Odor from Building Air Conditioners: Emission Characteristics, Odor Compounds and Influencing Factors
Abstract
1. Introduction
2. Methods
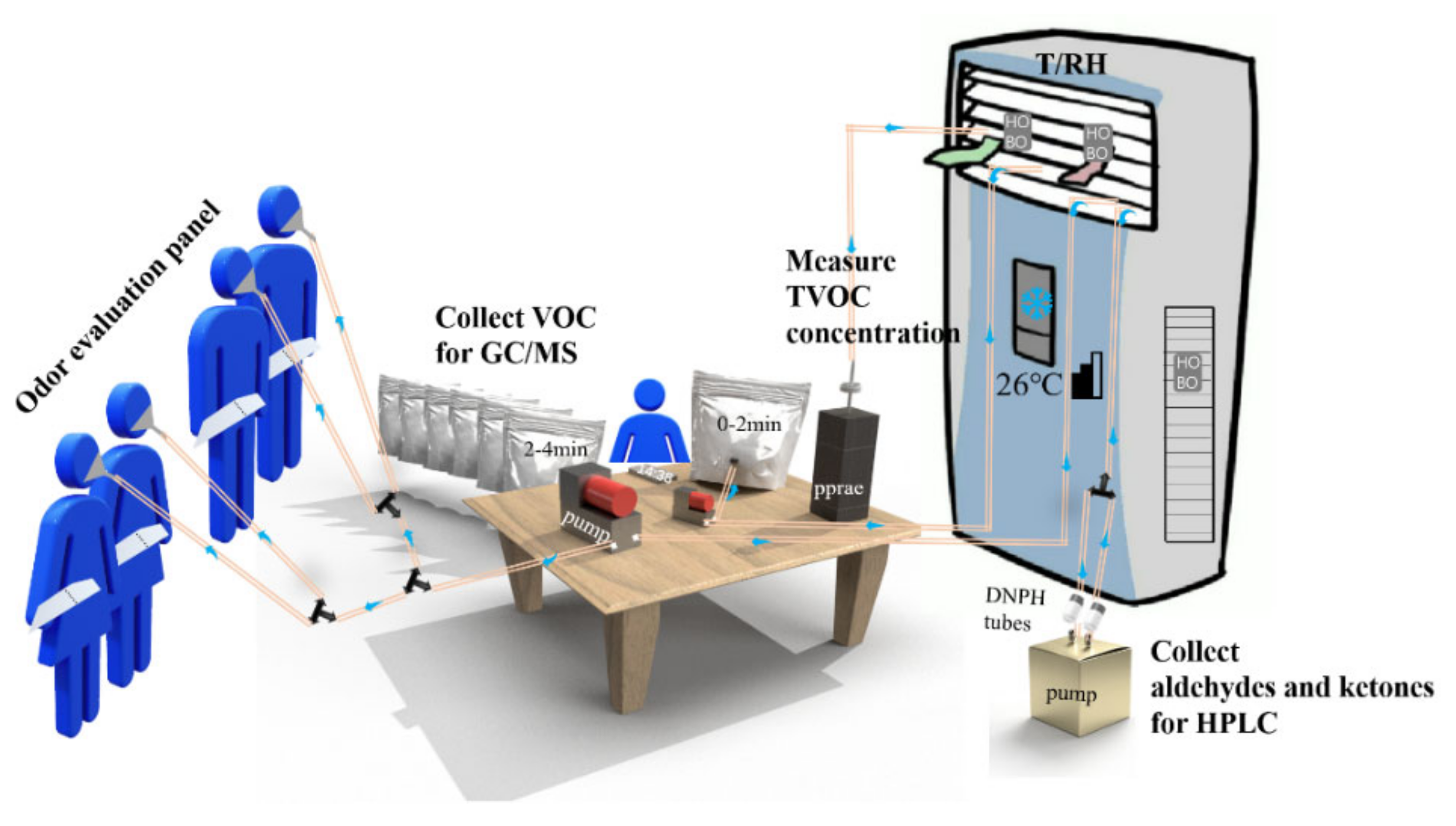
2.1. Samples and Experimental Design
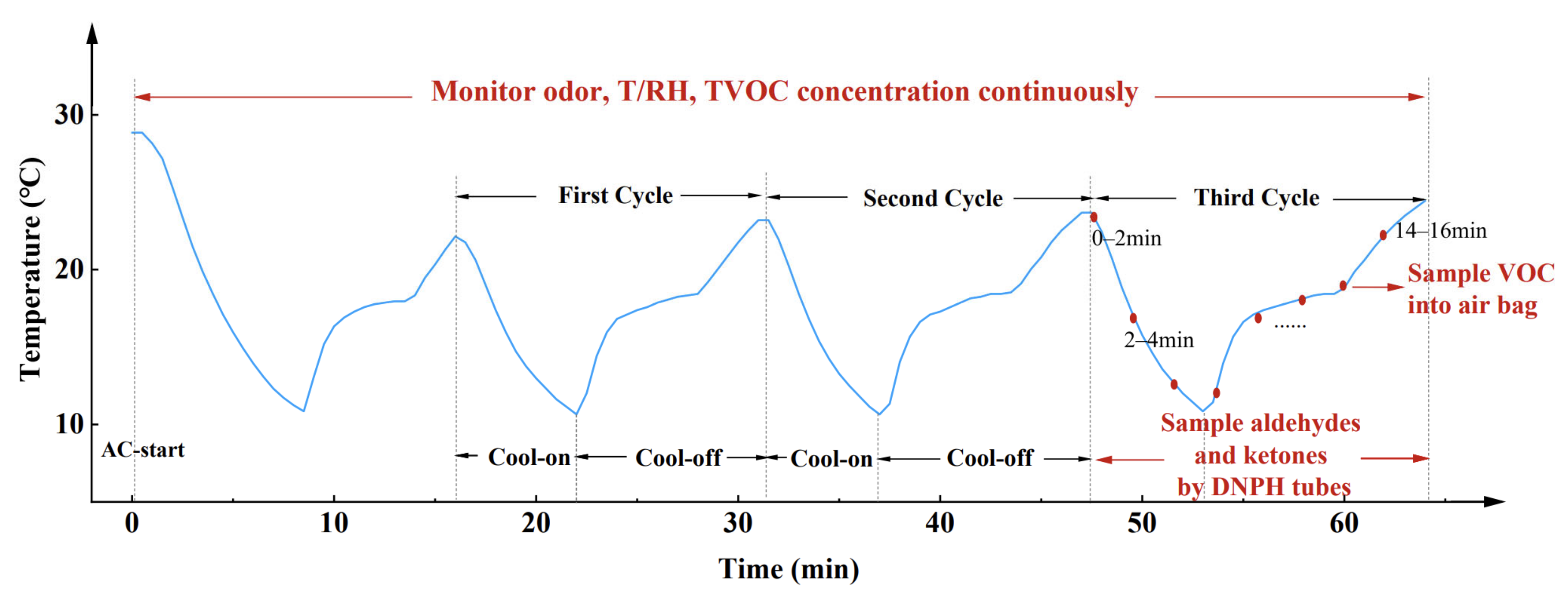
2.2. Test Procedure
2.2.1. Field Test of Odor Emission from Air Conditioner Outlet
2.2.2. Laboratory Test of Odor Emission from Air Filter
2.2.3. Tenax-TA, DNPH and Supelco Tube Sampling and Analysis Method
2.3. Methods to Identify Odor Compounds
3. Results and Analysis
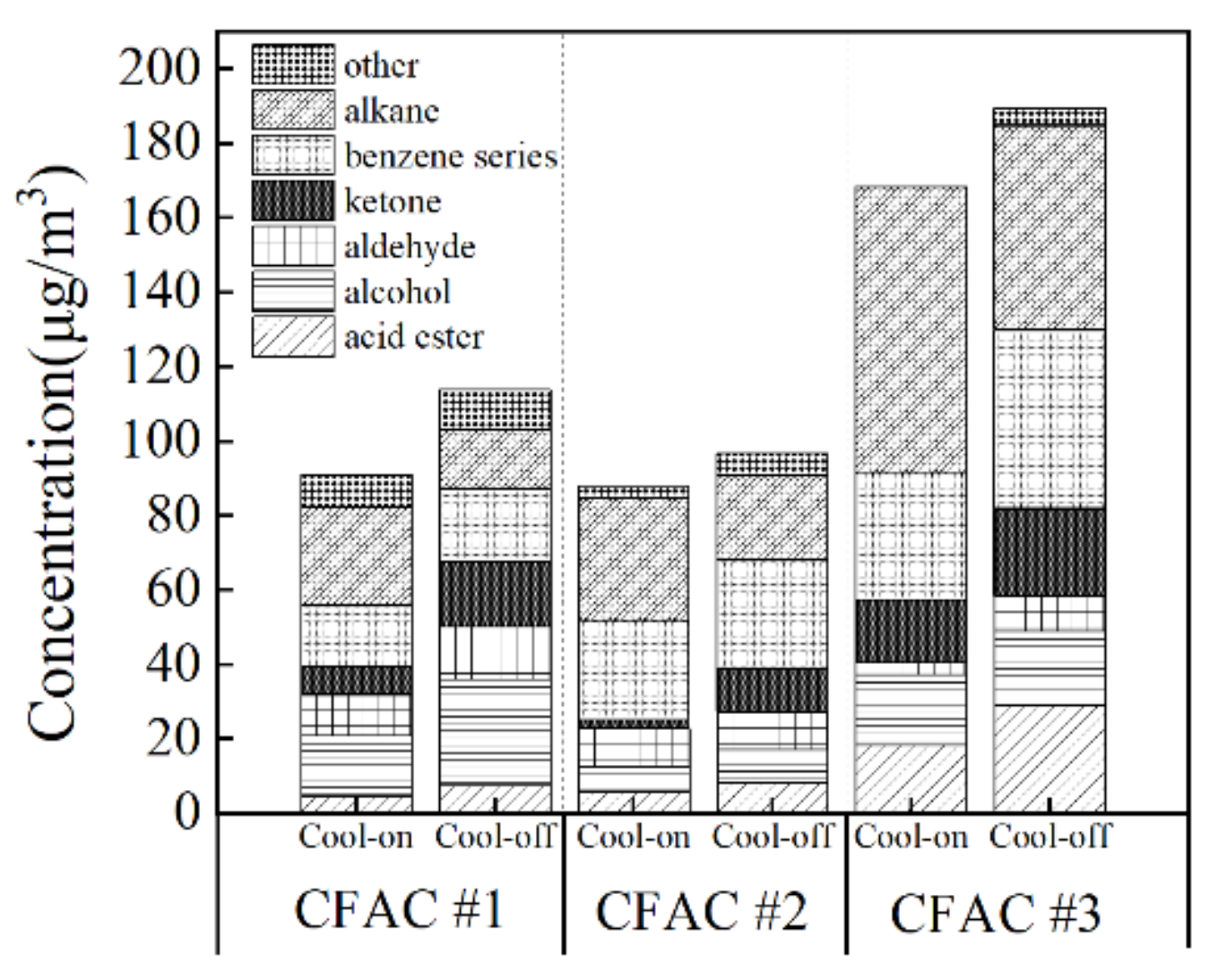
3.1. Correlation between Odor Emission Characteristics and Operation State of Air Conditioner
3.2. Identification of Odor Compounds
3.2.1. Odorants from Air Conditioner Outlet
3.2.2. Odorants from Used Air Filter
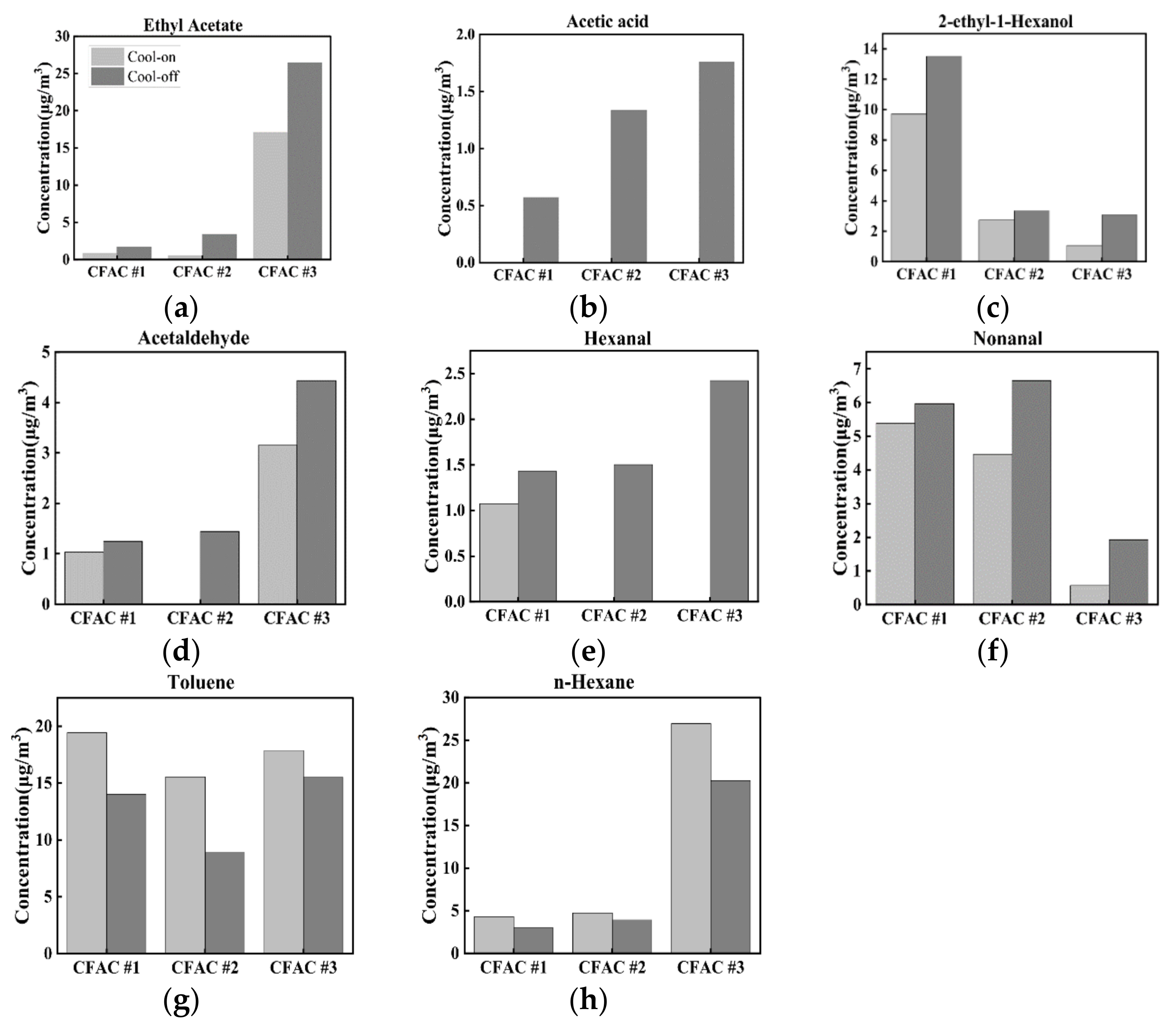
3.2.3. Variation of Odor Compounds with Cooling Status of CFAC
3.3. Effect of Cooling Setpoint Temperature on Emitted Odor Intensity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zadi, T.; Azizi, M.; Nasrallah, N.; Bouzaza, A.; Maachi, R.; Wolbert, D.; Rtimi, S.; Assadi, A.A. Indoor air treatment of refrigerated food chambers with synergetic association between cold plasma and photocatalysis: Process performance and photocatalytic poisoning. Chem. Eng. J. 2020, 382, 122951. [Google Scholar] [CrossRef]
