Investigation of Pyrolysis/Gasification Process Conditions and Syngas Production with Metal Catalysts Using Waste Bamboo Biomass: Effects and Insights
Abstract
:1. Introduction
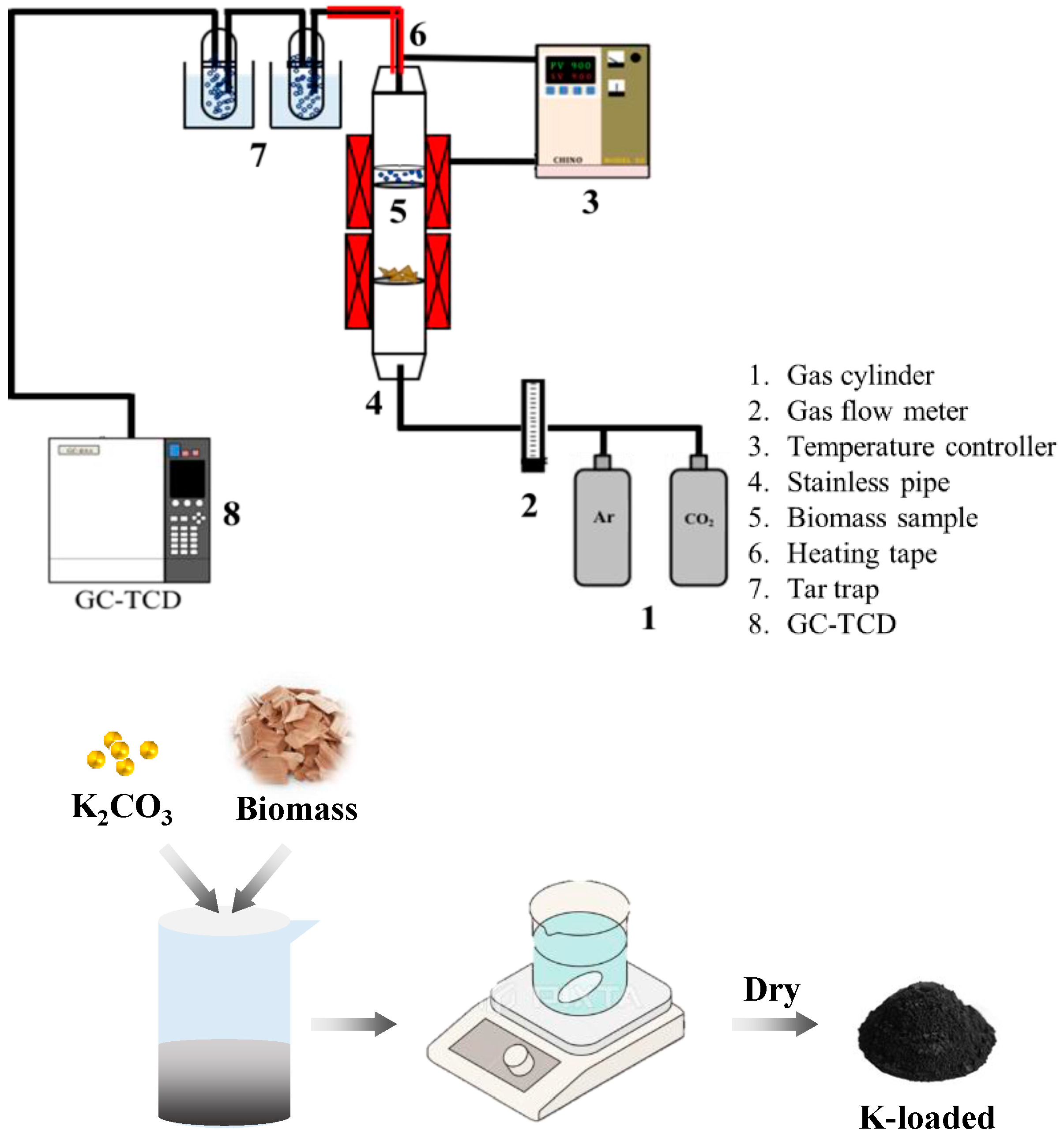
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Reaction Behaviors with Different Metal Catalysts
2.3. Analysis of Gas Components
3. Results and Discussions
3.1. Reaction Behaviors
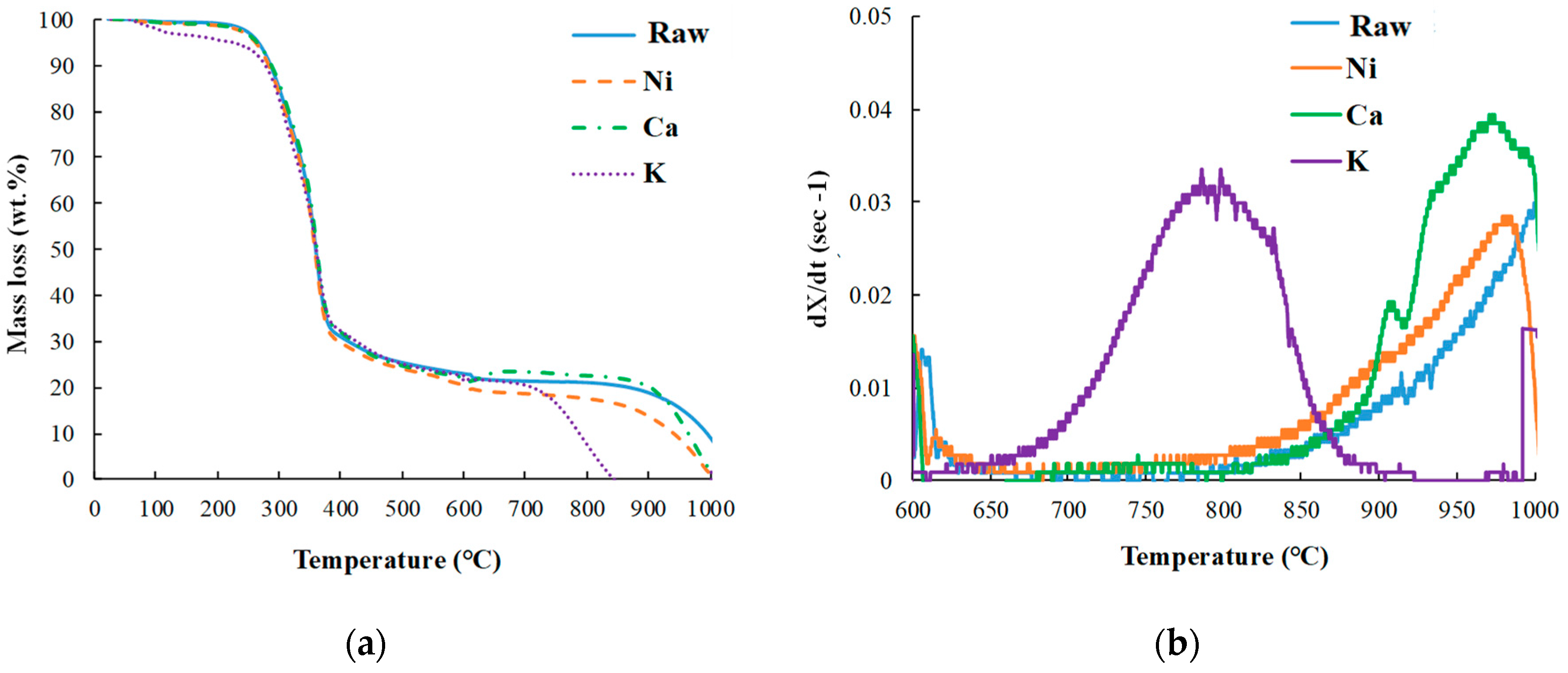
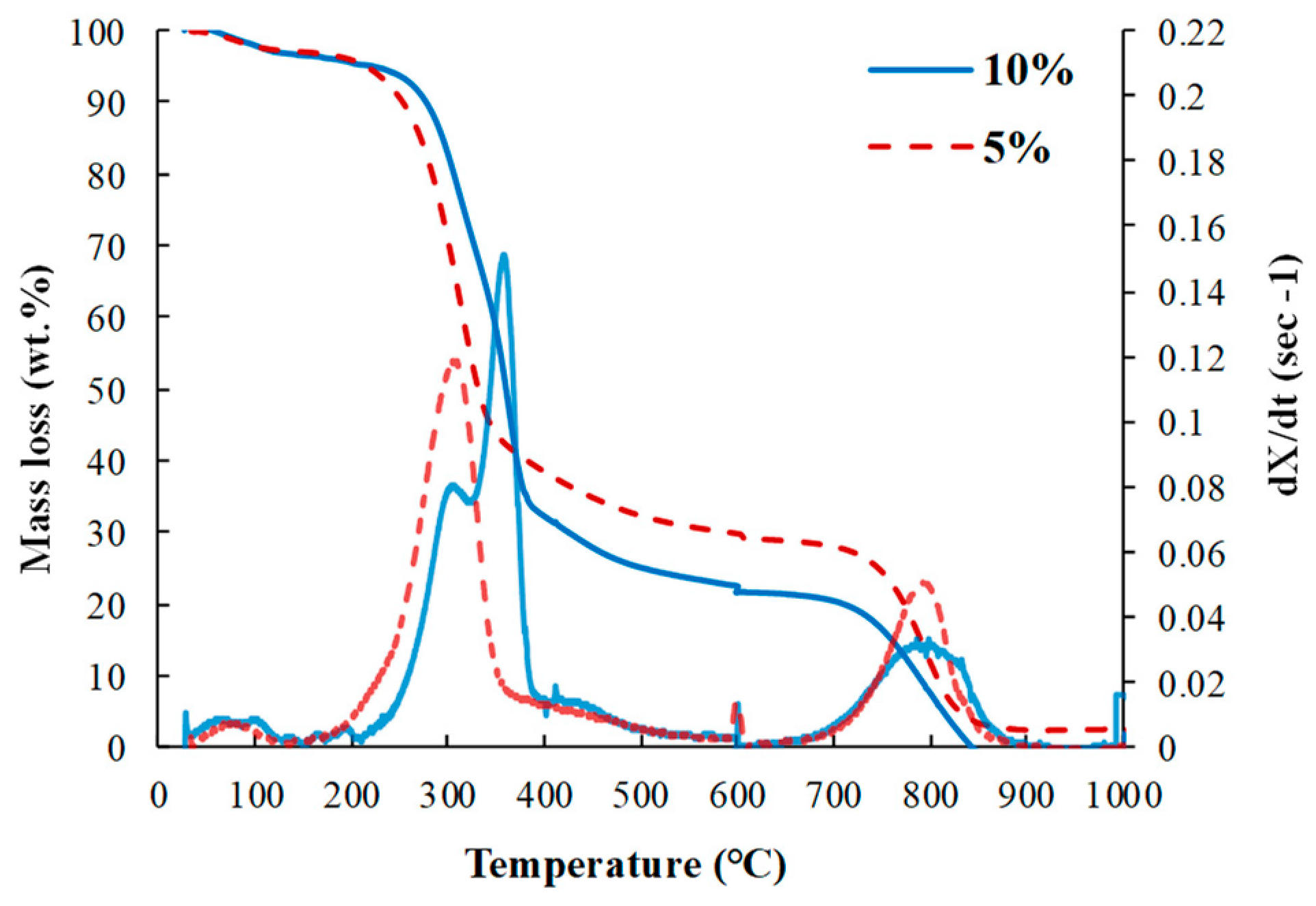
3.1.1. Pyrolysis and Gasification Behaviors of Bamboo Samples
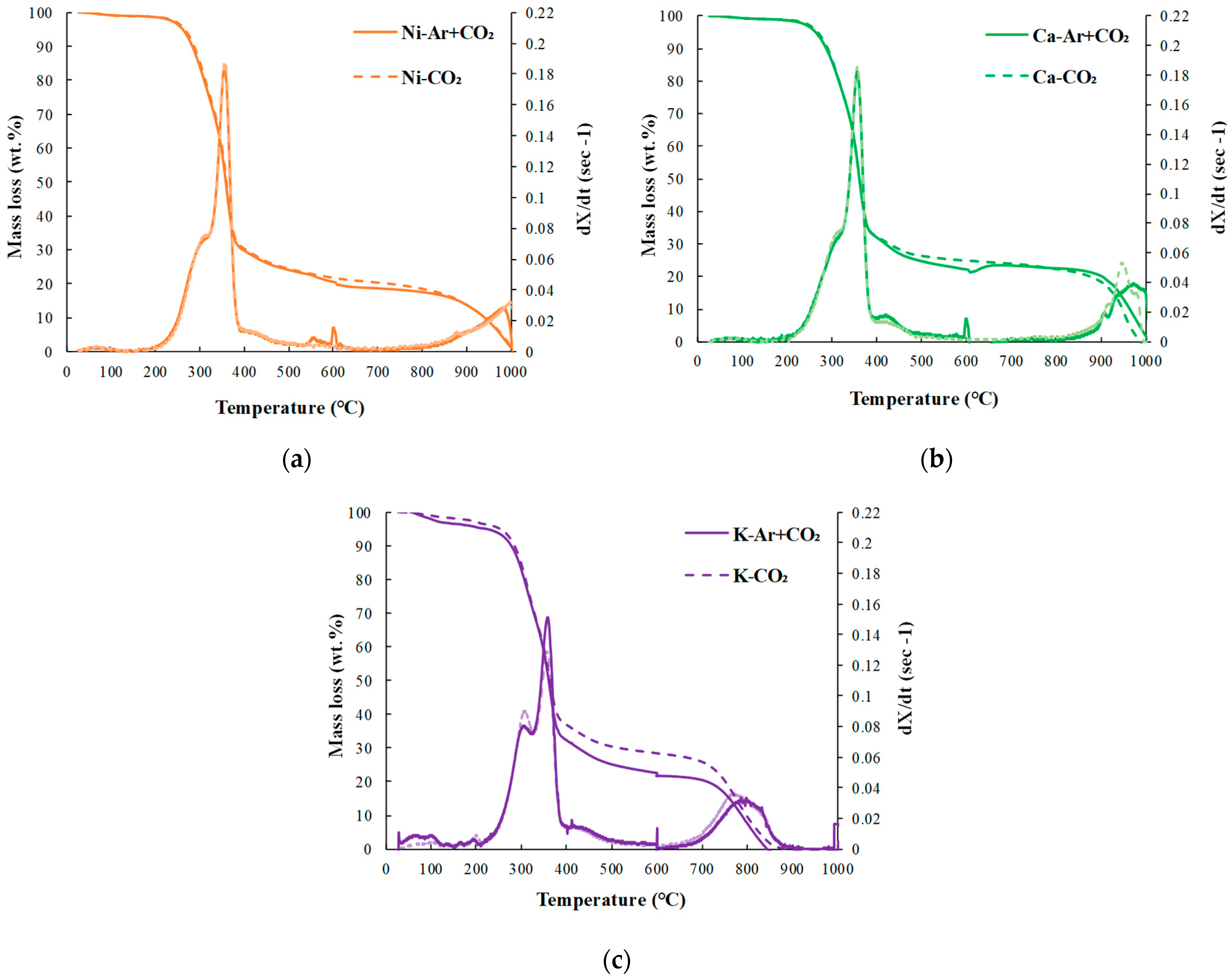
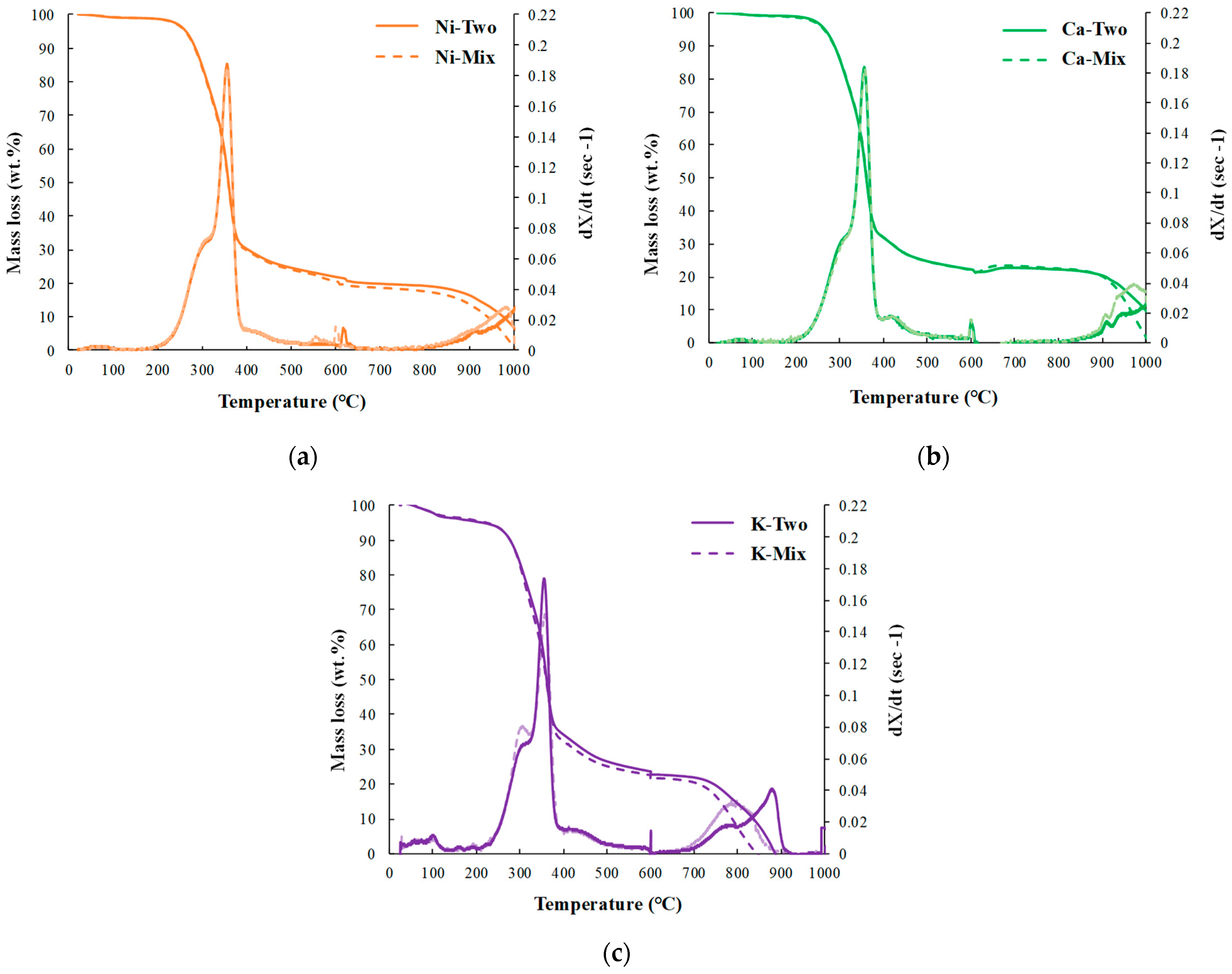
3.1.2. Effects of Pyrolysis Atmosphere and Catalyst Addition Method
3.1.3. Effect of Metal Catalysts
3.1.4. Effect of Catalyst Dose
3.2. Pyrolysis/Gasification Gas Production Components
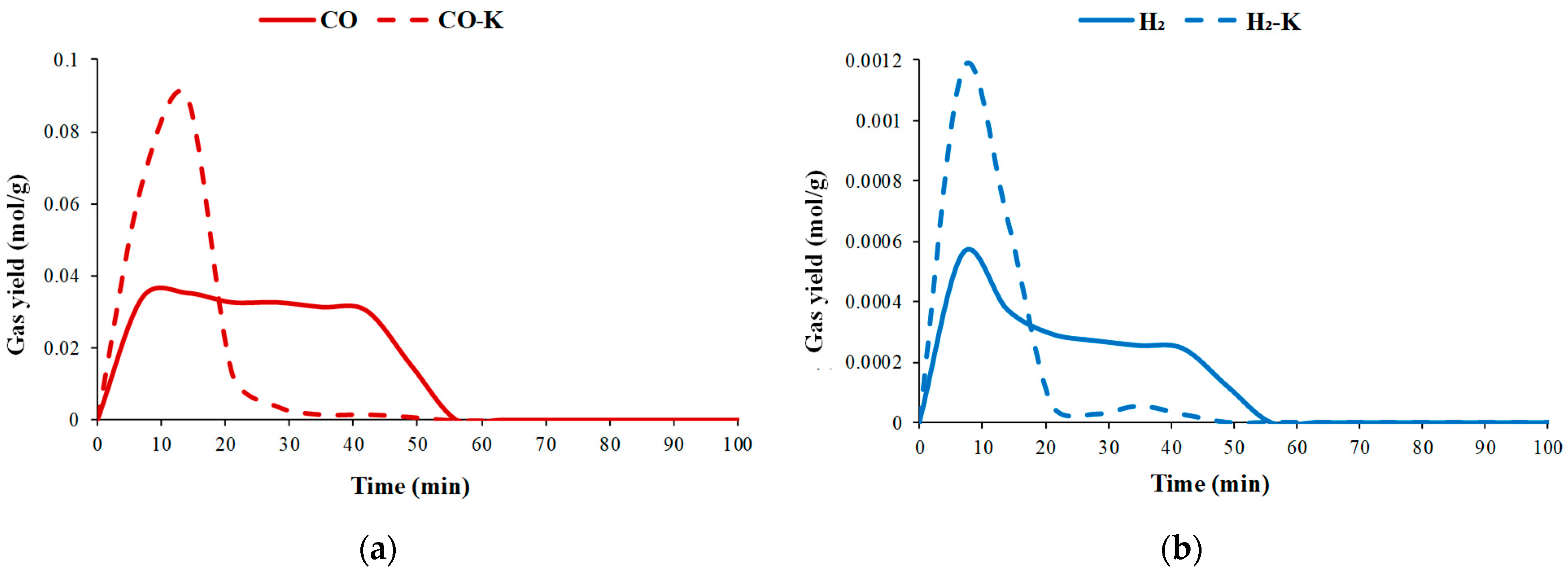
3.2.1. Pyrolysis Gas Production
