Heavy Metal and Metalloid Contamination in Food and Emerging Technologies for Its Detection
Abstract
1. Introduction
2. Metals in Food Toxicity
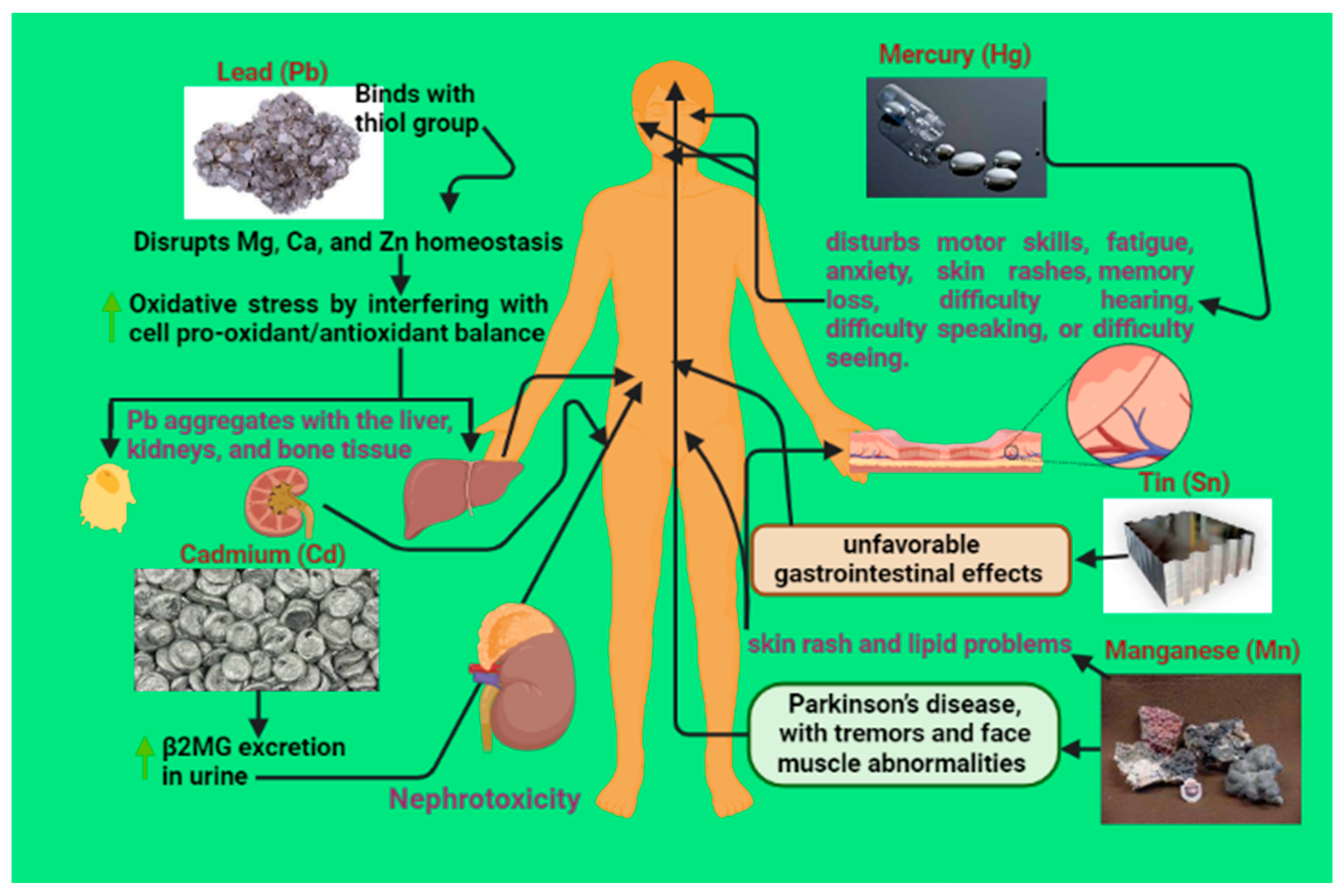
2.1. Cadmium (Cd) Toxicity
2.2. Lead (Pb) Toxicity
2.3. Mercury (Hg) Toxicity
2.4. Tin (Sn) Toxicity
2.5. Manganese (Mn) Toxicity
2.6. Aluminum (Al) Toxicity
2.7. Chromium (Cr) Toxicity
2.8. Iron (Fe) Toxicity
2.9. Nickel (Ni)Toxicity
| Metal | Sources | Recommendation of Intake | Side Effects | Reference |
|---|---|---|---|---|
| Cadmium (Cd) | staple food, rice, and several specific foods, such as animal kidneys, blood cockle, and local vegetables | The European Food Safety Authority’s (EFSA) panel on contaminants in the food chain recommended in 2009 that the PTWI be lowered to the tolerable weekly intake (TWI) level of 2.5 μg/kg body weight. | Kidney disease, osteoporosis, diabetes, cardiovascular disease, and cancer | [21] |
| Lead (Pb) | wind, consumption of Pb-contaminated soils, oral intake of Pb-contaminated water, and consumption of food cultivated in Pb-contaminated areas | The WHO and the FAO have approved daily Pb intakes of up to 7 µg/kg body weight or 490 µg of Pb for adults. | liver, kidneys, and bone tissue | [32,33] |
| Mercury (Hg) | ingestion of fish, whale sharks, sailfish, marlin, other billfish, and mercury amalgams | - | impaired motor skills, fatigue, anxiety, skin rashes, memory loss, difficulty hearing, difficulty speaking, difficulty seeing, Minamata disease, and acrodynia | [42,43] |
| Tin (Sn) | Cans for drinks are commonly made from tinplate. Over fifteen billion internally lacquered tinplate beverage cans are made and used annually in Europe | Maximum allowable levels of Sn in food are usually 250 mg/kg (200 mg/kg in the UK) for solid foods and 150 mg/kg (2.5 mmol/L) for beverages, with a PTWI of 14 mg/kg body weight. | unfavorable gastrointestinal effects | [47,48,49,50] |
| Manganese (Mn) | foods, with some vegans consuming >10 mg daily | Recommended intake of Mn is 1.8 (women) or 2.3 (men) mg/d, while the upper limit is 11 mg/d, according to the National Academy of Sciences. | Parkinson’s disease, with tremors and facial muscle problems | [64] |
| Aluminum (Al) | Corn, yellow cheese, salt, herbs, spices, tea, and tap water | Al intake should be kept below 1 milligram per kilogram of body weight per week, as determined by the European Food Safety Authority. Food Additives reported in 1989 that the average daily intake of aluminum for children is 2–6 mg/kg, and for adults, 6–14 mg/kg. Al has a PTWI value of 7 mg/kg. | pathogenesis of Alzheimer’s disease, impaired skeletal system, reduced collagen synthesis, slowed-down mineralization, weakening resistance, and a propensity toward breaking | [77] |
| Chromium (Cr) | canned and other processed foods, brown sugar, and molasses | Dietary intake above 25 µg (0.5 µmol)/d for adults and between 0.1 and 1.0 µg/kg/d (2 and 19 nmol/k/d) for children and adolescents are considered safe and appropriate. However, the Panel has not established reference nutrient intakes (RNI) for Cr compounds. | carcinogenic, corrosive, and delayed contact sensitizers | [98,99] |
| Iron (Fe) | lowland rice | A safe upper threshold of 25–50 mg Fe/day can be calculated by applying a safety factor of 2 to prevent this common iron toxicity endpoint. | stomach upset, primary and secondary hemochromatosis, hepatic fibrosis, diabetes, and heart failure | [104] |
| Nickel (Ni) | marine fish and plants watered with untreated sewage | Consumption of 325 mg causes vomiting, dizziness, and a slowing heart rate. | respiratory and digestive damage, cerebral edema, or diffuse interstitial pneumonitis | [107,108,109] |
3. Metalloids in Food Toxicity
3.1. Arsenic (As) Toxicity
3.2. Antimony (Sb) Toxicity
3.3. Selenium (Se) Toxicity
4. Detection of Heavy Metal and Metalloid Contaminants in Food Toxicity
4.1. Atomic Absorption Spectrometry (AAS)
4.2. Spectrofluorimetry
4.3. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
4.4. Liquid Chromatography–Inductively Coupled Plasma Mass Spectrometry (LC–ICP-MS)
4.5. E-Tongues
4.6. Electrochemical Aptasensors
4.7. Raman Spectroscopy
4.8. Fluorescence Sensors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement:
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reilly, C. Metal Contamination of Food: Its Significance for Food Quality and Human Health; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Massoud, R.; Hadiani, M.R.; Hamzehlou, P.; Khosravi-Darani, K. Bioremediation of heavy metals in food industry: Application of Saccharomyces cerevisiae. Electron. J. Biotechnol. 2019, 37, 56–60. [Google Scholar] [CrossRef]
- Fowler, B.A.; Alexander, J.; Oskarsson, A. Toxic metals in food. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2015; pp. 123–140. [Google Scholar]
- Borocz-Szabo, M. Effects of metals on sensory qualities of food. Acta Aliment. 1980, 9, 341–356. [Google Scholar]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, S.; Shi, Y.; Wang, C.; Li, B.; Li, Y.; Wu, S. Heavy metals in food crops, soil, and water in the Lihe River Watershed of the Taihu Region and their potential health risks when ingested. Sci. Total Environ. 2018, 615, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Onakpa, M.M.; Njan, A.A.; Kalu, O.C. A review of heavy metal contamination of food crops in Nigeria. Ann. Glob. Health 2018, 84, 488. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.G.; Wanjari, U.R.; Renu, K.; Vellingiri, B.; Gopalakrishnan, A.V. Heavy metal and metalloid-induced reproductive toxicity. Environ. Toxicol. Pharmacol. 2022, 92, 103859. [Google Scholar] [CrossRef] [PubMed]
- Nkwunonwo, U.C.; Odika, P.O.; Onyia, N.I. A review of the health implications of heavy metals in food chain in Nigeria. Sci. World J. 2020, 2020, 6594109. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Marfaing, H.; Desnica, N.; Jónsdóttir, R.; Skjermo, J.; Rebours, C.; Nitschke, U. Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: Health risk assessment and implication for food applications. Food Control 2019, 95, 121–134. [Google Scholar] [CrossRef]
- Goutam Mukherjee, A.; Ramesh Wanjari, U.; Eladl, M.A.; El-Sherbiny, M.; Elsherbini, D.M.A.; Sukumar, A.; Kannampuzha, S.; Ravichandran, M.; Renu, K.; Vellingiri, B.; et al. Mixed Contaminants: Occurrence, Interactions, Toxicity, Detection, and Remediation. Molecules 2022, 27, 2577. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bhattacharyya, S.; Ghosh, K.; Pal, S.; Halder, A.; Naseri, M.; Mohammadniaei, M.; Sarkar, S.; Ghosh, A.; Sun, Y. Sensory development for heavy metal detection: A review on translation from conventional analysis to field-portable sensor. Trends Food Sci. Technol. 2021, 109, 674–689. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Mao, W.; Sui, H.; Yong, L.; Yang, D.; Jiang, D.; Zhang, L.; Gong, Y. Dietary cadmium exposure assessment among the Chinese population. PLoS ONE 2017, 12, e0177978. [Google Scholar] [CrossRef] [PubMed]
- Chunhabundit, R. Cadmium exposure and potential health risk from foods in contaminated area, Thailand. Toxicol. Res. 2016, 32, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Suwatvitayakorn, P.; Ko, M.-S.; Kim, K.-W.; Chanpiwat, P. Human health risk assessment of cadmium exposure through rice consumption in cadmium-contaminated areas of the Mae Tao sub-district, Tak, Thailand. Environ. Geochem. Health 2020, 42, 2331–2344. [Google Scholar] [CrossRef] [PubMed]
- Aendo, P.; De Garine-Wichatitsky, M.; Mingkhwan, R.; Senachai, K.; Santativongchai, P.; Krajanglikit, P.; Tulayakul, P. Potential health effects of heavy metals and carcinogenic health risk estimation of pb and cd contaminated eggs from a closed gold mine area in northern Thailand. Foods 2022, 11, 2791. [Google Scholar] [CrossRef]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.-a. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135. [Google Scholar] [CrossRef]
