Characteristics of Silica Fume Nano Alumina Ternary Blended Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
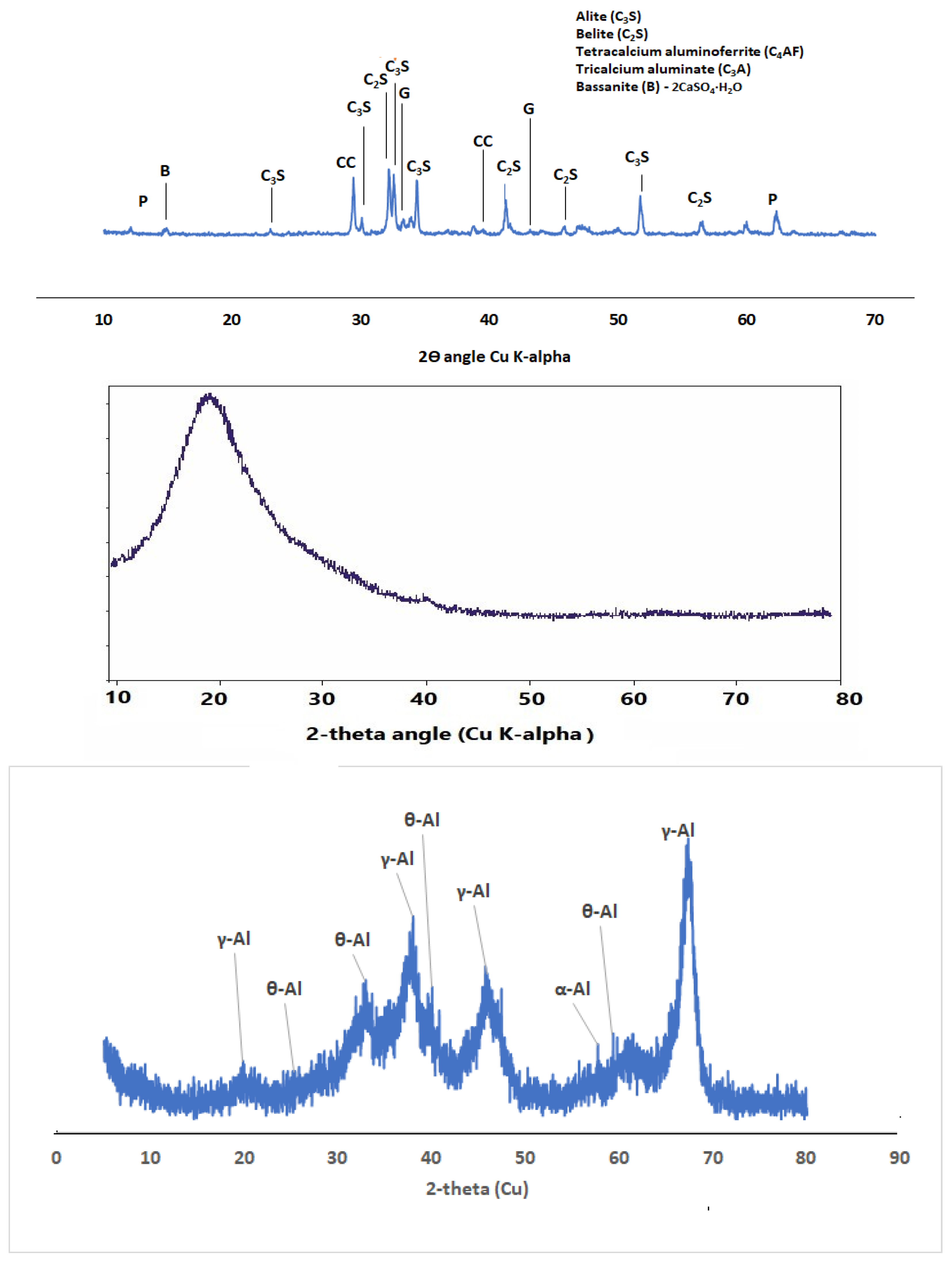
2.1.1. Nano Aluminum Oxide
2.1.2. Cement
2.1.3. Silica Fume
2.1.4. Aggregates:
2.1.5. Superplasticizer
2.1.6. Water
2.2. Experimental Design
2.2.1. Mix Design
2.2.2. Sample Designation
2.2.3. Mixing Procedure
2.3. Experimental Tests
2.3.1. Setting Time
2.3.2. Workability