- Rappert, S.; Müller, R. Odor compounds in waste gas emissions from agricultural operations and food industries. Waste Manage. 2005, 25, 887–907. [Google Scholar] [CrossRef] [PubMed]
- Gostelow, P.; Parsons, S.A.; Stuetz, R.M. Odour measurements for sewage treatment works. Water Sci. Technol. 2001, 35, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Vakalis, D.; Touchie, M.; Tzekova, E.; MacLean, H.L.; Siegel, J.A. Indoor environmental quality perceptions of social housing residents. Build. Environ. 2019, 150, 135–143. [Google Scholar] [CrossRef]
- Ferreira, M.H.; Renovato, T.P.; Barrichello, C.R.; Gualtieri, M. Olfactory interference on the emotional processing speed of visual stimuli: The influence of facial expressions intensities. PLoS One 2022, 17, e0264261. [Google Scholar] [CrossRef] [PubMed]
- Kalender, S.; Kukec, A.; Dovjak, M. The problem of indoor environmental quality at a general Slovenian hospital and its contribution to sick building syndrome. Build. Environ. 2022, 214, 108908. [Google Scholar] [CrossRef]
- Ng, T.W.; Chan, P.Y.; Chan, T.T.; Wu, H.; Lai, K.M. Skin squames contribute to ammonia and volatile fatty acid production from bacteria colonizing in air-cooling units with odor complaints. Indoor Air 2018, 28, 258–265. [Google Scholar] [CrossRef]
