Uranium and Fluoride Accumulation in Vegetable and Cereal Crops: A Review on Current Status and Crop-Wise Differences
Abstract
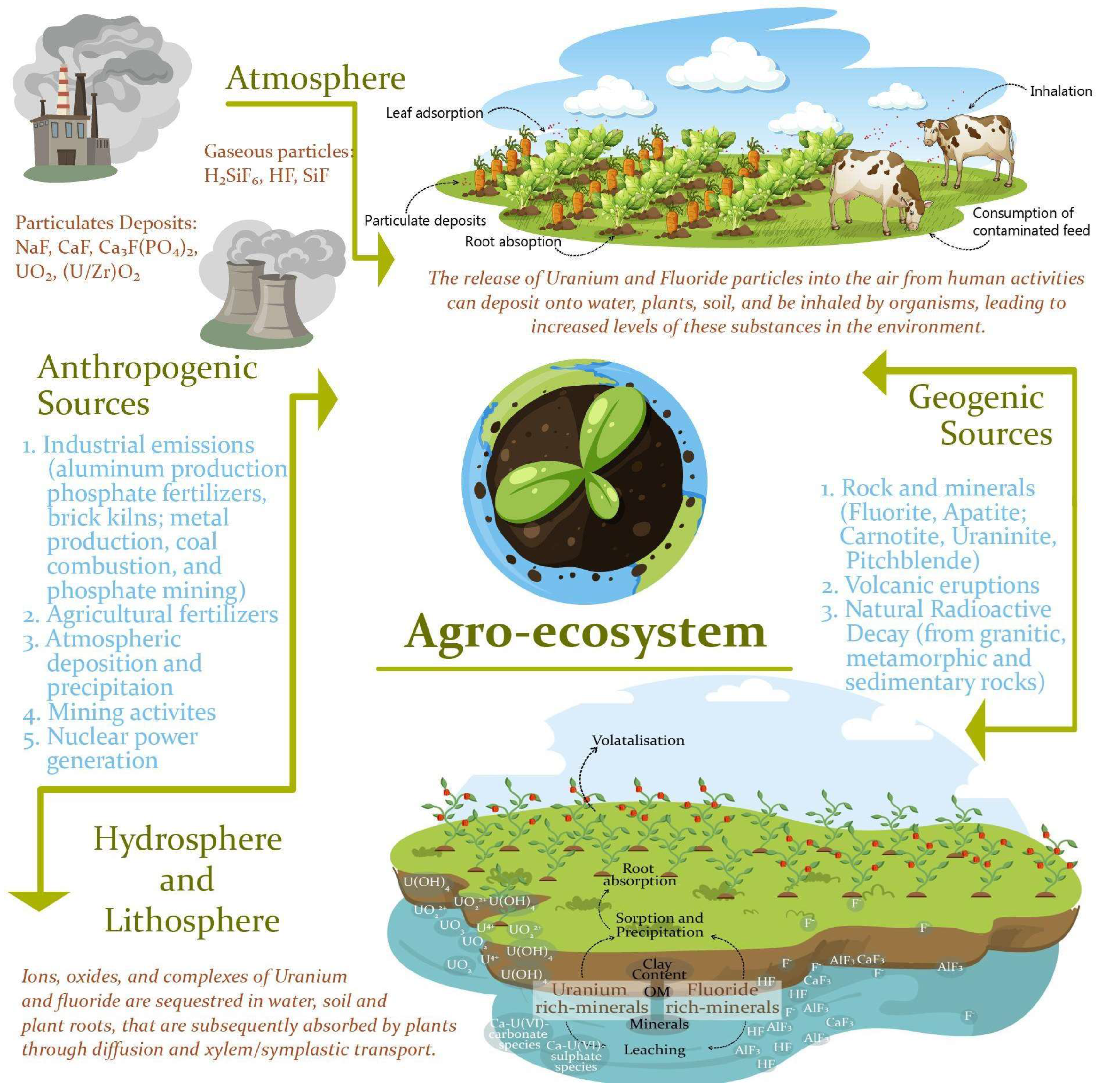
1. Introduction
2. Methodology
2.1. Data Collection and Processing
2.2. Statistical Analysis
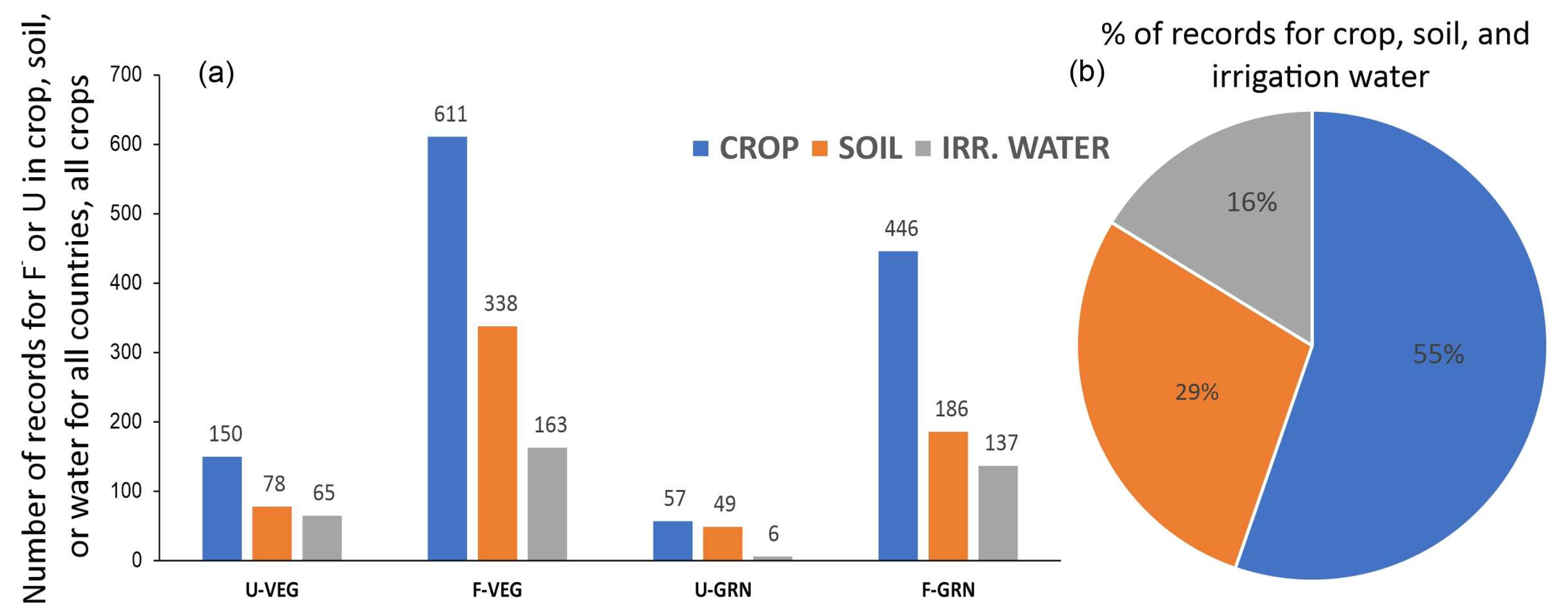
2.3. Basic Summary of the Compiled Data
3. Results and Discussion
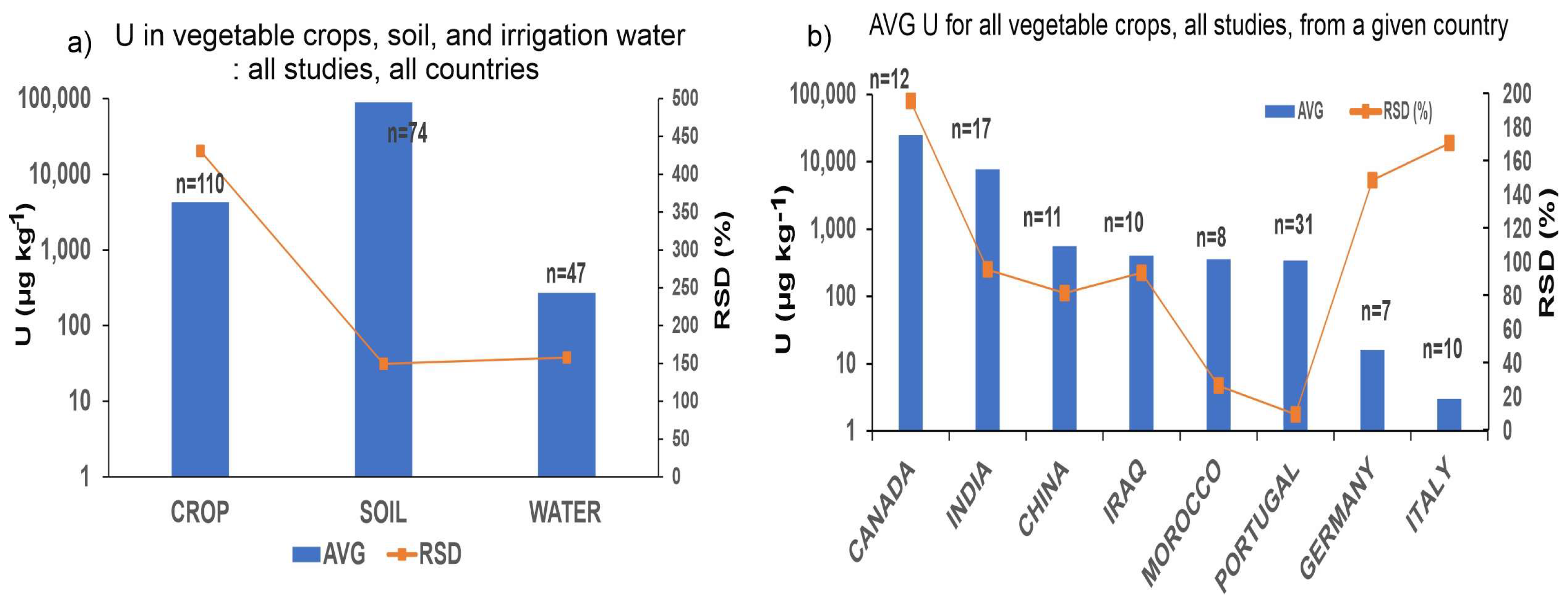
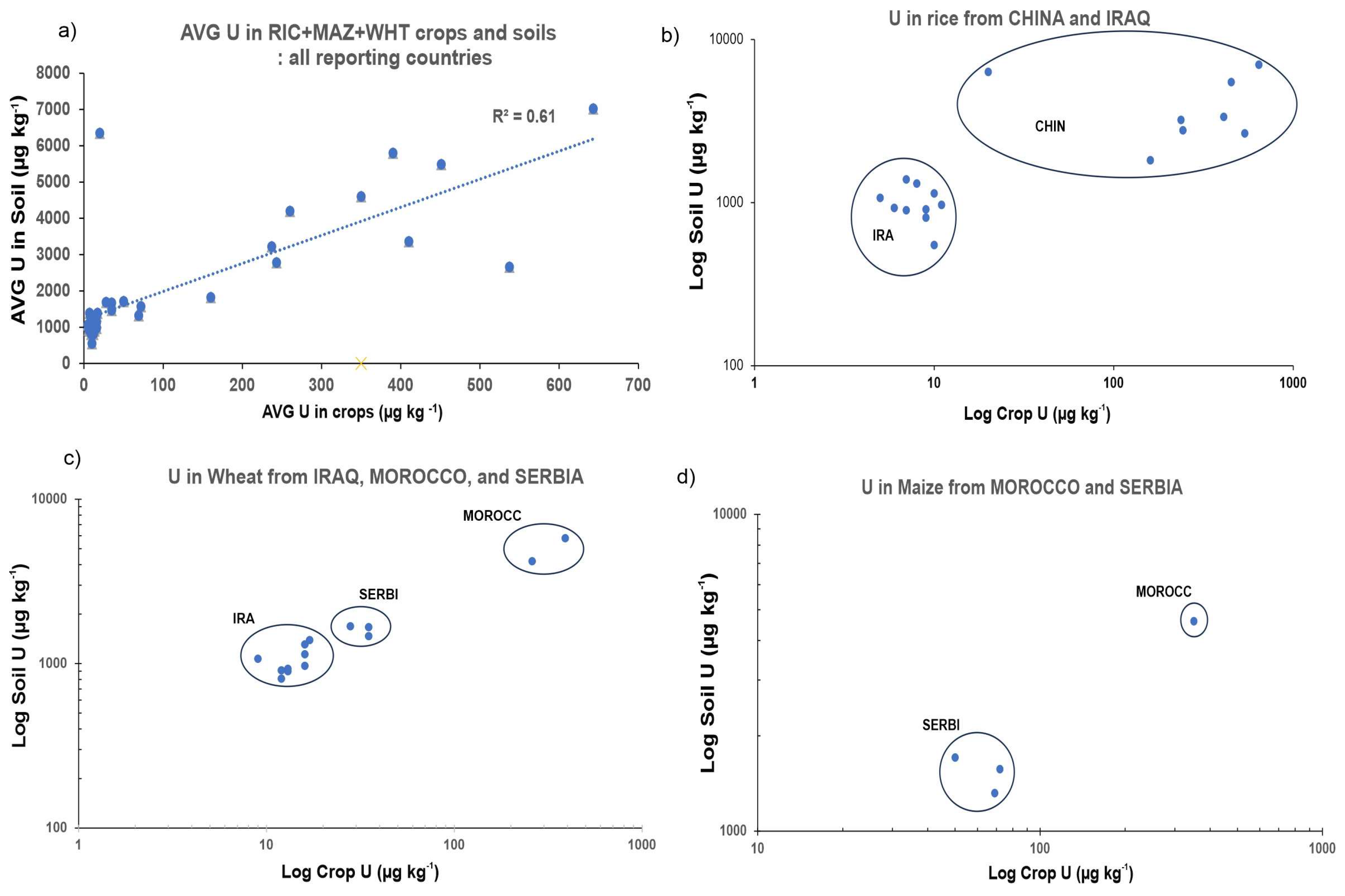
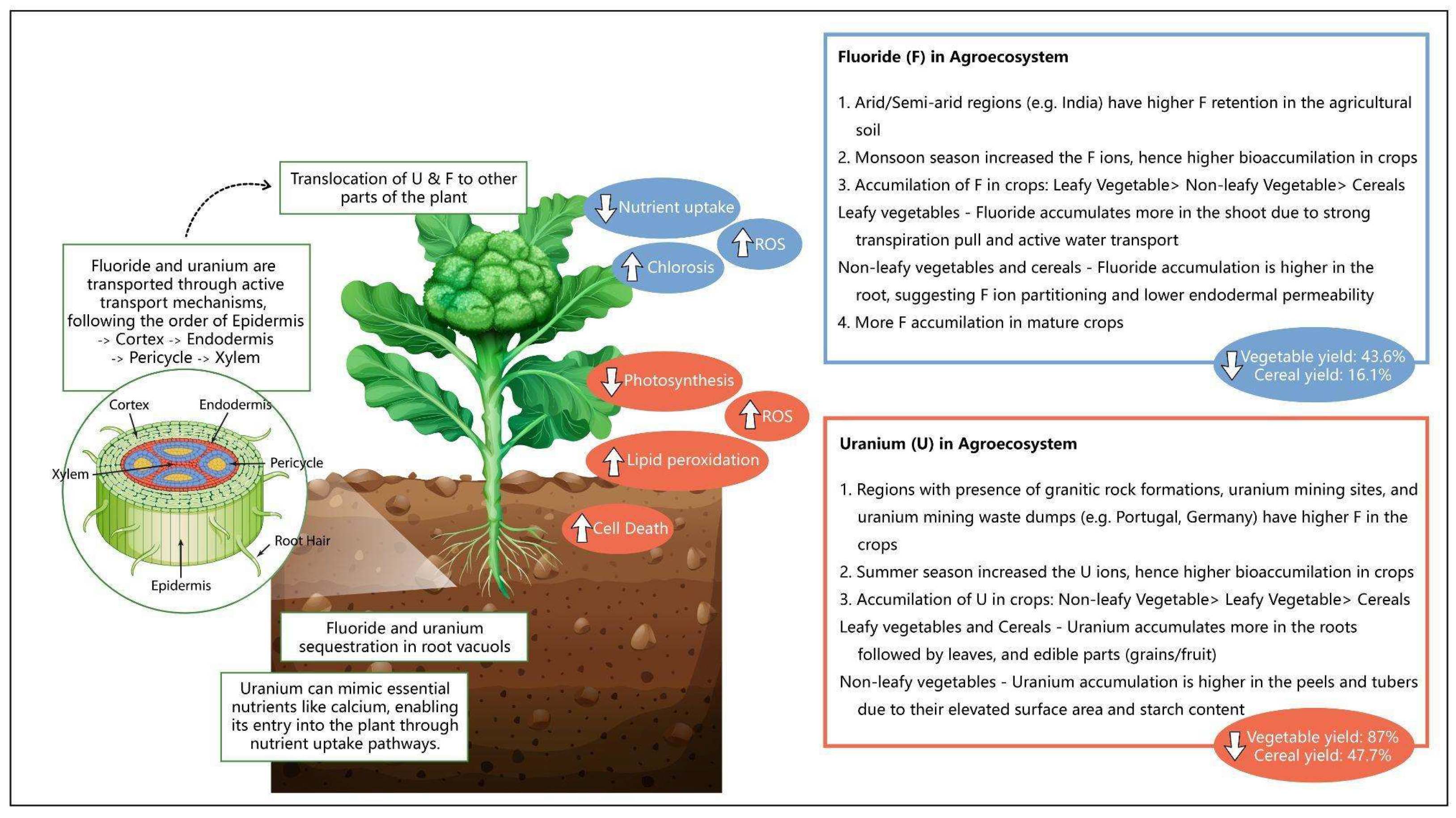
3.1. Occurrence of Uranium in Soil–Plant System
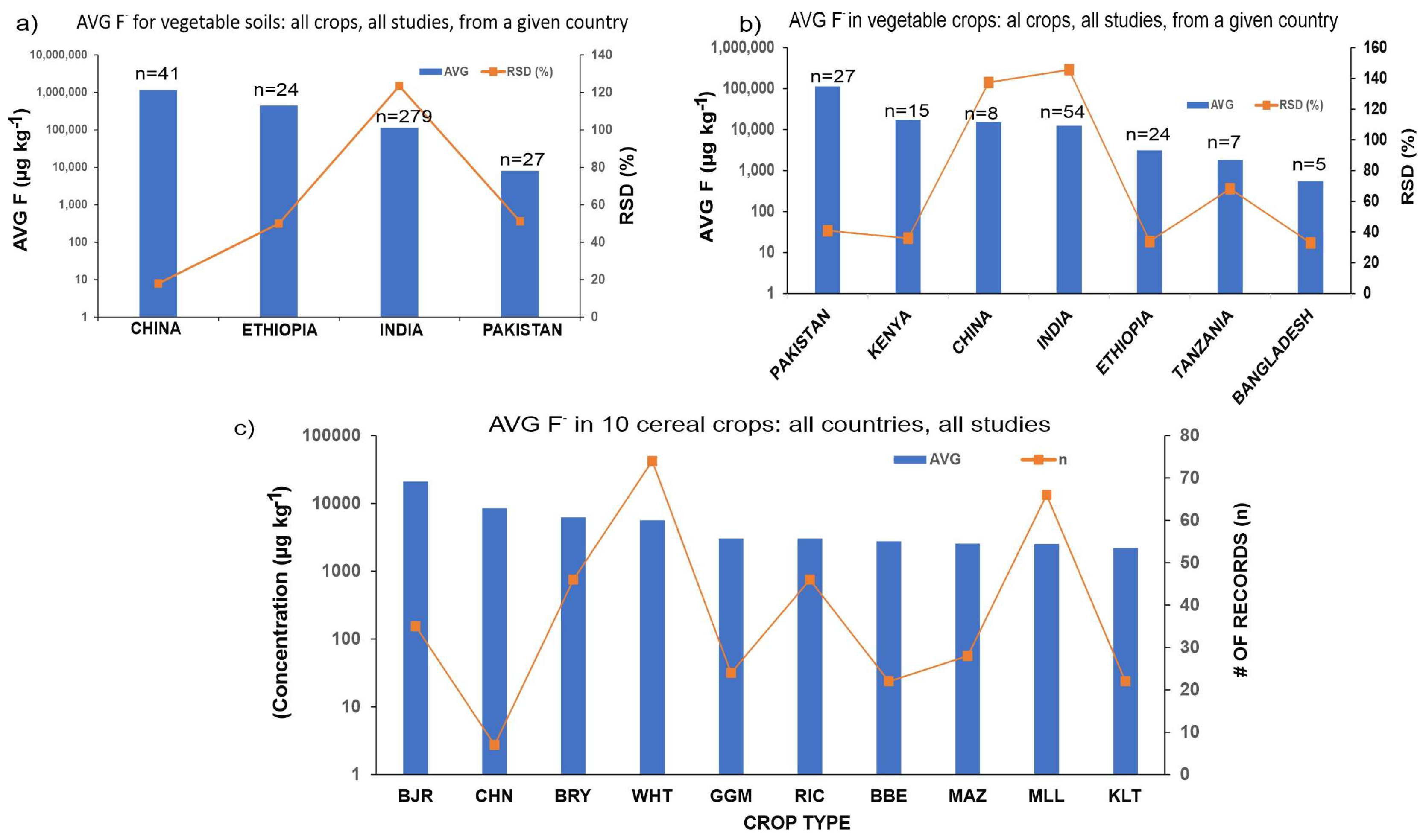
3.2. Occurrence of Fluoride in Soil–Plant System
3.3. Comparison between Vegetable and Grain Crops
4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viana, C.M.; Freire, D.; Abrantes, P.; Rocha, J.; Pereira, P. Agricultural Land Systems Importance for Supporting Food Security and Sustainable Development Goals: A Systematic Review. Sci. Total Environ. 2022, 806, 150718. [Google Scholar] [CrossRef] [PubMed]
- Bathaei, A.; Štreimikienė, D. A Systematic Review of Agricultural Sustainability Indicators. Agriculture 2023, 13, 241. [Google Scholar] [CrossRef]
- Lynch, J.; Cain, M.; Frame, D.; Pierrehumbert, R. Agriculture’s Contribution to Climate Change and Role in Mitigation Is Distinct From Predominantly Fossil CO2-Emitting Sectors. Front. Sustain. Food Syst. 2021, 4, 518039. [Google Scholar] [CrossRef]
- Piñeiro, V.; Arias, J.; Dürr, J.; Elverdin, P.; Ibáñez, A.M.; Kinengyere, A.; Opazo, C.M.; Owoo, N.; Page, J.R.; Prager, S.D.; et al. A Scoping Review on Incentives for Adoption of Sustainable Agricultural Practices and Their Outcomes. Nat. Sustain. 2020, 3, 809–820. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Shi, T.; Ma, J.; Wu, X.; Ju, T.; Lin, X.; Zhang, Y.; Li, X.; Gong, Y.; Hou, H.; Zhao, L.; et al. Inventories of Heavy Metal Inputs and Outputs to and from Agricultural Soils: A Review. Ecotoxicol. Environ. Saf. 2018, 164, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Jiang, L.; Zhang, W. A review on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques. Environ. Skept. Crit. 2014, 3, 24–38. [Google Scholar]
- Li, Y.; Wang, S.; Nan, Z.; Zang, F.; Sun, H.; Zhang, Q.; Huang, W.; Bao, L. Accumulation, Fractionation and Health Risk Assessment of Fluoride and Heavy Metals in Soil-Crop Systems in Northwest China. Sci. Total Environ. 2019, 663, 307–314. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy Metals in Food Crops: Health Risks, Fate, Mechanisms, and Management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 2014, 752708. [Google Scholar] [CrossRef]
