1. Introduction
The use of biomass in the production of electric energy has been gaining ground over the last decades, under the obligation of the EU’s 2030 and 2050 directives. By the year 2030, renewable energy resources should contribute at least 32% of the gross energy consumption, with the ambition to increase this share to 90% by 2050 [
1]. Within this framework the use of biomass in the energy sector has been promoted (17%), mainly in the production of electricity (70%) [
2]. In Greece, renewable energy resources are aimed to contribute up to 55% of total electrical energy produced. It is estimated that by 2030 about 1700 GWh will be produced from biomass and biogas [
3]. In 2019, 655 TWh of global electricity was generated from biomass combustion, accounting for 10% of total renewable energy production.
The combustion of biomass has a neutral atmospheric CO
2 fingerprint, since overall produced CO
2 emissions are balanced by the CO
2 uptake from the plants during their growth. Woody biomass is the main feedstock in EU power-plants, comprising mainly bark, woodchips, and sawdust. However, biomass combustion generates large amounts of by-products mainly bottom and fly ash. It is estimated that ~170 Mt/yr of biomass ash are produced annually from the combustion of biomass in power-plants. which is potentially going to increase up to ~1000 Mt/yr [
4]. The chemical composition of biomass ashes depends on the type of feedstock and the combustion conditions. Woody biomass ashes may contain up to 68% wt SiO
2, 84% CaO, 15% wt Al
2O
3, and 9% wt Fe
2O
3. These ashes are also rich in plant nutrients such as MgO (< 19% wt), P
2O
5 (< 17% wt) and K
2O (< 35% wt), while Na
2O and Cl
2O may reach 4% wt and 8% wt respectively. Finally a majority of trace elements are also found in solid biomass ashes, such as Cr, Cu, Ni, Mn, Fe, Pb, Zn and Se [
4,
5,
6]. In a recent published work comparing the properties of biomass (sawdust, olive kernel residuals and reed) and coal fuels (lignite) from Greece, the researchers reached the conclusion that all studied biomass samples exhibited higher calorific values than the coal used in electricity production in Greece [
7].
The total elemental compositions of ashes have been used in various classification systems in order to assess their efficiency in different applications [
5,
8,
9,
10] so as to minimize the amounts of wastes that end up in landfills [
11]. However, the water-leachable elemental concentrations are more appropriate to assess both the potential environmental hazard and efficiency of the ashes. Biomass ashes have a higher water-leachable fraction compared with coal ashes (up to 61% wt), due to the presence of soluble alkali salts (KCl, K
2SO
4, etc.). The water-leachable concentrations of Cl, S, K, Na, and Mg vary from 0 to 99% wt. They also exhibit a wider pH range, from 4.5 to 13.4, owing to the different combustion temperature and storage conditions [
12]. Consequently, the mobility of trace elements also varies. The mean water-leachable concentrations of these elements do not exceed 10%wt of the total concentrations [
12,
13]. Furthermore, the high water-soluble content of macro- and micro-nutrient in solid biomass ashes makes them appropriate as soil fertilizers and amendments. Specifically, the high content in K, P, S, Ca, etc. aids the growth of plants, whilst their alkaline character enhances the biological activities of soils and improves the texture aeration and water-holding capacity of the soils. Despite these advantages, few countries (Denmark, Finland, Germany) have established regulations for the recycling of biomass ashes back into soils as fertilizers [
10].
In southern Europe, biomass originates mainly from the by-products of olive-oil production, such as kernels, peels, and wastewater [
14]. The world average yield of olive groves is about 11.44 million ha, whereas Europe has 4.6 million ha [
15]. Moreover, as in Greece, olive residues display a significant allocation among different agro-industrial residues, with an annual (2020 data) share of ca. 9.5 Mt [
16,
17]. The physicochemical, chemical and morphological characteristics of these ashes have been studied for their utilization in the construction industry [
18,
19,
20] and in civil engineering [
21]. Most studies focus on the bulk chemical composition of the biomass ashes for the evaluation of their utilization in various applications. Few studies have been conducted regarding the leachability of major and trace elements from olive by-product biomasses and their effect on the respective amended soils [
13].
On the other hand, extraction activities for aggregates, usually, come with negative effects on the various environmental components of the quarry areas. Extraction activities may lead to a complete loss of topsoil, compaction of the remaining soil, nutrient depletion and high pH (e.g., in the case of a limestone quarry) that makes natural establishment of vegetation very unlikely [
22]. Therefore, revegetation requires the addition of amendments for soil improvement. It is noted that both EU and Greek legislation require the environmental restoration and rehabilitation of any exhausted quarry.