2.3.3. Compressive Strength
2.3.4. Residual Compressive Strength after Thermal Exposure
2.3.5. Characterization and Morphology of the Specimens
3. Discussion of Results
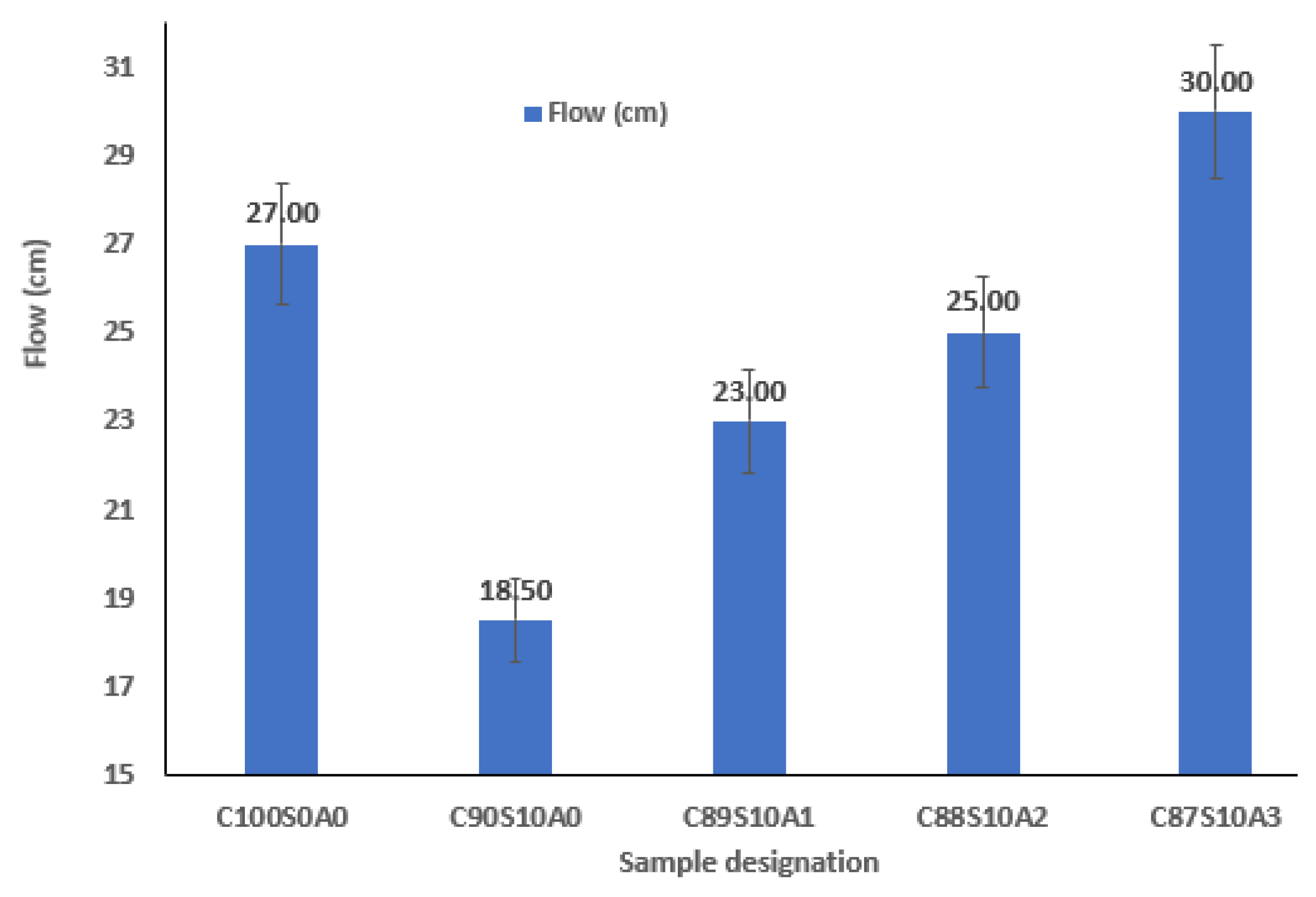
3.1. Workability of Nano Alumina/Silica Fume Ternary Blended Mortar
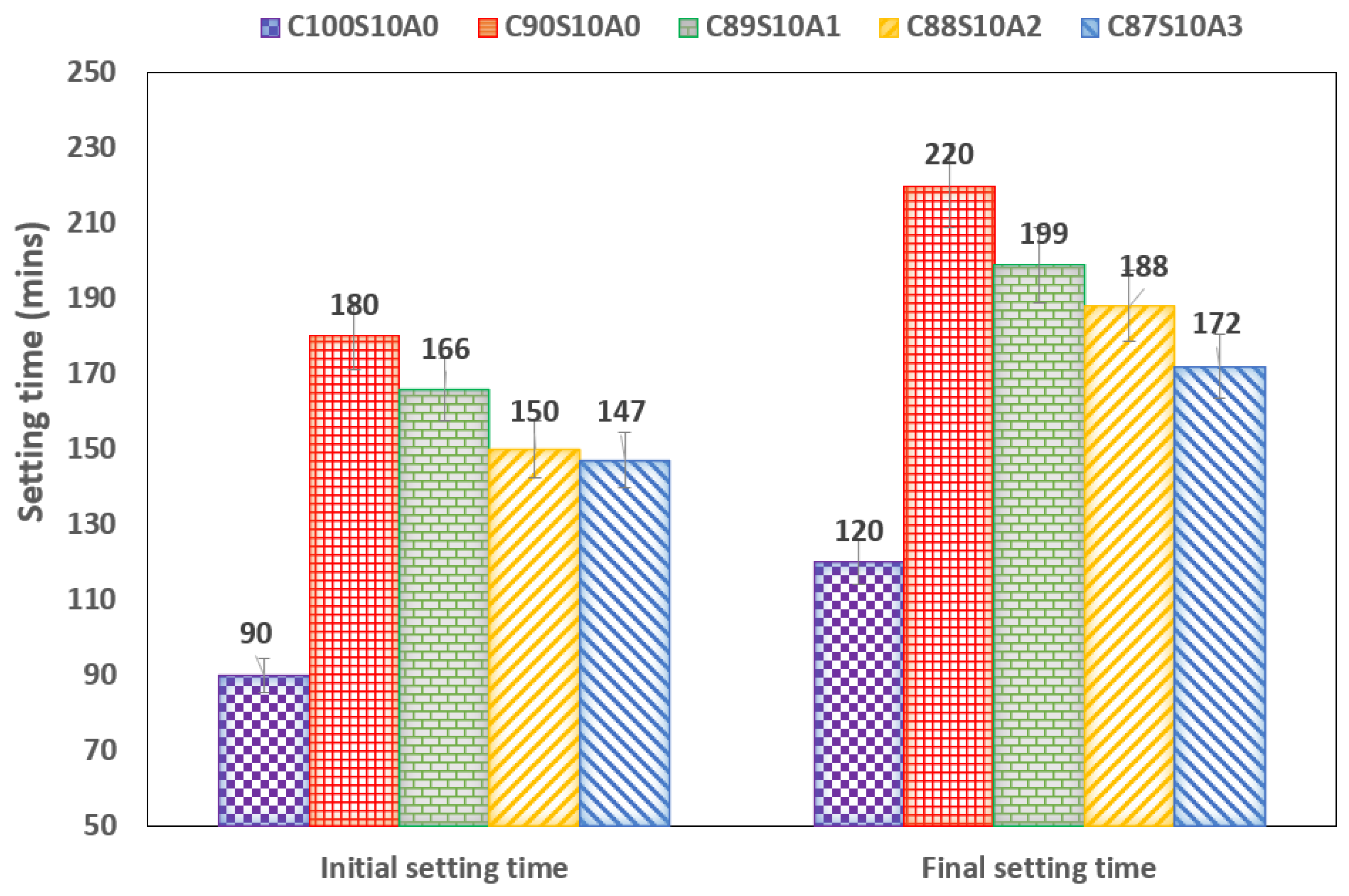
3.2. Setting Time of the SF Blended and Nano Alumina Silica Fume Blended Mortar
3.3. Compressive Strength of SF–nA Ternary Blended Mortar