- Faraji, M.; Alizadeh, I.; Oliveri Conti, G.; Mohammadi, A. Investigation of Health and Ecological Risk Attributed to the Soil Heavy Metals in Iran: Systematic Review and Meta-Analysis. Sci. Total Environ. 2023, 857, 158925. [Google Scholar] [CrossRef]
- Xu, P.; Liu, A.; Li, F.; Tinkov, A.A.; Liu, L.; Zhou, J.-C. Associations between Metabolic Syndrome and Four Heavy Metals: A Systematic Review and Meta-Analysis. Environ. Pollut. 2021, 273, 116480. [Google Scholar] [CrossRef]
- Ramteke, L.P.; Soni, R.S.; Rebary, B.; Patwardhan, A.W.; Sarode, D.D.; Ghosh, P.K. Analyses of uranium and fluoride in diammonium phosphate fertilizers marketed in India during 2021–2022. Int. J. Manures Fertil. 2022, 10, 1–10. [Google Scholar]
- Wang, Z.; Qin, H.; Liu, X. Health Risk Assessment of Heavy Metals in the Soil-Water-Rice System around the Xiazhuang Uranium Mine, China. Environ. Sci. Pollut. Res. 2019, 26, 5904–5912. [Google Scholar] [CrossRef]
- Shi, S.; Tang, X.; Yang, Y.; Liu, Z. Biological Effects of Uranium in Water, Soil and Rice in Uranium Deposits in Southern China. J. Radioanal. Nucl. Chem. 2021, 328, 507–517. [Google Scholar] [CrossRef]
- Ma, M.; Wang, R.; Xu, L.; Xu, M.; Liu, S. Emerging Health Risks and Underlying Toxicological Mechanisms of Uranium Contamination: Lessons from the Past Two Decades. Environ. Int. 2020, 145, 106107. [Google Scholar] [CrossRef] [PubMed]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.-H.; Epe, B.; Grune, T.; et al. Toxicity of Fluoride: Critical Evaluation of Evidence for Human Developmental Neurotoxicity in Epidemiological Studies, Animal Experiments and in Vitro Analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.R.; Strobel, S.A. Principles of Fluoride Toxicity and the Cellular Response: A Review. Arch. Toxicol. 2020, 94, 1051–1069. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.K.; Tripathi, P. Arsenic and Fluoride Contamination in Groundwater: A Review of Global Scenarios with Special Reference to India. Groundw. Sustain. Dev. 2021, 13, 100576. [Google Scholar] [CrossRef]
- Strunecka, A.; Strunecky, O. Mechanisms of Fluoride Toxicity: From Enzymes to Underlying Integrative Networks. Appl. Sci. 2020, 10, 7100. [Google Scholar] [CrossRef]