- Joint FAO; WHO Expert Committee on Food Additives; World Health Organization. Safety Evaluation of Certain Food Additives and Contaminants: Prepared by The Seventy-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain. Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Cadmium toxicity and treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef]
- Egger, A.E.; Grabmann, G.; Gollmann-Tepeköylü, C.; Pechriggl, E.J.; Artner, C.; Türkcan, A.; Hartinger, C.G.; Fritsch, H.; Keppler, B.K.; Brenner, E. Chemical imaging and assessment of cadmium distribution in the human body. Metallomics 2019, 11, 2010–2019. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; Taylor & Francis: London, UK, 2015. [Google Scholar]
- WHO. Safety Evaluation of Certain Food Additives and Contaminants; WHO Food Additives Series: Geneva, Switzerland, 1998. [Google Scholar]
- Hsu, P.-C.; Guo, Y.L. Antioxidant nutrients and lead toxicity. Toxicology 2002, 180, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; MMS, C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef] [PubMed]
- WHO. Childhood Lead Poisoning; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Commission, E. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Bellinger, D.C.; Malin, A.; Wright, R.O. The neurodevelopmental toxicity of lead: History, epidemiology, and public health implications. In Advances in Neurotoxicology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 1–26. [Google Scholar]
- Wani, A.; Ara, A.; Usmani, J. Lead toxicity: A review. Interdiscip Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Rumbeiha, W.K.; Braselton, W.E.; Donch, D. A retrospective study on the disappearance of blood lead in cattle with accidental lead toxicosis. J. Vet. Diagn. Investig. 2001, 13, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.; Livesey, C. Lead poisoning in cattle and its implications for food safety. Vet. Rec. 2006, 159, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Flora, S. Nutritional components modify metal absorption, toxic response and chelation therapy. J. Nutr. Environ. Med. 2002, 12, 53–67. [Google Scholar] [CrossRef]
- Yuan, G.; Dai, S.; Yin, Z.; Lu, H.; Jia, R.; Xu, J.; Song, X.; Li, L.; Shu, Y.; Zhao, X. Toxicological assessment of combined lead and cadmium: Acute and sub-chronic toxicity study in rats. Food Chem. Toxicol. 2014, 65, 260–268. [Google Scholar] [CrossRef]
- Cobbina, S.J.; Chen, Y.; Zhou, Z.; Wu, X.; Zhao, T.; Zhang, Z.; Feng, W.; Wang, W.; Li, Q.; Wu, X. Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. J. Hazard. Mater. 2015, 294, 109–120. [Google Scholar] [CrossRef]
- El-Neweshy, M.S.; El-Sayed, Y.S. Influence of vitamin C supplementation on lead-induced histopathological alterations in male rats. Exp. Toxicol. Pathol. 2011, 63, 221–227. [Google Scholar] [CrossRef]
- Abdou, H.M.; Hassan, M.A. Protective role of omega-3 polyunsaturated fatty acid against lead acetate-induced toxicity in liver and kidney of female rats. BioMed. Res. Int. 2014, 2014, 435857. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Catalano, A.; Lauria, G.; Sinicropi, M.S.; Genchi, G. Lead toxicity, antioxidant defense and environment. Rev. Environ. Contam. Toxicol. 2016, 238, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Tarrago, O.; Brown, M. Case Studies in Environmental Medicine (CSEM) Lead Toxicity. Agency Toxic Subst. Dis. Regist. 2017. Available online: https://www.atsdr.cdc.gov/csem/lead/docs/CSEM-Lead_toxicity_508.pdf (accessed on 30 October 2022).
- Loomis, D.; Guha, N.; Hall, A.L.; Straif, K. Identifying occupational carcinogens: An update from the IARC Monographs. Occup. Environ. Med. 2018, 75, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, E.G.; Pacyna, J.M.; Steenhuisen, F.; Wilson, S. Global anthropogenic mercury emission inventory for 2000. Atmos. Environ. 2006, 40, 4048–4063. [Google Scholar] [CrossRef]
- Sattar, T. Brief Discussion on Mercury Poisoning, Its Sources and Remedies to Cure It. J. Chem. Health Risks 2020, 12, 131–142. [Google Scholar] [CrossRef]
- Renzoni, A.; Zino, F.; Franchi, E. Mercury levels along the food chain and risk for exposed populations. Environ. Res. 1998, 77, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Derban, L.K. Outbreak of food poisoning due to alkyl-mercury fungicide. Arch. Environ. Health Int. J. 1974, 28, 49–52. [Google Scholar] [CrossRef]
- George, T.S. Minamata: Pollution and the Struggle for Democracy in Postwar JAPAN; Harvard University Asia Center: Cambridge, MA, USA, 2001; Volume 194. [Google Scholar]
- Joint, F.A.O.; WHO Expert Committee on Food Additives. Toxicological Evaluation of Certain Food Additives and Contaminants in Foods; WHO Food Additives Series: Geneva, Switzerland, 1996. [Google Scholar]
- Joint, F.A.O.; WHO Expert Committee on Food Additives. Toxicological Evaluation of Certain Food Additives and Contaminants; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Blunden, S.; Wallace, T. Tin in canned food: A review and understanding of occurrence and effect. Food Chem. Toxicol. 2003, 41, 1651–1662. [Google Scholar] [CrossRef]
- Codex Committee on Contaminants in Foods (CCCF). Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods; 5th session; Codex Committee on Contaminants in Foods (CCCF): Washington, DC, USA, 2011; pp. 21–25. [Google Scholar]
- Adams, F.; Blunden, S.J.; van Cleuvenbergen, R.; Evans, C.; Fishbein, L.; Rickenbacher, U.-J.; Schlatter, C.; Steinegger, A. Anthropogenic Compounds; Springer: Berlin, Germany, 2013; Volume 3. [Google Scholar]
- Weber, G. Speciation of tin in lemon juice: An example of trace metal speciation in food. Anal. Chim. Acta 1987, 200, 79–88. [Google Scholar] [CrossRef]
- Horio, T.; Iwamoto, Y.; Miyazaki, M. Studies on the internal corrosion of plain tinplate cans. In Proceedings of the 6th International Congress on Canned Foods, Tangerang, Indonesia, 4–5 August 2021. [Google Scholar]
- Biégo, G.; Joyeux, M.; Hartemann, P.; Debry, G. Determination of dietary tin intake in an adult French citizen. Arch. Environ. Contam. Toxicol. 1999, 36, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Rojas, E.; Herrera, L.A.; Poirier, L.A.; Ostrosky-Wegman, P. Are metals dietary carcinogens? Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 443, 157–181. [Google Scholar] [CrossRef]
- Fišera, M.; Kráčmar, S.; Velichová, H.; Fišerová, L.; Burešová, P.; Tvrzník, P. Tin compounds in food-their distribution and determination. Potravin. Slovak J. Food Sci. 2019, 13, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ostrakhovitch, E.A. Tin. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 807–856. [Google Scholar]
- Alamri, M.; Qasem, A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food packaging’s materials: A food safety perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kumar, P. Biochemical alteration of mustard grown under tin contaminated soil. Plant Arch. 2020, 20, 3487–3492. [Google Scholar]
- Greger, J.L. Tin and aluminum. In Trace Minerals in Foods; CRC Press: Boca Raton, FL, USA, 2020; pp. 291–323. [Google Scholar]
- Markesbery, W.R. Elevated manganese levels associated with dementia and extrapyramidal signs. Neurology 1977, 27, 213. [Google Scholar] [CrossRef]
- Calne, D.; Chu, N.; Huang, C.; Lu, C.; Olanow, W. Manganism and idiopathic parkinsonism: Similarities and differences. Neurology 1994, 44, 1583–1586. [Google Scholar] [CrossRef]
- Chia, S.-E.; Gan, S.-L.; Chua, L.-H.; Foo, S.-C.; Jeyaratnam, J. Postural stability among manganese exposed workers. Neurotoxicology 1995, 16, 519–526. [Google Scholar]
- Cook, D.G.; Fahn, S.; Brait, K.A. Chronic manganese intoxication. Arch. Neurol. 1974, 30, 59–64. [Google Scholar] [CrossRef]
- Russell, R.; Beard, J.L.; Cousins, R.J.; Dunn, J.T.; Ferland, G.; Hambidge, K.; Lynch, S.; Penland, J.; Ross, A.; Stoecker, B. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. 2001; p. 797. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222310/?report=reader (accessed on 30 October 2022).
