Bacterial Biodegradation of Perfluorooctanoic Acid (PFOA) and Perfluorosulfonic Acid (PFOS) Using Pure Pseudomonas Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Pseudomonas Strain Culture Growth
2.3. Bacterial Growth Inhibition Evaluation
2.4. Chemical Analysis
2.5. Sample Preparation for Analytical Detection
2.6. QA and QC Section
2.7. Statistical Analysis
3. Results
3.1. Decrease of PFOA and PFOS in the Presence of Pseudomonas Strains
3.2. Bacterial Growth Inhibition in Presence of PFOA and PFOS
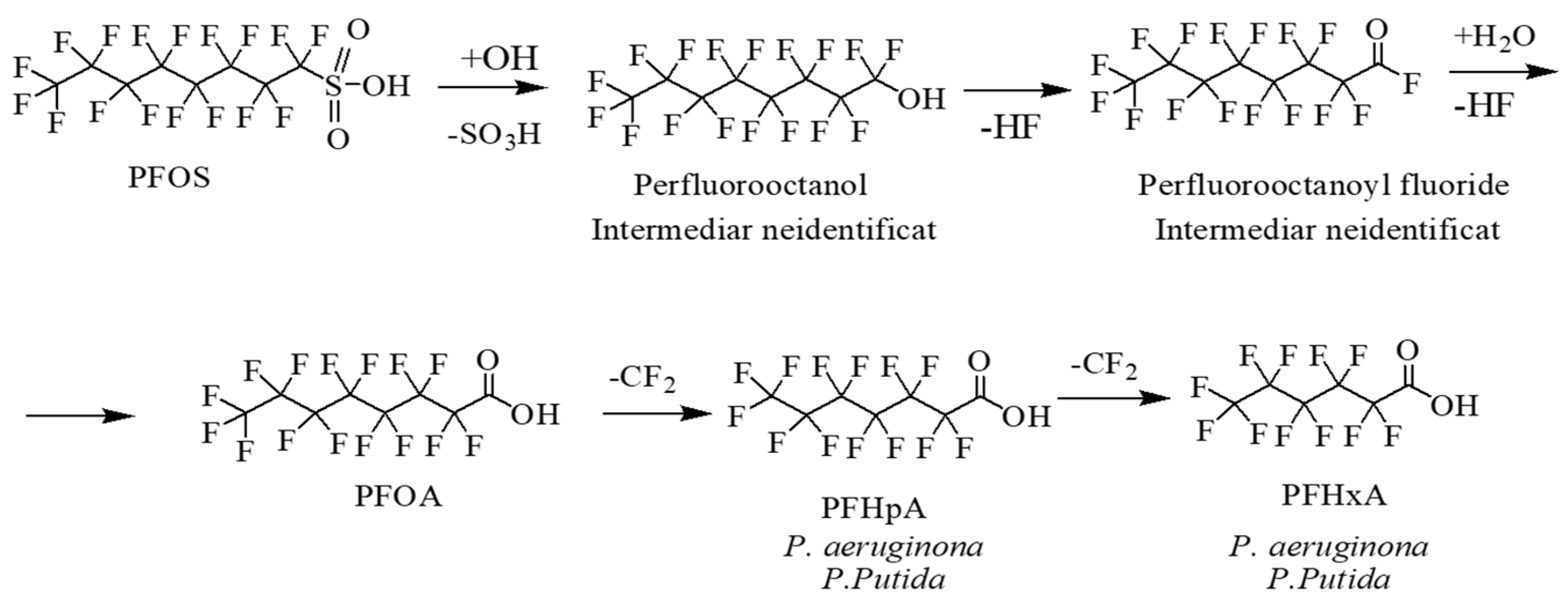
3.3. Biotransformation Products Sustain the PFOA and PFOS Biodegradation by Pseudomonas Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parsons, J.R.; Sáez, M.; Dolfing, J.; de Voogt, P. Biodegradation of Perfluorinated Compounds. Rev. Environ. Contam. Toxicol. 2008, 196, 53–71. [Google Scholar]
- Butt, C.M.; Muir, D.C.G.; Mabury, S.A. Biotransformation Pathways of Fluorotelomer-Based Polyfluoroalkyl Substances: A Review. Environ. Toxicol. Chem. 2014, 33, 243–267. [Google Scholar] [CrossRef]
- Liu, J.; Avendaño, S.M. Microbial degradation of polyfluoroalkyl chemicals in the environment: A review. Environ. Int. 2013, 61, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, O.; Snyder, S.A. Occurrence of Perfluoroalkyl Carboxylates and Sulfonates in Drinking Water Utilities and Related Waters from the United States. Environ. Sci. Technol. 2009, 43, 9089–9095. [Google Scholar] [CrossRef] [PubMed]
- Espana, V.A.A.; Mallavarapu, M.; Naidu, R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA): A critical review with an emphasis on field testing. Environ. Technol. Innov. 2015, 4, 168–181. [Google Scholar] [CrossRef]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef]