- Li, D.T.; Shi, Q.Q.; Sui, M.; Xie, B.Z.; Lai, Z.F. Gas Chromatography/Mass Spectrometry Determination and Control of Air Conditioning and Refrigeration ‘Outlet odor’. Electr. Appl. 2019, 9, 6. [Google Scholar]
- Lu, C.; Xiao, F.; Norbäck, D.; Yang, X.; Zhang, Y.P.; Li, B.Z.; Zhao, Z.H.; Huang, C.; Zhang, X.; Qian, H.; et al. Long-term exposure to mould/damp stains and mouldy odour increases low birth weight. Build. Environ. 2022, 222, 109418. [Google Scholar] [CrossRef]
- Sabiniewicz, A.; Hoffmann, L.; Haehner, A.; Hummel, T. Symptoms of depression change with olfactory function. Sci Rep 2022, 12, 5656. [Google Scholar] [CrossRef]
- Kohli, P.; Soler, Z.M.; Nguyen, S.A.; Muus, J.S.; Schlosser, R.J. The Association Between Olfaction and Depression: A Systematic Review. Chem. Senses 2016, 41, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Feilberg, A.; Liu, D.Z.; Adamsen, A.P.S.; Hansen, M.J.; Jonassen, K.E.N. Odorant Emissions from Intensive Pig Production Measured by Online Proton-Transfer-Reaction Mass Spectrometry. Environ. Sci. Technol. 2010, 44, 5894–5900. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.J.; Adamsen, A.P.S.; Wu, C.; Feilberg, A. Additivity between Key Odorants in Pig House Air. Atmosphere 2021, 12, 1008. [Google Scholar] [CrossRef]