- Gibson, R.S. Content and bioavailability of trace elements in vegetarian diets. Am. J. Clin. Nutr. 1994, 59, 1223S–1232S. [Google Scholar] [CrossRef]
- Hurley, L.S. Teratogenic aspects of manganese, zinc, and copper nutrition. Physiol. Rev. 1981, 61, 249–295. [Google Scholar] [CrossRef] [PubMed]
- Friedman, B.; Freeland-Graves, J.H.; Bales, C.W.; Behmardi, F.; Shorey-Kutschke, R.L.; Willis, R.A.; Crosby, J.B.; Trickett, P.C.; Houston, S.D. Manganese balance and clinical observations in young men fed a manganese-deficient diet. J. Nutr. 1987, 117, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Freeland-Graves, J.; Llanes, C. Models to Study Manganese Deficiency; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Freeland-Graves, J.; Mertz, W.; Abernathy, C.; Olin, S. Derivation of manganese safe and adequate daily dietary intakes. In Risk Assessment of Essential Elements; ILSI Press: Washington, DC, USA, 1994; pp. 237–252. [Google Scholar]
- Klimis-Tavantzis, D.; Leach, R., Jr.; Kris-Etherton, P. The effect of dietary manganese deficiency on cholesterol and lipid metabolism in the Wistar rat and in the genetically hypercholesterolemic RICO rat. J. Nutr. 1983, 113, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Schoenthaler, S.J.; Bier, I.D. The effect of vitamin-mineral supplementation on juvenile delinquincy among American schoolchildren: A randomized, double-blind placebo-controlled trial. J. Altern. Complement. Med. 2000, 6, 7–17. [Google Scholar] [CrossRef]
- Cromwell, P.F.; Abadie, B.R.; Stephens, J.T.; Kyler, M. Hair mineral analysis: Biochemical imbalances and violent criminal behavior. Psychol. Rep. 1989, 64, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, L.A.; Rebello, T.; Buchsbaum, M.S.; Tucker, H.G.; Hodges, E.L. Abnormalities in hair trace elements as indicators of aberrant behavior. Compr. Psychiatry 1991, 32, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W.; Penland, J.G.; Pettit, R.E.; Davis, C.D. Dietary manganese intake and type of lipid do not affect clinical or neuropsychological measures in healthy young women. J. Nutr. 2003, 133, 2849–2856. [Google Scholar] [CrossRef]
- Greger, J. Dietary standards for manganese: Overlap between nutritional and toxicological studies. J. Nutr. 1998, 128, 368S–371S. [Google Scholar] [CrossRef]
- Ochmański, W.; Barabasz, W. Aluminum—occurrence and toxicity for organisms. Przegla̧d Lek. 2000, 57, 665–668. [Google Scholar]
- Bratakos, S.M.; Lazou, A.E.; Bratakos, M.S.; Lazos, E.S. Aluminium in food and daily dietary intake estimate in Greece. Food Addit. Contam. Part B 2012, 5, 33–44. [Google Scholar] [CrossRef]
- Sato, K.; Suzuki, I.; Kubota, H.; Furusho, N.; Inoue, T.; Yasukouchi, Y.; Akiyama, H. Estimation of daily aluminum intake in Japan based on food consumption inspection results: Impact of food additives. Food Sci. Nutr. 2014, 2, 389–397. [Google Scholar] [CrossRef]
- Al Zubaidy, E.A.; Mohammad, F.S.; Bassioni, G. Effect of pH, salinity and temperature on aluminum cookware leaching during food preparation. Int. J. Electrochem. Sci. 2011, 6, 6424–6441. [Google Scholar] [CrossRef]
- Ieggli, C.V.; Bohrer, D.; do Nascimento, P.C.; de Carvalho, L.M.; Gobo, L.A. Determination of aluminum, copper and manganese content in chocolate samples by graphite furnace atomic absorption spectrometry using a microemulsion technique. J. Food Compos. Anal. 2011, 24, 465–468. [Google Scholar] [CrossRef]
- Nanda, B.; Biswal, R.; Acharya, R.; Rao, J.; Pujari, P. Determination of aluminium contents in selected food samples by instrumental neutron activation analysis. J. Radioanal. Nucl. Chem. 2014, 302, 1471–1474. [Google Scholar] [CrossRef]
- Stahl, T.; Taschan, H.; Brunn, H. Aluminium content of selected foods and food products. Environ. Sci. Eur. 2011, 23, 37. [Google Scholar] [CrossRef]
- Pennington, J.A. Aluminium content of foods and diets. Food Addit. Contam. 1988, 5, 161–232. [Google Scholar] [CrossRef] [PubMed]
- López, F.F.; Cabrera, C.; Lorenzo, M.L.; López, M.C. Aluminium levels in spices and aromatic herbs. Sci. Total Environ. 2000, 257, 191–197. [Google Scholar] [CrossRef]
- Aguilar, F.; Autrup, H.; Barlow, S.; Castle, L.; Crebelli, R.; Dekant, W.; Engel, K.-H.; Gontard, N.; Gott, D.; Grilli, S. Annex of the opinion on Safety of aluminium from dietary intake Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA J. 2008, 754, 1–34. [Google Scholar]
- Stahl, T.; Falk, S.; Rohrbeck, A.; Georgii, S.; Herzog, C.; Wiegand, A.; Hotz, S.; Boschek, B.; Zorn, H.; Brunn, H. Migration of aluminum from food contact materials to food—A health risk for consumers? Part I of III: Exposure to aluminum, release of aluminum, tolerable weekly intake (TWI), toxicological effects of aluminum, study design, and methods. Environ. Sci. Eur. 2017, 29, 19. [Google Scholar] [CrossRef]
- Soni, M.G.; White, S.M.; Flamm, W.G.; Burdock, G.A. Safety evaluation of dietary aluminum. Regul. Toxicol. Pharmacol. 2001, 33, 66–79. [Google Scholar] [CrossRef]
- Yokel, R.A. Aluminum in Food–the Nature and Contribution of Food Additives; Intech: Rijeka, Croatia, 2012; Volume 203. [Google Scholar]
- Greger, J. Aluminum metabolism. Annu. Rev. Nutr. 1993, 13, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T.; Falk, S.; Rohrbeck, A.; Georgii, S.; Herzog, C.; Wiegand, A.; Hotz, S.; Boschek, B.; Zorn, H.; Brunn, H. Migration of aluminum from food contact materials to food—A health risk for consumers? Part III of III: Migration of aluminum to food from camping dishes and utensils made of aluminum. Environ. Sci. Eur. 2017, 29, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.Y.; Wang, H.L.; Luo, P.J.; Zhang, J.B. The revision of aluminum-containing food additive provisions in China. Biomed. Environ. Sci. 2016, 29, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T.; Falk, S.; Taschan, H.; Boschek, B.; Brunn, H. Evaluation of human exposure to aluminum from food and food contact materials. Eur. Food Res. Technol. 2018, 244, 2077–2084. [Google Scholar] [CrossRef]
- Starska, K. Aluminum in food. Rocz. Panstw. Zakl. Hig. 1993, 44, 55–63. [Google Scholar]
- Doisy, R.; Streeten, D.; Freiberg, J.; Schneider, A. Chromium metabolism in man and biochemical effects. Trace Elem. Hum. Health Dis. 2013, 2, 79–104. [Google Scholar]
- Brown, M.L. Present knowledge in nutrition. In International Life Science Institute-Nutrition Foundation; John Wiley & Sons: Hoboken, NJ, USA, 1990. [Google Scholar]
- Kumpulainen, J. Chromium content of foods and diets. Biol. Trace Elem. Res. 1992, 32, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Mutuma, S.; Amuna, P.; Shukla, H.; Sumar, S. Chromium in food, nutrition and health-an introduction. Nutrition & Food Science 1999, 99, 81–88. [Google Scholar] [CrossRef]
- Values, G.B.P.o.D.R.; Health, G.B.D.o. Dietary Reference Values for Food Energy and Nutrients for the United Kingdom: Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy; HM Stationery Office: Richmond, UK, 1991; Volume 41. [Google Scholar]
- Gad, S.C. Acute and chronic systemic chromium toxicity. Sci. Total Environ. 1989, 86, 149–157. [Google Scholar] [CrossRef]
- Fageria, N.; Santos, A.; Barbosa Filho, M.; Guimarães, C. Iron toxicity in lowland rice. J. Plant Nutr. 2008, 31, 1676–1697. [Google Scholar] [CrossRef]
- Audebert, A.; Sahrawat, K.L. Mechanisms for iron toxicity tolerance in lowland rice. J. Plant Nutr. 2000, 23, 1877–1885. [Google Scholar] [CrossRef]
- Becker, M.; Asch, F. Iron toxicity in rice—Conditions and management concepts. J. Plant Nutr. Soil Sci. 2005, 168, 558–573. [Google Scholar] [CrossRef]
