Cytotoxic Evaluation and Elucidation of Dammarane-Type Triterpenoids Isolated from the Exocarp of Aglaia cucullata (Meliaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Extracts Preparations
2.4. Isolation of Compounds from Aglaia Cucullata
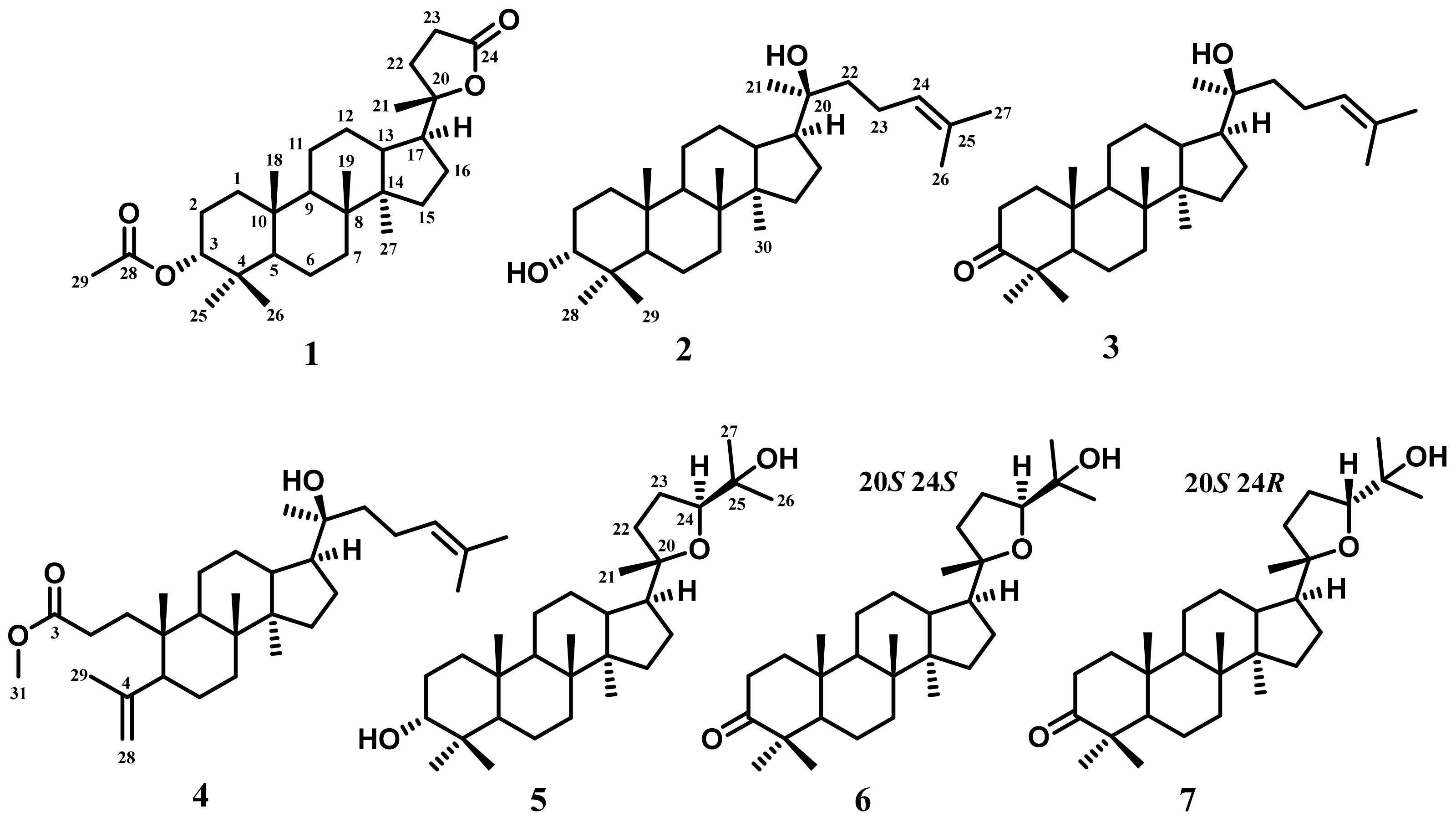
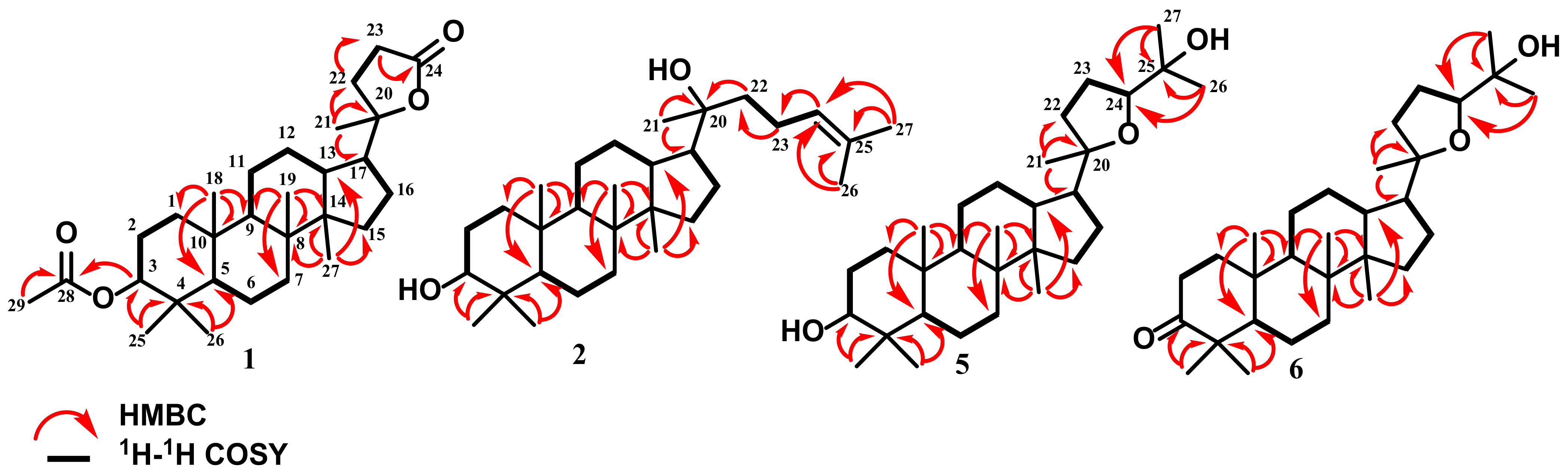
2.4.1. Cabraleahydroxylactone 3α-Acetate (1)
2.4.2. (20S)-20-Hydroxydammar,24-en-3α-ol (2)
2.4.3. (20S)-20-Hydroxydammar,24-en-3-on (3)
2.4.4. Methyl 20(S)-Hydroxy-3,4 secodammar-4(28),24-diene-3-oic Acid (4)
2.4.5. 3-Epi Ocotillol II (5)
2.4.6. Cabraleone (6)
2.4.7. Ocotillone (7)
2.5. Cytotoxic Activity Test by the PrestoBlue Assay
3. Results and Discussion
3.1. Compound Identification
3.2. Cytotoxic Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Harneti, D.; Supratman, U. Phytochemistry and Biological Activities of Aglaia Species. Phytochemistry 2021, 181, 112540. [Google Scholar] [CrossRef]
- Sholikhah, E.N. Indonesian Medicinal Plants as Sources of Secondary Metabolites for Pharmaceutical Industry. J. Med. Sci. 2016, 48, 226–239. [Google Scholar] [CrossRef]
- Malgaonkar, M.; Shirolkar, A.; Murthy, S.N.; Pawar, S. Ayurvedic Plants with Antidiabetic Potential. In Medicinal Plants—Recent Advances in Research and Development; Tsay, H.S., Shyur, L.F., Agrawal, D., Wu, Y.C., Wang, S.Y., Eds.; Springer: Singapore, 2016. [Google Scholar]
- Liu, B.; Xu, Y.K. Cytotoxicity and Synergistic Effect of the Constituents from Roots of Aglaia Odorata (Meliaceae). Nat. Prod. Res. 2015, 30, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Berning, L.; Scharf, L.; Aplak, E.; Stucki, D.; Von Montfort, C.; Reichert, A.S.; Stahl, W.; Brenneisen, P. In Vitro Selective Cytotoxicity of the Dietary Chalcone Cardamonin (CD) on Melanoma Compared to Healthy Cells Is Mediated by Apoptosis. PLoS ONE 2019, 14, e0222267. [Google Scholar] [CrossRef] [PubMed]
