Abstract
Aglaia cucullata is a mangrove plant with a tropical Asian distribution. It is used as traditional medicine for the treatment of diarrhea, inflammation, skin diseases, and heart diseases. Several compounds isolated from A. cucullata have demonstrated cytotoxic activity against various human cancer cells. Cancer therapies such as surgery, chemo-, and radiotherapy have many side effects. However, the use of natural bioactive compounds such as triterpenoid in cancer treatment can be used as an alternative to reduce these side effects. Therefore, the discovery of bioactive compounds from plants is very important to improve aspects of discovery and development of sustainable new anticancer drug candidates. Here, we report the chemical structures of seven known dammarane-type triterpenoids (1–7) isolated from A. cucullata exocarp and evaluate their cytotoxicity against B16-F10 melanoma skin cancer cells. The isolated compounds included cabraleahydroxylactone 3α-acetate (1), (20S)-20-hydroxydammar,24-en-3α-ol (2), (20S)-20-hydroxydammar,24-en-3-on (3), methyl 20(S)-hydroxy-3,4-secodammar-4(28),24-diene-3-oic acid (4), 3-epi ocotillol II (5), cabraleone (6), and ocotillone (7). The n-hexane extract was found to be active against B16-F10 cells, exhibiting an IC50 value of 7.85 ± 0.22 µg/mL. Fractionation of this extract subsequently identified the compound (20S)-20-hydroxydammar 24-en-3-on (3) as an active substance with an IC50 value of 21.55 ± 0.25 µM, comparing favorably with the positive control cisplatin (12.90 µg/mL; 43.00 µM). These results provide further evidence of the genus Aglaia as a source of cytotoxic cancer drug leads. In addition, compound 3 has potential as a convincing therapeutic agent for further research in the context of sustainable drug development, especially the development of new safe cancer chemotherapeutic agents.
1. Introduction
In ethnobotany, plants are widely used as traditional medicine for treating various diseases such as fever, cough, and diarrhea [1,2,3]. These plants contain several natural compounds with diverse chemical structures and biological activities, particularly cytotoxic activity against various human cancer cells [4,5,6]. Therefore, natural compounds in plants are commonly used in the development of drugs for various diseases, including the discovery and development of new cancer chemotherapeutic agents.
Aglaia is a large source of natural compounds in the Meliaceae family, comprising 150 plants distributed in subtropical and tropical rainforests in south-eastern and southern Asia, the Pacific region [7], and northern Australia [8]. In Asia, plants of the Aglaia genus are found in India, Malaysia, Thailand, Indonesia, and Vietnam [9], with eight species present in the south of China. Approximately 65 species of Aglaia plants grow in Indonesia, thriving on the islands of Java, Sumatra, Bali, Sulawesi, and Kalimantan [10]. These plants are widely used as indigenous medicine for the treatment of cough, diarrhea, fever [6], skin diseases, heart disease, and bruises [11]. Furthermore, their biological activities that have been investigated include antioxidant [12,13], antibacterial [12,13], anti-inflammation [14], insecticidal [15,16], molluscicidal [17], antiviral [18], antifungal [19], and cytotoxic activities [20,21]. A total of 96 triterpenoids have been reported from the Aglaia genus.
Aglaia cucullata is a mangrove plant that grows in mangrove swamps and is the only species from the Aglaia genus with pneumatophore roots [22,23]. A. cucullata is distributed in the rainforests of Southeast Asia, India, Bangladesh, Nepal, Pakistan, Myanmar, Malaysia, Thailand, Vietnam, and the Solomon Islands, as well as in Indonesia in the islands of Borneo and Sumatra [12]. A total of 29 compounds from A. cucullata have been reported, consisting of diterpenoids, triterpenoids [24], alkaloids [25], bisamides [24,26], rocaglamides [27], and flavonoids [26]. Amocurin C isolated from the root of A. cucullata showed cytotoxicity against KB cells and MCF-7 breast cancer cells, with IC50 values of 4.2 and 3.5 µM, respectively [25]. Furthermore, rocaglamide derivatives from the fruit of A. cucullata showed strong cytotoxicity against KB, BC, and NCI-H187 cells [27].
Melanoma skin cancer is characterized by a high mortality rate [28] and occurs in pigment-producing cells known as melanocytes [29]. This cancer can also occur in the eyes, meninges, and surface mucosa of the body that contain melanin. Previous investigations have highlighted that commonly used cancer therapies, including surgery, chemo-, and radiotherapy cause several side effects, such as sunburn, difficulty swallowing, weakness, hair loss, headache, nausea, vomiting, weight loss, and redness of the skin [30]. As such, investigations of active compounds from the Indonesian Aglaia plant are warranted to develop new melanoma skin cancer chemotherapy agents that can reduce these side effects. Consequently, integrating sustainable drug discovery and development research by utilizing active compounds from plants to encourage a sustainable flow of new anticancer drug candidates is an innovative solution to reduce the side effects from the previous drugs. Therefore, the initial stage research of drug discovery and development needs to be strengthened, as this is important to discover new cancer therapy agent candidates that are more effective and safer without providing adverse side effects to patients so that the quality of life of patients improves. This research aims to investigate the isolation and structural elucidation of triterpenoids from the exocarp of A. cucullata, as well as evaluate their cytotoxicity against B16-F10 melanoma skin cancer cells.