- Choubisa, S.L.; Choubisa, D.; Choubisa, A. Fluoride Contamination of Groundwater and Its Threat to Health of Villagers and Their Domestic Animals and Agriculture Crops in Rural Rajasthan, India. Environ. Geochem. Health 2022, 45, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Sahu, G.; Bag, A.G.; Plal, B.; Hazra, G.C. Role of fluoride on soil, plant and human health: A review on its sources, toxicity and mitigation strategies. Int. J. Environ. Clim. Chang. 2020, 10, 77–90. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, W.; Zhou, J.; Luo, D.; Li, Z. Uranium (U) Source, Speciation, Uptake, Toxicity and Bioremediation Strategies in Soil-Plant System: A Review. J. Hazard. Mater. 2021, 413, 125319. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Zhang, Z.; Beiyuan, J.; Cui, Y.; Chen, L.; Chen, H.; Fang, L. A Critical Review of Uranium in the Soil-Plant System: Distribution, Bioavailability, Toxicity, and Bioremediation Strategies. Crit. Rev. Environ. Sci. Technol. 2022, 53, 340–365. [Google Scholar] [CrossRef]
- Sarthou, M.C.M.; Devime, F.; Baggio, C.; Figuet, S.; Alban, C.; Bourguignon, J.; Ravanel, S. Calcium-Permeable Cation Channels Are Involved in Uranium Uptake in Arabidopsis Thaliana. J. Hazard. Mater. 2022, 424, 127436. [Google Scholar] [CrossRef]
- Croteau, M.-N.; Fuller, C.C.; Cain, D.J.; Campbell, K.M.; Aiken, G. Biogeochemical Controls of Uranium Bioavailability from the Dissolved Phase in Natural Freshwaters. Environ. Sci. Technol. 2016, 50, 8120–8127. [Google Scholar] [CrossRef]
- Nie, X.; Dong, F.; Liu, N.; Liu, M.; Zhang, D.; Kang, W.; Sun, S.; Zhang, W.; Yang, J. Subcellular Distribution of Uranium in the Roots of Spirodela Punctata and Surface Interactions. Appl. Surf. Sci. 2015, 347, 122–130. [Google Scholar] [CrossRef]
- Baker, M.R.; Coutelot, F.M.; Seaman, J.C. Phosphate Amendments for Chemical Immobilization of Uranium in Contaminated Soil. Environ. Int. 2019, 129, 565–572. [Google Scholar] [CrossRef]
- Huang, Z.; Tang, S.; Zhang, L.; Ma, L.; Ding, S.; Du, L.; Zhang, D.; Jin, Y.; Wang, R.; Huang, C.; et al. Interaction between U and Th on Their Uptake, Distribution, and Toxicity in V S. Alfredii Based on the Phytoremediation of U and Th. Environ. Sci. Pollut. Res. 2016, 24, 2996–3005. [Google Scholar] [CrossRef]
- Duhan, S.S.; Khyalia, P.; Solanki, P.; Laura, J.S. Uranium Sources, Uptake, Translocation in the Soil-Plant System and Its Toxicity in Plants and Humans: A Critical Review. Orient. J. Chem. 2023, 39, 303–319. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Xiao, P.; Ma, Q.; Li, Y.; Lai, J.; Luo, X.; Ji, X.; Xia, J.; Yang, X. A New Perspective on the Inhibition of Plant Photosynthesis by Uranium: Decrease of Root Activity and Stomatal Closure. Int. J. Phytoremed. 2021, 24, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, C.; Zhou, Y.; Li, S.; Hayat, T.; Alsaedi, A.; Wang, X. Effects of Uranium Stress on Physiological and Biochemical Characteristics in Seedlings of Six Common Edible Vegetables. J. Radioanal. Nucl. Chem. 2018, 316, 1001–1010. [Google Scholar] [CrossRef]