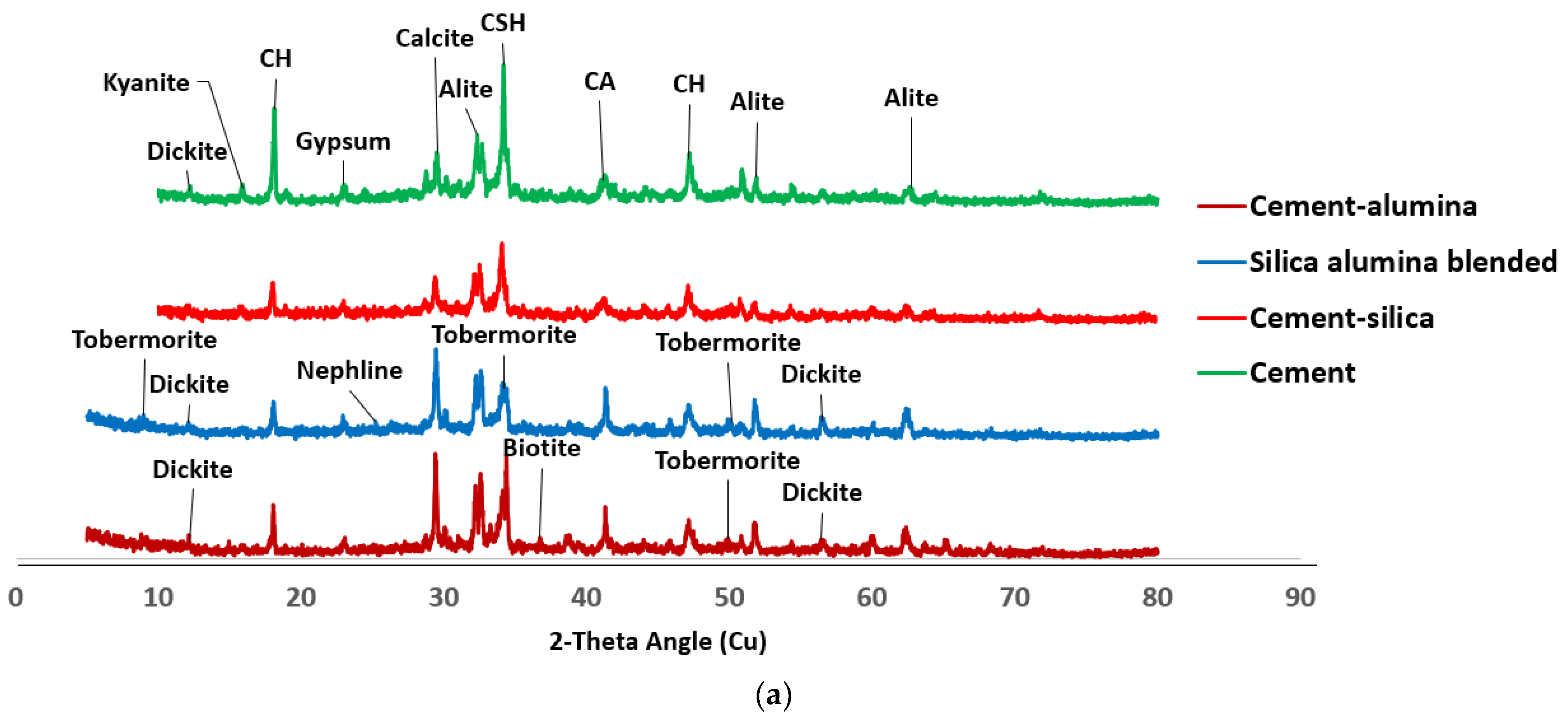
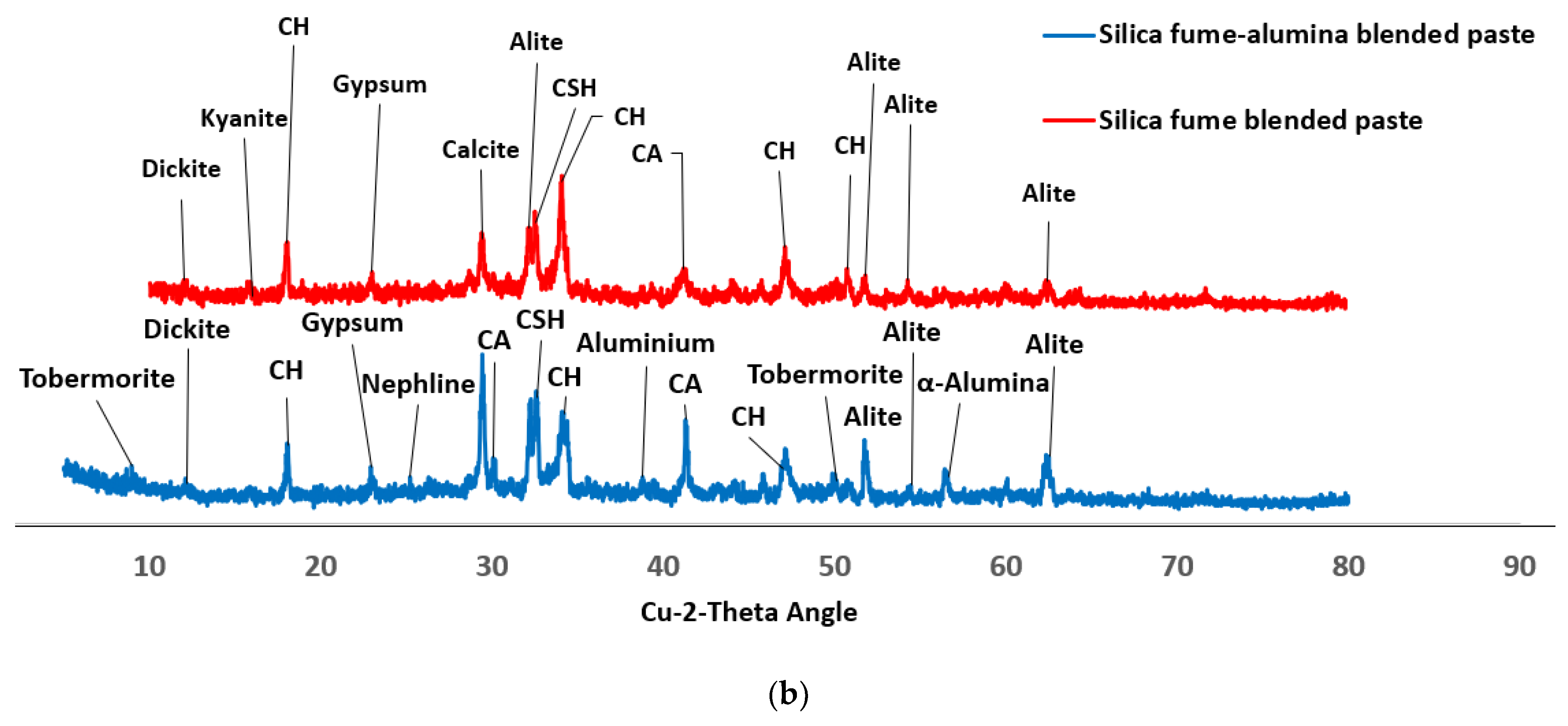
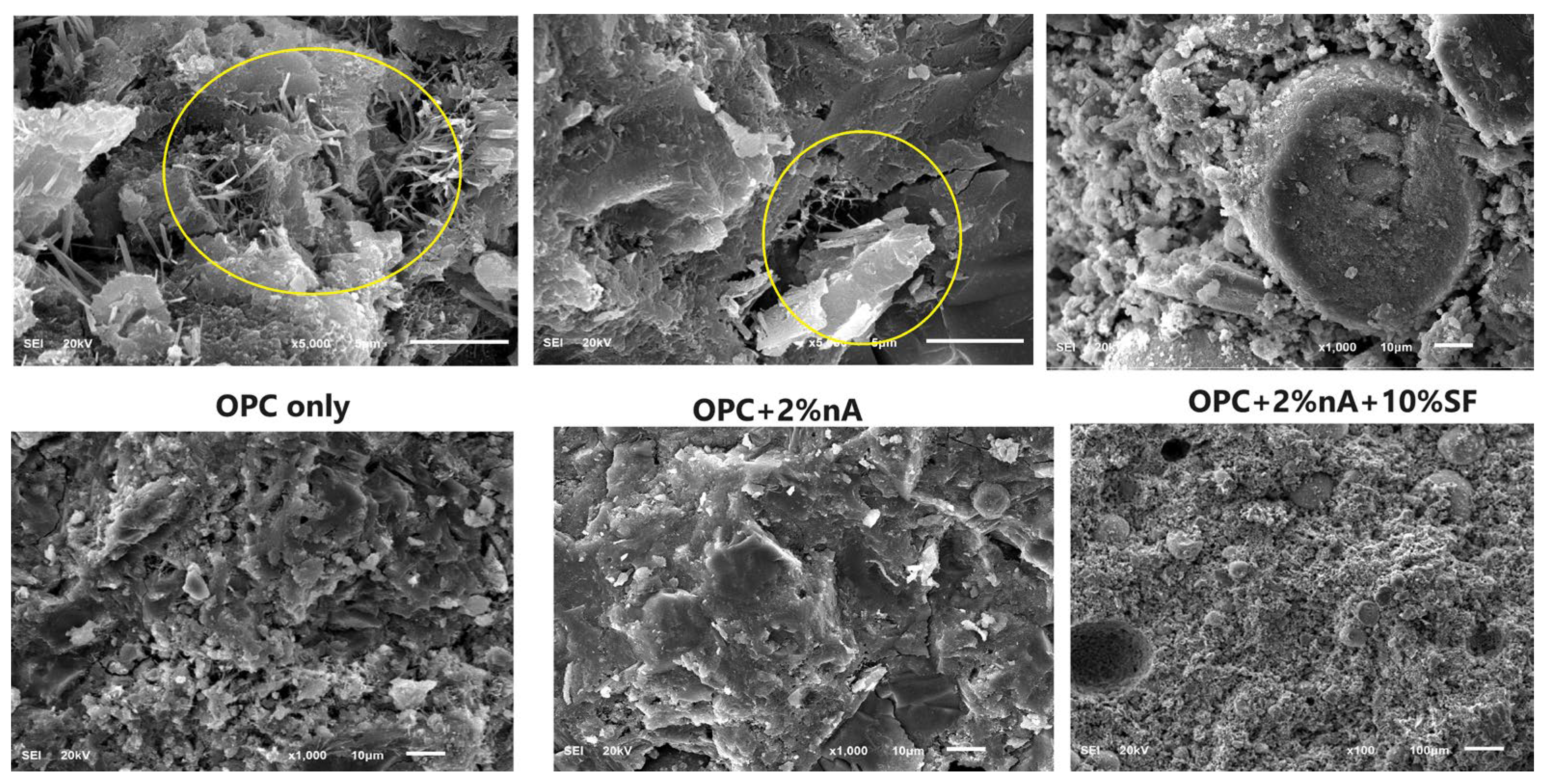
3.4. Morphology of SF Blended Binary and SF–nA Ternary Blended Pastes
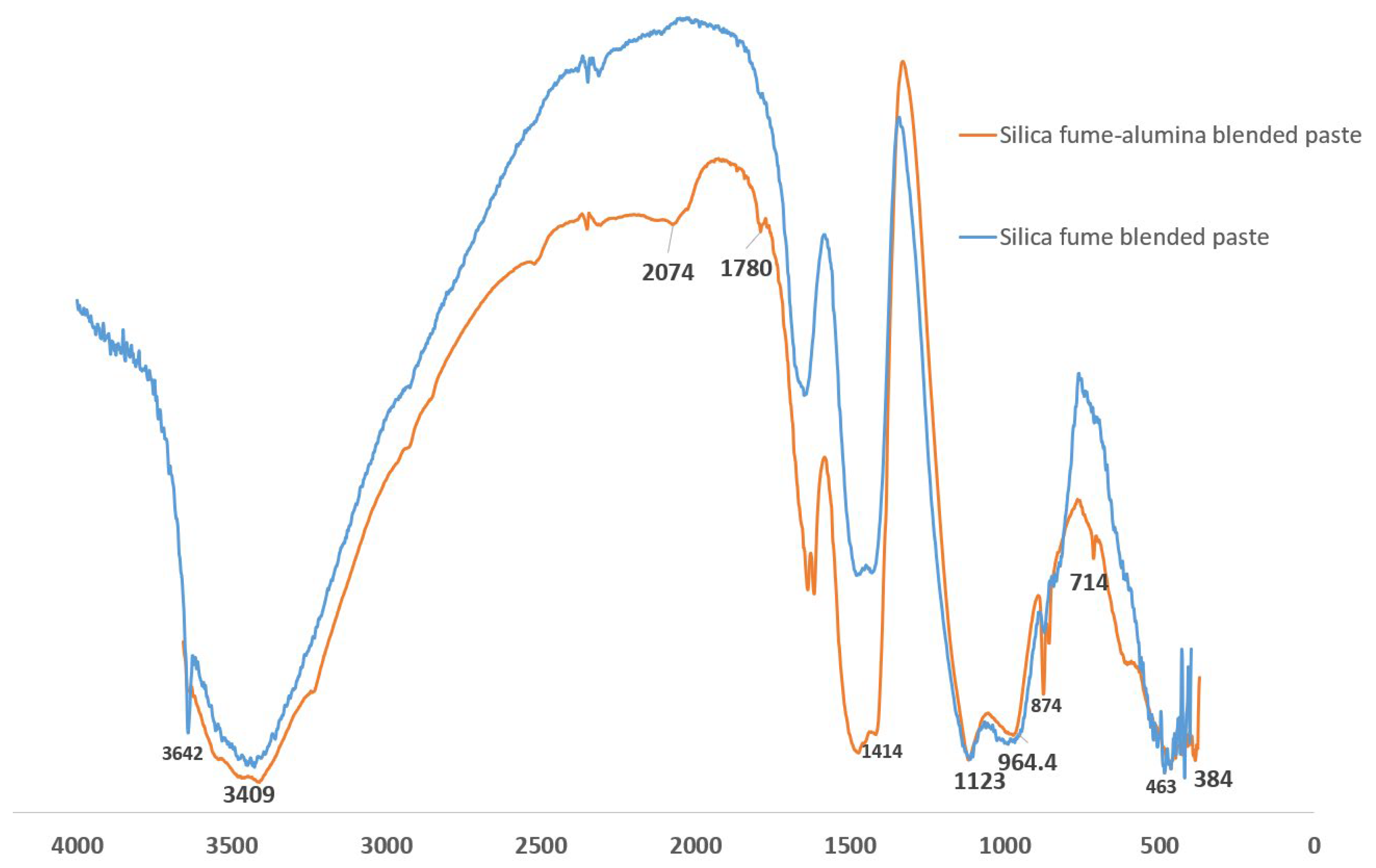
3.5. FTIR of SF Binary and SF–nA Ternary Binder
3.6. Thermal Performance of SF Binary and SF–nA Ternary Blended Mortar
4. Conclusions