- Schümann, K. Safety aspects of iron in food. Ann. Nutr. Metab. 2001, 45, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Arias, V.O.; Som, L.V.; Rodríguez, V.Q.; Romero, R.G.; Muñoz, N.; Alarcón, M.N.; Vique, C.C. Nickel in food and influencing factors in its levels, intake, bioavailability and toxicity: A review. CyTA J. Food 2015, 13, 87–101. [Google Scholar] [CrossRef]
- Haber, L.T.; Bates, H.K.; Allen, B.C.; Vincent, M.J.; Oller, A.R. Derivation of an oral toxicity reference value for nickel. Regul. Toxicol. Pharmacol. 2017, 87, S1–S18. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G.; Barceloux, D. Nickel. J. Toxicol. Clin. Toxicol. 1999, 37, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.M. Observations on the Salts of Nickel, Especially the Bromide of Nickel; Sidney Kimmel Medical College: Philadelphia, PA, USA, 1883. [Google Scholar]
- Sunderman, F.W., Jr.; Dingle, B.; Hopfer, S.M.; Swift, T. Acute nickel toxicity in electroplating workers who accidently ingested a solution of nickel sulfate and nickel chloride. Am. J. Ind. Med. 1988, 14, 257–266. [Google Scholar] [CrossRef]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef]
- Duxbury, J.; Mayer, A.; Lauren, J.; Hassan, N. Food chain aspects of arsenic contamination in Bangladesh: Effects on quality and productivity of rice. J. Environ. Sci. Health Part A 2003, 38, 61–69. [Google Scholar] [CrossRef]
- Rehman, M.U.; Khan, R.; Khan, A.; Qamar, W.; Arafah, A.; Ahmad, A.; Ahmad, A.; Akhter, R.; Rinklebe, J.; Ahmad, P. Fate of arsenic in living systems: Implications for sustainable and safe food chains. J. Hazard. Mater. 2021, 417, 126050. [Google Scholar] [CrossRef]
- Mondal, D.; Rahman, M.M.; Suman, S.; Sharma, P.; Siddique, A.B.; Rahman, M.A.; Bari, A.F.; Kumar, R.; Bose, N.; Singh, S.K.; et al. Arsenic exposure from food exceeds that from drinking water in endemic area of Bihar, India. Sci. Total Environ. 2021, 754, 142082. [Google Scholar] [CrossRef] [PubMed]
- Halder, D.; Bhowmick, S.; Biswas, A.; Chatterjee, D.; Nriagu, J.; Guha Mazumder, D.N.; Šlejkovec, Z.; Jacks, G.; Bhattacharya, P. Risk of arsenic exposure from drinking water and dietary components: Implications for risk management in rural Bengal. Environ. Sci. Technol. 2013, 47, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- WHO. Guidelines for Drinking Water Quality; WHO: Geneva, Switzerland, 2011; Volume 4. [Google Scholar]
- Tyagi, N.; Raghuvanshi, R.; Upadhyay, M.K.; Srivastava, A.K.; Suprasanna, P.; Srivastava, S. Elemental (As, Zn, Fe and Cu) analysis and health risk assessment of rice grains and rice based food products collected from markets from different cities of Gangetic basin, India. J. Food Compos. Anal. 2020, 93, 103612. [Google Scholar] [CrossRef]
- Liu, J.; Song, W.; Li, Y.; Wang, Y.; Cui, Y.; Huang, J.; Wang, Q.; Wei, S. Burden of Coronary Heart Disease and Cancer from Dietary Exposure to Inorganic Arsenic in Adults in China, 2016. Ann. Glob. Health 2022, 88, 28. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Barrachina, Á.A.; Burló, F.; Valero, D.; López, E.; Martínez-Romero, D.; Martínez-Sánchez, F. Arsenic toxicity and accumulation in turnip as affected by arsenic chemical speciation. J. Agric. Food Chem. 1999, 47, 2288–2294. [Google Scholar] [CrossRef]
- Snedeker, S.M. Antimony in food contact materials and household plastics: Uses, exposure, and health risk considerations. In Toxicants in Food Packaging and Household Plastics; Springer: Berlin, Germany, 2014; pp. 205–230. [Google Scholar]
- FDA. List of Indirect Additives Used in Food Contact Substances; FDA: Silver Spring, MA, USA, 2011. [Google Scholar]
- Pearson, A.J.; Ashmore, E. Risk assessment of antimony, barium, beryllium, boron, bromine, lithium, nickel, strontium, thallium and uranium concentrations in the New Zealand diet. Food Addit. Contam. Part A 2020, 37, 451–464. [Google Scholar] [CrossRef]
- Ekhator, O.; Udowelle, N.; Igbiri, S.; Asomugha, R.; Igweze, Z.; Orisakwe, O. Safety evaluation of potential toxic metals exposure from street foods consumed in mid-west Nigeria. J. Environ. Public Health 2017, 2017, 8458057. [Google Scholar] [CrossRef]
- Chapa-Martínez, C.; Hinojosa-Reyes, L.; Hernández-Ramírez, A.; Ruiz-Ruiz, E.; Maya-Treviño, L.; Guzmán-Mar, J. An evaluation of the migration of antimony from polyethylene terephthalate (PET) plastic used for bottled drinking water. Sci. Total Environ. 2016, 565, 511–518. [Google Scholar] [CrossRef]
- Carneado, S.; Hernández-Nataren, E.; López-Sánchez, J.; Sahuquillo, A. Migration of antimony from polyethylene terephthalate used in mineral water bottles. Food Chem. 2015, 166, 544–550. [Google Scholar] [CrossRef]
- Tinggi, U. Selenium toxicity and its adverse health effects. In Reviews in Food and Nutrition Toxicity; CRC Press: Boca Raton, FL, USA, 2005; pp. 29–56. [Google Scholar]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Reilly, C. Selenium in Food and Health; Kluwer Academic/Plenum Publishers: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Kieliszek, M. Selenium–fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed]
- Thiry, C.; Ruttens, A.; De Temmerman, L.; Schneider, Y.-J.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784. [Google Scholar] [CrossRef]
- Board, F.N. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Natl. Acad. Sci. 2000, 19, 95–185. [Google Scholar]
- García, R.; Báez, A. Atomic absorption spectrometry (AAS). At. Absorpt. Spectrosc. 2012, 1, 1–13. [Google Scholar]
- Jorhem, L. Determination of metals in foods by atomic absorption spectrometry after dry ashing: NMKL1 collaborative study. J. AOAC Int. 2000, 83, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Shokrollahi, A.; Kianfar, A.; Mirsadeghi, A.; Pourfarokhi, A.; Soylak, M. The determination of some heavy metals in food samples by flame atomic absorption spectrometry after their separation-preconcentration on bis salicyl aldehyde, 1, 3 propan diimine (BSPDI) loaded on activated carbon. J. Hazard. Mater. 2008, 154, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Soylak, M.; Aydin, A. Determination of some heavy metals in food and environmental samples by flame atomic absorption spectrometry after coprecipitation. Food Chem. Toxicol. 2011, 49, 1242–1248. [Google Scholar] [CrossRef]
- Bagheri, H.; Afkhami, A.; Saber-Tehrani, M.; Khoshsafar, H. Preparation and characterization of magnetic nanocomposite of Schiff base/silica/magnetite as a preconcentration phase for the trace determination of heavy metal ions in water, food and biological samples using atomic absorption spectrometry. Talanta 2012, 97, 87–95. [Google Scholar] [CrossRef]
- Tüzen, M. Determination of heavy metals in fish samples of the middle Black Sea (Turkey) by graphite furnace atomic absorption spectrometry. Food Chem. 2003, 80, 119–123. [Google Scholar] [CrossRef]
- Wagner, K.; Batchelor, J.; Jones, B. A Rowland Circle, multielement graphite furnace atomic absorption spectrometer. Spectrochim. Acta Part B At. Spectrosc. 1998, 53, 1805–1813. [Google Scholar] [CrossRef]
- Harvey, D. Modern Analytical Chemistry; McGraw-Hill: New York, NY, USA, 2000; Volume 1. [Google Scholar]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis, 5th ed.; Thomson Brooks: Sydney, Australia, 1998. [Google Scholar]
- Ferreira, S.L.; Bezerra, M.A.; Santos, A.S.; dos Santos, W.N.; Novaes, C.G.; de Oliveira, O.M.; Oliveira, M.L.; Garcia, R.L. Atomic absorption spectrometry–A multi element technique. TrAC Trends Anal. Chem. 2018, 100, 1–6. [Google Scholar] [CrossRef]
- Gore, M. Spectrophotometry and Spectrofluorimetry: A Practical Approach; OUP: Oxford, UK, 2000; Volume 225. [Google Scholar]
- Andrade-Eiroa, A.; de-Armas, G.; Estela, J.-M.; Cerda, V. Critical approach to synchronous spectrofluorimetry. II. TrAC Trends Anal. Chem. 2010, 29, 902–927. [Google Scholar] [CrossRef]