The current study investigates the potential use of biomass ash originating from the combustion of olive-kernel residuals for electricity production in the restoration of depleted quarries as substrate or soil amendment in the frame of the circular economy. In earlier studies lignite fly ash has been successfully utilized for depleted mine remediation and clean up [e.g., [
23]]. Olive-kernel residuals are used in Mediterranean regions as feedstock for power-plant boilers to produce electricity. Such a power plant exists in central Greece that provides energy up to 5 MW, using olive-kernel residuals imported from north Africa (due to logistics issues) and the environmental management of waste from the combustion is required by EU and Greek regulations. For the scope of this study, the produced bottom ash will be mixed with soil and used for the backfilling of a depleted aggregates quarry that is located nearby the power-plant. The specific objectives of the study are: (a) the environmental characterization of the bottom ash regarding its disposal in a landfill, (b) the determination of the leachability rate of trace and major elements (inorganic nutrients) from the bottom ash and the respective amended soil and (c) the effect of the bottom ash application on the growth of different plant species suitable for the restoration and rehabilitation of a depleted aggregates quarry.
2. Materials and Methods
2.1. Preparation and Characterization of Solid Samples
The bottom ash (BA) samples were collected from a combustion power plant with a capacity of 5 MW that uses olive-kernel residuals as a feedstock for the production of electricity. In order to ensure the representativeness of the samples, sampling took place from the power-plant boilers (operating in