- An, F.L.; Wang, X.B.; Wang, H.; Li, Z.R.; Yang, M.H.; Luo, J.; Kong, L.Y. Cytotoxic Rocaglate Derivatives from Leaves amof Aglaia perviridis. Sci. Rep. 2016, 6, 20045. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Woodard, J.L.; Lucas, D.M.; Fuchs, J.R.; Kinghorn, A.D. Rocaglamide, Silvestrol and Structurally Related Bioactive Compounds from Aglaia Species. Nat. Prod. Rep. 2014, 31, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fu, W.X.; Zeng, C.X.; Zhou, L.; Bao, M.F.; Cai, X.H. Two New Lignans from Twigs of Aglaia odorata. J. Asian Nat. Prod. Res. 2015, 18, 147–152. [Google Scholar] [CrossRef]
- Joycharat, N.; Greger, H.; Hofer, O.; Saifah, E. Flavaglines and Triterpenoids from the Leaves of Aglaia forbesii. Phytochemistry 2008, 69, 206–211. [Google Scholar] [CrossRef]
- Xie, B.J.; Yang, S.P.; Chen, H.D.; Yue, J.M. Agladupols A–E, Triterpenoids from Aglaia duperreana. J. Nat. Prod. 2007, 70, 1532–1535. [Google Scholar] [CrossRef]
- Yodsaoue, O.; Sonprasit, J.; Karalai, C.; Ponglimanont, C.; Tewtrakul, S.; Chantrapromma, S. Diterpenoids and Triterpenoids with Potential Anti-Inflammatory Activity from the Leaves of Aglaia odorata. Phytochemistry 2012, 76, 83–91. [Google Scholar] [CrossRef]
- Pervin, R.; Afrin, S.; Sabrin, F.; Zohora, U.S.; Rahman, M.S.; Islam, K.D.; Billah, M.M. Antioxidant, Antibacterial and Brine Shrimp Lethality Bioassay of Amoora cucullata, a Mangrove Plant. J. Young Pharm. 2016, 8, 33–38. [Google Scholar] [CrossRef]
- Rahman, M.S.; Rashid, M.A. Preliminary Antimicrobial and Cytotoxic Activities of Amoora cucullata Extractives. Orient. Pharm. Exp. Med. 2009, 9, 182–185. [Google Scholar] [CrossRef]
- An, F.L.; Xu, W.J.; Yang, M.H.; Luo, J.; Kong, L.Y. Anti-Inflammatory Flavagline Glycosides and Putrescine Bisamides from Aglaia perviridis Leaves. Tetrahedron 2020, 76, 131257. [Google Scholar] [CrossRef]
- Schneider, C.; Bohnenstengel, F.I.; Nugroho, B.W.; Wray, V.; Witte, L.; Hung, P.D.; Kiet, L.C.; Proksch, P. Insecticidal Rocaglarnide Derivatives from Aglaia spectabilis (Meliaceae). Phytochemistry 2000, 54, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Chaidir; Hiort, J.; Bohnenstengel, F.I.; Nugroho, B.W.; Schneider, C.; Wray, V.; Witte, L.; Hung, P.D.; Kiet, L.C.; Proksch, P. New Insecticidal Rocaglamide Derivatives from the Roots of Aglaia duperreana. J. Nat. Prod. 1999, 62, 1632–1635. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.H.; Song, Z.J.; Chen, L.Y.; Wen, H.J. Molluscicidal Activity of Aglaia duperreana and the Constituents of Its Twigs and Leaves. Fitoterapia 2012, 83, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Esimone, C.O.; Eck, G.; Nworu, C.S.; Hoffmann, D.; Überla, K.; Proksch, P. Dammarenolic Acid, a Secodammarane Triterpenoid from Aglaia sp. Shows Potent Anti-Retroviral Activity in Vitro. Phytomedicine 2010, 17, 540–547. [Google Scholar] [CrossRef]
- Joycharat, N.; Plodpai, P.; Panthong, K.; Yingyongnarongkul, B.E.; Voravuthikunchai, S. Terpenoid Constituents and Antifungal Activity of Aglaia forbesii Seed against Phytopathogens. Can. J. Chem. 2010, 88, 937–944. [Google Scholar] [CrossRef]
- Ngo, N.T.N.; Lai, N.T.D.D.T.; Le, H.C.; Nguyen, L.T.T.; Trinh, B.T.D.; Nguyen, H.D.; Pham, P.D.; Dang, S.V.; Nguyen, L.H.D. Chemical Constituents of Aglaia elaeagnoidea and Aglaia odorata and Their Cytotoxicity. Nat. Prod. Res. 2021, 36, 1494–1502. [Google Scholar] [CrossRef]
- Phongmaykin, J.; Kumamoto, T.; Ishikawa, T.; Saifah, E.; Suttisri, R. Biologically Active Constituents of Aglaia erythrosperma. Nat. Prod. Res. 2011, 25, 1621–1628. [Google Scholar] [CrossRef]
- Heads, M. Biogeography and Ecology in a Pantropical Family, the Meliaceae. Gard. Bull. Singap. 2019, 71, 335–461. [Google Scholar] [CrossRef]
- Meepol, W.; Maxwell, G.S.; Havanond, S. Aglaia cucullata: A Little-Known Mangrove with Big Potential for Research. ISME/GLOMIS Electron. J. 2020, 18, 4–9. [Google Scholar]
- Ahmed, F.; Toume, K.; Sadhu, S.K.; Ohtsuki, T.; Arai, M.A.; Ishibashi, M. Constituents of Amoora cucullata with TRAIL Resistance-Overcoming Activity. Org. Biomol. Chem. 2010, 8, 3696–3703. [Google Scholar] [CrossRef] [PubMed]
- Chumkaew, P.; Teerapongpisan, P.; Pechwang, J.; Srisawat, T. New Oxoprotoberberine and Aporphine Alkaloids from the Roots of Amoora cucullata with Their Antiproliferative Activites. Rec. Nat. Prod. 2019, 13, 491–498. [Google Scholar] [CrossRef]