2. Materials and Methods
2.1. Plant Material
The exocarp of Aglaia cucullata (Roxb.) Pellegr. was collected from the Manggar River, Balikpapan, East Kalimantan, Indonesia in December 2020. The plant was identified at the Herbarium Wanariset (WAN), 1°12′46.6″ S 116°57′42.1″ E, Balikapapan (No. FF7.20), and deposited at the Faculty of Forestry, Mulawarman University by a botanical expert.
2.2. Chemicals and Reagents
Solvents, including n-butanol, ethanol, ethyl acetate, methanol, n-hexane, methylene chloride, acetone, and chloroform in distilled and technical quality. were obtained from Kristata Gemilang Company, Bandung, Indonesia, Purified water was acquired using the Milli-Qplus185 system (Millipore, Billerica, MA, USA). Rosewell Park Memorial Institute (RPMI) provided dimethyl sulfoxide (DMSO), PrestoBlue® reagents, cisplatin, and B16-F10 cancer cells (Manassas, VA, USA).
2.3. Extracts Preparations
The dried exocarp of A. cucullata (1.0 kg) was macerated using ethanol 70% repeatedly, followed by filtration, and the solvent evaporated to obtain a concentrated ethanol extract. The ethanol extract was suspended in water/methanol (8:2) and consecutively partitioned with n-hexane, ethyl acetate, and n-butanol. Furthermore, the organic layer was evaporated with a rotary evaporator at a temperature of 37 °C to produce the extract of n-hexane (250.7 g), ethyl acetate (40.9 g), and n-butanol (5.9 g).
2.4. Isolation of Compounds from Aglaia Cucullata
The orangish n-hexane extract was separated by vacuum liquid chromatography (VLC) with a 10% gradient eluent system of n-hexane-EtOAc (100:0–0:100) on silica gel to obtain five (A–E) fractions that were collected based on the polarity properties of each fraction guided by thin-layer chromatography (TLC). Next, 9.4 g of the B fraction was column-chromatographed on silica gel of 70–230 mesh with a 1% gradient of n-hexane-EtOAc (100:0–90:10) to obtain seven fractions (B1–B7). The B5 fraction (2.71 g) was separated by column chromatography (CC) on silica gel (70–230 mesh) with n-hexane/dichloromethane/EtOAc (25:25:1) eluent to obtain nine combined fractions (B5A–B5I). Fraction B5B (269.0 mg) was further separated on silica gel (230–400 mesh) using n-hexane/EtOAc (15:1) eluent to obtain five subfractions (B5B1–B5B5). Subsequently, the subfraction B5B3 (52.0 mg) was purified on ODS (C18, 100–200 mesh) column chromatography and eluted with methanol/water (9:1) to obtain compound 4 (5.3 mg). Each fraction was physically collected in vials of varying sizes.
B5C fraction (452 mg) was CC on silica gel (230–400 mesh) by n-hexane/chloroform/EtOAc (30:2:1) eluent to obtain 14 fractions (B5C1–B5C14). Subsequently, B5C9 (43.8 mg) and B5C11 (51.1 mg) fractions were purified by ODS (C18, 100–200 mesh) column chromatography using methanol/water (9:1) to obtain compound 3 (10.3 mg) from B5C9 fraction and compound 1 (10.4 mg) from B5C11 fraction.
C fraction (30.8 g) was separated by VLC using a 2.5% gradient eluent system of n-hexane-ethyl acetate (10:0–8:2) on silica gel to obtain six (C1–C6) fractions. Subsequently, the C3 fraction (6.25 g) was further separated by column chromatography on silica gel (70–230 mesh) using n-hexane/dichloromethane/EtOAc (15:4:1) as eluent to yield 12 fractions, namely C3B–C3L. Fraction of C3B (211 mg), C3C (423 mg), and C3E (302 mg) was purified by ODS (C18, 100–200 mesh) column chromatography using methanol/water (9:1) to obtain compound 2 (10.7 mg) from C3B fraction, compound 6 (10.2 mg) and 7 (9.9 mg) from C3C fraction, as well as compound 5 (9.3 mg) from C3E fraction.
The chemical structure of all purified compounds was elucidated by NMR (nuclear magnetic resonance) spectrometry. Compound 4 was analyzed using a JEOL JNM-ECX500R/S1 spectrometer (Tokyo, Japan), while compounds 1–3 and 5–7 were evaluated using a Bruker Av-500 spectrometer (Karlsruhe, Germany). The NMR spectrometry was performed at 500 MHz for 1H and 125 MHz for 13C, using CDCl3 as solvent and TMS as an internal standard. Furthermore, HR-TOFMS (Waters, Milford, MA, USA), and FT-IR (Waltham, MA, USA) analysis was conducted using a KBR plate. The HR-TOFMS, FT-IR, 1H, 13C-NMR, and DEPT 135° spectral data of these seven dammarane-type triterpenoids are presented in the Supplementary Materials.