3.2.2. Gasification Gas Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yusuf, A.A.; Inambao, F.L. Effect of low bioethanol fraction on emissions, performance, and combustion behavior in a modernized electronic fuel injection engine. Biomass Convers. Biorefinery 2021, 11, 885–893. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Chica, E.L.; Pérez, J.F. Parametric Analysis of a Gasification-Based Cookstove as a Function of Biomass Density, Gasification Behavior, Airflow Ratio, and Design. ACS Omega 2022, 7, 7481–7498. [Google Scholar] [CrossRef] [PubMed]
- George, O.S.; Dennison, M.S.; Yusuf, A.A. Characterization and energy recovery from biomass wastes. Sustain. Energy Technol. Assess. 2023, 58, 103346. [Google Scholar] [CrossRef]
- Yao, D.; Hu, Q.; Wang, D.; Yang, H.; Wu, C.; Wang, X.; Chen, H. Hydrogen production from biomass gasification using biochar as a catalyst/support. Bioresour. Technol. 2016, 216, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Zhou, N.; Dai, L.; Deng, W.; Liu, C.; Cheng, Y.; Liu, Y.; Cobb, K.; Chen, P.; et al. Applications of calcium oxide–based catalysts in biomass pyrolysis/gasification—A review. J. Clean. Prod. 2021, 291, 125826. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Inambao, F.L. Characterization of Ugandan biomass wastes as the potential candidates towards bioenergy production. Renew. Sustain. Energy Rev. 2020, 117, 109477. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, C.; Hu, S.; Wang, Y.; Xu, K.; Su, S.; Xiang, J. Catalytic behaviors of alkali metal salt involved in homogeneous volatile and heterogeneous char reforming in steam gasification of cellulose. Energy Convers. Manag. 2018, 158, 147–155. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Gong, Y.; Ding, L.; Wang, J.; Yu, G. A review of the effects of alkali and alkaline earth metal species on biomass gasification. Fuel Process. Technol. 