- Hansen, M.J.; Jonassen, K.E.N.; Løkke, M.M.; Adamsen, A.P.S.; Feilberg, A. Multivariate prediction of odor from pig production based on in-situ measurement of odorants. Atmos. Environ. 2016, 135, 50–58. [Google Scholar] [CrossRef]
- Cometto-Muñiz, J.E.; Abraham, M.H. Compilation and analysis of types and concentrations of airborne chemicals measured in various indoor and outdoor human environments. Chemosphere 2015, 127, 70–86. [Google Scholar] [CrossRef]
- Dobrzyniewski, D.; Szulczyński, B.; Gębicki, J. Determination of Odor Air Quality Index (OAQII) Using Gas Sensor Matrix. Molecules 2022, 27, 4180. [Google Scholar] [CrossRef]
- Wang, M.; Fan, G.; Qian, W. The Real-time Assessment of Food Freshness in Refrigerator Based on Miniaturized Electronic Nose. Anal. Methods 2018, 10, 4741–4749. [Google Scholar] [CrossRef]
- Jo, S.H.; Kim, K.H.; Kim, Y.H.; Lee, M.H.; Kim, B.W.; Ahn, J.H. Deodorization of food-related nuisances from a refrigerator: The feasibility test of photocatalytic system. Chem. Eng. J. 2015, 277, 260–268. [Google Scholar] [CrossRef]
- ASHRAE. ASHRAE 62.1, Ventilation for Acceptable Indoor Air Quality. ASHRAE J. 1989, 31, 40–48. [Google Scholar]
- GB18883; Standards for indoor air quality. Standards Press of China: Beijing, China, 2022.