- Serre, N.B.C.; Alban, C.; Bourguignon, J.; Ravanel, S. Uncovering the Physiological and Cellular Effects of Uranium on the Root System of Arabidopsis Thaliana. Environ. Exp. Bot. 2019, 157, 121–130. [Google Scholar] [CrossRef]
- Parvaiz, A.; Khattak, J.A.; Hussain, I.; Masood, N.; Javed, T.; Farooqi, A. Salinity Enrichment, Sources and Its Contribution to Elevated Groundwater Arsenic and Fluoride Levels in Rachna Doab, Punjab Pakistan: Stable Isotope (δ2H and δ18O) Approach as an Evidence. Environ. Pollut. 2021, 268, 115710. [Google Scholar] [CrossRef] [PubMed]
- Wehr, J.B.; Dalzell, S.A.; Menzies, N.W. Predicting and Modelling Availability of Fluoride in Soil from Sorption Properties. Soil Use Manag. 2022, 39, 521–534. [Google Scholar] [CrossRef]
- Senkondo, Y. Immobilization of Fluoride in Soils through Soil Properties—A Review. J. Exp. Agric. Int. 2017, 19, 1–8. [Google Scholar] [CrossRef]
- Jha, S.K.; Nayak, A.K.; Sharma, Y.K. Site Specific Toxicological Risk from Fluoride Exposure through Ingestion of Vegetables and Cereal Crops in Unnao District, Uttar Pradesh, India. Ecotoxicol. Environ. Saf. 2011, 74, 940–946. [Google Scholar] [CrossRef]
- Battaleb-Looie, S.; Moore, F.; Malde, M.K.; Jacks, G. Fluoride in Groundwater, Dates and Wheat: Estimated Exposure Dose in the Population of Bushehr, Iran. J. Food Compos. Anal. 2013, 29, 94–99. [Google Scholar] [CrossRef]
- Hong, B.-D.; Chung, D.-Y.; Song, S.-G.; Min, S.; Rhie, J.-H.; Lee, D.-S.; Lee, K.-S.; Joo, R.-N. Fluoride in soil and plant. Korean J. Agric. Sci. 2016, 43, 522–536. [Google Scholar]
- Gadi, B.R.; Kumar, R.; Goswami, B.; Rankawat, R.; Rao, S.R. Recent Developments in Understanding Fluoride Accumulation, Toxicity, and Tolerance Mechanisms in Plants: An Overview. J. Soil Sci. Plant Nutr. 2020, 21, 209–228. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Samal, A.C.; Banerjee, S.; Pyne, J.; Santra, S.C. Assessment of Potential Health Risk of Fluoride Consumption through Rice, Pulses, and Vegetables in Addition to Consumption of Fluoride-Contaminated Drinking Water of West Bengal, India. Environ. Sci. Pollut. Res. 2017, 24, 20300–20314. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kaur, R. Insights into fluoride-induced oxidative stress and antioxidant defences in plants. Acta Physiol. Plant. 2018, 40, 181. [Google Scholar] [CrossRef]
- Staven, L.H.; Napier, B.A.; Rhoads, K.; Strenge, D.L. A Compendium of Transfer Factors for Agricultural and Animal Products; U.S. Department of Energy: Washington, DC, USA, 2003.