2.4.1. Cabraleahydroxylactone 3α-Acetate (1)
Colorless crystals with molecular formula C29H46O4 (HR-TOFMS m/z 481.3289, [M + Na]+); FT-IR, νmax (cm−1): 2947 (sp3), 1741 and 1765 (C=O ester), 1449 and 1374 (gem-dimethyl); 1H-NMR (500 MHz in CDCl3) δH (ppm): 1.45 (H-1a), 1.14 (H-1b; H-22b), 1.87 (H-2a), 1.60 (H-2b), 4.61 (t, J = 2.4 Hz, H-3), 1.22 (H-5), 1.43 (H-6; H-9), 1.27 (H-7a), 1.59 (H-7b, H-13), 1.50 (H-11a), 1.19 (H-11b), 1.75 (H-12a), 1.24 (H-12b), 1.94 (H-15a), 1.51 (H-15b), 1.84 (H-16a), 1.26 (H-16b), 2.00 (H-17), 0.95 (CH3-18), 0.84 (CH3-19), 1.35 (CH3-21), 2.11 (H-22a), 2.63 (H-23a), 2.55 (H-23b), 0.82 (CH3-25), 0.87 (CH3-26), 0.92 (CH3-27), 2.07 (CH3-29); 13C-NMR shown in Table S1.
2.4.2. (20S)-20-Hydroxydammar,24-en-3α-ol (2)
White crystals with molecular formula C30H52O2 (HR-TOFMS m/z 467.3848, [M + Na]+); FT-IR, νmax (cm−1): 3353 (O-H), 2940 (sp3), 1650 (C=C), 1449 and 1378 (gem-dimethyl); 1H-NMR (500 MHz in CDCl3) δH (ppm): 1.35 (H-1a), 1.42 (H-1b), 1.85 (H-2a), 1.50 (H-2b; H-11a), 3.35 (t, J = 3.0 Hz, H-3), 1.20 (H-5), 1.36 (H-6a), 1.46 (H-6b), 1.22 (H-7a), 1.54 (H-7b), 1.40 (H-9), 1.55 (H-11b), 1.52 (H-12a; H-22b), 1.91 & 1.58 (H-12b & H-13), 1.02 & 1.46 (H-15a & H-15b), 1.70 & 1.28 (H-16a & H16b), 1.78 (H-17), 0.95 (CH3-18), 0.85 (CH3-19), 1.13 (CH3-21), 1.43 (H-22a), 1.86 (H-23a), 2.03 (H-23b), 5.11 (t, J = 7.1 Hz, H-24), 1.68 (CH3-26), 1.62 (CH3-27), 0.93 (CH3-28), 0.83 (CH3-29), 0.88 (CH3-30); 13C-NMR shown in Table S1.
2.4.3. (20S)-20-Hydroxydammar,24-en-3-on (3)
White crystals with molecular formula C30H50O2 (HR-TOFMS m/z 465.3737, [M + Na]+); FT-IR, νmax (cm−1): 3481 (O-H), 2958 (sp3), 1704 (C=O ketone), 1457 and 1377 (gem-dimethyl); 1H-NMR (500 MHz in CDCl3) δH (ppm) 1.88 (H-1), 2.38 & 2.42 (H-2a & H-2b), 1.30 (H-5), 1.51 & 1.42 (H-6a & H-6b), 1.49 (H-7a), 1.28 (H-7b), 1.37 (H-9), 1.44 (H-11a; H-16b), 1.26 (H-11b), 1.80 (H-12a), 1.24 (H-12b), 1.63 (H-13), 1.39 (H-15a), 1,00 (H-15b), 1.67 (H-16a), 1.65 (H-17), 0.99 (CH3-18), 0.93 (CH3-19), 1.14 (CH3-21), 1.41 (H-22), 1.99 (H-23), 5.11 (tt, J = 7.1, 1.4 Hz, H-24), 1.68 (CH3-26), 1.61 (CH3-27), 1.07 (CH3-28), 1.02 (CH3-29), 0.87 (CH3-30); 13C-NMR shown in Table S1.
2.4.4. Methyl 20(S)-Hydroxy-3,4 secodammar-4(28),24-diene-3-oic Acid (4)
Colorless crystals with molecular formula C31H52O3 (HR-TOFMS m/z 473.3978 [M + H]+); FT-IR νmax (cm−1): 3452 (O-H), 2959 (sp3), 1727 (C=O), 1456 and 1385 (gem-dimethyl); 1H-NMR (500 MHz in CDCl3) δH (ppm): 1.55 (H-1), 2.31; 2.16 (H-2a; H-2b), 1.34; 1.77 (H-6a & H-6b), 1.92 (H-5), 1.51 (H-7a; H-15a), 1.20 (H-7b), 1.45 (H-9), 1.31 (H-11), 1.16; 1.92 (H-12a; H-12b), 1.67 (H-13), 1.04 (H-15b), 1.67 (H-16), 1.70 (H-17), 0.83 (CH3-18), 0.98 (CH3-19), 1.13 (CH3-21), 1.42 (H-22), 2.01 (H-23), 5.10 (t, J = 7.1 Hz, H-24), 1.67 (CH3-26), 1.61 (CH3-27), 4.83, 4.64 (H-28a, H-28b), 1.72 (CH3-29), 0.87 (CH3-30); 3.62 (CH3-31); 13C-NMR shown in Table S1.