- Tabrizi, A.B. Cloud point extraction and spectrofluorimetric determination of aluminium and zinc in foodstuffs and water samples. Food Chem. 2007, 100, 1698–1703. [Google Scholar] [CrossRef]
- Zeeb, M.; Ganjali, M.R.; Norouzi, P.; Kalaee, M.R. Separation and preconcentration system based on microextraction with ionic liquid for determination of copper in water and food samples by stopped-flow injection spectrofluorimetry. Food Chem. Toxicol. 2011, 49, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Afrin, A.; Akhtar, Y. A Highly Sensitive and Selective Spectrofluorimetric Method for the Determination of Vanadium at Pico-Trace Levels in Some Real, Environmental, Biological, Soil and Food Samples Using 2-(α-Pyridyl)-Thioquinaldinamide. Am. J. Anal. Chem. 2019, 10, 528–561. [Google Scholar] [CrossRef]
- López-López, J.A.; Borrego-Corchado, C.; Mánuel, M.P.; Espada-Bellido, E. A simple and economical spectrofluorimetric alternative for Al routine analysis in seafood. Talanta 2018, 182, 210–217. [Google Scholar] [CrossRef]
- Nahata, A. Spectrofluorimetry as an analytical tool. Pharm Anal Acta 2011, 2, 1–2. [Google Scholar] [CrossRef]
- Ammann, A.A. Inductively coupled plasma mass spectrometry (ICP MS): A versatile tool. J. Mass Spectrom. 2007, 42, 419–427. [Google Scholar] [CrossRef]
- Pröfrock, D.; Prange, A. Inductively coupled plasma-mass spectrometry (ICP-MS) for quantitative analysis in environmental and life sciences: A review of challenges, solutions, and trends. Appl. Spectrosc. 2012, 66, 843–868. [Google Scholar] [CrossRef]
- Vincevica-Gaile, Z.; Klavins, M.; Rudovica, V.; Viksna, A. Research review trends of food analysis in Latvia: Major and trace element content. Environ. Geochem. Health 2013, 35, 693–703. [Google Scholar] [CrossRef]
- Stasinos, S.; Nasopoulou, C.; Tsikrika, C.; Zabetakis, I. The bioaccumulation and physiological effects of heavy metals in carrots, onions, and potatoes and dietary implications for Cr and Ni: A review. J. Food Sci. 2014, 79, R765–R780. [Google Scholar] [CrossRef] [PubMed]
- Ebbs, S.; Talbott, J.; Sankaran, R. Cultivation of garden vegetables in Peoria Pool sediments from the Illinois River: A case study in trace element accumulation and dietary exposures. Environ. Int. 2006, 32, 766–774. [Google Scholar] [CrossRef]
- Şenol, F. Determination of Arsenic, Selenium and Cadmium in Some Turkish Spices by Inductively Coupled Plasma Mass Spectrometry. Ph.D. Thesis, Middle East Technical University, Çankaya/Ankara, Turkey, 2010. [Google Scholar]
- Nuttall, K.L.; Gordon, W.H.; Ash, K.O. Inductively coupled plasma mass spectrometry for trace element analysis in the clinical laboratory. Ann. Clin. Lab. Sci. 1995, 25, 264–271. [Google Scholar] [PubMed]
- Waldron, K.J.; Rutherford, J.C.; Ford, D.; Robinson, N.J. Metalloproteins and metal sensing. Nature 2009, 460, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.P.; Hare, D.J.; Clases, D.; Doble, P.A. Applications of liquid chromatography-inductively coupled plasma-mass spectrometry in the biosciences: A tutorial review and recent developments. TrAC Trends Anal. Chem. 2018, 104, 11–21. [Google Scholar] [CrossRef]
- Montes-Bayón, M.a.; DeNicola, K.; Caruso, J.A. Liquid chromatography–inductively coupled plasma mass spectrometry. J. Chromatogr. A 2003, 1000, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, T. Liquid chromatography–inductively coupled plasma mass spectrometry (LC–ICP–MS). J. Liq. Chromatogr. Relat. Technol. 2007, 30, 807–831. [Google Scholar] [CrossRef]
- Batista, B.L.; Rodrigues, J.L.; De Souza, S.S.; Souza, V.C.O.; Barbosa, F., Jr. Mercury speciation in seafood samples by LC–ICP-MS with a rapid ultrasound-assisted extraction procedure: Application to the determination of mercury in Brazilian seafood samples. Food Chem. 2011, 126, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.; Lemos, V.A.; Silva, L.O.; Queiroz, A.F.; Souza, A.S.; da Silva, E.G.; dos Santos, W.N.; das Virgens, C.F. Analytical strategies of sample preparation for the determination of mercury in food matrices—A review. Microchem. J. 2015, 121, 227–236. [Google Scholar] [CrossRef]
- Lorenc, W.; Hanć, A.; Sajnóg, A.; Barałkiewicz, D. LC/ICP-MS and complementary techniques in bespoke and nontargeted speciation analysis of elements in food samples. Mass Spectrom. Rev. 2022, 41, 32–50. [Google Scholar] [CrossRef]
- Batista, B.; Nacano, L.; De Souza, S.; Barbosa, F., Jr. Rapid sample preparation procedure for As speciation in food samples by LC-ICP-MS. Food Addit. Contam. Part A 2012, 29, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ryu, K.Y.; Choi, J.Y.; Nho, E.Y.; Habte, G.; Choi, H.; Kim, M.H.; Park, K.S.; Kim, K.S. Determination of toxic heavy metals and speciation of arsenic in seaweeds from South Korea. Food Chem. 2015, 169, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Koplik, R.; Klimešová, I.; Mališová, K.; Mestek, O. Determination of Mercury Species in Foodstuffs using LC-ICP-MS: The Applicability and Limitations of the Method. Czech J. Food Sci. 2014, 32, 249–259. [Google Scholar] [CrossRef]
- Jackson, B.P.; Liba, A.; Nelson, J. Advantages of reaction cell ICP-MS on doubly charged interferences for arsenic and selenium analysis in foods. J. Anal. At. Spectrom. 2015, 30, 1179–1183. [Google Scholar] [CrossRef]
- Hwang, I.M.; Lee, H.M.; Lee, H.-W.; Jung, J.-H.; Moon, E.W.; Khan, N.; Kim, S.H. Determination of Toxic Elements and Arsenic Species in Salted Foods and Sea Salt by ICP–MS and HPLC–ICP–MS. ACS Omega 2021, 6, 19427–19434. [Google Scholar] [CrossRef]
- Zmozinski, A.V.; Llorente-Mirandes, T.; López-Sánchez, J.F.; da Silva, M.M. Establishment of a method for determination of arsenic species in seafood by LC-ICP-MS. Food Chem. 2015, 173, 1073–1082. [Google Scholar] [CrossRef]
- Shimizu, F.M.; Braunger, M.L.; Riul, A., Jr. Heavy metal/toxins detection using electronic tongues. Chemosensors 2019, 7, 36. [Google Scholar] [CrossRef]
- Riul, A., Jr.; Dantas, C.A.; Miyazaki, C.M.; Oliveira, O.N., Jr. Recent advances in electronic tongues. Analyst 2010, 135, 2481–2495. [Google Scholar] [CrossRef]
- Wadehra, A.; Patil, P.S. Application of electronic tongues in food processing. Anal. Methods 2016, 8, 474–480. [Google Scholar] [CrossRef]
- Wardencki, W.; Chmiel, T.; Dymerski, T. Gas chromatography-olfactometry (GC-O), electronic noses (e-noses) and electronic tongues (e-tongues) for in vivo food flavour measurement. Instrum. Assess. Food Sens. Qual. 2013, 195–229. [Google Scholar] [CrossRef]
- Mourzina, Y.G.; Schubert, J.; Zander, W.; Legin, A.; Vlasov, Y.G.; Lüth, H.; Schöning, M.J. Development of multisensor systems based on chalcogenide thin film chemical sensors for the simultaneous multicomponent analysis of metal ions in complex solutions. Electrochim. Acta 2001, 47, 251–258. [Google Scholar] [CrossRef]
- Di Natale, C.; Macagnano, A.; Davide, F.; D’amico, A.; Legin, A.; Vlasov, Y.; Rudnitskaya, A.; Selezenev, B. Multicomponent analysis on polluted waters by means of an electronic tongue. Sens. Actuators B Chem. 1997, 44, 423–428. [Google Scholar] [CrossRef]
- Vlasov, Y.G.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D’AMICO, A. Multisensor system with an array of chemical sensors and artificial neural networks (electronic tongue) for quantitative analysis of multicomponent aqueous solutions. Russ. J. Appl. Chem. 1996, 69, 848–853. [Google Scholar]
- Legin, A.; Rudnitskaya, A.; Vlasov, Y.; Di Natale, C.; Davide, F.; D’Amico, A. Tasting of beverages using an electronic tongue. Sens. Actuators B Chem. 1997, 44, 291–296. [Google Scholar] [CrossRef]