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; pp. 303–304. [Google Scholar]
- WHO. World Health Statistics 2017: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Chowdhury, A.; Adak, M.K.; Mukherjee, A.; Dhak, P.; Khatun, J.; Dhak, D. A Critical Review on Geochemical and Geological Aspects of Fluoride Belts, Fluorosis and Natural Materials and Other Sources for Alternatives to Fluoride Exposure. J. Hydrol. 2019, 574, 333–359. [Google Scholar] [CrossRef]
- Ijumulana, J.; Ligate, F.; Irunde, R.; Bhattacharya, P.; Ahmad, A.; Tomašek, I.; Maity, J.P.; Mtalo, F. Spatial variability of the sources and distribution of fluoride in groundwater of the Sanya alluvial plain aquifers in northern Tanzania. Sci. Total. Environ. 2022, 810, 152153. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Sinam, G.; Kumari, B.; Gautam, A.; Patnaik, S.; Mallick, S. Spatio-Temporal Variation of Fluoride in Groundwater and Agricultural Soil and Crops of Unnao District, UP: Monitoring and Assessment. Environ. Res. 2022, 210, 112927. [Google Scholar] [CrossRef]
- Shahandeh, H.; Hossner, L.R. Role of soil properties in phytoaccumulation of uranium. Water Air Soil Pollut. 2002, 141, 165–180. [Google Scholar] [CrossRef]
- Abreu, M.M.; Neves, O.; Marcelino, M. Yield and uranium concentration in two lettuce (Lactuca sativa L.) varieties influenced by soil and irrigation water composition, and season growth. J. Geochem. Explor. 2014, 142, 43–48. [Google Scholar] [CrossRef]
- Van Netten, C.; Morley, D.R. Uptake of Uranium, Molybdenum, Copper, and Selenium by the Radish from Uranium-Rich Soils. Arch. Environ. Health Int. J. 1983, 38, 172–175. [Google Scholar] [CrossRef]
- Tracy, B.L.; Prantl, F.A.; Quinn, J.M. Transfer of 226Ra, 210Pb and Uranium from Soil to Garden Produce. Health Phys. 1983, 44, 469–477. [Google Scholar] [CrossRef]
- Lakshmanan, A.R.; Venkateswarlu, K.S. Uptake of Uranium by Vegetables and Rice. Water Air Soil Pollut. 1988, 38, 151–155. [Google Scholar] [CrossRef]
- Chen, S.B.; Zhu, Y.G.; Hu, Q.H. Soil to plant transfer of 238U, 226RA and 232th on a uranium mining-impacted soil from southeastern China. J. Environ. Radioact. 2005, 82, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Muñoz, J.G.; Guillén, J.; Salas, A. Comparison of soil to plant transfer of uranium, radium and 210PO to wheat using three cultivation methods: Hydroponics, plantlet and pot cultures. J. Radioanal. Nucl. Chem. 2021, 328, 359–367. [Google Scholar] [CrossRef]
- Al-Hamzawi, A.A.; Kareem, N.A. Experimental investigation of uranium concentration, radium content and radon exhalation rates in food crops consumed in Babil Governorate, Iraq. Int. J. Radiat. Res. 2022, 20, 205–210. [Google Scholar] [CrossRef]
- Lazarova, R.; Yordanova, I.; Staneva, D. Plant Uptake of Radioactive Elements from Soils Contaminated by Uranium Mining Industry in Buhovo, Bulgaria. Bulg. J. Soil Sci. 2020, 5, 110–116. [Google Scholar]
- Al-Hamarneh, I.F.; Alkhomashi, N.; Almasoud, F.I. Study on the radioactivity and soil-to-plant transfer factor of 226RA, 234U and 238U radionuclides in irrigated farms from the northwestern Saudi Arabia. J. Environ. Radioact. 2016, 160, 1–7. [Google Scholar] [CrossRef]
- Pietrzak-Flis, Z.; Rosiak, L.; Suplinska, M.M.; Chrzanowski, E.; Dembinska, S. Daily intakes of 238U, 234U, 232th, 230th, 228th and 226Ra in the adult population of Central Poland. Sci. Total Environ. 2001, 273, 163–169. [Google Scholar] [CrossRef]
- Aswood, M.S.; Jaafar, M.S.; Bauk, S. Assessment of radionuclide transfer from soil to vegetables in farms from Cameron Highlands and Penang, (Malaysia) using Neutron Activation Analysis. Appl. Phys. Res. 2013, 5, 85–92. [Google Scholar] [CrossRef]
- Lai, J.; Liu, Z.; Li, C.; Luo, X. Analysis of accumulation and phytotoxicity mechanism of uranium and cadmium in two sweet potato cultivars. J. Hazard. Mater. 2021, 409, 124997. [Google Scholar] [CrossRef]
- Basha, A.M.; Yasovardhan, N.; Satyanarayana, S.V.; Reddy, G.V.; Kumar, A.V. Trace metals in vegetables and fruits cultivated around the surroundings of Tummalapalle uranium mining site, Andhra Pradesh, India. Toxicol. Rep. 2014, 1, 505–512. [Google Scholar] [CrossRef]
- Carvalho, F.P.; Oliveira, J.M.; Malta, M. Preliminary assessment of uranium mining legacy and environmental radioactivity levels in Sabugal region, Portugal. Int. J. Energy Environ. Eng. 2016, 7, 399–408. [Google Scholar] [CrossRef]
- Anke, M.; Seeber, O.; Müller, R.; Schäfer, U.; Zerull, J. Uranium transfer in the food chain from soil to plants, animals and man. Geochemistry 2009, 69, 75–90. [Google Scholar] [CrossRef]
- Esposito, M.; De Roma, A.; Cavallo, S.; Miedico, O.; Chiaravalle, E.; Soprano, V.; Baldi, L.; Gallo, P. Trace elements in vegetables and fruits cultivated in Southern Italy. J. Food Compos. Anal. 2019, 84, 103302. [Google Scholar] [CrossRef]
- Misdaq, M.A.; Bourzik, W. Determination of committed effective doses from annual intakes of 238U and 232Th from the ingestion of cereals, fruits and vegetables by using CR-39 and LR-115 II SSNTD. J. Radioanal. Nucl. Chem. 2002, 254, 551–555. [Google Scholar] [CrossRef]
- Jing, Z.; Diyun, C.; Juan, L. The Uranium Availability and Uptake in the Vegetables. In Proceedings of the 2012 International Conference on Biomedical Engineering and Biotechnology, Macau, Macao, 28–30 May 2012. [Google Scholar] [CrossRef]
- Lauria, D.C.; Ribeiro, F.C.A.; Silva, R. Phosphate Fertilizer Influence on 238U Content in Vegetables. In Proceedings of the 1st American Congress of the IRPA Mex, Acapulco, Mexico, 3–8 September 2006. [Google Scholar]
- Stojanović, M.; Mihajlović, M.; Lopičić, Z.; Milojković, J.; Šoštarić, T.; Petrović, M. The Influence of Soil Type on Maize and Wheat Uranium Uptake. Qual. Assur. Saf. Crops Foods 2013, 5, 237–242. [Google Scholar] [CrossRef]
- Choudhury, S.; Goswami, T.D. Estimation of uranium contents in different parts of the plants and soils. J. Phys. 1990, 64A, 399–404. [Google Scholar]
- Pearson, A.J.; Gaw, S.; Hermanspahn, N.; Glover, C.N. Natural and anthropogenic radionuclide activity concentrations in the new zealand diet. J. Environ. Radioact. 2016, 151, 601–608. [Google Scholar] [CrossRef]
- Neves, O.; Abreu, M.M.; Vicente, E.M. Uptake of Uranium by Lettuce (Lactuca sativa L.) in Natural Uranium Contaminated Soils in Order to Assess Chemical Risk for Consumers. Water Air Soil Pollut. 2008, 195, 73–84. [Google Scholar] [CrossRef]
- Neves, M.O.; Figueiredo, V.R.; Abreu, M.M. Transfer of U, Al and Mn in the Water–Soil–Plant (Solanum tuberosum L.) System near a Former Uranium Mining Area (Cunha Baixa, Portugal) and Implications to Human Health. Sci. Total Environ. 2012, 416, 156–163. [Google Scholar] [CrossRef]
- Amin, S.; Ayoub, A.; Jassim, A. Radioactivity Levels in Some Vegetables and Herbs. Eng. Technol. J. 2018, 36, 174–178. [Google Scholar]
- Gulati, K.L.; Oswal, M.C.; Nagpaul, K.K. Assimilation of uranium by wheat and Tomato Plants. Plant Soil 1980, 55, 55–59. [Google Scholar] [CrossRef]