2.4.5. 3-Epi Ocotillol II (5)
White crystals with molecular formula C30H52O3 (HR-TOFMS m/z 483.3827 [M + Na]+); FT-IR νmax (cm−1): 3353 (O-H), 2940 (sp3), 1650 (C=C), 1449 and 1378 (gem-dimethyl); 1H-NMR (500 MHz in CDCl3) δH (ppm): 1.45; 1.38 (H-1a; H-1b), 1.61; 1.95 (H-2a; H-2b), 1.40 (H-5), 3.38 (t, J = 2.9 Hz, H-3), 1.36 & 1.41 (H-6a & H-6b), 1.64; 1.82 (H-7a; H-7b), 1.22 (H-9), 1.50 & 1.56 (H-11a & H11-b), 1.77 & 1.85 (H-12a & H-12b), 1.65 (H-13), 1.47 & 1.04 (H-15a & H-15b), 1.88; 1.94 (H-16a & H-16b), 0.85 (CH3-18), 1.87 (H-17), 0.96 (CH3-19), 1.14 (CH3-21), 1.21; 1.29 (H-22a; H-22b), 1.76; 1.85 (H-23a; H-23b), 3.62 (dd, J = 5.3, 10.2 Hz, H-24), 1.18 (CH3-26), 1.10 (CH3-27), 0.93 (CH3-28), 0.83 (CH3-29), 0.88 (CH3-30); 13C-NMR shown in Table S1.
2.4.6. Cabraleone (6)
White crystals with molecular formula C30H50O3 (HR-TOFMS m/z 481.3639 [M + Na]+); FT-IR νmax (cm−1): 3525 (O-H), 2965 (sp3), 1691 (C=O), 1460 and 1381 (gem-dimethyl); 1H-NMR (500 MHz in CDCl3) δH (ppm): 1.88 & 1.41 (H-1a and H-1b), 2.41 (H-2), 1.33 (H-5), 1.50; 1.42 (H-6a; H-6b), 1.52; 1.26 (H-7a; H-7b), 1.38 (H-9), 1.47; 1.21 (H-11a; H-11b), 0.99 (H-12), 1.63 (H-13), 1.43; 1.04 (H-15a; H-15b), 1.74 (H-16), 1.82 (H-17; H-22a; H-23), 0.93 (CH3-18), 0.99 (CH3-19), 1.14 (CH3-21), 1.62 (H-22b), 3.63 (dd, J = 5.2, 10.1 Hz, H-24), 1.18 (CH3-26), 1.10 (CH3-27), 1.07 (CH3-28), 1.02 (CH3-29), 0.87 (CH3-30); 13C-NMR shown in Table S1.
2.4.7. Ocotillone (7)
White crystals with molecular formula C30H50O3 (HR-TOFMS m/z 481.3663 [M + Na]+); FT-IR νmax (cm−1): 3491 (O-H), 2963 (sp3), 1691 (C=O), 1459 and 1374 (gem-dimethyl); 1H-NMR (500 MHz in CDCl3) δH (ppm): 1.85 & 1.38 (H-1a; H-17 & H-1b), 2.38 (H-2), 1.31 (H-5), 1.52 & 1.42 (H-6a & H-6b; H-15b), 1.54 & 1.28 (H-7a & H-7b), 1.36 (H-9), 1.45 & 1.20 (H-11a & H-11b), 0.98 (H-12), 1.65 (H-13), 1.02 (H-15a), 1.78 (H-16), 0.92 (CH3-18), 0.97 (CH3-19), 1.12 (CH3-21), 1.84; 1.63 (H-22a; H-22b), 1.80 (H-23), 3.72 (dd, J = 5.3, 10.1 Hz, H-24), 1.20 (CH3-26), 1.11 (CH3-27), 1.06 (CH3-28), 1.02 (CH3-29), 0.86 (CH3-30); 13C-NMR shown in Table S1.
2.5. Cytotoxic Activity Test by the PrestoBlue Assay
The activity of compounds 1–7 was determined by the PretoBlue® method, which depended on the resazurin reagent obtained from Thermo Fisher Scientific, Uppsala, Sweden. In simple terms, resazurin (blue) acted as a viability indicator which passed through a reduction reaction with healthy cells to form resorufin (purple). The measurement of this interaction was used to determine cell viability [31]. In this method, the first stage involved seeding B16-F10 melanoma cancer cell lines (ATCC® CRL-6475™, Manassas, VA, USA) into 96-well plates on the RPMI (Rosewell Park Memorial Institute) medium with an initial cell amount of 200 µL of 1.7 × 104 cells/well. Furthermore, the cells were incubated for 24 h at a temperature of 37 °C and 5% CO2 gas, with a total of five passages.
The second stage was cell treatment with samples (compounds 1–7 and the extract of ethanol, n-hexane, ethyl acetate, and butanol), positive control, negative control, and solvent control. In this stage, the RPMI medium on the plates was discarded and the medium containing samples at the different concentrations of 250.00, 125.00, 62.50, 31.25, 15.63, 7.81, 3.91, and 1.95 µg/mL in PBS was added with DMSO 2% as a solvent and incubated for two days at a temperature of 37 °C and 5% CO2. In addition, cisplatin was used as positive control. In the final step, PrestoBlue® reagents were added and the absorbances were measured. The medium containing the sample on the plates was discarded and the PretoBlue® reagent (10 µL PrestoBlue in 90 µL RPMI medium) was added. Subsequently, the plates were incubated for 2 h until the color changed from blue to purple. The absorbance of each sample was read using a multimode reader at the wavelengths 570 nm and 600 nm. These wavelengths were used because resazurin and resorufin have a maximum absorbance of 600 nm and 570 nm, respectively. The median inhibitory concentration (IC50) values of each compound were determined by comparing the plotted graph of normalized percentage live cells and negative control with the tested compound concentration.