- The SF–nA ternary blended binder (C90-xS10Ax) had a better consistency than SF-OPC binary blended paste. Nano-alumina improved the interparticle lubrication and the workability of SF blended mortar.
- SF delayed the setting time of OPC due to the dilution effect, however, the more nA in C90-xS10Ax, the shorter the setting time. Hence, nA can be used to accelerate the delayed setting of SF blended binder (C90S10A0).
- The early compressive strength of SF blended mortar (C90S10A0) was lower than that of OPC (C100S0A0), while incorporation of nA significantly enhanced early and 28-d compressive strength; CSH, CASH, tobermorite (CSH of lower Ca/Si ratio), calcite, and mayenite dominated the SF–nA ternary binder phases as noted in the XRD.
- Introducing nA to the SF blended binder caused a reduction in Ca/Al and Si/Al ratios compared to those in the OPC binder.
- The calcite peak and CO vibration peak in the XRD and FTIRS indicate that the SF–nA ternary blended binder was more prone to carbonation compared to SF blended concrete due to the possible dual formation of aluminum and calcium carbonates.
- The optimum microstructural density and maximum 28-day compressive strength of 43.2 MPa were achieved in the 10% SF and 2% nA (C88S10A2) mixture, whereas 36 and 30.5 MPa were recorded in OPC (C100S0A0) and 10% SF blended mortar (C90S10A0), respectively.