2021, 214, 106723. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B.; Sharrock, P. A review of catalysts for the gasification of biomass char, with some reference to coal. Energy 2013, 58, 305–317. [Google Scholar] [CrossRef]
- Guo, F.; Li, X.; Liu, Y.; Peng, K.; Guo, C.; Rao, Z. Catalytic cracking of biomass pyrolysis tar over char-supported catalysts. Energy Convers. Manag. 2018, 167, 81–90. [Google Scholar] [CrossRef]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Characterization of Spanish biomass wastes for energy use. Bioresour. Technol. 2012, 103, 249–258. [Google Scholar] [CrossRef]
- Dhanuskar, S.Y.; Modak, A.; Mhatre, D.; Naik, S.N.; Pant, K.K. High Yield Synthesis of Green Pyrolytic Oil via Thermal Cracking of Ricinoleic Acid Methyl Ester. Chem. Select. 2023, 8, e202204680. [Google Scholar] [CrossRef]
- Wongsiriamnuay, T.; Kannang, N.; Tippayawong, N. Effect of Operating Conditions on Catalytic Gasification of Bamboo in a Fluidized Bed. Int. J. Chem. Eng. 2013, 2013, 297941. [Google Scholar] [CrossRef]
- Nguyen-Thi, T.X.; Nguyen, P.Q.P.; Tran, V.D.; Ağbulut, Ü.; Nguyen, L.H.; Balasubramanian, D.; Tarelko, W.; Bandh, S.A.; Pham, N.D.K. Recent advances in hydrogen production from biomass waste with a focus on pyrolysis and gasification. Int. J. Hydrogen Energy, 2023; in press. [Google Scholar] [CrossRef]
- Mohammed, H.I.; Garba, K.; Ahmed, S.I.; Abubakar, L.G. Recent advances on strategies for upgrading biomass pyrolysis vapor to value-added bio-oils for bioenergy and chemicals. Sustain. Energy Technol. Assess. 2023, 55, 102984. [Google Scholar] [CrossRef]
- Ghodke, P.K.; Sharma, A.K.; Jayaseelan, A.; Gopinath, K.P. Hydrogen-rich syngas production from the lignocellulosic biomass by catalytic gasification: A state of art review on advance technologies, economic challenges, and future prospectus. Fuel 2023, 342, 127800. [Google Scholar] [CrossRef]