their maximum capacity of 5 MW at approximately 1000 °C) on a daily basis for a week during a month. At the end of the month 17 L of bottom ash were collected. This bottom ash was homogenized manually by coning and quartering, and crushed to obtain particles with a size < 2 cm. A representative subsample was subtracted for the subsequent experiments and analysis.
An alkaline soil (pH 9.3) was collected from a nearby depleted calcareous aggregates quarry that was to be restored for use in batch, column and pot experiments. Moreover, an acidic soil (pH 5.7) was collected from Kyparissia town (SW Greece) and used only in pot experiments for comparison. Both soils were homogenized by quartering and coning and sieved to obtain the <2 mm grain fraction. Mixtures with bottom ash and soil were then prepared at a ratio 1:1 w/w.
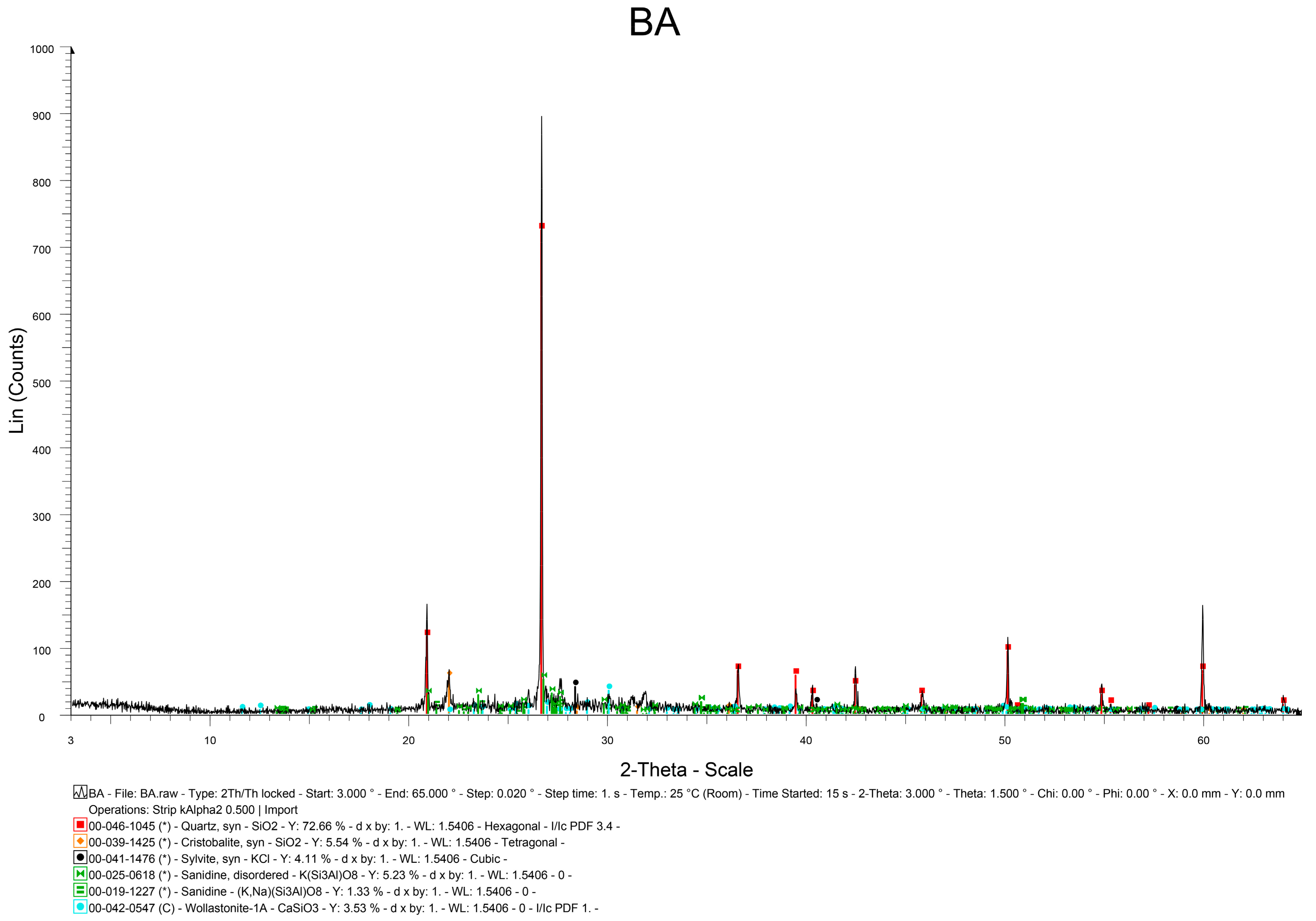
The solids (bottom ash and soils) were characterized regarding their mineralogical composition using X-ray diffraction in randomly oriented samples (SIEMENS D5005, CuKa, 40 mA, 40 mV). The samples were scanned from 5° to 65° (2theta) at a scanning rate of 0.18°/min. Zincite (ZnO) had been added as internal standard at a ratio 1:4 w/w. The mineralogical phases of the samples were identified using EVA v.2.2 (Bruker, Billerica, MA, USA) and the Rietvield quantitative analysis was performed using Topas V.5.0 (Bruker, Billerica, MA, USA).
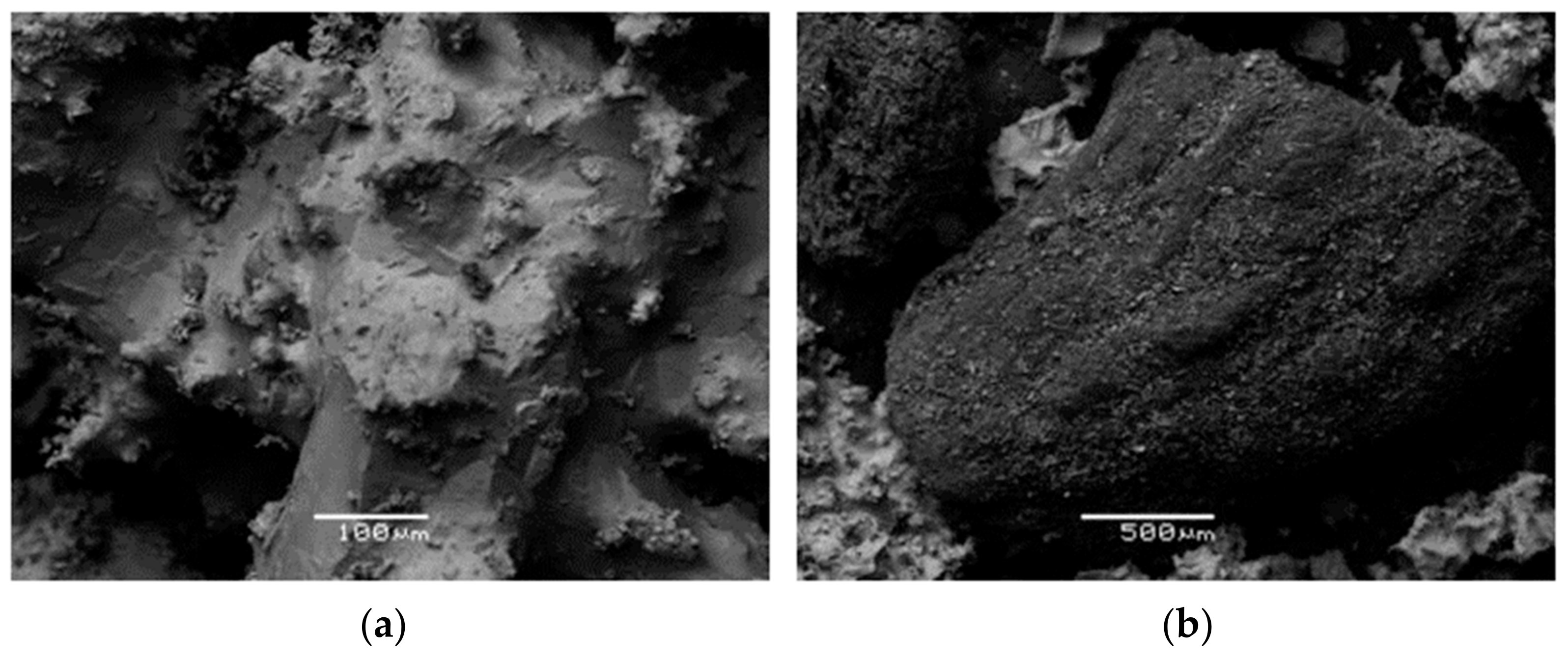
The morphology of the bottom ash samples was examined using scanning electron microscopy (SEM) on graphite-coated free surfaces. EDS spot analysis was also used to identify the chemical character of selected grains (JEOL JSM-5600 equipped with Oxford Link ISIS 300, with a beam diameter of 3 μm, operating at 0.5 nA and 20 kV).