- Tsuda, S. Differential toxicity between perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). J. Toxicol. Sci. 2016, 41, SP27–SP36. [Google Scholar] [CrossRef]
- Huang, S.; Jaff’e, P.R. Defluorination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef]
- Kwon, B.G.; Lim, H.-J.; Na, S.-H.; Choi, B.-I.; Shin, D.-S.; Chung, S.-Y. Biodegradation of perfluorooctanesulfonate (PFOS) as an emerging contaminant. Chemosphere 2014, 109, 221–225. [Google Scholar] [CrossRef]
- Yi, L.B.; Chai, L.Y.; Xie, Y.; Peng, Q.J.; Peng, Q.Z. Isolation, identification, and degradation performance of a PFOA-degrading strain. Genet. Mol. Res. 2016, 15, 235–246. [Google Scholar] [CrossRef]
- Chiavola, A.; Di Marcantonio, C.; Boni, M.R.; Biagioli, S.; Frugis, A.; Cecchini, G. PFOA and PFOS removal processes in activated sludge reactor at laboratory scale. In Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability; Naddeo, V., Balakrishnan, M., Choo, K.H., Eds.; Springer: Cham, Switzerland, 2020; pp. 375–377. [Google Scholar]
- Li, F.; Su, Q.; Zhou, Z.; Liao, X.; Zou, J.; Yuan, B.; Sun, W. Anaerobic biodegradation of 8:2 fluorotelomer alcohol in anaerobic activated sludge: Metabolic products and pathways. Chemosphere 2018, 200, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Lemal, D.M. Perspective on fluorocarbon chemistry. J. Org. Chem. 2004, 69, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.B.; O’Hagan, D. The fluorinated natural products. Nat. Prod. Rep. 1994, 11, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Azerad, R.; Badet, B.; Copin, E. Microbial cleavage of C-F bond. J. Fluor. Chem. 2005, 126, 424–435. [Google Scholar] [CrossRef]