- Xu, L.; Hu, Y.B.; Liang, W.N. Composition and correlation of volatile organic compounds and odor emissions from typical indoor building materials based on headspace analysis. Build. Environ. 2022, 221, 109321. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Tan, Y.; Liu, J.; Xiong, J. Measurement of the key parameters of VOC emissions from wooden furniture, and the impact of temperature. Atmos. Environ. 2021, 7, 118510. [Google Scholar] [CrossRef]
- Que, Z.L.; Wang, F.B.; Li, J.Z.; Furuno, T. Assessment on emission of volatile organic compounds and formaldehyde from building materials. Compos. Pt. B-Eng. 2013, 49, 36–42. [Google Scholar]
- Lenochova, P.; Roberts, S.C.; Havlicek, J. Methods of Human Body Odor Sampling: The Effect of Freezing. Chem. Senses 2008, 34, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. Biochemistry 2011, 150, 257–266. [Google Scholar] [CrossRef]
- Zou, Z.; He, J.; Yang, X. An experimental method for measuring VOC emissions from individual human whole-body skin under controlled conditions. Build. Environ. 2020, 181, 107137. [Google Scholar] [CrossRef]
- Lai, K.M.; Sung, Y.H.; Ma, K.K. Viable airborne microbial counts from air-cooling units with and without complaints of urine and body odors. Aerobiologia 2016, 33, 229–241. [Google Scholar] [CrossRef]
- Palanisamy, D.; Ayalur, B.K. Impact of condensate cooled air purging on indoor air quality in an air-conditioned laboratory. Build. Environ. 2021, 188, 107511. [Google Scholar] [CrossRef]
- Zuraimi, M.S.; Tham, K.W.; Sekhar, S.C. A study on the identification and quantification of sources of VOCs in 5 air-conditioned Singapore office buildings. Build. Environ. 2004, 39, 165–177. [Google Scholar] [CrossRef]
- Fanger, P.O.; Lauridsen, J.; Bluyssen, P.; Clausen, G. Air pollution sources in offices and assembly halls, quantified by the olf unit. Energy Build. 1988, 12, 7–19. [Google Scholar] [CrossRef]
- Jie, W.; Zhao, B.Q.; Li, W.H.; Zhang, Z.H. Analysis of the Mechanism of Breathing Odour in Heat Exchanger of Air Conditioning Unit and Its Manufacturing Control Method. Henan Sci. Technol. 2019, 666, 58. [Google Scholar]
- Kim, K.H.; Kim, S.H.; Jung, Y.R.; Kim, M.G. Evaluation of malodor for automobile air conditioner evaporator by using laboratory-scale test cooling bench. J. Chromatogr. A 2008, 1204, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, T. Analysis of Microorganism Causing Odor in an Air-Conditioning System; SAE Technical Paper 2015-01-0354; SAE International: Warrendale, PA, USA, 2015. [Google Scholar] [CrossRef]
- You, H.; Lee, T.; Sung, K. Estimation of VOCs Affecting a Used Car Air Conditioning Smell via PLSR. Trans. KSAE 2013, 21, 175–182. [Google Scholar] [CrossRef]
- Simmons, R.B.; Noble, J.A.; Rose, L.J.; Price, D.L.; Crow, S.A.; Ahearn, D.G. Fungal colonization of automobile air conditioning systems. J. Microbiol. Biotechnol. 1997, 19, 150–153. [Google Scholar] [CrossRef]
- Simmons, R.B.; Rose, L.J.; Crow, S.A.; Ahearn, D.G. Occurrence and persistence of mixed biofilms in automobile air conditioning systems. Curr. Microbiol. 1999, 34, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, M.; Kasebe, O.; Uchiyama, K.; Kobayashi, K.; Hasegawa, E.; Ohara, T. Reduction of Odor Adhesion to Evaporator. In SAE Technical Paper; SAE International: Warrendale, PA, USA, 2004. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.H.; Jung, Y.R.; Kim, M.G.; Kim, J.O.; Park, H.Y.; Ji, Y.J. Examination about evaluation method of odor active compounds in evaporator by using condensed water. Anal. Sci. Technol. 2007, 20, 361–369. [Google Scholar]
- Liu, R.; Wang, C.; Huang, A.; Lv, B. Characterization of odors of wood by gas chromatography-olfactometry with removal of extractives as attempt to control indoor air quality. Molecules 2018, 23, 203. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, J.; Shao, Y.; Dong, H.; Li, Z.; Shen, X. Volatile organic compounds and odor emissions from veneered particleboards coated with water-based lacquer detected by gas chromatography-mass spectrometry/olfactometry. Eur. J. Wood Wood Prod 2019, 77, 771–781. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; de Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds. Sensors 2014, 40, 121–126. [Google Scholar] [CrossRef]
- Liang, W.H. Volatile organic compounds, odor, and inhalation health risks during interior construction of a fully furnished residential unit in Nanjing, China. Build. Environ. 2020, 186, 107366. [Google Scholar] [CrossRef]
- Fang, J.J.; Yang, N.; Cen, D.Y.; Shao, L.M.; He, P.J. Odor compounds from different sources of landfill: Characterization and source identification. Waste Manage. 2012, 32, 1401–1410. [Google Scholar] [CrossRef]
- ISO16000-28; Indoor air–Part 28: Determination of odour emissions from building products using test chambers. International Organization for Standardization: Geneva, Switzerland, 2020.
- Salthammer, T.; Schulz, N.; Stolte, R.; Monegel, F.; Uhde, E. Sensory evaluation in test chambers: Influences of direct and indirect assessment. Build. Environ. 2020, 172, 106668. [Google Scholar] [CrossRef]
- ISO16000-30; Indoor Air–Part 30: Sensory Testing of Indoor Air. International Organization for Standardization: Geneva, Switzerland, 2012.