- Guo, F.; Jia, X.; Liang, S.; Zhou, N.; Chen, P.; Ruan, R. Development of biochar-based nanocatalysts for tar cracking/reforming during biomass pyrolysis and gasification. Bioresour. Technol. 2020, 298, 122263. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, J.; Ge, X.; Chen, M. By-products recycling for syngas cleanup in biomass pyrolysis—An overview. Renew. Sustain. Energy Rev. 2016, 59, 1246–1268. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q.; Ma, D.; Takahashi, F.; Yoshikawa, K. In-situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification. Appl. Catal. B Environ. 2014, 152–153, 140–151. [Google Scholar] [CrossRef]
- Shen, Y. Chars as carbonaceous adsorbents/catalysts for tar elimination during biomass pyrolysis or gasification. Renew. Sustain. Energy Rev. 2015, 43, 281–295. [Google Scholar] [CrossRef]
- French, R.J.; Iisa, K.; Orton, K.A.; Griffin, M.B.; Christensen, E.; Black, S.; Brown, K.; Palmer, S.E.; Schaidle, J.A.; Mukarakate, C.; et al. Optimizing Process Conditions during Catalytic Fast Pyrolysis of Pine with Pt/TiO2—Improving the Viability of a Multiple-Fixed-Bed Configuration. ACS Sustain. Chem. Eng. 2021, 9, 1235–1245. [Google Scholar] [CrossRef]
- Li, X.; Zhuo, C.; Peng, L.; Huang, S.; Wu, S.; Lei, T.; Wu, Y. Feasibility assessment of recycling waste aluminum dross as a basic catalyst for biomass pyrolysis to produce hydrogen-rich gas. Int. J. Hydrogen Energy, 2023; in press. [Google Scholar] [CrossRef]
- Huang, Y.; Yin, X.; Wu, C.; Wang, C.; Xie, J.; Zhou, Z.; Li, H. Effects of metal catalysts on CO2 gasification reactivity of biomass char. Biotechnol. Adv. 2009, 27, 568–572. [Google Scholar] [CrossRef] [PubMed]
- JIS M8812 Standard. Available online: https://kikakurui.com/m/M8812-2006-01.html (accessed on 5 October 2023).