2.2. Leaching Experiments
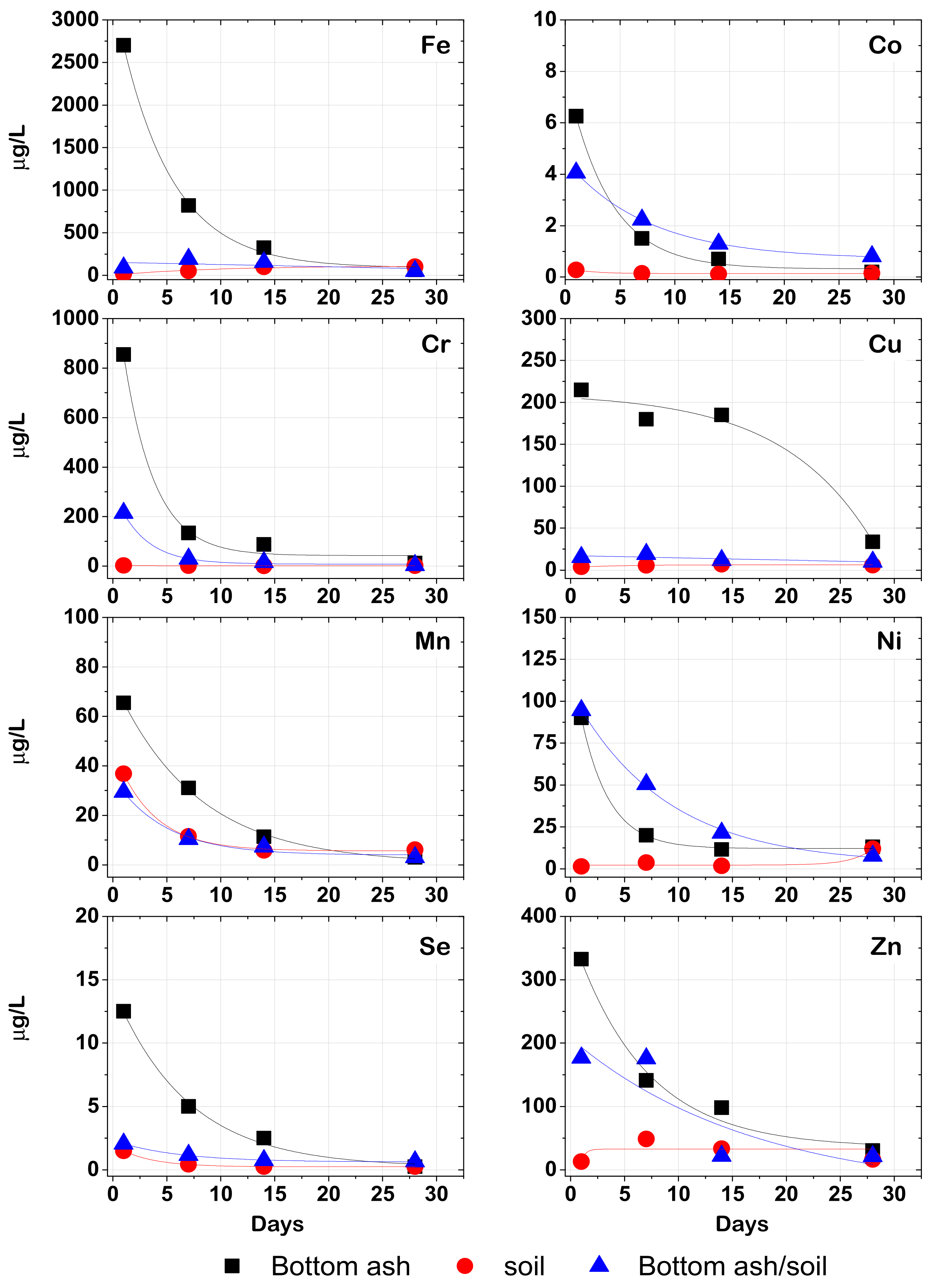
A series of batch and column leaching experiments were performed to assess the mobility of nutrients and potentially toxic elements (PTEs) in bottom ash amended soils. Duplicates of bottom ash, alkaline soil, acidic soil and their mixtures were mixed with deionized water in 10 L/kg ratio for 24 h in a rotary shaker, according to the EN 12457-4 protocol [
24]. The suspensions were vacuum filtered using 0.45 cellulose membranes.