- Abdelfattah, M.S.; Toume, K.; Ahmed, F.; Sadhu, S.K.; Ishibashi, M. Cucullamide, a New Putrescine Bisamide from Amoora cucullata. Chem. Pharm. Bull. 2010, 58, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Chumkaew, P.; Kato, S.; Chantrapromma, K. Potent Cytotoxic Rocaglamide Derivatives from the Fruits of Amoora cucullata. Chem. Pharm. Bull. 2006, 54, 1344–1346. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current State of Melanoma Diagnosis and Treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Sarasin, A.; Dessen, P. DNA Repair Pathways and Human Metastatic Malignant Melanoma. Curr. Mol. Med. 2010, 10, 413–418. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae. Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Chapter 2 of Cell Viability Assays. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays: Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1601, pp. 1–17. [Google Scholar]
- Khanh, P.N.; Tai, B.H.; Huong, T.T.; Cuong, T.D.; Hai, H.V.; Luong, N.X.; Kim, Y.H.; Cuong, N.M. Terpenoids from The Leaves and Stems of Dysoxylum tpongense. Vietnam. J. Sci. Technol. 2019, 57, 139–145. [Google Scholar] [CrossRef]
- Vedernikov, D.N.; Roshchin, V.I. Extractive Compounds of Betulaceae Family Birch Buds (Betula pendula Roth.): V. Composition of Triterpene Seco-Acids. Russ. J. Bioorg Chem. 2012, 38, 762–768. [Google Scholar] [CrossRef]
- Seger, C.; Pointinger, S.; Greger, H.; Hofer, O. Isoeichlerianic Acid from Aglaia silvestris and Revision of the Stereochemistry of Foveolin B. Tetrahedron Lett. 2008, 49, 4313–4315. [Google Scholar] [CrossRef]
- Hidayat, A.T.; Farabi, K.; Harneti, D.; Maharani, R.; Darwati; Nurlelasari; Mayanti, T.; Setiawan, A.S.; Supratman, U.; Shiono, Y. Cytotoxicity and Structure Activity Relationship of Dammarane-Type Triterpenoids from the Bark of Aglaia elliptica against P-388 Murine Leukemia Cells. Nat. Prod. Sci. 2017, 23, 291–298. [Google Scholar] [CrossRef]
- Golodov, V.A. Synergistic Phenomena in Chemistry. Eurasian Chem. Technol. J. 2000, 2, 29–34. [Google Scholar] [CrossRef]