- Saric, M.R.; Stojanovic, M.; Babic, M. Uranium in plant species grown on natural barren soil. J. Plant Nutr. 1995, 18, 1509–1518. [Google Scholar] [CrossRef]
- Neves, O.; Abreu, M.M. Are Uranium-Contaminated Soil and Irrigation Water a Risk for Human Vegetables Consumers? A Study Case with Solanum tuberosum L., Phaseolus vulgaris L. and Lactuca sativa L. Ecotoxicology 2009, 18, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G.; Voigt, K.-D. Gradual Accumulation of Heavy Metals in an Industrial Wheat Crop from Uranium Mine Soil and the Potential Use of the Herbage. Agriculture 2016, 6, 51. [Google Scholar] [CrossRef]
- Rejah, B.K.; Aljanabi, A.T.; Fzaa, W.T.; Hussein, A.A.; Ali, A.H. Calculation of Concentrations and Transfer Factors of Uranium from Soil to Plants Using Nuclear Track Detector CR-39. J. Phys. Conf. Ser. 2019, 1178, 012012. [Google Scholar] [CrossRef]
- Saroja, R.R.M.; Sreedevi, A. Thorium and uranium in vegetables and fruits from a high background radiation region–along the south west coast of India. World J. Pharm. Sci. 2017, 5, 1–80. [Google Scholar]
- Thien, B.N.; Ba, V.N.; Vy, N.T.; Loan, T.T. Estimation of the soil to plant transfer factor and the annual organ equivalent dose due to ingestion of food crops in Ho Chi Minh City, Vietnam. Chemosphere 2020, 259, 127432. [Google Scholar] [CrossRef] [PubMed]
- Shtangeeva, I. Uptake of uranium and thorium by native and cultivated plants. J. Environ. Radioact. 2010, 101, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Aydın, M.F.; Söğüt, Ö.; Kara, A. Radiological health risks assessment of vegetable and fruit samples taken from the provincial borders of Adıyaman in the south-eastern anatolia region, in Turkey. J. Radiat. Res. Appl. Sci. 2022, 15, 100491. [Google Scholar] [CrossRef]
- Carvalho, F.P.; Oliveira, J.M.; Neves, M.O.; Abreu, M.M.; Vicente, E.M. Soil to plant (Solanum tuberosum L.) radionuclide transfer in the vicinity of an old uranium mine. Geochem. Explor. Environ. Anal. 2009, 9, 275–278. [Google Scholar] [CrossRef]
- Samuel-Nakamura, C.; Hodge, F.S.; Sokolow, S.; Ali, A.-M.S.; Robbins, W.A. Metal(Loid)s in Cucurbita Pepo in a Uranium Mining Impacted Area in Northwestern New Mexico, USA. Int. J. Environ. Res. Public Health 2019, 16, 2569. [Google Scholar] [CrossRef]
- Lai, J.; Deng, Z.; Ji, X.; Luo, X. Absorption and Interaction Mechanisms of Uranium & Cadmium in Purple Sweet Potato(Ipomoea batatas L.). J. Hazard. Mater. 2020, 400, 123264. [Google Scholar] [CrossRef] [PubMed]
- Shanthi, G.; Maniyan, C.G.; Raj, G.A.G.; Kumaran, J.T.T. Radioactivity in food crops from high-background radiation area in southwest India. Curr. Sci. 2009, 97, 1331–1335. [Google Scholar]
- Kadhim, A.Y.; Al-Ataya, K.H.; Aswood, M.S. Distribution and Uptake of Uranium in Rice and Wheat from Soil Samples Collected from Al- Diwaniyah, Iraq. J. Phys. Conf. Ser. 2021, 1897, 012065. [Google Scholar] [CrossRef]
- Neves, M.O.; Abreu, M.M.; Figueiredo, V. Uranium in Vegetable Foodstuffs: Should Residents near the Cunha Baixa Uranium Mine Site (Central Northern Portugal) Be Concerned? Environ. Geochem. Health 2011, 34, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Liu, Z.; Ye, T.; Zhang, L. Uranium Pollution Status and Speciation Analysis in the Farmland-Rice System around a Uranium Tailings Mine in Southeastern China. J. Radioanal. Nucl. Chem. 2019, 322, 1011–1022. [Google Scholar] [CrossRef]
- Ouyang, J.; Liu, Z.; Zhang, L.; Wang, Y.; Zhou, L. Analysis of Influencing Factors of Heavy Metals Pollution in Farmland-Rice System around a Uranium Tailings Dam. Process Saf. Environ. Prot. 2020, 139, 124–132. [Google Scholar] [CrossRef]
- Hakonson-Hayes, A.C.; Fresquez, P.R.; Whicker, F.W. Assessing potential risks from exposure to natural uranium in well water. J. Environ. Radioact. 2002, 59, 29–40. [Google Scholar] [CrossRef]
- Giri, S.; Singh, G.; Jha, V.N.; Tripathi, R.M. Ingestion of u(nat),226ra,230th and210po in vegetables by adult inhabitants of Bagjata uranium mining area, Jharkhand, India. Radioprotection 2010, 45, 183–199. [Google Scholar] [CrossRef][Green Version]
- Hashim, A.K.; Najam, L.A. Radium and uranium concentrations measurements in vegetables samples of Iraq. Detection 2015, 3, 21–28. [Google Scholar] [CrossRef]
- WHO. Uranium in Drinking Water; Background Document for Development of WHO, World Health Organization, Guidelines for Drinking Water Quality (WHO/SDE/03.04/118); World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Bellés, M.; Linares, V.; Perelló, G.; Domingo, J.L. Human dietary exposure to uranium in Catalonia, Spain. Biol. Trace Elem. Res. 2013, 152, 1–8. [Google Scholar] [CrossRef]
- Haikel, Y.; Voegel, J.C.; Frank, R.M. Fluoride Content of Water, Dust, Soils and Cereals in the Endemic Dental Fluorosis Area of Khouribga (Morocco). Arch. Oral Biol. 1986, 31, 279–286. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.K. Fluoride Exposure and Its Potential Health Risk Assessment through Ingestion of Food in the Mica Mining Areas of Jharkhand, India. Hum. Ecol. Risk Assess. Int. J. 2022, 28, 507–520. [Google Scholar] [CrossRef]
- Gevera, P.K.; Cave, M.; Dowling, K.; Gikuma-Njuru, P.; Mouri, H. Potential Fluoride Exposure from Selected Food Crops Grown in High Fluoride Soils in the Makueni County, South-Eastern Kenya. Environ. Geochem. Health 2022, 44, 4703–4717. [Google Scholar] [CrossRef]
- Mishra, P.C.; Sahu, S.K.; Bhoi, A.K.; Mohapatra, S.C. Fluoride Uptake and Net Primary Productivity of Selected Crops. Open J. Soil Sci. 2014, 04, 388–398. [Google Scholar] [CrossRef]
- Jha, S.K.; Nayak, A.K.; Sharma, Y.K.; Mishra, V.K.; Sharma, D.K. Fluoride Accumulation in Soil and Vegetation in the Vicinity of Brick Fields. Bull. Environ. Contam. Toxicol. 2008, 80, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, L.; Chen, D.; Hamid, Y.; Shan, A.; Chen, Z.; Yu, S.; Feng, Y.; Yang, X. Fluorine in 20 Vegetable Species and 25 Lettuce Cultivars Grown on a Contaminated Field Adjacent to a Brick Kiln. Environ. Geochem. Health 2022, 45, 1655–1667. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, W.; Zeng, Y.; Liao, Y.; Wang, L.; Lu, G. Industrial Fluoride Pollution of Vegetables in Hubei Province, China. Fluoride 2006, 39, 31–34. [Google Scholar]
- Owuor, P.O. Fluoride Content of Common Vegetables from Different Parts of Kenya. Food Chem. 1985, 18, 283–289. [Google Scholar] [CrossRef]
- Jha, S.K.; Damodaran, T.; Verma, C.L.; Mishra, V.K.; Sharma, D.K.; Sah, V.; Rai, R.B.; Dhama, K. Fluoride Partitioning in Rice (Oryza Sativa) and Wheat (Triticum Aestivum) upon Irrigation with Fluorideâ€contaminated Water and Its Risk Assessment. South Asian J. Exp. Biol. 2013, 3, 137–144. [Google Scholar] [CrossRef]
- Devi, G.; Kushwaha, A.; Goswami, L.; Chakrabarty, S.; Kaur, H.; Sathe, S.S.; Bahukhandi, K.; Bhan, U.; Sarma, H.P. Toxicity Assessment of Fluoride-Contaminated Soil and Wastewater in Solanum Tuberosum. Water Air Soil Pollut. 2022, 233, 232. [Google Scholar] [CrossRef]