- Murphy, C.D. Microbial degradation of fluorinated drugs: Biochemical pathways, impacts on the environment and potential applications. Appl. Microbiol. Biotechnol. 2016, 100, 2617–2627. [Google Scholar] [CrossRef]
- Schennen, U.; Braun, K.; Knackmuss, H.J. Anaerobic degradation of 2-fluorobenzoate by benzoate-degrading, denitrifying bacteria. J. Bacteriol. 1985, 161, 321–325. [Google Scholar] [CrossRef]
- Vargas, C.; Song, B.; Camps, M.; Häggblom, M.M. Anaerobic degradation of fluorinated aromatic compounds. Appl. Microbiol. Biotechnol. 2000, 53, 342–347. [Google Scholar] [CrossRef]
- Tiedt, O.; Mergelsberg, M.; Boll, K.; Müller, M.; Adrian, L.; Jehmlich, N.; von Bergen, M.; Boll, M. ATP-dependent C-F bond cleavage allows the complete degradation of 4-fluoroaromatics without oxygen. MBio 2016, 7, 00990. [Google Scholar] [CrossRef]
- Kiel, M.; Engesser, K.-H. The biodegradation vs. biotransformation of fluorosubstituted aromatics. Appl. Microbiol. Biotechnol. 2015, 99, 7433–7464. [Google Scholar] [CrossRef]
- Tiedt, O.; Mergelsberg, M.; Eisenreich, W.; Boll, M. Promiscuous defluorinating enoyl-CoA hydratases/hydrolases allow for complete anaerobic degradation of 2-fluorobenzoate. Front. Microbiol. 2017, 8, 2579. [Google Scholar] [CrossRef]
- Goldman, P.; Milne, G.W. Carbon-fluorine bond cleavage. II. Studies on the mechanism of the defluorination of fluoroacetate. J. Biol. Chem. 1966, 241, 5557–5559. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Herrera, V.; Field, J.A.; Luna-Velasco, A.; Sierra-Alvarez, R. Microbial toxicity and biodegradability of perfluorooctane sulfonate (PFOS) and shorter chain perfluoroalkyl and polyfluoroalkyl substances (PFASs). Environ. Sci. Process. Impacts 2016, 18, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Cui, J.; Guo, J.; Zhai, Z.; Zuo, P.; Zhang, J. Fluorochemicals biodegradation as a potential source of trifluoroacetic acid (TFA) to the environment. Chemosphere 2020, 254, 126894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, X.; Wang, N.; Buck, R.C. Biotransformation potential of 6:2 fluorotelomer sulfonate (6:2 FTSA) in aerobic and anaerobic sediment. Chemosphere 2016, 154, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Merino, N.; Wang, M.; Ambrocio, R.; Mak, K.; O’Connor, E.; Gao, A.N.; Hawley, E.L.; Deeb, R.A.; Tseng, L.Y.; Mahendra, S. Fungal biotransformation of 6:2 fluorotelomer alcohol. Biorem. J. 2018, 28, 59–70. [Google Scholar] [CrossRef]
- Kim, M.H.; Wang, N.; Chu, K.H. 6:2 Fluorotelomer alcohol (6:2 FTOH) biodegradation by multiple microbial species under different physiological conditions. Appl. Microbiol. Biotechnol. 2014, 98, 1831–1840. [Google Scholar] [CrossRef]
- Meesters, R.J.; Schroder, H.F. Perfluorooctane sulfonate-a quite mobile anionic anthropogenic surfactant, ubiquitously found in the environment. Water Sci. Technol. 2004, 50, 235–242. [Google Scholar] [CrossRef]
- Chetverikov, S.P.; Sharipov, D.A.; Korshunova, T.Y.; Loginov, O.N. Degradation of perfluorooctanyl sulfonate by strain Pseudomonas plecoglossicida 2.4-D. Appl. Biochem. Microbiol. 2017, 53, 533–538. [Google Scholar] [CrossRef]
- Presentato, A.; Lampis, S.; Vantini, A.; Manea, F.; Daprà, F.; Zuccoli, S.; Vallini, G. On the ability of perfluorohexane sulfonate (PFHxS) bioaccumulation by two Pseudomonas sp. strains isolated from PFAS-contaminated environmental matrices. Microorganisms 2020, 8, 92. [Google Scholar] [CrossRef]