- Zhang, Y.C.; Lin, Q.B.; Huang, Z.Y.; Li, Z.; Li, T. Determination of volatile odor compounds from food packaging paper by headspace gas chromatography-mass spectrometry coupled with retention indices. Food Ferment. Ind. 2021, 47, 268–273. [Google Scholar]
- Xue, M.; Liu, J.J.; Zhao, L.; Pei, J.J. Identification of odour compounds emitted by wooden boards with the presence of indoor ozone. Build. Environ. 2022, 221, 109341. [Google Scholar] [CrossRef]
- Nagata, E.; Yoshio, Y. Measurement of odor threshold by triangle odor bag method. Jpn. Environ. Sanit. Cent. 2003, 118, 118–127. [Google Scholar]
- Polizzi, V.; Adams, A.; Saeger, S.D.; Peteghem, C.V.; Moretti, A.; Kimpe, N.D. Influence of various growth parameters on fungal growth and volatile metabolite production by indoor molds. Sci. Total Environ. 2012, 414, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.S.; Shaw, B.R.; Wu, A.H.B. The clinical effects of plasticizers, antioxidants, and other contaminants in medical polyvinylchloride tubing during respiratory and non-respiratory exposure. Clin. Chim. Acta 2001, 304, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, H.J.; Yang, C.P.; Zeng, G.M.; Li, X.; Chen, H.; Yu, G.L. Challenges and solutions for biofiltration of hydrophobic volatile organic compounds. Biotechnol. Adv. 2016, 34, 1091–1102. [Google Scholar] [CrossRef]
- Kim, S.S.; Rhyu, M.; Lee, S.H. Use of sensory evaluation for measuring deodorizing effects of an air conditioner. J. Sens. Stud. 2003, 18, 33–46. [Google Scholar] [CrossRef]
- Niinemets, Ü. Controls on the emission of plant volatiles through stomata: Differential sensitivity of emission rates to stomatal closure explained. J. Geophys. Res. 2003, 108, 4208. [Google Scholar] [CrossRef]
- Cheng, W.H.; Chou, M.S.; Perng, C.H.; Chu, F.S. Determining the equilibrium partitioning coefficients of volatile organic compounds at an air-water interface. Chemosphere 2004, 54, 935–942. [Google Scholar] [CrossRef]
- Chen, L.P.; Xuan, K.Y.; Zhou, B.; Deng, G.F. Effects of thermodynamics parameters on mass transfer of volatile pollutants at air-water interface. Water Sci. Eng. 2015, 8, 211–216. [Google Scholar] [CrossRef]
- Abdullahi, M.E.; Zainura, Z.N.; Hassan, M.A.; Raja, K.R.I. Temperature and air–water ratio influence on the air stripping of benzene, toluene and xylene. Desalin. Water Treat. 2014, 54, 2832–2839. [Google Scholar] [CrossRef]
- Greenberg, M.I.; Curtis, J.A.; Vearrier, D. The perception of odor is not a surrogate marker for chemical exposure: A review of factors influencing human odor perception. Clin. Toxicol. 2013, 51, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Fang, Z.S.; Liu, W.W. Decreased humidity improves cognitive performance at extreme high indoor temperature. Indoor Air 2020, 31, 608–627. [Google Scholar] [CrossRef]
- Wu, Z.; Li, N.; Lan, L.; Wargocki, P. The effect of inhaled air temperature on thermal comfort, perceived air quality, acute health symptoms and physiological responses at two ambient temperatures. Indoor Air 2022, 32, e13092. [Google Scholar] [CrossRef]
- Fang, L.; Wyon, D.P.; Clausen, G.; Fanger, P.O. Impact of indoor air temperature and humidity in an office on perceived air quality, SBS symptoms and performance. Indoor Air 2010, 14, 74–81. [Google Scholar] [CrossRef]
- Lan, L.; Wargocki, P.; Wyon, D.P.; Lian, Z. Effects of thermal discomfort in an office on perceived air quality, SBS symptoms, physiological responses, and human performance. Indoor Air 2011, 21, 376–390. [Google Scholar] [CrossRef]
- He, M.L.; Li, N.P.; He, Y.D.; He, D.; Wang, K. Influences of Temperature and Humidity on Perceived Air Quality with Radiant Panel Workstation. Procedia Eng. 2017, 205, 765–772. [Google Scholar] [CrossRef]
- Wu, Y.F.; Sun, H.L.; Duan, M.F.; Lin, B.R.; Zhao, H.X. Dehumidification-adjustable cooling of radiant cooling terminals based on a flat heat pipe. Build. Environ. 2021, 194, 107716. [Google Scholar] [CrossRef]
- Zhao, W.X.; Kilpeläinen, S.; Kosonen, R.; Jokisalo, J.; Lestinen, S.; Wu, Y.X.; Mustakallio, P. Human response to thermal environment and perceived air quality in an office with individually controlled convective and radiant cooling systems. Build. Environ. 2021, 195, 107736. [Google Scholar] [CrossRef]