3. Results and Discussion
3.1. Compound Identification
A total of seven known dammarane-type triterpenoids have been identified from the n-hexane extract of A. cucullata exocarp: cabraleahydroxylactone 3α-acetate (1), (20S)-20-hydroxydammar,24-en-3α-ol (2), (20S)-20-hydroxydammar,24-en-3-on (3), methyl 20(S)-hydroxy-3,4 secodammar-4(28),24-diene-3-oic acid (4), 3-epi ocotillol II (5), cabraleone (6), and ocotillone (7). The chemical structures of these compounds are shown in Figure 1.
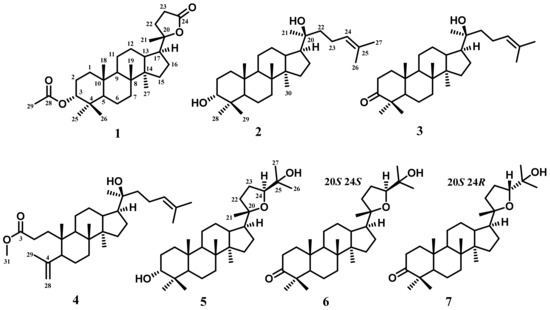
Figure 1.
Structure of compounds 1–7 isolated from the exocarp of A. cucullata: cabraleahydroxylactone 3α-acetate (1), (20S)-20-hydroxydammar,24-en-3α-ol (2), (20S)-20-hydroxydammar,24-en-3-on (3), methyl 20(S)-hydroxy-3,4 secodammar-4(28),24-diene-3-oic acid (4), 3-epi ocotillol II (5), cabraleone (6), and ocotillone (7).
Compound 1 was identified as cabraleahydroxylactone 3α-acetate [32] and included in the trisnor–triterpenoid group. 13C-NMR (Table S1) displayed the presence of 29 carbon atoms, where the presence of the acetyl group at δH 2.07 ppm indicated the absence of three carbon atoms in the trisnor–triterpenoid group. Furthermore, the characteristic of the trisnor–triterpenoid group was supported by the appearance of a lactone ring system on the side chain, showing a distinctive ester chemical shift at δC 176.8 ppm and oxygenated quaternary carbon at δC 90.2 ppm. This compound also exhibited a chemical shift for dammarane-type triterpenoid at 15.5, 15.9, 21.7, 16.4, and 16.4 ppm. This evaluation revealed that the spectroscopic data of 1 are similar to cabraleahydroxylactone 3α-acetate from Dysoxylum tpongense leaves and stem [32]. The 2D-NMR analysis of compound 1 (Figure 2) confirmed the exact chemical structure of this compound. Therefore, compound 1 was exactly obtained as cabraleahydroxylactone 3α-acetate.
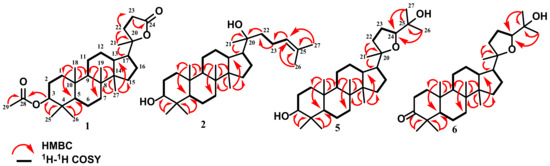
Figure 2.
Selected HMBC and 1H-1H COSY correlations for 1, 2, 5, and 6.
Compounds 2 and 3 were also obtained as triterpenoids. Based on 13C-NMR (Table S1), these triterpenoids had a similar chemical shift from C-8 to C-30, indicating a similar structure, particularly on the side chain. There was one double bond at chemical shifts 124.7/131.6 (C-24/25) and 124.6/131.6 (C-24/25) for compounds 2 and 3, respectively, as well as a hydroxylated quaternary carbon at 25.4 ppm for both. Meanwhile, the main difference between these two compounds was in the presence of oxygenated methine carbon at 76.3 ppm (C-3) in compound 2 and the ketone group at 218.2 ppm (C-3) in compound 3. This showed that compound 3 was a derivative of compound 2 that passed through further oxidation at C-3. The 13C-NMR, DEPT 135°, and 1H-NMR (Figures S11, S12, S19 and S20) of compounds 2 and 3 have a high similarity with (20S)-20-hydroxydammar,24-en-3α-ol (2) and (20S)-20-hydroxydammar,24-en-3-one (3) isolated from the stembarks of A. elliptica. Therefore, these compounds were identified as (20S)-20-hydroxydammar,24-en-3α-ol and (20S)-20-hydroxydammar,24-en-3-one, respectively, and were included in the dammarane-type triterpenoid group.
Compound 4 was also included in the dammarane-type triterpenoid group and had the same side chain as compounds 2 and 3. The 13C-NMR (Table S1) showed the presence of 31 carbon atoms, indicating that there was one additional carbon atom which was the methoxy group at 51.7 ppm (C-31). Apart from the functional group in the side chain, there was one double bond and one ester group at 174.7 ppm that bonded to the methoxy group on the main skeleton. Furthermore, compound 4 was classified as a seco-A -type triterpenoid which had three aliphatic quaternary carbons, indicating a modification ring in the form of an A-ring opening. The spectroscopic data of compound 4 were identical to methyl 20(S)-hydroxy-3,4 secodammar-4(28),24-diene-3-oic acid isolated from the leaf buds of Betula pendula Roth [33].