- Shaw, D.M.J.; Munoz, G.; Bottos, E.M.; Duy, S.V.; Sauve, S.; Liu, J.; Van Hamme, J.D. Degradation and defluorination of 6:2 fluorotelomer sulfonamidoalkyl betaine and 6:2 fluorotelomer sulfonate by Gordonia sp. strain NB4-1Y under sulfur-limiting conditions. Sci. Total Environ. 2019, 647, 690–698. [Google Scholar] [CrossRef]
- Singh, R.K.; Fernando, S.; Baygi, S.F.; Multari, N.; Thagard, S.M.; Holsen, T.M. Breakdown Products from Perfluorinated Alkyl Substances (PFAS) Degradation in a Plasma-Based Water Treatment Process. Environ. Sci. Technol. 2019, 53, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
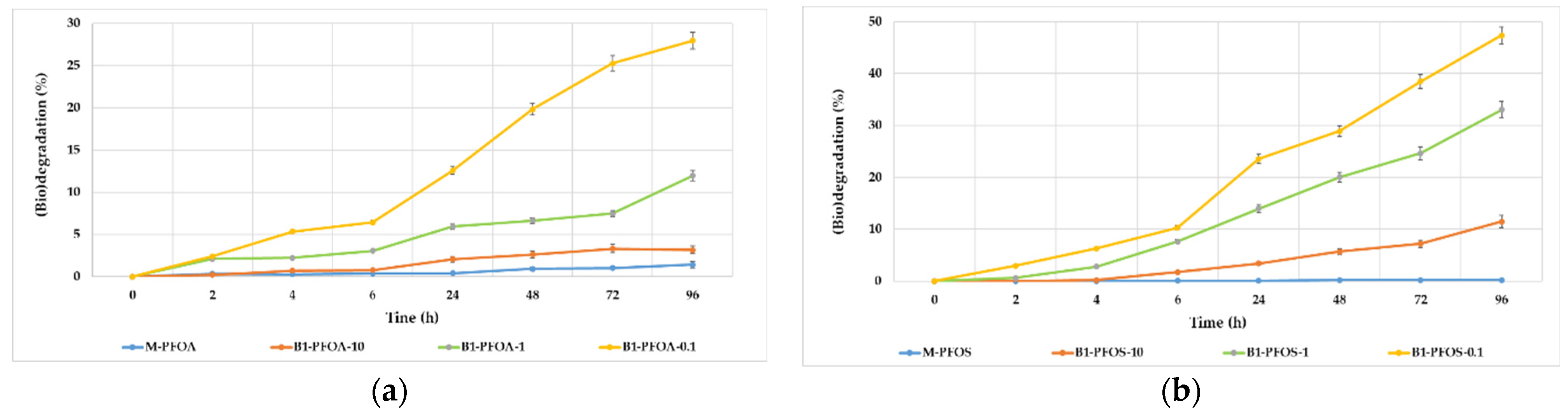

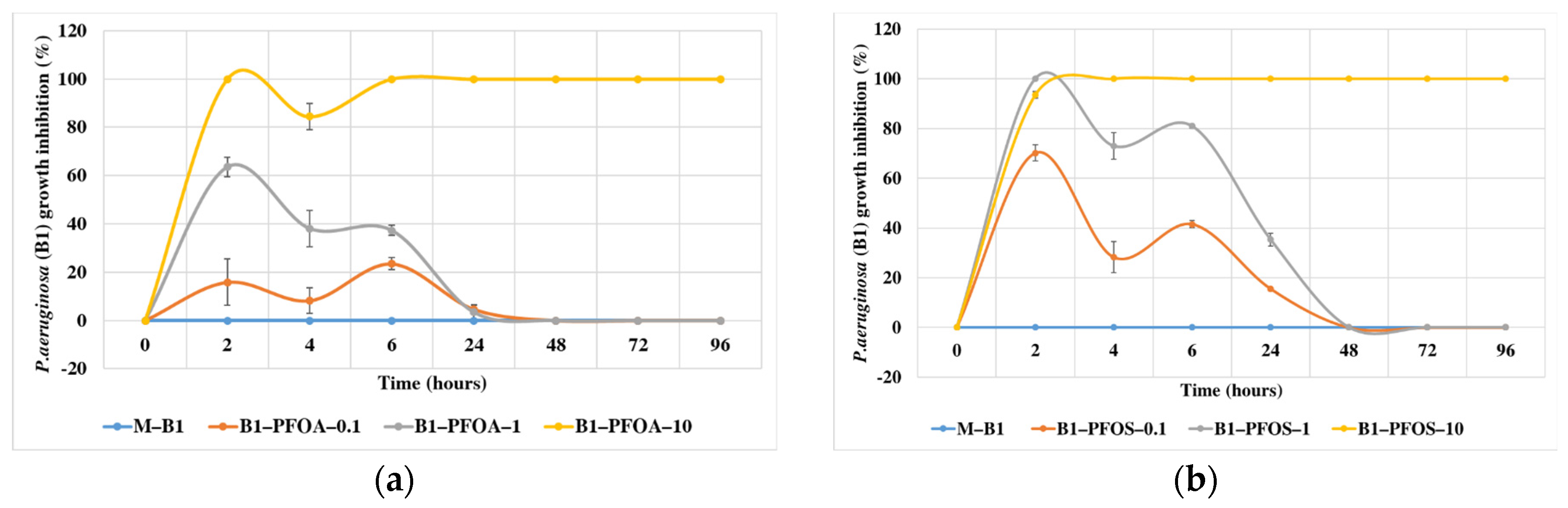
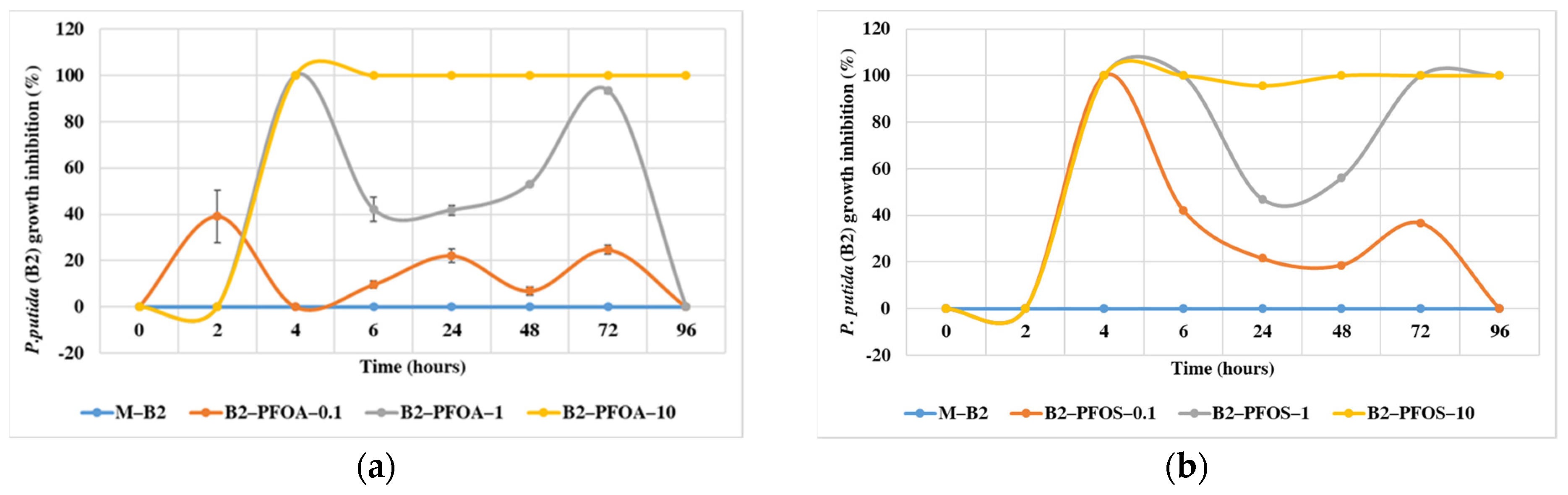
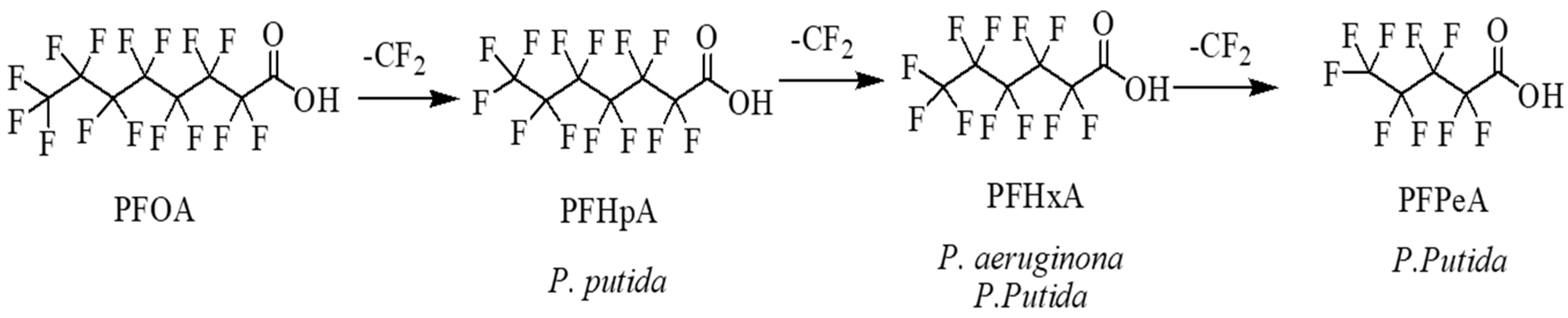
| Compound (Conc, mg/L) | Bacterial Strains | Conditions | Biotransformation Products | Biodegradation Efficiency | Test Time (Days) | References |
|---|---|---|---|---|---|---|
| PFOA (0.1/100) | Acidimicrobium sp. strain A6 | Anaerobe | PFBA; PFPeA; PFHxA; PFHpA | 63% (0.1 mg/L) 50% (100 mg/L) | 100 | [8] |
| PFOS (0.1/100) | Acidimicrobium sp. strain A6 | Anaerobe | PFBA; PFBS | 60% (0.1 mg/L) 47% (100 mg/L) | 100 | [8] |
| PFOA/PFOS (5 each) | Mixed culture | Aerobe/anaerobe sludge | Not reported | 0% (aerobe) 100% (anaerobe) | 30 | [28] |
| PFOA (500) | Pseudomonas Parafulva strain YAB1 | Aerobe | Not reported | 48% | 5 | [10] |
| PFOS (1.8) | Pseudomonas aeruginosa strain HJ4 | Aerobe | PFBS; PFHxS | 67% | 2 | [9] |
| PFOS (1000) | Pseudomonas plecoglossicida 2.4-D | Aerobe soil | Not reported | 75% | 90 | [29] |
| PFOS (1000) | Pseudomonas plecoglossicida 2.4-D | Aerobic mineral medium | PFHpA | 100% | 6 | [29] |