- The SF–nA ternary blended binder (C88S10A2) had the best thermal resistance—with a 3.7% loss of its 28-day strength, which became 8.85 and 33.3% in the SF binary blended binder (C90S10A0) and OPC binder (C100S0A0), respectively.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quercia, G.; Brouwers, H.J.H. Application of nano-silica (nS) in concrete mixtures. In Proceedings of the 8th Fib PhD Symposium in Kgs, Lyngby, Denmark, 20–23 June 2010; pp. 431–436. [Google Scholar] [CrossRef]
- Ghasemi, A.R.; Parhizkar, T.; Ramezanianpour, A.A. Influence of Colloidal Nano-SiO2 Addition as Silica Fume Replacement Material in Properties of Concrete. In Proceedings of the Second International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; pp. 28–30. [Google Scholar]
- Björnström, J.; Martinelli, A.; Matic, A.; Börjesson, L.; Panas, I. Accelerating effects of colloidal nano-silica for beneficial calcium-silicate-hydrate formation in cement. Chem. Phys. Lett. 2004, 392, 242–248. [Google Scholar] [CrossRef]
- Peerzada, M.; Adnan, K.; Bilal, B.; Janees, R.; Qazi, B.; Javed, N.A. Aptness of nano alumina (Al2O3) with high range water reducer-incorporated concrete. World J. Eng. 2023, 20, 746–753. [Google Scholar] [CrossRef]
- Orakzai, M.A. Hybrid effect of nano-alumina and nano-titanium dioxide on Mechanical properties of concrete. Case Stud. Constr. Mater. 2021, 14, e00483. [Google Scholar] [CrossRef]
- Krishnaveni, C.; Selvan, S.S. Nano-alumina incorporation into concrete by various methods. Mater. Today Proc. 2022, 68, 1926–1929. [Google Scholar] [CrossRef]
- Ashok, K.; Rao, B.K.; Kumar, B.S.C. Experimental Investigation on Nano Alumina based Concrete. ARPN J. Eng. Appl. Sci. 2021, 16, 76–87. [Google Scholar]
- Sadik, C.; El Amrani, I.; Albizane, A. Recent advances in silica-alumina refractory: A review. J. Asian Ceram. Soc. 2014, 2, 83–96. [Google Scholar] [CrossRef]
- Yusuf, M.O. Performance of Aluminium Shaving Waste and Silica fume Blended Mortar. Mag. Civ. Eng. 2023, 122, 12209. [Google Scholar]
- Yu, J.; Song, H.; Jeon, D.; Sim, S.; Kim, D.; Lee, H.; Yoon, S.; Yum, W.S.; Oh, J.E. Influence of the degree of crystallinity of added nano-alumina on strength and reaction products of the CaO-activated GGBFS system. Constr. Build. Mater. 2021, 296, 123647. [Google Scholar] [CrossRef]
- Prabaharan, J.S.; Sophia, M.; Babu, O.G.; Raja, M.A. High temperature effects on the compressive strength and UPV of nano alumina modified concrete containing sillimanite. Mater. Today Proc. 2022, 62, 5613–5619. [Google Scholar] [CrossRef]
- Shao, Q.; Zheng, K.; Zhou, X.; Zhou, J.; Zeng, X. Enhancement of nano-alumina on long-term strength of portland cement and the relation to its influences on compositional and microstructural aspects. Cem. Concr. Compos. 2019, 98, 39–48. [Google Scholar] [CrossRef]
- Yu, J.; Sim, S.; Song, H.; Kim, D.; Jang, K.; Jeon, D.; Oh, J.E. Examination of sulfate resistance of nano-alumina added ordinary Portland cement paste, focusing on the two different crystallinity of nano-aluminas. Int. J. Concr. Struct. Mater. 2023, 17, 32. [Google Scholar] [CrossRef]
- Dyer, T. Concrete Durability; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Mehta, P.K.; Klein, A. Formation of ettringite in pastes containing calcium aluminoferrites and gypsum. Highw. Res. Rec. 1967, 192, 32–45. [Google Scholar]
- Weather in the Middle East. Available online: http://climateandweather.com/weather-in-middle-east (accessed on 11 September 2023).
- Li, Z.; Wang, H.; He, S.; Lu, Y.; Wang, M. Investigations on the preparation and mechanical properties of the nano-alumina reinforced cement composite. Mater. Lett. 2006, 60, 356–359. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S.; Riahi, S.; Shamekhi, S.F.; Khademno, A. Influence of Al2O3 Nanoparticles on the Compressive Strength and Workability of Blended Concrete. 2010. Available online: http://www.americanscience.orgeditor@americanscience.org (accessed on 1 August 2023).
- Nazari, A.; Riahi, S. The effect of aluminium oxide nanoparticles on the compressive strength and structure of self-compacting concrete. Mag. Concr. Res. 2012, 64, 71–82. [Google Scholar] [CrossRef]
- Carmo, R.N.F.; Costa, H.; Júlio, E. Influence of nanoparticles additions on the bond between steel fibres and the binding paste. Constr. Build. Mater. 2017, 151, 312–318. [Google Scholar] [CrossRef]
- Krishnaveni, C.; Selvan, S.S. Study on nano-alumina in concrete. Mater. Today Proc. 2020, 46, 3648–3652. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S. The effect of nanoalumina on early hydration and mechanical properties of cement pastes. Constr. Build. Mater. 2019, 202, 169–176. [Google Scholar] [CrossRef]
- Shabbar, R.; Nedwell, P.; Wu, Z. Mechanical properties of lightweight aerated concrete with different aluminium powder content. MATEC Web Conf. 2017, 120, 02010. [Google Scholar] [CrossRef]
- Younus, S.J.; Mosaberpanah, M.A.; Alzeebaree, R. The Performance of Alkali-Activated Self-Compacting Concrete with and without Nano-Alumina. Sustainability 2023, 15, 2811. [Google Scholar] [CrossRef]
- ASTM C157; Standard Test Method for Length Change of Hardened Hydraulic-Cement Mortar and Concrete. America Standard Method of Testing: West Conshohocken, PA, USA, 2008.