- Men, H.; Zou, S.; Li, Y.; Wang, Y.; Ye, X.; Wang, P. A novel electronic tongue combined MLAPS with stripping voltammetry for environmental detection. Sens. Actuators B Chem. 2005, 110, 350–357. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A. Cross-sensitivity evaluation of chemical sensors for electronic tongue: Determination of heavy metal ions. Sens. Actuators B Chem. 1997, 44, 532–537. [Google Scholar] [CrossRef]
- Mortensen, J.; Legin, A.; Ipatov, A.; Rudnitskaya, A.; Vlasov, Y.; Hjuler, K. A flow injection system based on chalcogenide glass sensors for the determination of heavy metals. Anal. Chim. Acta 2000, 403, 273–277. [Google Scholar] [CrossRef]
- Cetó, X.; Del Valle, M. Phenolic compounds analyzed with an electronic tongue. In Electronic Noses and Tongues in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 235–244. [Google Scholar]
- Wang, L.; Peng, X.; Fu, H.; Huang, C.; Li, Y.; Liu, Z. Recent advances in the development of electrochemical aptasensors for detection of heavy metals in food. Biosens. Bioelectron. 2020, 147, 111777. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, C.; Ma, T.; Liu, X.; Chen, Z.; Li, S.; Deng, Y. Advances in aptamer screening and aptasensors’ detection of heavy metal ions. J. Nanobiotechnol. 2021, 19, 166. [Google Scholar] [CrossRef]
- Duan, N.; Wu, S.; Dai, S.; Gu, H.; Hao, L.; Ye, H.; Wang, Z. Advances in aptasensors for the detection of food contaminants. Analyst 2016, 141, 3942–3961. [Google Scholar] [CrossRef]
- Ye, B.-F.; Zhao, Y.-J.; Cheng, Y.; Li, T.-T.; Xie, Z.-Y.; Zhao, X.-W.; Gu, Z.-Z. Colorimetric photonic hydrogel aptasensor for the screening of heavy metal ions. Nanoscale 2012, 4, 5998–6003. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Wang, W.; Cao, J.; Gan, N.; Han, H. Application of multiplexed aptasensors in food contaminants detection. ACS Sens. 2020, 5, 3721–3738. [Google Scholar] [CrossRef]
- Poolsup, S.; Kim, C.-Y. Therapeutic applications of synthetic nucleic acid aptamers. Curr. Opin. Biotechnol. 2017, 48, 180–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent advances in aptamer discovery and applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Yan, J.; Xiong, H.; Cai, S.; Wen, N.; He, Q.; Liu, Y.; Peng, D.; Liu, Z. Advances in aptamer screening technologies. Talanta 2019, 200, 124–144. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Han, P.; Feng, X. Fluorescent aptasensing of chlorpyrifos based on the assembly of cationic conjugated polymer-aggregated gold nanoparticles and luminescent metal–organic frameworks. Analyst 2019, 144, 6025–6032. [Google Scholar] [CrossRef]
- Komarova, N.; Andrianova, M.; Glukhov, S.; Kuznetsov, A. Selection, characterization, and application of ssDNA aptamer against furaneol. Molecules 2018, 23, 3159. [Google Scholar] [CrossRef]
- Ho, L.S.J.; Fogel, R.; Limson, J.L. Generation and screening of histamine-specific aptamers for application in a novel impedimetric aptamer-based sensor. Talanta 2020, 208, 120474. [Google Scholar] [CrossRef]
- Luo, Z.; He, L.; Wang, J.; Fang, X.; Zhang, L. Developing a combined strategy for monitoring the progress of aptamer selection. Analyst 2017, 142, 3136–3139. [Google Scholar] [CrossRef]
- Boussebayle, A.; Groher, F.; Suess, B. RNA-based capture-SELEX for the selection of small molecule-binding aptamers. Methods 2019, 161, 10–15. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, J.; Yang, Z.; Mou, Q.; Ma, Y.; Xiong, Y.; Lu, Y. Functional DNA regulated CRISPR-Cas12a sensors for point-of-care diagnostics of non-nucleic-acid targets. J. Am. Chem. Soc. 2019, 142, 207–213. [Google Scholar] [CrossRef]
- Babu, E.; Bhuvaneswari, J.; Mareeswaran, P.M.; Thanasekaran, P.; Lee, H.-M.; Rajagopal, S. Transition metal complexes based aptamers as optical diagnostic tools for disease proteins and biomolecules. Coord. Chem. Rev. 2019, 380, 519–549. [Google Scholar] [CrossRef]
- Ding, J.; Qin, W. Recent advances in potentiometric biosensors. TrAC Trends Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Liu, M.; Khan, A.; Wang, Z.; Liu, Y.; Yang, G.; Deng, Y.; He, N. Aptasensors for pesticide detection. Biosens. Bioelectron. 2019, 130, 174–184. [Google Scholar] [CrossRef]
- Alkhamis, O.; Canoura, J.; Yu, H.; Liu, Y.; Xiao, Y. Innovative engineering and sensing strategies for aptamer-based small-molecule detection. TrAC Trends Anal. Chem. 2019, 121, 115699. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Sensors and biosensors based on metal oxide nanomaterials. TrAC Trends Anal. Chem. 2019, 121, 115690. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, H.; Wang, Z.; Cao, H.; Zhang, K.; Li, X.; Yang, Z. Nanomaterial-based aptamer sensors for arsenic detection. Biosens. Bioelectron. 2020, 148, 111785. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.; Dong, H.; Liu, K.; He, N. Progress on sensors based on nanomaterials for rapid detection of heavy metal ions. Sci. China Chem. 2017, 60, 329–337. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent progress in nanomaterial-based optical aptamer assay for the detection of food chemical contaminants. ACS Appl. Mater. Interfaces 2017, 9, 23287–23301. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Q.; Zhou, X.; Wang, R.; Yang, Z. Reusable, facile, and rapid aptasensor capable of online determination of trace mercury. Environ. Int. 2021, 146, 106181. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, B.; Cui, X.; Li, Y.; Tang, J.; Wang, H.; Zhang, D.; Li, Z. Recent advances in aptasensors for mycotoxin detection: On the surface and in the colloid. Talanta 2021, 223, 121729. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Shorie, M. Nanomaterial based aptasensors for clinical and environmental diagnostic applications. Nanoscale Adv. 2019, 1, 2123–2138. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Y.; Zhao, X.; Wu, J.; Zhu, L.; Zhang, X.; Wei, Z.; Liu, Y.; He, P. Selective pressures of heavy metals on microbial community determine microbial functional roles during composting: Sensitive, resistant and actor. J. Hazard. Mater. 2020, 398, 122858. [Google Scholar] [CrossRef]
- Li, L.; Xing, H.; Zhang, J.; Lu, Y. Functional DNA molecules enable selective and stimuli-responsive nanoparticles for biomedical applications. Acc. Chem. Res. 2019, 52, 2415–2426. [Google Scholar] [CrossRef]
- Sahai, N.; Gogoi, M.; Ahmad, N. Mathematical modeling and simulations for developing nanoparticle-based cancer drug delivery systems: A review. Curr. Pathobiol. Rep. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Arghya, S.; Suradip, D.; Pragya, S.; Utpal, B. Aptasensors in health, environment and food safety monitoring. Open J. Appl. Biosens. 2012, 2012, 21880. [Google Scholar] [CrossRef]
- Ullah, S.; Zahra, Q.u.a.; Mansoorianfar, M.; Hussain, Z.; Ullah, I.; Li, W.; Kamya, E.; Mehmood, S.; Pei, R.; Wang, J. Heavy Metal Ions Detection Using Nanomaterials-Based Aptasensors. Crit. Rev. Anal. Chem. 2022, 1–17. [Google Scholar] [CrossRef]
- Li, Z.; Mohamed, M.A.; Vinu Mohan, A.; Zhu, Z.; Sharma, V.; Mishra, G.K.; Mishra, R.K. Application of electrochemical aptasensors toward clinical diagnostics, food, and environmental monitoring. Sensors 2019, 19, 5435. [Google Scholar] [CrossRef]
- Rapini, R.; Marrazza, G. Electrochemical aptasensors for contaminants detection in food and environment: Recent advances. Bioelectrochemistry 2017, 118, 47–61. [Google Scholar] [CrossRef]
- Mishra, G.K.; Sharma, V.; Mishra, R.K. Electrochemical Aptasensors for Food and Environmental Safeguarding: A Review. Biosensors 2018, 8, 28. [Google Scholar] [CrossRef]