| PFHxS (0.2) | Pseudomonas sp. strain PS27 | Aerobe | Not reported | 32% | 10 | [30] |
| PFHxS (0.2) | Pseudomonas sp. strain PDMF10 | Aerobe | Not reported | 28% | 10 | [30] |
| Time (h) | B1-FFOA-0.1 | B1-FFOA-1 | B2-PFOA-0.1 | B2-PFOA-1 | ||||
|---|---|---|---|---|---|---|---|---|
| PFHxA | PFHxA | PFPeA | PFHxA | PFHpA | PFPeA | PFHxA | PFHpA | |
| 0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 2 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4 | 0.12 ± 0.004 | 0.56 ± 0.024 | 0.39 ± 0.014 | 0.10 ± 0.003 | 0.17 ± 0.007 | <LOQ | 0.12 ± 0.004 | 0.22 ± 0.009 |
| 24 | 0.19 ± 0.008 | 0.60 ± 0.025 | 1.47 ± 0.057 | 0.12 ± 0.004 | 0.19 ± 0.008 | <LOQ | 0.16 ± 0.006 | 0.31 ± 0.013 |
| 48 | 0.35 ± 0.013 | 0.52 ± 0.022 | 28.0 ± 1.092 | 0.12 ± 0.004 | 0.23 ± 0.010 | <LOQ | 0.19 ± 0.007 | 0.25 ± 0.011 |
| 72 | 0.42 ± 0.016 | 0.41 ± 0.017 | 23.5 ± 0.917 | 0.10 ± 0.003 | 0.19 ± 0.008 | <LOQ | 0.15 ± 0.006 | 0.21 ± 0.009 |
| 96 | 0.57 ± 0.021 | 0.36 ± 0.015 | 16.3 ± 0.636 | 0.05 ± 0.002 | 0.16 ± 0.007 | <LOQ | 0.13 ± 0.005 | 0.17 ± 0.007 |
| Time (h) | B1-FFOS-0.1 | B1-FFOS-1 | B1-FFOS-0.1 | B1-FFOS-1 | ||||
|---|---|---|---|---|---|---|---|---|
| PFHxA | PFHpA | PFHxA | PFHpA | PFHxA | PFHpA | PFHxA | PFHpA | |
| 0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 2 | 0.16 ± 0.006 | 0.11 ± 0.005 | 0.13 ± 0.005 | 0.10 ± 0.004 | <LOQ | <LOQ | <LOQ | <LOQ |
| 4 | 0.15 ± 0.006 | 0.22 ± 0.009 | 0.23 ± 0.009 | 0.14 ± 0.006 | <LOQ | <LOQ | <LOQ | <LOQ |
| 6 | 0.34 ± 0.013 | 0.24 ± 0.010 | 0.36 ± 0.014 | 0.22 ± 0.008 | 0.12 ± 0.005 | <LOQ | 0.12 ± 0.004 | 0.19 ± 0.008 |
| 24 | 0.44 ± 0.016 | 0.26 ± 0.011 | 0.64 ± 0.025 | 0.24 ± 0.009 | 0.13 ± 0.005 | 0.11 ± 0.005 | 0.14 ± 0.005 | 0.26 ± 0.011 |