- Saini, P.; Khan, S.; Baunthiyal, M.; Sharma, V. Mapping of Fluoride Endemic Area and Assessment of F−1 Accumulation in Soil and Vegetation. Environ. Monit. Assess. 2013, 185, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Parikh, R. In-Vivo Uptake of Fluoride by Pearl Millet [Pennisetum Typhoideum Rich.] Irrigated with Fluorinated Groundwater. Int. J. Allied Pract. Res. Rev. 2015, II, 50–54. [Google Scholar]
- Gautam, R.; Bharadwaj, N.; Saini, Y. Fluoride Accumulation by Vegetables and Crops Grown in Nawa Tehsil of Nagaur District (Rajasthan, India). J. Phytol. 2010, 2, 80–85. [Google Scholar]
- Arora, G.; Bhateja, S. Estimating the Fluoride Concentration in Soil and Crops Grown over It in and around Mathura, Uttar Pradesh, India. Am. J. Ethnomed. 2014, 1, 36–41. [Google Scholar]
- Pal, K.C.; Mondal, N.K.; Bhaumik, R.; Banerjee, A.; Datta, J.K. Incorporation of fluoride in vegetation and associated biochemical changes due to fluoride contamination in water and soil: A comparative field study. Ann. Environ. Sci. 2012, 6, 123–139. [Google Scholar]
- Okibe, F.G.; Ekanem, E.J.; Paul, E.D.; Shallangwa, G.A.; Ekwumemgbo, P.A.; Sallau, M.S.; Abanka, O.C. Fluoride Content of Soil and Vegetables from Irrigation Farms on the Bank of River Galma, Zaria, Nigeria. Aust. J. Basic Appl. Sci. 2010, 4, 779–784. [Google Scholar]
- Pandey, J.; Pandey, U. Fluoride contamination and fluorosis in rural community in the vicinity of a phosphate fertilizer factory in India. Bull. Environ. Contam. Toxicol. 2011, 87, 245–249. [Google Scholar] [CrossRef]
- Mustofa, S.; Chandravanshi, B.S.; Zewge, F. Levels of fluoride in staple cereals and legumes produced in selected areas of Ethiopia. SINET Ethiop. J. Sci. 2014, 37, 43–52. [Google Scholar]
- Dagnaw, L.A.; Chandravanshi, B.S.; Zewge, F. Fluoride content of leafy vegetables, irrigation water, and farmland soil in the rift valley and in non-rift valley areas of ethiopia. Int. Soc. Fluoride Res. 2017, 50, 409–429. [Google Scholar]
- Wang, M.; Zhang, L.; Liu, Y.; Chen, D.; Liu, L.; Li, C.; Kang, K.J.; Wang, L.; He, Z.; Yang, X. Spatial Variation and Fractionation of Fluoride in Tobacco-Planted Soils and Leaf Fluoride Concentration in Tobacco in Bijie City, Southwest China. Environ. Sci. Pollut. Res. 2021, 28, 26112–26123. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Prete, D.; Xue, S.; Nan, Z.; Zang, F.; Zhang, Q. Accumulation and Interaction of Fluoride and Cadmium in the Soil-Wheat Plant System from the Wastewater Irrigated Soil of an Oasis Region in Northwest China. Sci. Total Environ. 2017, 595, 344–351. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Tu, C.; He, S.; Long, J.; Sun, Y.; Sun, Y.; Lin, C. Fluorine Enrichment of Vegetables and Soil around an Abandoned Aluminium Plant and Its Risk to Human Health. Environ. Geochem. Health 2020, 43, 1137–1154. [Google Scholar] [CrossRef] [PubMed]
- Havale, R.; Rao, D.; Shrutha, S.; Taj, K.; Raj, S.; Tharay, N.; Tuppadmath, K.; Mathew, I. Estimation of Fluoride Uptake in Soil and Staple Food Crops Produced in Highly Fluoridated and Non-Fluoridated Regions of Raichur District, Karnataka. J. Fam. Med. Prim. Care 2022, 11, 3546. [Google Scholar] [CrossRef]
- Khandare, A.L.; Rao, G.S. Uptake of Fluoride, Aluminum and Molybdenum by Some Vegetables from Irrigation Water. J. Hum. Ecol. 2006, 19, 283–288. [Google Scholar] [CrossRef]
- Lakshmi, D.V.; Rao, K.J.; Ramprakash, T.; Reddy, A.P.K. Bioaccumulation of Fluoride in Different Plant Parts of Food Crops Grown in Narkatpally Mandal of Nalgonda District, Telangana. Environ. Ecol. 2017, 35, 1753–1758. [Google Scholar]
- Mondal, D.; Gupta, S. Influence of fluoride contaminated irrigation water on biochemical constituents of different crops and vegetables with an implication to human risk through diet. J. Mater. Environ. Sci. 2015, 6, 3134–3142. [Google Scholar]
- Gupta, S.; Banerjee, S.; Burdwan, I. Fluoride accumulation in crops and vegetables and dietary intake in a fluoride-endemic area of West Bengal. Fluoride 2011, 44, 153–157. [Google Scholar]
- Devi, G.; Sarma, H.P. Fluoride Incorporation on Selected Vegetables in Cultivated Areas of Kamrup District of Assam. IOSR J. Environ. Sci. Toxicol. Food Technol. (IOSR-JESTFT) 2020, 14, 12–17. [Google Scholar]
- De, A.; Mridha, D.; Ray, I.; Joardar, M.; Das, A.; Chowdhury, N.R.; Roychowdhury, T. Fluoride Exposure and Probabilistic Health Risk Assessment through Different Agricultural Food Crops From Fluoride Endemic Bankura and Purulia Districts of West Bengal, India. Front. Environ. Sci. 2021, 9, 713148. [Google Scholar] [CrossRef]
- Kazi, T.G.; Brahman, K.D.; Baig, J.A.; Afridi, H.I. Bioaccumulation of Arsenic and Fluoride in Vegetables from Growing Media: Health Risk Assessment among Different Age Groups. Environ. Geochem. Health 2018, 41, 1223–1234. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A. Health Risk from Fluoride Exposure of a Population in Selected Areas of Tamil Nadu South India. Food Sci. Hum. Wellness 2013, 2, 75–86. [Google Scholar] [CrossRef]
- Ranjan, S.; Yasmin, S. Assessment of Fluoride Intake through Food Chain and Mapping of Endemic Areas of Gaya District, Bihar, India. Bull. Environ. Contam. Toxicol. 2014, 94, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liao, S.; Zhao, Y.; Li, X.; Wang, Z.; Liao, C.; Sun, D.; Zhang, Q.; Lu, Q. Soil Exposure Is the Major Fluoride Exposure Pathways for Residents from the High-Fluoride Karst Region in Southwest China. Chemosphere 2023, 310, 136831. [Google Scholar] [CrossRef] [PubMed]
- Mridha, D.; Priyadarshni, P.; Bhaskar, K.; Gaurav, A.; De, A.; Das, A.; Joardar, M.; Chowdhury, N.R.; Roychowdhury, T. Fluoride Exposure and Its Potential Health Risk Assessment in Drinking Water and Staple Food in the Population from Fluoride Endemic Regions of Bihar, India. Groundw. Sustain. Dev. 2021, 13, 100558. [Google Scholar] [CrossRef]
- Kerketta, A.; Kumar, H.; Powell, M.A.; Sahoo, P.K.; Kapoor, H.S.; Mittal, S. Trace element occurrence in vegetable and cereal crops from parts of Asia: A meta-data analysis of crop-wise differences. Curr. Pollut. Rep. 2023, 9, 1–21. [Google Scholar] [CrossRef]
- Atamaleki, A.; Yazdanbakhsh, A.; Fakhri, Y.; Salem, A.; Ghorbanian, M.; Mousavi Khaneghah, A. A systematic review and meta-analysis to investigate the correlation vegetable irrigation with wastewater and concentration of potentially toxic elements (PTES): A case study of spinach (Spinacia oleracea) and radish (Raphanus raphanistrum subsp. sativus). Biol. Trace Elem. Res. 2020, 199, 792–799. [Google Scholar] [CrossRef]
- Malde, M.K.; Maage, A.; Macha, E.; Julshamn, K.; Bjorvatn, K. Fluoride Content in Selected Food Items from Five Areas in East Africa. J. Food Compos. Anal. 1997, 10, 233–245. [Google Scholar] [CrossRef]
- Bhargava, D.; Bharadwaj, N. Study of Fluoride Contribution Through Water and Food to Human Population in Fluorosis Endemic Villages of North-Eastern Rajasthan. Afr. J. Basic Appl. Sci. 2009, 1, 55–58. [Google Scholar]