- Chan, H.C.S.; Pan, L.; Li, Y.; Yuan, S. Rationalization of Stereoselectivity in Enzyme Reactions. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2019, 9, e1403. [Google Scholar] [CrossRef]
| Compounds | IC50 ± SD (µM) |
|---|---|
| Cabraleahydroxylactone 3α-acetate (1) | 153.38 ± 0.19 |
| (20S)-20-hydroxydammar,24-en-3α-ol (2) | 41.08 ± 0.23 |
| (20S)-20-hydroxydammar,24-en-3-on (3) | 21.55 ± 0.25 |
| Methyl 20(S)-hydroxy-3,4 secodammar-4(28),24-diene-3-oic acid (4) | 71.04 ± 0.19 |
| 3-epi ocotillol II (5) | 195.07 ± 0.32 |
| Cabraleone (6) | 545.01 ± 0.22 |
| Ocotillone (7) | 303.68 ± 0.24 |
| Cisplatin (positive control) | 43.00 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purnama; Anjari, I.H.; Farabi, K.; Runadi, D.; Mayanti, T.; Nurlelasari; Naini, A.A.; Harneti, D.; Harizon; Kuncoro, H.; et al. Cytotoxic Evaluation and Elucidation of Dammarane-Type Triterpenoids Isolated from the Exocarp of Aglaia cucullata (Meliaceae). Sustainability 2023, 15, 13565. https://doi.org/10.3390/su151813565
Purnama, Anjari IH, Farabi K, Runadi D, Mayanti T, Nurlelasari, Naini AA, Harneti D, Harizon, Kuncoro H, et al. Cytotoxic Evaluation and Elucidation of Dammarane-Type Triterpenoids Isolated from the Exocarp of Aglaia cucullata (Meliaceae). Sustainability. 2023; 15(18):13565. https://doi.org/10.3390/su151813565
Chicago/Turabian StylePurnama, Intan Hawina Anjari, Kindi Farabi, Dudi Runadi, Tri Mayanti, Nurlelasari, Al Arofatus Naini, Desi Harneti, Harizon, Hadi Kuncoro, and et al. 2023. "Cytotoxic Evaluation and Elucidation of Dammarane-Type Triterpenoids Isolated from the Exocarp of Aglaia cucullata (Meliaceae)" Sustainability 15, no. 18: 13565. https://doi.org/10.3390/su151813565
APA StylePurnama, Anjari, I. H., Farabi, K., Runadi, D., Mayanti, T., Nurlelasari, Naini, A. A., Harneti, D., Harizon, Kuncoro, H., Prescott, T. A. K., Azmi, M. N., & Supratman, U. (2023). Cytotoxic Evaluation and Elucidation of Dammarane-Type Triterpenoids Isolated from the Exocarp of Aglaia cucullata (Meliaceae). Sustainability, 15(18), 13565. https://doi.org/10.3390/su151813565







