV50/pBI121 | Cysteine synthase | Tolerance to Cd | Dominguez-Solis et al. [132] |
| Arabidopsis thaliana | BnPCS | A. tumefaciens CV50/pBI121 | Phytochelatin | Tolerance to Cd | Bai et al. [133] |
| Brassica napus | CKX2 | A. tumefaciens GV3101 | Cytokinin content | Tolerance to Cd, Zn | Nehnevajova et al. [134] |
| Plants | Antigen/Virus | Diseases | Method of Administration | Reference |
|---|---|---|---|---|
| Transgenic potatoes | Hepatitis B surface antigen (HBsAg) | Hepatitis B | Oral | Richter et al. [142] |
| N. tabacum cv. Samsun | Virus glycoprotein and nucleoprotein fused with A1Mvcoat protein | Rabies | Parenteral | Yusibov et al. [143] |
| Potato, Maize kernels Potato | E. coli LT-B | Diarrhea | Oral | Tacket et al. [144] |
| Potato | Norwalk virus like particles (rNV) | Diarrhea, nausea | Oral | Mason et al. [145] |
| N. benthamiana | D antigen (PV3)/Poliovirus | polio | Intraperitoneal injections | Marsian, et al. [141] |
| N. benthamiana | H1, H5/Influenza virus | Influenza | NA | Makarkov et al. [146] |
| Peanut and tobacco | Glycoproteins hemaglutinin (H), Hemaglutinin neuraminidase (HS) | “cattle plague” and “Goat plague” | NA | Abha Khandelwal et al. [147] |
| N. benthamiana | VP2,VP3,VP5,VP7/African horse sickness virus (AHSV) | African horse | Intramuscular | Dennis et al. [148] |
| N. benthamiana | influenza HAC1 | H1N1 “swine” influenza | Intramuscular | Yusibov et al. [149] |
| N. benthamiana | Protective antigen (PA) | Anthrax | Subcutaneous | Watson et al. [150] |
| Maize | Spike protein | Swine transmissible gastroenteritis virus | Oral | Lamphear et al. [151] |
| Potato | CTB-gpl20 (HIV-1 gp 120V3 cholera toxin B subunit fusion gene) | Cholera | Kim et al. [152] | |
| Potato | HEV CP (HEV capsid proteins) | Hepatitis E | Oral | Maloney et al. [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, B.K.; Yu, C.Y.; Kim, W.-R.; Moon, H.-S.; Lee, J.; Kim, S.H.; Chung, I.M. Assessment of Benefits and Risk of Genetically Modified Plants and Products: Current Controversies and Perspective. Sustainability 2023, 15, 1722. https://doi.org/10.3390/su15021722
Ghimire BK, Yu CY, Kim W-R, Moon H-S, Lee J, Kim SH, Chung IM. Assessment of Benefits and Risk of Genetically Modified Plants and Products: Current Controversies and Perspective. Sustainability. 2023; 15(2):1722. https://doi.org/10.3390/su15021722
Chicago/Turabian StyleGhimire, Bimal Kumar, Chang Yeon Yu, Won-Ryeol Kim, Hee-Sung Moon, Joohyun Lee, Seung Hyun Kim, and Ill Min Chung. 2023. "Assessment of Benefits and Risk of Genetically Modified Plants and Products: Current Controversies and Perspective" Sustainability 15, no. 2: 1722. https://doi.org/10.3390/su15021722
APA StyleGhimire, B. K., Yu, C. Y., Kim, W.-R., Moon, H.-S., Lee, J., Kim, S. H., & Chung, I. M. (2023). Assessment of Benefits and Risk of Genetically Modified Plants and Products: Current Controversies and Perspective. Sustainability, 15(2), 1722. https://doi.org/10.3390/su15021722