| 48 | 0.45 ± 0.017 | 0.16 ± 0.007 | 0.35 ± 0.014 | 0.22 ± 0.008 | 0.18 ± 0.008 | 0.16 ± 0.007 | 0.23 ± 0.009 | 0.29 ± 0.012 |
| 72 | 0.44 ± 0.017 | 0.13 ± 0.005 | 0.16 ± 0.006 | 0.11 ± 0.004 | 0.41 ± 0.017 | 0.36 ± 0.015 | 0.64 ± 0.024 | 0.19 ± 0.008 |
| 96 | 0.38 ± 0.014 | <LOQ | <LOQ | <LOQ | 0.26 ± 0.009 | 0.21 | 0.46 ± 0.017 | <LOQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiriac, F.L.; Stoica, C.; Iftode, C.; Pirvu, F.; Petre, V.A.; Paun, I.; Pascu, L.F.; Vasile, G.G.; Nita-Lazar, M. Bacterial Biodegradation of Perfluorooctanoic Acid (PFOA) and Perfluorosulfonic Acid (PFOS) Using Pure Pseudomonas Strains. Sustainability 2023, 15, 14000. https://doi.org/10.3390/su151814000
Chiriac FL, Stoica C, Iftode C, Pirvu F, Petre VA, Paun I, Pascu LF, Vasile GG, Nita-Lazar M. Bacterial Biodegradation of Perfluorooctanoic Acid (PFOA) and Perfluorosulfonic Acid (PFOS) Using Pure Pseudomonas Strains. Sustainability. 2023; 15(18):14000. https://doi.org/10.3390/su151814000
Chicago/Turabian StyleChiriac, Florentina Laura, Catalina Stoica, Cristina Iftode, Florinela Pirvu, Valentina Andreea Petre, Iuliana Paun, Luoana Florentina Pascu, Gabriela Geanina Vasile, and Mihai Nita-Lazar. 2023. "Bacterial Biodegradation of Perfluorooctanoic Acid (PFOA) and Perfluorosulfonic Acid (PFOS) Using Pure Pseudomonas Strains" Sustainability 15, no. 18: 14000. https://doi.org/10.3390/su151814000
APA StyleChiriac, F. L., Stoica, C., Iftode, C., Pirvu, F., Petre, V. A., Paun, I., Pascu, L. F., Vasile, G. G., & Nita-Lazar, M. (2023). Bacterial Biodegradation of Perfluorooctanoic Acid (PFOA) and Perfluorosulfonic Acid (PFOS) Using Pure Pseudomonas Strains. Sustainability, 15(18), 14000. https://doi.org/10.3390/su151814000