- Zhou, J.M.; Noor, N.; Wang, F.M.; Zhang, X.S. Simulation and experiment on direct contact membrane distillation regenerator in the liquid dehumidification air-conditioning system. Build. Environ. 2020, 168, 106496. [Google Scholar] [CrossRef]
- Chen, W.H.; Yin, Y.G.; Zhao, X.W.; Xu, G.Y.; Cao, B.W.; Ji, Q. Experimental investigation on condensation characteristics of a novel radiant terminal based on sepiolite composite humidity-conditioning coating. Build. Environ. 2020, 223, 109488. [Google Scholar] [CrossRef]
- Alabi, W.O.; Krishnan, E.N.; Karoyo, A.H.; Dehabadi, L.; Wilson, L.D.; Simonson, C.J. Suitability of bio-desiccants for energy wheels in HVAC applications. Build. Environ. 2021, 206, 108369. [Google Scholar] [CrossRef]
- Yang, H.; Yang, C.X. Analysis of a novel pressurized dehumidification air conditioning system for urban underground space. Build. Environ. 2021, 206, 108400. [Google Scholar] [CrossRef]





| Sample No. | Type | Using Environment | Refrigeration Power (W) | Stated Air Flow Rate (m3/h) | Time after Last Clean (Years) |
|---|---|---|---|---|---|
| #1 | Constant frequency air conditioner | Meeting room | 7250 | 1200 | 2–3 |
| #2 | Office | 7120 | 1100 | 1–2 | |
| #3 | Kitchen | 7200 | 1100 | 2 | |
| #4 | Variable frequency air conditioner | Office | 7200 (1800–8500) | 1000 | 2 |
| #5 | Used Air filter | Bedroom | - | - | 2–3 |
| #6 | Study | - | - | 2–3 | |
| #7 | Bedroom | - | - | 1 |
| Samples | Tenax-TA Tubes | Supelco Tubes | DNPH Tubes |
|---|---|---|---|
| Analysis method | TD-GC/MS (TD-100 a, Markes; Agilent 7890 B/5975 B, USA) | GC/MS (Agilent 7890 B/5975B, USA); ODP (Gerstel C200, Germany) | HPLC (Agilent 1260, USA) c |
| Column | DB-624UI (60 m × 0.25 mm × 1.4 μm) | UA-1 (60 m × 320 μm × 0.5 μm) | Poroshell 120 EC-C18 (4.6 × 100 mm, 2.7 μm) |
| Column flow | 1.2 mL/min, He | MS: 3.0 mL/min, N2 ODP: 1.5 mL/min | 1.5 mL/min, Acetonitrile |
| Oven temp. | 50 °C (2) b−9 °C/min-130 °C (1) b−10 °C/min-240 °C (10) b, 32.89 min analysis time | 40 °C (5) b−3℃/min−92 °C−5 °C/min−160 °C−10 °C/min−280 °C (15) b-300 °C | 65% (0–4.2), 55% (at 14.6 min), 30%(25–31 min), 65% (31.1–34 min) |
| Injector temp. | 200 °C | - | - |
| Detector | MS | - | UV spectra (360 nm) |
| Quadrupole temp. | 230 °C | - | - |
| Ion source temp. | 230 °C | 230 °C | - |
| Trans line temp. | 250 °C | 250 °C | - |
| Scan range | 40–300 m/z | 33–550 m/z | - |
| Category | Compound | CAS | CFAC #1 C(μg/m3) | CFAC #2 C(μg/m3) | CFAC #3 C(μg/m3) | Odor Description |
|---|---|---|---|---|---|---|
| Acid ester | Ethyl Acetate | 141-78-6 | 1.99 | 2.83 | 21.77 | pineapple |
| Ethyl iso-allocholate | 101230-69-7 | 1.21 | 1.14 | - | / | |
| Androstane-17-carboxylic acid, methyl ester | 15173-52-1 | 2.29 | - | - | / | |
| 10,13-Eicosadienoic acid, methyl ester | 30223-50-8 | 1.81 | - | - | / | |
| 1,3-benzene dicarboxylic acid | 112043-90-0 | - | 5.24 | - | / | |
| Acetic acid | 64-19-7 | 1.14 | 1.33 | 1.76 | sour | |
| Alcohol | Ethanol | 64-17-5 | - | 5.03 | 12.12 | sweet, alcohol |
| 2-ethyl-1-Hexanol | 104-76-7 | 11.60 | 3.04 | 2.08 | rose, green | |
| 2-methyl-1-Propanol | 78-83-1 | 5.24 | - | 3.00 | wine, solvent, bitter | |
| 1-Butanol | 71-36-3 | 1.29 | - | 1.75 | medicine, fruit | |
| Aldehyde | Acetaldehyde | 75-07-0 | 1.17 | 0.72 | 3.79 | pungent, ether |
| Hexanal | 66-25-1 | 1.25 | 0.75 | 1.21 | grass, tallow, fat | |
| Nonanal | 124-19-6 | 5.67 | 5.55 | 0.96 | fat, citrus | |
| Decanal | 112-31-2 | 4.60 | 3.63 | - | soap, orange peel, tallow | |
| Octanal | 124-13-0 | - | 1.45 | - | fat, soap, lemon, green | |
| Ketone | Acetone | 67-64-1 | 8.85 | 6.20 | 12.11 | / |
| Cyclohexanone | 108-94-1 | 4.25 | - | 1.00 | / | |
| 2-Oxetanone, 4-methyl- | 3068-88-0 | - | - | 6.22 | / | |
| Benzene series | Toluene | 108-88-3 | 16.73 | 12.22 | 16.70 | aroma, paint |
| o-Xylene | 95-47-6 | - | 6.88 | 13.80 | aroma, geranium | |
| Ethylbenzene | 100-41-4 | 0.95 | 2.15 | 6.57 | / | |
| Benzene | 71-43-2 | 0.57 | 0.73 | 2.72 | / | |
| Styrene | 100-42-5 | - | 6.69 | 8.81 | balsamic, gasoline | |
| Alkane | n-Hexane | 110-54-3 | 3.65 | 4.32 | 23.59 | alkane |
| Methylene chloride | 75-09-2 | 12.62 | 2.55 | 16.33 | / | |
| Methane, oxybis [dichloro- | 20524-86-1 | 1.56 | 1.78 | 0.79 | / | |
| Heptane | 142-82-5 | - | 9.05 | 1.73 | alkane | |
| Bicyclo [3.1.1] hept-2-ene | 23978-81-6 | 1.81 | - | 3.08 | / | |