Compounds 5–7 were also classified into a dammarane-type triterpenoid group, specifically 20,24 epoxy dammarane-type. Detailed 1H and 13C-NMR with DEPT 135° (Figures S25–S44) spectra of 5–7 showed the appearance of a tetrahydrofuran ring, indicating their similar structure. The main difference in chemical shift between compounds 5 and 6 was shown at C-3. Compound 5 had a hydroxylated methine at 76.2 ppm, while compound 6 had a ketone group at 218.3 ppm. Furthermore, compound 7 had the same molecular formula and functional group as 6. The comparison of the spectroscopic data of compounds 6 and 7 showed that compound 7 was the epimer of 6 that had only stereochemical differences in one carbon atom at C-24. The results also indicated that compound 6 showed 24S at 86.4 ppm, while compound 7 displayed 24R configuration at 83.3 ppm. The NMR data of compounds 5–7 showed a high similarity with 3-epi ocotillol II (5), cabraleone (6), and ocotillone (7) that were isolated from A. argentea, A. elliptica, and A. silvestris, respectively [34,35]. All exact structures of triterpenoids isolated from A. cucullata peel fruit were confirmed by 2D-NMR analysis, as shown in Figure 2. Moreover, all dammarane-type triterpenoids identified in this research were obtained from A. cucullata exocarp for the first time.
3.2. Cytotoxic Activity Evaluation
The cytotoxic activity of all A. cucullata peel fruit extracts including ethanol, n-hexane, ethyl acetate, and n-butanol extract as well as compounds 1–7 was evaluated against B16-F10 melanoma skin cancer cells corresponded to a method explained previously. The extracts of ethanol, n-hexane, ethyl acetate, and n-butanol showed varying cytotoxicity with IC50 values of 21.90 ± 0.12, 7.85 ± 0.22, 85.03 ± 0.16, and 120.22 ± 0.09 µg/mL, respectively. The other results of cytotoxicity are shown in Table 1. Based on the results, (20S)-20-hydroxydammar,24-en-3-on (3) had a potential activity with an IC50 value of 21.55 ± 0.25 µM. Meanwhile, the positive control, cisplatin, had an IC50 value of 43.00 ± 0.10 µM.

Table 1.
Cytotoxicity evaluation of novel compounds in B16-F10 melanoma cell lines using the PrestoBlue assay (IC50 ± SD, µM).
Among all A. cucullata exocarp extracts, the n-hexane extract exhibited the highest cytotoxic activity, followed by the extracts of ethanol, ethyl acetate, and n-butanol. To identify the active compound in A. cucullata exocarp, triterpenoid compounds were isolated from n-hexane extract. All dammarane-type triterpenoids isolated from n-hexane extract showed various cytotoxicity from moderate level to inactive. Furthermore, the IC50 value of n-hexane extract showed the strongest value compared to the compounds 1–7. This result showed the synergistic effect that occurred when the combined product in the form of extract had a greater effect than a single compound [36]. The other active constituents in the extracts gave more significant cytotoxicity.
Compounds 2 and 3 showed moderate levels of cytotoxicity with an IC50 value close to that of cisplatin. Meanwhile, compound 3 with the ketone group at C-3 was more active than compound 2 with the hydroxyl at C-3. The comparison of the IC50 value of compounds 2 and 3 indicated that the presence of the ketone group at C-3 increased the cytotoxicity. Compound 3 showed higher activity than cisplatin, indicating its potential as an anticancer agent. Compound 4 showed weak activity with an IC50 value of 33.56 µg/mL. This indicated that the modification of the A-ring in the form of an opening ring in the dammarane-type triterpenoid reduced the cytotoxic activity.
Compounds 5–7 had the same skeleton that was classified as 20,24 epoxy dammarane-type. Meanwhile, compound 5 showed the highest cytotoxicity among these three triterpenoids with moderate levels, while others were categorized as inactive. Based on morphological analysis, the cells that were treated by compound 6 and 7 did not show cell death. Compared to the dammarane-type triterpenoids with an acyclic side chain (compounds 2 and 3), the tetrahydrofuran ring as a side chain showed lower activity. This indicated that the form of the side chain affected cytotoxicity, where the acyclic form had a better effect than the tetrahydrofuran ring form.
Based on the chemical structure, compounds 5 and 6 only showed differences in the substituents at C-3, which were the hydroxyl and ketone groups, respectively. Compound 5 was more active than 6, indicating that the change of hydroxyl groups in C-3 to ketones decreased cytotoxic activity. In triterpenoids with tetrahydrofuran ring side chains, ketone groups did not effectively contribute, thereby making the compounds inactive. This phenomenon occurred due to the difference in the shape of the side chain. In addition, compounds 6 and 7 were epimers that only had stereochemical differences in one asymmetric carbon, which was at C-24. Meanwhile, compound 7 was more active than 6, indicating that the stereochemical differences influenced the cytotoxicity. In this case, the 24R configuration contributed more to increasing the cytotoxicity compared to the 24S configuration. These results supported the theory of stereoselectivity [37].