- ASTM C191-21; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. America Standard Method of Testing: West Conshohocken, PA, USA, 2021.
- ASTM C1437-20; Standard Test Method for Flow of Hydraulic Cement Mortar. America Standard Method of Testing: West Conshohocken, PA, USA, 2020.
- ASTM C109/C109M-20; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). America Standard Method of Testing: West Conshohocken, PA, USA, 2020.
- Yusuf, M.O. Bond Characterization in Cementitious Material Binders Using Fourier-Transform Infrared Spectroscopy. Appl. Sci. 2023, 13, 3353. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, K.; Liu, Z.; He, F. Chemical effect of nano-alumina on early-age hydration of Portland cement. Cem. Concr. Res. 2019, 116, 159–167. [Google Scholar] [CrossRef]
- Zahedi, R.; Mirmohammadi, S.J. Sulfate removal from chemical industries’ wastewater using ettringite precipitation process with recovery of Al(OH)3. Appl. Water Sci. 2022, 12, 226. [Google Scholar] [CrossRef]
- Lecomte, I.; Henrist, C.; Liégeois, M.; Maseri, F.; Rulmont, A.; Cloots, R. (Micro)-structural comparison between geopolymers, alkali-activated slag cement and Portland cement. J. Eur. Ceram. Soc. 2006, 26, 3789–3797. [Google Scholar] [CrossRef]




| Oxide Composition | OPC | SF | Nano Alumina |
|---|---|---|---|
| SiO2 | 19.01 | 95.85 | 0.00 |
| Al2O3 | 4.68 | 0.26 | 99.87 |
| Fe2O3 | 3.20 | 0.05 | 0.03 |
| CaO | 66.89 | 0.21 | 0.07 |
| MgO | 0.81 | 0.45 | 0.03 |
| Na2O | 0.09 | 0.4 | - |
| TiO2 | 0.22 | - | - |
| K2O | 1.17 | 1.22 | - |
| P2O5 | 0.08 | - | - |
| SO3 | 3.66 | 1.00 | - |
| MnO | 0.19 | 0.00 | - |
| SiO2 + Al2O3 + Fe2O3 | 26.89 | 96.16 | 99.90 |
| SG | 3.14 | 2.25 | 3.38 |
| LOI (%) | 2.80 | 2.48 | 1.05 |
| Surface area (m2/g) | 0.33 | 22.8 | 440 |
| Samples | Cement (Kg/m3) | Silica Fume (Kg/m3) | Alumina (Kg/m3) | Sand (Kg/m3) | Water (Kg/m3) | SP (Kg/m3) | Wet Density |
|---|---|---|---|---|---|---|---|
| C100S0A0 | 350.00 | 0.00 | 0.00 | 1809.84 | 157.50 | 3.50 | 2320.84 |
| C90S10A0 | 315.00 | 35.00 | 0.00 | 1798.64 | 157.50 | 3.50 | 2309.64 |
| C89S10A1 | 311.50 | 35.00 | 3.50 | 1798.84 | 157.50 | 3.50 | 2309.84 |
| C88S10A2 | 308.00 | 35.00 | 7.00 | 1799.04 | 157.50 | 3.50 | 2310.04 |
| C87S10A3 | 304.50 | 35.00 | 10.50 | 1799.24 | 157.50 | 3.50 | 2310.24 |
| Element | OPC | SF + OPC | SF + OPC + nA | OPC + nA |
|---|---|---|---|---|
| C | 8.8 | 15.1 | 11.1 | 15.5 |
| O | 49.9 | 44.6 | 44.2 | 44.6 |
| Al | 1.04 | 1.4 | 1.6 | 1.7 |
| Si | 6.4 | 10.8 | 8.2 | 9.7 |
| K | 1.6 | 0 | 1.6 | 0 |
| Ca | 30.8 | 26.5 | 32.2 | 27.1 |
| Fe | 1.1 | 1.7 | 2.1 | 1.5 |
| Ca/Si | 4.8 | 2.5 | 3.9 | 2.8 |
| Ca/Al | 29.6 | 18.9 | 20.1 | 15.9 |
| Si/Al | 6.2 | 7.7 | 5.1 | 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusuf, M.O. Characteristics of Silica Fume Nano Alumina Ternary Blended Mortar. Sustainability 2023, 15, 14615. https://doi.org/10.3390/su151914615
Yusuf MO. Characteristics of Silica Fume Nano Alumina Ternary Blended Mortar. Sustainability. 2023; 15(19):14615. https://doi.org/10.3390/su151914615
Chicago/Turabian StyleYusuf, Moruf Olalekan. 2023. "Characteristics of Silica Fume Nano Alumina Ternary Blended Mortar" Sustainability 15, no. 19: 14615. https://doi.org/10.3390/su151914615
APA StyleYusuf, M. O. (2023). Characteristics of Silica Fume Nano Alumina Ternary Blended Mortar. Sustainability, 15(19), 14615. https://doi.org/10.3390/su151914615