| Limonene | 138-86-3 | - | 0.96 | 1.28 | lemon, orange | |
| Tetrachloroethylene | 127-18-4 | 3.55 | - | - | / | |
| Cyclobutane, methyl- | 598-61-8 | - | 11.58 | - | / | |
| Other | Acetonitrile | 75-05-8 | 7.29 | 4.67 | 4.63 | / |
| N, N, O-Triacetylhydroxylamine | 17720-63-7 | 2.03 | - | - | / |
| Compound | OT a (μg/m3) | CFAC #1 | CFAC #2 | CFAC #3 | |||
|---|---|---|---|---|---|---|---|
| C(μg/m3) | OAV | C(μg/m3) | OAV | C(μg/m3) | OAV | ||
| Formaldehyde | 670.31 | 67.36 | 0.10 | 52.53 | 0.08 | 30.97 | 0.05 |
| Acetaldehyde | 2.95 | 15.21 | 5.15 | 12.34 | 4.18 | 15.20 | 5.15 |
| Acetone | 108900 | 27.95 | 0.00 | 19.48 | 0.00 | 21.01 | 0.00 |
| Hexanal | 1.25 | 12.68 | 10.12 | 10.51 | 8.39 | 8.51 | 6.80 |
| Propanal | 2.59 | 2.63 | 1.01 | - | - | 1.23 | 0.47 |
| Crotonaldehyde | 71.97 | 1.36 | 0.02 | - | - | - | - |
| 2-Butanone | 1416.45 | 1.34 | 0.00 | - | - | - | - |
| Group | Compound | Filter #5 | Filter #6 | Filter #7 | Odor Description | |||
|---|---|---|---|---|---|---|---|---|
| C (μg/m3) | OI | C (μg/m3) | OI | C (μg/m3) | OI | |||
| Acid ester | Octadecanoic acid, 2-propenyl ester | 2.07 | / | - | / | - | / | / |
| Acetic acid ethenyl ester | - | / | - | / | 2.11 | 3 | yogurt, hawthorn | |
| Ethyl Acetate | - | / | - | / | 2.42 | 3 | soap, freshener | |
| Alcohol | 2-methyl-1-Propanol | 2.37 | / | - | / | - | / | / |
| 2-ethyl-1-Hexanol | 1.40 | 0.5 | 5.05 | 1 | 17.00 | 2 | slightly sour | |
| Aldehyde | Benzaldehyde | - | / | 3.29 | / | - | / | / |
| Octanal | - | / | 1.34 | 1 | 7.63 | 3.5 | freshener, soap | |
| Nonanal | 6.32 | 2 | 5.04 | 2 | 11.49 | 3.5 | almond, sour | |
| Hexanal | 1.06 | 0.75 | 7.04 | 2.5 | 9.14 | 3.5 | grass, alcohol | |
| Butanal, 3-methyl- | - | / | - | / | 2.76 | 3 | alcohol | |
| Ketone | Acetone | 2.18 | / | 1.43 | / | 1.63 | / | / |
| Acetylacetone | - | / | 12.71 | / | - | / | / | |
| Benzene series | Benzene | 1.99 | / | 1.99 | / | 0.93 | / | / |
| Toluene | 16.25 | 1.5 | 13.61 | 1 | 24.55 | 2.5 | paint, leather, plastic | |
| Ethylbenzene | 1.91 | / | - | / | 1.34 | / | / | |
| o-Xylene | 2.88 | / | 1.57 | / | 3.00 | / | / | |
| Styrene | 5.98 | 1.5 | 2.58 | / | 6.11 | 1.5 | garbage, charred | |
| Benzene, 1,4-dichloro- | 195.22 | 4 | 20.03 | 1.5 | - | / | hawthorn, irritation | |
| Alkane | n-Hexane | 2.58 | 2 | 1.78 | 1 | 1.06 | 0.75 | metal, grass |
| Heptadecane, 9-hexyl- | 1.08 | / | 2.35 | / | - | / | / | |
| Limonene | 2.93 | / | 10.82 | / | 2.31 | / | / | |
| Decane, 2,6,7-trimethyl- | - | / | 3.39 | / | - | / | / | |
| 3-Ethyl-3-methyl heptane | - | / | 7.58 | / | 6.56 | / | / | |
| Heptane | - | / | - | / | 9.39 | 3 | metal | |
| Tetrachloroethylene | - | / | - | / | 2.64 | / | / | |
| Octane, 4-methyl- | - | / | - | / | 2.13 | 1.5 | cucumber | |
| Decane, 2-methyl- | - | / | - | / | 2.70 | / | / | |
| Category | Odorants | Air Conditioner Outlet | Air Filter | Odor Description | |
|---|---|---|---|---|---|
| GC/MS | HPLC | GC/O/MS | |||
| Acid ester | Ethyl Acetate | √ 3 | pineapple, soap, freshener | ||
| Acetic acid | √3 | sour | |||
| Alcohol | 2-ethyl-1-Hexanol | √ | √ 2 | resin, flower, slight sour | |
| Aldehyde | Acetaldehyde | √ | √ 1 | pungent, ether | |
| Hexanal | √ | √ 1 | √ | grass, tallow, fat, alcohol | |
| Nonanal | √ | √2 | fat, citrus, almond, sour | ||
| Benzene series | Toluene | √ | √2 | aroma, paint, leather, plastic | |
| Alkane | n-Hexane | √ | √2 | alkane, metal, grass | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, J.; Sun, L. Odor from Building Air Conditioners: Emission Characteristics, Odor Compounds and Influencing Factors. Sustainability 2023, 15, 1495. https://doi.org/10.3390/su15021495
Pei J, Sun L. Odor from Building Air Conditioners: Emission Characteristics, Odor Compounds and Influencing Factors. Sustainability. 2023; 15(2):1495. https://doi.org/10.3390/su15021495
Chicago/Turabian StylePei, Jingjing, and Luyao Sun. 2023. "Odor from Building Air Conditioners: Emission Characteristics, Odor Compounds and Influencing Factors" Sustainability 15, no. 2: 1495. https://doi.org/10.3390/su15021495
APA StylePei, J., & Sun, L. (2023). Odor from Building Air Conditioners: Emission Characteristics, Odor Compounds and Influencing Factors. Sustainability, 15(2), 1495. https://doi.org/10.3390/su15021495