- Li, Y.; Wang, Y.; Chai, M.; Li, C.; Yellezuome, D.; Liu, R. Pyrolysis kinetics and thermodynamic parameters of bamboo residues and its three main components using thermogravimetric analysis. Biomass Bioenergy 2023, 170, 106705. [Google Scholar] [CrossRef]
- Tay, T.; Ucar, S.; Karagöz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Thow, Z.; Wang, C.H. Biomass gasification with CO2 in a fluidized bed. Powder Technol. 2016, 296, 87–101. [Google Scholar] [CrossRef]
- Mitsuoka, K.; Hayashi, S.; Amano, H.; Kayahara, K.; Sasaoaka, E.; Uddin, M.A. Gasification of woody biomass char with CO2: The catalytic effects of K and Ca species on char gasification reactivity. Fuel Process. Technol. 2011, 92, 26–31. [Google Scholar] [CrossRef]
- Sarkar, J.K.; Wang, Q. Characterization of Pyrolysis Products and Kinetic Analysis of Waste Jute Stick Biomass. Processes 2020, 8, 837. [Google Scholar] [CrossRef]
- Santamaría Moreno, L.; López Zabalbeitia, G.; Fernández Sáenz, E.; Cortázar Dueñas, M.; Arregi Joaristi, A.; Olazar Aurrecoechea, M.; Bilbao Elorriaga, J. Progress on Catalyst Development for the Steam Reforming of Biomass and Waste Plastics Pyrolysis Volatiles: A Review. Energy Fuels 2021, 35, 17051–17084. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Sun, H.; Wu, C.; Ling, H.; Jiang, Y.; Williams, P.T.; Huang, J. Effect of calcium addition on Mg-AlOx supported Ni catalysts for hydrogen production from pyrolysis-gasification of biomass. Catal. Today 2018, 309, 2–10. [Google Scholar] [CrossRef]
- Chan, F.L.; Tanksale, A. Review of recent developments in Ni-based catalysts for biomass gasification. Renew. Sustain. Energy Rev. 2014, 38, 428–438. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Collard, F.X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]



| Samples | Proximate Analysis | Ultimate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| M | VM | A | FC | C | H | N | O+S | |
| Madake | 4.59 | 80.2 | 0.648 | 14.5 | 44.8 | 5.82 | 0.260 | 48.5 |
| Moso bamboo | 4.85 | 80.6 | 0.229 | 14.3 | 45.4 | 6.00 | 0.270 | 48.1 |
| K-loaded moso | 4.85 | 71.6 | 3.11 | 20.5 | 35.9 | 5.58 | 0.440 | 54.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wang, Q. Investigation of Pyrolysis/Gasification Process Conditions and Syngas Production with Metal Catalysts Using Waste Bamboo Biomass: Effects and Insights. Sustainability 2023, 15, 14588. https://doi.org/10.3390/su151914588
Guo Y, Wang Q. Investigation of Pyrolysis/Gasification Process Conditions and Syngas Production with Metal Catalysts Using Waste Bamboo Biomass: Effects and Insights. Sustainability. 2023; 15(19):14588. https://doi.org/10.3390/su151914588
Chicago/Turabian StyleGuo, Yue, and Qingyue Wang. 2023. "Investigation of Pyrolysis/Gasification Process Conditions and Syngas Production with Metal Catalysts Using Waste Bamboo Biomass: Effects and Insights" Sustainability 15, no. 19: 14588. https://doi.org/10.3390/su151914588
APA StyleGuo, Y., & Wang, Q. (2023). Investigation of Pyrolysis/Gasification Process Conditions and Syngas Production with Metal Catalysts Using Waste Bamboo Biomass: Effects and Insights. Sustainability, 15(19), 14588. https://doi.org/10.3390/su151914588