- Abd-Ellatief, R.; Abd-Ellatief, M.R. Electrochemical Aptasensors: Current Status and Future Perspectives. Diagnostics 2021, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, T.; Sun, D.-W.; Cheng, J.-H. Raman imaging for food quality and safety evaluation: Fundamentals and applications. Trends Food Sci. Technol. 2017, 62, 177–189. [Google Scholar] [CrossRef]
- Lorenz, B.; Wichmann, C.; Stöckel, S.; Rösch, P.; Popp, J. Cultivation-Free Raman Spectroscopic Investigations of Bacteria. Trends Microbiol. 2017, 25, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.R.; Hooper, D.C.; Zhang, L.; Wolverson, D.; Valev, V.K. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Bodelón, G.; Montes-García, V.; Pérez-Juste, J.; Pastoriza-Santos, I. Surface-Enhanced Raman Scattering Spectroscopy for Label-Free Analysis of P. aeruginosa Quorum Sensing. Front. Cell Infect. Microbiol. 2018, 8, 143. [Google Scholar] [CrossRef]
- Petersen, M.; Yu, Z.; Lu, X. Application of Raman Spectroscopic Methods in Food Safety: A Review. Biosensors 2021, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, D.-W.; Pu, H.; Wei, Q. Surface enhanced Raman spectroscopy (SERS): A novel reliable technique for rapid detection of common harmful chemical residues. Trends Food Sci. Technol. 2018, 75, 10–22. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Lai, K.; Fan, Y.; Rasco, B.A. Trace analysis of organic compounds in foods with surface-enhanced Raman spectroscopy: Methodology, progress, and challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 622–642. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, T.; Wu, Z.; Yu, R. Novel ratiometric surface-enhanced raman spectroscopy aptasensor for sensitive and reproducible sensing of Hg(2). Biosens. Bioelectron. 2018, 99, 646–652. [Google Scholar] [CrossRef]
- Fan, M.; Andrade, G.F.S.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal Chim Acta 2020, 1097, 1–29. [Google Scholar] [CrossRef]
- Song, D.; Yang, R.; Long, F.; Zhu, A. Applications of magnetic nanoparticles in surface-enhanced Raman scattering (SERS) detection of environmental pollutants. J. Environ. Sci. 2019, 80, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.; Mangino, S.; Portesi, C.; Vittone, E.; Rossi, A.M. Novel Approaches in Tip-Enhanced Raman Spectroscopy: Accurate Measurement of Enhancement Factors and Pesticide Detection in Tip Dimer Configuration. J. Phys. Chem. C 2019, 123, 24723–24730. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.R.; Zou, X.; Yang, H. Detection of heavy metals in food and agricultural products by surface-enhanced Raman spectroscopy. Food Rev. Int. 2021, 1–22. [Google Scholar] [CrossRef]
- Yang, T.; Wang, P.; Guo, H.; He, L. Surface-Enhanced Raman Spectroscopy: A Tool for All Classes of Food Contaminants. 2017. Available online: https://www.tandfonline.com/doi/abs/10.1080/87559129.2021.1934005?journalCode=lfri20 (accessed on 10 June 2021).
- Xu, G.; Song, P.; Xia, L. Examples in the detection of heavy metal ions based on surface-enhanced Raman scattering spectroscopy. Nanophotonics 2021, 10, 4419–4445. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, X.; Wang, H.; Song, B.; Zhu, Y.; Wang, H.; Su, Y.; He, Y. Surface-enhancement Raman scattering sensing strategy for discriminating trace mercuric ion (II) from real water samples in sensitive, specific, recyclable, and reproducible manners. Anal. Chem. 2015, 87, 1250–1256. [Google Scholar] [CrossRef]
- Kang, T.; Yoo, S.M.; Yoon, I.; Lee, S.; Choo, J.; Lee, S.Y.; Kim, B. Au nanowire-on-film SERRS sensor for ultrasensitive Hg2+ detection. Chemistry 2011, 17, 2211–2214. [Google Scholar] [CrossRef]
- Ding, X.; Kong, L.; Wang, J.; Fang, F.; Li, D.; Liu, J. Highly sensitive SERS detection of Hg2+ ions in aqueous media using gold nanoparticles/graphene heterojunctions. ACS Appl. Mater. Interfaces 2013, 5, 7072–7078. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Zong, S.; Chen, H.; Zhu, D.; Wu, L.; Hu, G.; Cui, Y. SERS detection and removal of mercury(II)/silver(I) using oligonucleotide-functionalized core/shell magnetic silica sphere@Au nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 7371–7379. [Google Scholar] [CrossRef]
- Cecchini, M.P.; Turek, V.A.; Demetriadou, A.; Britovsek, G.; Welton, T.; Kornyshev, A.A.; Wilton-Ely, J.D.; Edel, J.B. Heavy metal sensing using self-assembled nanoparticles at a liquid–liquid interface. Adv. Opt. Mater. 2014, 2, 966–977. [Google Scholar] [CrossRef]
- Sarkar, S.; Pradhan, M.; Sinha, A.K.; Basu, M.; Pal, T. Selective and sensitive recognition of Cu2+ in an aqueous medium: A surface-enhanced Raman scattering (SERS)-based analysis with a low-cost Raman reporter. Chemistry 2012, 18, 6335–6342. [Google Scholar] [CrossRef]
- Ndokoye, P.; Ke, J.; Liu, J.; Zhao, Q.; Li, X. L-cysteine-modified gold nanostars for SERS-based copper ions detection in aqueous media. Langmuir ACS J. Surf. Colloids 2014, 30, 13491–13497. [Google Scholar] [CrossRef]
- Tang, W.; Chase, D.B.; Sparks, D.L.; Rabolt, J.F. Selective and Quantitative Detection of Trace Amounts of Mercury(II) Ion (Hg²⁺) and Copper(II) Ion (Cu²⁺) Using Surface-Enhanced Raman Scattering (SERS). Appl. Spectrosc. 2015, 69, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Zamarion, V.M.; Timm, R.A.; Araki, K.; Toma, H.E. Ultrasensitive SERS nanoprobes for hazardous metal ions based on trimercaptotriazine-modified gold nanoparticles. Inorg. Chem. 2008, 47, 2934–2936. [Google Scholar] [CrossRef] [PubMed]
- Szlag, V.M.; Rodriguez, R.S.; He, J.; Hudson-Smith, N.; Kang, H.; Le, N.; Reineke, T.M.; Haynes, C.L. Molecular Affinity Agents for Intrinsic Surface-Enhanced Raman Scattering (SERS) Sensors. ACS Appl. Mater. Interfaces 2018, 10, 31825–31844. [Google Scholar] [CrossRef]
- Cheng, T.; Xu, Y.; Zhang, S.; Zhu, W.; Qian, X.; Duan, L. A highly sensitive and selective OFF-ON fluorescent sensor for cadmium in aqueous solution and living cell. J. Am. Chem. Soc. 2008, 130, 16160–16161. [Google Scholar] [CrossRef] [PubMed]
- Szabó, L.; Leopold, L.F.; Cozar, B.I.; Leopold, N.; Herman, K.; Chiş, V. SERS approach for Zn (II) detection in contaminated soil. Cent. Eur. J. Chem. 2011, 9, 410–414. [Google Scholar] [CrossRef]
- Jin, L.; She, G.; Mu, L.; Shi, W. Highly uniform indicator-mediated SERS sensor platform for the detection of Zn2+. RSC Adv. 2016, 6, 16555–16560. [Google Scholar] [CrossRef]
- Yin, J.; Wu, T.; Song, J.; Zhang, Q.; Liu, S.; Xu, R.; Duan, H. SERS-active nanoparticles for sensitive and selective detection of cadmium ion (Cd2+). Chem. Mater. 2011, 23, 4756–4764. [Google Scholar] [CrossRef]
- Tian, A.; Liu, Y.; Gao, J. Sensitive SERS detection of lead ions via DNAzyme based quadratic signal amplification. Talanta 2017, 171, 185–189. [Google Scholar] [CrossRef]
- Fu, C.; Xu, W.; Wang, H.; Ding, H.; Liang, L.; Cong, M.; Xu, S. DNAzyme-based plasmonic nanomachine for ultrasensitive selective surface-enhanced Raman scattering detection of lead ions via a particle-on-a-film hot spot construction. Anal. Chem. 2014, 86, 11494–11497. [Google Scholar] [CrossRef]
- Wang, Y.; Irudayaraj, J. A SERS DNAzyme biosensor for lead ion detection. Chem. Commun. 2011, 47, 4394–4396. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Jing, C. Preparation of Fe3O4@Ag SERS substrate and its application in environmental Cr(VI) analysis. J. Colloid Interface Sci. 2011, 358, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Sun, Z.; Zhang, J.; Jing, C. Multifunctional satellite Fe3O4-Au@ TiO2 nano-structure for SERS detection and photo-reduction of Cr (VI). Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 234–240. [Google Scholar] [CrossRef]