4. Conclusions
In conclusion, seven known dammarane-type triterpenoids, namely cabraleahydroxylactone 3α-acetate (1), (20S)-20-hydroxydammar,24-en-3α-ol (2), (20S)-20-hydroxydammar,24-en-3-on (3), methyl 20(S)-hydroxy-3,4 secodammar-4(28),24-diene-3-oic acid (4), 3-epi ocotillol II (5), cabraleone (6), and ocotillone (7), were isolated from the n-hexane extract of A. cucullata (Meliaceae) exocarp. The cytotoxic activity of these triterpenoids were tested against B16-F10 melanoma skin cancer cell lines. Among all dammarane-type triterpenoids, compound 3 showed the highest cytotoxicity at a moderate level with IC50 21.55 ± 0.25 µM. This indicated that the presence of the ketone group increased the cytotoxicity in acyclic side chain form, compared to compound 2. These results open up opportunities for the sustainable discovery of new cancer drug candidates. To ensure safe cancer chemotherapeutic agents in the sustainable drug discovery and development process, more efforts are needed not only to find compounds that are active against melanoma skin cancer cells but also to study the mechanisms of action of these active compounds on target proteins and their selectivity against normal cells. Therefore, we suggest that further work should be carried out, especially on compound 3 to determine its effects on a panel of cell lines and to investigate its cytotoxic mechanism of action.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su151813565/s1.
Author Contributions
Conceptualization, M.N.A. and U.S.; methodology, Purnama and D.R.; validation, Nurlelasari; formal analysis, K.F. and T.M.; investigation, I.H.A. and Purnama; resources, H.K. and Harizon; software, K.F.; visualization, D.H.; data curation, A.A.N.; writing—original draft preparation, I.H.A. and Purnama; writing—review and editing, U.S., T.A.K.P. and M.N.A.; project administration, D.H.; supervision, T.M. and U.S.; funding acquisition, U.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the facilities provided by Universitas Padjadjaran, Indonesia through the Academic Leadership Grant, Universitas Padjadjaran. No: 1959/UN6.3.1/PT.00/2023 awarded to Unang Supratman and Master Thesis Research Grant, (No. 1318/UN6.3.1/PT.00/2022) awarded to Desi Harneti. This investigation was funded by the Ministry of Education and Culture, Innovative and Research Council, Indonesia, Master Thesis Research (PTM) Grant and Universitas Padjadjaran under Academic Leadership Grant.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data can be found in this article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harneti, D.; Supratman, U. Phytochemistry and Biological Activities of Aglaia Species. Phytochemistry 2021, 181, 112540. [Google Scholar] [CrossRef]
- Sholikhah, E.N. Indonesian Medicinal Plants as Sources of Secondary Metabolites for Pharmaceutical Industry. J. Med. Sci. 2016, 48, 226–239. [Google Scholar] [CrossRef]
- Malgaonkar, M.; Shirolkar, A.; Murthy, S.N.; Pawar, S. Ayurvedic Plants with Antidiabetic Potential. In Medicinal Plants—Recent Advances in Research and Development; Tsay, H.S., Shyur, L.F., Agrawal, D., Wu, Y.C., Wang, S.Y., Eds.; Springer: Singapore, 2016. [Google Scholar]
- Liu, B.; Xu, Y.K. Cytotoxicity and Synergistic Effect of the Constituents from Roots of Aglaia Odorata (Meliaceae). Nat. Prod. Res. 2015, 30, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Berning, L.; Scharf, L.; Aplak, E.; Stucki, D.; Von Montfort, C.; Reichert, A.S.; Stahl, W.; Brenneisen, P. In Vitro Selective Cytotoxicity of the Dietary Chalcone Cardamonin (CD) on Melanoma Compared to Healthy Cells Is Mediated by Apoptosis. PLoS ONE 2019, 14, e0222267. [Google Scholar] [CrossRef] [PubMed]
- An, F.L.; Wang, X.B.; Wang, H.; Li, Z.R.; Yang, M.H.; Luo, J.; Kong, L.Y. Cytotoxic Rocaglate Derivatives from Leaves amof Aglaia perviridis. Sci. Rep. 2016, 6, 20045. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Woodard, J.L.; Lucas, D.M.; Fuchs, J.R.; Kinghorn, A.D. Rocaglamide, Silvestrol and Structurally Related Bioactive Compounds from Aglaia Species. Nat. Prod. Rep. 2014, 31, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fu, W.X.; Zeng, C.X.; Zhou, L.; Bao, M.F.; Cai, X.H. Two New Lignans from Twigs of Aglaia odorata. J. Asian Nat. Prod. Res. 2015, 18, 147–152. [Google Scholar] [CrossRef]
- Joycharat, N.; Greger, H.; Hofer, O.; Saifah, E. Flavaglines and Triterpenoids from the Leaves of Aglaia forbesii. Phytochemistry 2008, 69, 206–211. [Google Scholar] [CrossRef]