- Jha, S.K.; Nayak, A.K.; Sharma, Y.K. Fluoride Toxicity Effects in Onion (Allium cepa L.) Grown in Contaminated Soils. Chemosphere 2009, 76, 353–356. [Google Scholar] [CrossRef]
| Species | Total Count | Min. | Max. | Mean | Median |
|---|---|---|---|---|---|
| Leafy Vegetables | |||||
| CBB | 8 | 0.0008 | 17.28 | 2.24 | 0.06 |
| SPN | 3 | 0.13 | 0.497 | 0.28 | 0.20 |
| LTT | 20 | 0.008 | 5.37 | 0.74 | 0.22 |
| CLF | 3 | 0.003 | 12.46 | 4.26 | 0.32 |
| SWC | 1 | 12.22 | 12.22 | 12.22 | 12.22 |
| MGR | 1 | 17.28 | 17.28 | 17.28 | 17.28 |
| Tuber/Root Vegetables | |||||
| PTT | 20 | 0.0006 | 1.35 | 0.16 | 0.04 |
| CRR | 13 | 0.0008 | 0.67 | 0.19 | 0.06 |
| ONN | 6 | 0.11 | 0.279 | 0.19 | 0.19 |
| BET | 3 | 0.192 | 0.37 | 0.30 | 0.33 |
| TNP | 4 | 0.0038 | 17.95 | 4.81 | 0.65 |
| SPT | 1 | 0.76 | 0.76 | 0.76 | 0.76 |
| RDH | 14 | 0.01324 | 14 | 3.40 | 1.15 |
| Stem Vegetables | |||||
| GBT | 3 | 0.0044 | 0.49 | 0.17 | 0.004 |
| PMK | 1 | 0.006 | 0.006 | 0.01 | 0.01 |
| CLL | 2 | 0.0044 | 0.008 | 0.01 | 0.01 |
| GBL | 2 | 0.006 | 0.00713 | 0.01 | 0.01 |
| BRN | 4 | 0.0005 | 0.46 | 0.12 | 0.01 |
| OKR | 3 | 0.01723 | 0.536 | 0.19 | 0.02 |
| PEA | 4 | 0.0005 | 0.26 | 0.07 | 0.01 |
| TMT | 14 | 0.0012 | 0.34 | 0.09 | 0.03 |
| BNS | 10 | 0.0081 | 0.645 | 0.19 | 0.03 |
| BPP | 1 | 0.16 | 0.16 | 0.16 | 0.16 |
| CCM | 3 | 0.0085 | 0.56 | 0.33 | 0.42 |
| Cereals | |||||
| RIC | 22 | 0.003 | 0.643 | 0.14 | 0.01 |
| WHT | 23 | 0.0012 | 3.01 | 0.20 | 0.03 |
| MAZ | 9 | 0.0043 | 9.69 | 1.21 | 0.11 |
| GGR | 1 | 0.45 | 0.45 | 0.45 | 0.45 |
| BRY | 2 | 0.3 | 5.48 | 2.89 | 2.89 |
| Species | Total Count | Min. | Max. | Mean | Median |
|---|---|---|---|---|---|
| Leafy Vegetables | |||||
| LTT | 9 | 0.013 | 11.2 | 1.86 | 0.110 |
| CBB | 1 | 1.07 | 1.07 | 1.07 | 1.070 |
| SCH | 1 | 1.63 | 1.63 | 1.63 | 1.630 |
| CLR | 1 | 5.55 | 5.55 | 5.55 | 5.550 |
| SRR | 1 | 6.290 | 6.290 | 6.290 | 6.290 |
| INS | 1 | 11.9 | 11.9 | 11.90 | 11.900 |
| SWC | 1 | 13.540 | 13.540 | 13.540 | 13.540 |
| SPN | 1 | 13.600 | 13.600 | 13.600 | 13.600 |
| MGR | 2 | 21 | 27 | 24.00 | 24.000 |
| Root/Stem Vegetables | |||||
| PMP | 3 | 0.009 | 1.9 | 0.64 | 0.017 |
| OCH | 1 | 0.025 | 0.025 | 0.03 | 0.025 |
| BRN | 3 | 0.028 | 0.032 | 0.03 | 0.029 |
| TMT | 15 | 0.003 | 1.3 | 0.34 | 0.034 |
| GBT | 1 | 0.051 | 0.051 | 0.05 | 0.051 |
| CRR | 3 | 0.05 | 1.26 | 0.49 | 0.154 |
| OKR | 6 | 0.023 | 2.5 | 0.81 | 0.250 |
| INN | 1 | 0.43 | 0.43 | 0.43 | 0.430 |
| DST | 2 | 0.15 | 0.81 | 0.48 | 0.480 |
| CMM | 7 | 0.031 | 6.25 | 1.42 | 0.680 |
| ONN | 5 | 0.012 | 3.12 | 1.07 | 0.900 |
| CLF | 1 | 1.96 | 1.96 | 1.96 | 1.960 |
| BPP | 1 | 2.16 | 2.16 | 2.16 | 2.160 |
| PTT | 3 | 0.005 | 4.72 | 2.32 | 2.240 |
| BNS | 2 | 0.012 | 5.2 | 2.61 | 2.606 |
| BET | 1 | 2.98 | 2.98 | 2.98 | 2.980 |
| Cereals | |||||
| WHT | 2 | 2.49 | 2.76 | 2.63 | 2.625 |
| BRY | 3 | 3.27 | 4.97 | 4.40 | 4.960 |
| MAZ | 4 | 0.03 | 30 | 14.34 | 13.665 |
| Species | Total Count | Min. | Max. | Mean | Median |
|---|---|---|---|---|---|
| Leafy Vegetables | |||||
| SCH | 22 | 0.88 | 5.43 | 2.07 | 1.49 |
| SRR | 22 | 1.02 | 4.52 | 2.06 | 1.62 |
| INS | 23 | 0.62 | 6.68 | 2.14 | 1.66 |
| SBR | 22 | 0.96 | 5.79 | 2.40 | 1.91 |
| ABS | 6 | 2.08 | 2.59 | 2.29 | 2.24 |
| SWC | 6 | 2.74 | 5.4 | 3.68 | 3.16 |
| CBB | 30 | 0.054 | 29.8 | 5.79 | 3.30 |
| INN | 21 | 0.58 | 6.95 | 3.46 | 3.48 |
| LTT | 8 | 0.096 | 71.62 | 12.06 | 3.94 |
| MST | 7 | 0.73 | 43.6 | 10.88 | 4.40 |
| CLF | 14 | 1.11 | 78.9 | 11.55 | 4.88 |
| RDL | 9 | 3.21 | 81.2 | 19.95 | 11.94 |
| KAL | 11 | 7 | 29 | 17.27 | 17.00 |
| CRN | 4 | 15 | 26.94 | 20.62 | 20.27 |
| MYL | 3 | 10.1 | 24.86 | 19.65 | 24.00 |
| SPN | 51 | 0.52 | 87.5 | 27.93 | 29.80 |
| MNT | 24 | 34.5 | 102.3 | 51.64 | 44.34 |
| BTH | 17 | 0.67 | 98.42 | 62.68 | 72.01 |
| Tuber/Root Vegetables | |||||
| TNP | 2 | 0.89 | 1.2 | 1.05 | 1.05 |
| SPT | 4 | 2.2 | 13.43 | 6.54 | 5.27 |
| CRR | 13 | 1.7 | 62 | 18.17 | 5.88 |
| BET | 5 | 0.16 | 20.6 | 8.04 | 9.10 |
| PTT | 21 | 0.96 | 17 | 7.56 | 9.75 |
| ONN | 13 | 3.19 | 43 | 19.32 | 17.61 |
| RDH | 10 | 4.21 | 63 | 27.11 | 21.81 |
| Stem Vegetables | |||||
| GRD | 1 | 0.21 | 0.21 | 0.21 | 0.21 |
| BPP | 8 | 0.27 | 0.77 | 0.52 | 0.50 |
| GPT | 6 | 0.35 | 1.47 | 0.83 | 0.78 |
| BNS | 13 | 1 | 15.26 | 2.98 | 1.47 |
| CLL | 5 | 0.23 | 7.52 | 2.92 | 1.56 |
| GBL | 8 | 1.9 | 14 | 4.79 | 2.30 |
| PMPn | 5 | 2.1 | 3.2 | 2.56 | 2.50 |
| TMT | 24 | 0.12 | 75 | 9.02 | 6.35 |
| BRN | 15 | 1.35 | 75.9 | 13.35 | 7.29 |
| PEA | 3 | 1.6 | 27.1 | 12.35 | 8.34 |
| GBT | 4 | 0.97 | 22.7 | 11.17 | 10.50 |
| OKR | 12 | 0.14 | 75.3 | 18.91 | 17.60 |
| GRD | 7 | 12.8 | 25.3 | 19.06 | 18.50 |
| OCH | 6 | 16.8 | 26.6 | 20.68 | 19.20 |
| BCL | 9 | 61.4 | 68.5 | 64.69 | 64.20 |
| CMM | 12 | 9.23 | 113 | 78.00 | 96.25 |
| SIN | 9 | 158 | 186 | 174.00 | 177.00 |
| Others | |||||
| BPP | 1 | 0.0009 | 0.0009 | 0.001 | 0.001 |
| AMR | 47 | 0.74 | 7.542 | 2.71 | 2.05 |
| FRG | 3 | 0.94 | 18.24 | 7.09 | 2.10 |
| DST | 23 | 1.45 | 33.139 | 4.31 | 2.30 |
| BKC | 1 | 9.69 | 9.69 | 9.69 | 9.69 |
| CLR | 1 | 10.83 | 10.83 | 10.83 | 10.83 |
| MBK | 18 | 8.96 | 24.86 | 13.53 | 12.49 |
| BLS | 1 | 19 | 19 | 19.00 | 19.00 |
| Cereals | |||||
| RIC | 74 | 0.07 | 17.44 | 2.56 | 1.19 |
| GGM | 38 | 0.17 | 71.2 | 4.21 | 2.06 |
| MGR | 26 | 0.21 | 2.54 | 1.87 | 2.08 |
| KLT | 22 | 1.3 | 3.44 | 2.19 | 2.20 |
| MAZ | 31 | 0.68 | 72.5 | 5.97 | 2.32 |
| WIP | 21 | 2.06 | 5.09 | 3.03 | 2.60 |
| MFN | 22 | 1.42 | 4.29 | 2.68 | 2.61 |
| BBE | 22 | 1.16 | 4.21 | 2.76 | 2.64 |
| MPR | 23 | 1.51 | 61.3 | 5.34 | 2.76 |
| WHT | 75 | 0.32 | 66.9 | 6.79 | 3.63 |
| ARH | 2 | 2.58 | 4.68 | 3.63 | 3.63 |
| BRY | 45 | 0.9 | 28.4 | 6.25 | 3.65 |
| GGR | 2 | 0.97 | 7.3 | 4.14 | 4.14 |
| CHN | 7 | 3.26 | 15.88 | 8.49 | 7.80 |
| BJR | 36 | 1.88 | 41.04 | 18.77 | 15.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachdeva, S.; Powell, M.A.; Nandini, G.; Kumar, H.; Kumar, R.; Sahoo, P.K. Uranium and Fluoride Accumulation in Vegetable and Cereal Crops: A Review on Current Status and Crop-Wise Differences. Sustainability 2023, 15, 13895. https://doi.org/10.3390/su151813895
Sachdeva S, Powell MA, Nandini G, Kumar H, Kumar R, Sahoo PK. Uranium and Fluoride Accumulation in Vegetable and Cereal Crops: A Review on Current Status and Crop-Wise Differences. Sustainability. 2023; 15(18):13895. https://doi.org/10.3390/su151813895
Chicago/Turabian StyleSachdeva, Saloni, Mike A. Powell, Girish Nandini, Hemant Kumar, Rakesh Kumar, and Prafulla Kumar Sahoo. 2023. "Uranium and Fluoride Accumulation in Vegetable and Cereal Crops: A Review on Current Status and Crop-Wise Differences" Sustainability 15, no. 18: 13895. https://doi.org/10.3390/su151813895
APA StyleSachdeva, S., Powell, M. A., Nandini, G., Kumar, H., Kumar, R., & Sahoo, P. K. (2023). Uranium and Fluoride Accumulation in Vegetable and Cereal Crops: A Review on Current Status and Crop-Wise Differences. Sustainability, 15(18), 13895. https://doi.org/10.3390/su151813895