- Ji, W.; Wang, Y.; Tanabe, I.; Han, X.; Zhao, B.; Ozaki, Y. Semiconductor-driven “turn-off” surface-enhanced Raman scattering spectroscopy: Application in selective determination of chromium(vi) in water. Chem. Sci. 2015, 6, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, P.; Li, J.; Sun, Y.; Shi, H.; Chen, N.; Zhang, X.; Chen, H. Colorimetric and SERS dual-mode detection of lead Ions based on Au-Ag core-shell nanospheres: Featuring quick screening with ultra-high sensitivity. Opt. Express 2019, 27, 29248–29260. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.; Ma, W.; Kuang, H.; Wang, L.; Xu, C. Triple Raman Label-Encoded Gold Nanoparticle Trimers for Simultaneous Heavy Metal Ion Detection. Small 2015, 11, 3435–3439. [Google Scholar] [CrossRef]
- Li, D.; Ma, Y.; Duan, H.; Jiang, F.; Deng, W.; Ren, X. Fluorescent/SERS dual-sensing and imaging of intracellular Zn(2). Anal Chim Acta 2018, 1038, 148–156. [Google Scholar] [CrossRef]
- Eberhardt, K.; Stiebing, C.; Matthäus, C.; Schmitt, M.; Popp, J. Advantages and limitations of Raman spectroscopy for molecular diagnostics: An update. Expert Rev. Mol. Diagn. 2015, 15, 773–787. [Google Scholar] [CrossRef]
- Baranska, M.; Schütze, W.; Schulz, H. Determination of lycopene and beta-carotene content in tomato fruits and related products: Comparison of FT-Raman, ATR-IR, and NIR spectroscopy. Anal. Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nuriman; Huskens, J.; Verboom, W. Optical sensing systems for microfluidic devices: A review. Anal Chim Acta 2007, 601, 141–155. [Google Scholar] [CrossRef]
- Aiestaran, P.; Dominguez, V.; Arrue, J.; Zubia, J. A fluorescent linear optical fiber position sensor. Opt. Mater. 2009, 31, 1101–1104. [Google Scholar] [CrossRef]
- Vendrell, M.; Zhai, D.; Er, J.C.; Chang, Y.T. Combinatorial strategies in fluorescent probe development. Chem. Rev. 2012, 112, 4391–4420. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; He, Y.; Xing, X.; Zhao, Y.; Tang, H.; Pang, D. Aptamer functionalized gold nanoparticles based fluorescent probe for the detection of mercury (II) ion in aqueous solution. Talanta 2013, 113, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin, Germany, 2006. [Google Scholar]
- Elosua, C.; De Acha, N.; Lopez-Torres, D.; Matias, I.; Arregui, F. Luminescent optical fiber oxygen sensor following layer-by-layer method. Procedia Eng. 2014, 87, 987–990. [Google Scholar] [CrossRef]
- Caselli, M. Porphyrin-based electrostatically self-assembled multilayers as fluorescent probes for mercury (ii) ions: A study of the adsorption kinetics of metal ions on ultrathin films for sensing applications. RSC Adv. 2015, 5, 1350–1358. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Li, Y.; Zhuang, Q.; Zhao, W.; Gu, J. Porphyrinic MOFs for reversible fluorescent and colorimetric sensing of mercury (II) ions in aqueous phase. RSC Adv. 2016, 6, 69807–69814. [Google Scholar] [CrossRef]
- He, J.-L.; Zhu, S.-L.; Wu, P.; Li, P.-P.; Li, T.; Cao, Z. Enzymatic cascade based fluorescent DNAzyme machines for the ultrasensitive detection of Cu (II) ions. Biosens. Bioelectron. 2014, 60, 112–117. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Wang, Y.S.; Zhou, B.; Yu, J.H.; Peng, L.L.; Huang, Y.Q.; Li, X.J.; Chen, S.H.; Tang, X.; Wang, X.F. A multifunctional fluorescent aptamer probe for highly sensitive and selective detection of cadmium(II). Anal. Bioanal. Chem. 2017, 409, 4951–4958. [Google Scholar] [CrossRef]
- Xu, H.; Miao, R.; Fang, Z.; Zhong, X. Quantum dot-based “turn-on” fluorescent probe for detection of zinc and cadmium ions in aqueous media. Anal. Chim. Acta 2011, 687, 82–88. [Google Scholar] [CrossRef]
- Cai, S.; Lu, Y.; He, S.; Wei, F.; Zhao, L.; Zeng, X. A highly sensitive and selective turn-on fluorescent chemosensor for palladium based on a phosphine-rhodamine conjugate. Chem. Commun. 2013, 49, 822–824. [Google Scholar] [CrossRef]
- Saleem, M.; Rafiq, M.; Hanif, M. Organic Material Based Fluorescent Sensor for Hg(2+): A Brief Review on Recent Development. J. Fluoresc. 2017, 27, 31–58. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.B.; Xu, P.; Zhang, J.; Qiao, R.; Chen, M.Y.; Mei, M.Y.; Wei, B.; Wang, C.; Zhang, L.; Chen, S.S. Long-Wavelength Fluorescent Chemosensors for Hg(2+) based on Pyrene. ACS Omega 2019, 4, 14621–14625. [Google Scholar] [CrossRef]
- Bai, C.B.; Wang, W.G.; Zhang, J.; Wang, C.; Qiao, R.; Wei, B.; Zhang, L.; Chen, S.S.; Yang, S. A Fluorescent and Colorimetric Chemosensor for Hg(2+) Based on Rhodamine 6G With a Two-Step Reaction Mechanism. Front. Chem. 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Xu, Q.; Wang, Y.; Meng, F. Recent Developments in Fluorescent Materials for Heavy Metal Ions Analysis From the Perspective of Forensic Chemistry. Front. Chem. 2020, 8, 593291. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Köhler, G.; Knapp, M.; Gaubitzer, E.; Puchinger, M.; Edetsberger, M. Emerging applications of fluorescence spectroscopy in medical microbiology field. J. Transl. Med. 2009, 7, 99. [Google Scholar] [CrossRef]
- Abernethy, D.R.; DeStefano, A.J.; Cecil, T.L.; Zaidi, K.; Williams, R.L. Metal impurities in food and drugs. Pharm. Res. 2010, 27, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Buledi, J.A.; Amin, S.; Haider, S.I.; Bhanger, M.I.; Solangi, A.R. A review on detection of heavy metals from aqueous media using nanomaterial-based sensors. Environ. Sci. Pollut. Res. Int. 2021, 28, 58994–59002. [Google Scholar] [CrossRef]
- Hao, Y.; Wu, X.; Guo, Y. Study on test and detection method of mechanical properties of heavy metal contaminated soil. Soil Sediment Contam. Int. J. 2020, 29, 929–939. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, D.; Liang, R.-P.; Qiu, J.-D. Graphene-based optical nanosensors for detection of heavy metal ions. TrAC Trends Anal. Chem. 2018, 102, 280–289. [Google Scholar] [CrossRef]
- Liu, X.; Yu, K.; Zhang, H.; Zhang, X.; Zhang, H.; Zhang, J.; Gao, J.; Li, N.; Jiang, J. A portable electromagnetic heating-microplasma atomic emission spectrometry for direct determination of heavy metals in soil. Talanta 2020, 219, 121348. [Google Scholar] [CrossRef]
- Pyo, J.; Hong, S.M.; Kwon, Y.S.; Kim, M.S.; Cho, K.H. Estimation of heavy metals using deep neural network with visible and infrared spectroscopy of soil. Sci. Total Environ. 2020, 741, 140162. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, A.G.; Renu, K.; Gopalakrishnan, A.V.; Veeraraghavan, V.P.; Vinayagam, S.; Paz-Montelongo, S.; Dey, A.; Vellingiri, B.; George, A.; Madhyastha, H.; et al. Heavy Metal and Metalloid Contamination in Food and Emerging Technologies for Its Detection. Sustainability 2023, 15, 1195. https://doi.org/10.3390/su15021195
Mukherjee AG, Renu K, Gopalakrishnan AV, Veeraraghavan VP, Vinayagam S, Paz-Montelongo S, Dey A, Vellingiri B, George A, Madhyastha H, et al. Heavy Metal and Metalloid Contamination in Food and Emerging Technologies for Its Detection. Sustainability. 2023; 15(2):1195. https://doi.org/10.3390/su15021195
Chicago/Turabian StyleMukherjee, Anirban Goutam, Kaviyarasi Renu, Abilash Valsala Gopalakrishnan, Vishnu Priya Veeraraghavan, Sathishkumar Vinayagam, Soraya Paz-Montelongo, Abhijit Dey, Balachandar Vellingiri, Alex George, Harishkumar Madhyastha, and et al. 2023. "Heavy Metal and Metalloid Contamination in Food and Emerging Technologies for Its Detection" Sustainability 15, no. 2: 1195. https://doi.org/10.3390/su15021195
APA StyleMukherjee, A. G., Renu, K., Gopalakrishnan, A. V., Veeraraghavan, V. P., Vinayagam, S., Paz-Montelongo, S., Dey, A., Vellingiri, B., George, A., Madhyastha, H., & Ganesan, R. (2023). Heavy Metal and Metalloid Contamination in Food and Emerging Technologies for Its Detection. Sustainability, 15(2), 1195. https://doi.org/10.3390/su15021195