The column experiments were set using glass burets filled with 300 g of bottom ash, alkaline soil, and their mixtures in duplicates. Glass wool was added at the bottom of the column to support the solids bed. The solids were kept at saturation by adding 250 mL of water once per week from the top of the column. The leachates (150–250 mL) were collected from the bottom of the column in the 1st, 7th, 14th and 28th day of the experiment and were immediately vacuum filtrated using 0.45 μm cellulose membranes. The pH and total dissolved solids were measured in the batch and column solutions immediately after their collection.
2.3. Pot Experiments
To investigate the efficiency of bottom ash as a substrate for quarry restoration, pot experiments were carried out using Cupressus sempervirens (cypress) and Dichondra repens (alfalfa) plant species. Cupressus sempervirens is suitable for the restoration of the quarry area while the rapid growth rate of Dichondra repens gives the opportunity to assess the results of the experiment in a relatively short time. Bottom ash was added in different pots containing alkaline and acidic soil in ratio 1:1 w/w. The plants of Cupressus sempervirens used for the experiments were about 12-months old. Pots containing only each different soil were also used as controls. The pots (containing about 2.5 kg of solids) were watered daily for a week using deionized water. After a week, an amount of cypress plants or 1.5 g of alfalfa grains were planted in the pots. Then, the pots were watered in a daily basis and the development of the plants was monitored.
2.4. Methods of Chemical Analysis
Bulk chemical analysis of the solids was carried out using X-ray fluorescence (XRF) after fusion with lithium tetraborate (SGS labs., Mississauga, ON, Canada). Sulfur and total carbon content were estimated using LECO analyzer (SGS labs., Mississauga, ON, Canada). Trace elements were determined after digestion with Na2O2/NaOH using ICP-AES/MS (SGS labs., Mississauga, ON, Canada). The concentration of Cl− and SO42− ions in batch and column leachates was determined by Mercuric Thiocyanate (Method 8113) and SulfaVer4 (Method 8051) using HACH DR6000 UV-Vis spectrophotometer in the Laboratory of Economic Geology and Geochemistry of the National and Kapodistrian University of Athens. Aliquots were oxidized and stored at 4 °C for chemical analysis by ICP-AES/MS techniques (Bureau Veritas Lab., Vancouver, BC, Canada). The obtained analytical relative percent difference between the duplicates was < 10%.
5. Conclusions
During this study we evaluated the biomass ash wastes originating from the combustion of olive-kernel residuals for electricity production in accordance with Directive EE/2003 and investigated the potential use of this waste in the restoration of depleted calcareous aggregate quarries as substrate or soil amendment, in the frame of the circular economy.
The results of the chemical and environmental characterization of the olive-kernel residuals bottom ash, based on Directive 33/2003, indicate that the water-leachable concentrations of controlled elements (except Cr) are, generally, within the acceptable limits for the disposal of that ash, as inert waste in landfills.
However, bottom ash from electricity production through combustion of olive-kernel residuals was found to contain significant amounts of K, Ca and Mg, which are macro-nutrients for the growth of plants.
Both batch and column experiments showed that the leachability of major and trace elements decreased in biomass ash-amended soils, implying the retention of these elements by the soil components.
Bottom ash serves as a slow-release fertilizer by adding nutrients in the soil.
The application of bottom ash in the alkaline soil had a minor positive effect in plant growth which is mainly attributed to the release of nutrients from the bottom ash and their retention by the soil components.
The addition of the bottom ash in the acidic soil exhibited considerable effect in the growth of Dichondra repens and Cupressus sempervirens due to the release of nutrients from the bottom ash and due to the pH conditioning by the amendment.
Therefore, olive-kernel residual bottom ash is appropriate as a soil amendment and is suitable to be used as a soil substrate for the restoration of depleted quarries decreasing the requirement for commercial inorganic fertilizers.