- Xie, B.J.; Yang, S.P.; Chen, H.D.; Yue, J.M. Agladupols A–E, Triterpenoids from Aglaia duperreana. J. Nat. Prod. 2007, 70, 1532–1535. [Google Scholar] [CrossRef]
- Yodsaoue, O.; Sonprasit, J.; Karalai, C.; Ponglimanont, C.; Tewtrakul, S.; Chantrapromma, S. Diterpenoids and Triterpenoids with Potential Anti-Inflammatory Activity from the Leaves of Aglaia odorata. Phytochemistry 2012, 76, 83–91. [Google Scholar] [CrossRef]
- Pervin, R.; Afrin, S.; Sabrin, F.; Zohora, U.S.; Rahman, M.S.; Islam, K.D.; Billah, M.M. Antioxidant, Antibacterial and Brine Shrimp Lethality Bioassay of Amoora cucullata, a Mangrove Plant. J. Young Pharm. 2016, 8, 33–38. [Google Scholar] [CrossRef]
- Rahman, M.S.; Rashid, M.A. Preliminary Antimicrobial and Cytotoxic Activities of Amoora cucullata Extractives. Orient. Pharm. Exp. Med. 2009, 9, 182–185. [Google Scholar] [CrossRef]
- An, F.L.; Xu, W.J.; Yang, M.H.; Luo, J.; Kong, L.Y. Anti-Inflammatory Flavagline Glycosides and Putrescine Bisamides from Aglaia perviridis Leaves. Tetrahedron 2020, 76, 131257. [Google Scholar] [CrossRef]
- Schneider, C.; Bohnenstengel, F.I.; Nugroho, B.W.; Wray, V.; Witte, L.; Hung, P.D.; Kiet, L.C.; Proksch, P. Insecticidal Rocaglarnide Derivatives from Aglaia spectabilis (Meliaceae). Phytochemistry 2000, 54, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Chaidir; Hiort, J.; Bohnenstengel, F.I.; Nugroho, B.W.; Schneider, C.; Wray, V.; Witte, L.; Hung, P.D.; Kiet, L.C.; Proksch, P. New Insecticidal Rocaglamide Derivatives from the Roots of Aglaia duperreana. J. Nat. Prod. 1999, 62, 1632–1635. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.H.; Song, Z.J.; Chen, L.Y.; Wen, H.J. Molluscicidal Activity of Aglaia duperreana and the Constituents of Its Twigs and Leaves. Fitoterapia 2012, 83, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Esimone, C.O.; Eck, G.; Nworu, C.S.; Hoffmann, D.; Überla, K.; Proksch, P. Dammarenolic Acid, a Secodammarane Triterpenoid from Aglaia sp. Shows Potent Anti-Retroviral Activity in Vitro. Phytomedicine 2010, 17, 540–547. [Google Scholar] [CrossRef]
- Joycharat, N.; Plodpai, P.; Panthong, K.; Yingyongnarongkul, B.E.; Voravuthikunchai, S. Terpenoid Constituents and Antifungal Activity of Aglaia forbesii Seed against Phytopathogens. Can. J. Chem. 2010, 88, 937–944. [Google Scholar] [CrossRef]
- Ngo, N.T.N.; Lai, N.T.D.D.T.; Le, H.C.; Nguyen, L.T.T.; Trinh, B.T.D.; Nguyen, H.D.; Pham, P.D.; Dang, S.V.; Nguyen, L.H.D. Chemical Constituents of Aglaia elaeagnoidea and Aglaia odorata and Their Cytotoxicity. Nat. Prod. Res. 2021, 36, 1494–1502. [Google Scholar] [CrossRef]
- Phongmaykin, J.; Kumamoto, T.; Ishikawa, T.; Saifah, E.; Suttisri, R. Biologically Active Constituents of Aglaia erythrosperma. Nat. Prod. Res. 2011, 25, 1621–1628. [Google Scholar] [CrossRef]
- Heads, M. Biogeography and Ecology in a Pantropical Family, the Meliaceae. Gard. Bull. Singap. 2019, 71, 335–461. [Google Scholar] [CrossRef]
- Meepol, W.; Maxwell, G.S.; Havanond, S. Aglaia cucullata: A Little-Known Mangrove with Big Potential for Research. ISME/GLOMIS Electron. J. 2020, 18, 4–9. [Google Scholar]
- Ahmed, F.; Toume, K.; Sadhu, S.K.; Ohtsuki, T.; Arai, M.A.; Ishibashi, M. Constituents of Amoora cucullata with TRAIL Resistance-Overcoming Activity. Org. Biomol. Chem. 2010, 8, 3696–3703. [Google Scholar] [CrossRef] [PubMed]
- Chumkaew, P.; Teerapongpisan, P.; Pechwang, J.; Srisawat, T. New Oxoprotoberberine and Aporphine Alkaloids from the Roots of Amoora cucullata with Their Antiproliferative Activites. Rec. Nat. Prod. 2019, 13, 491–498. [Google Scholar] [CrossRef]
- Abdelfattah, M.S.; Toume, K.; Ahmed, F.; Sadhu, S.K.; Ishibashi, M. Cucullamide, a New Putrescine Bisamide from Amoora cucullata. Chem. Pharm. Bull. 2010, 58, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Chumkaew, P.; Kato, S.; Chantrapromma, K. Potent Cytotoxic Rocaglamide Derivatives from the Fruits of Amoora cucullata. Chem. Pharm. Bull. 2006, 54, 1344–1346. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current State of Melanoma Diagnosis and Treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Sarasin, A.; Dessen, P. DNA Repair Pathways and Human Metastatic Malignant Melanoma. Curr. Mol. Med. 2010, 10, 413–418. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae. Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Chapter 2 of Cell Viability Assays. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays: Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1601, pp. 1–17. [Google Scholar]
- Khanh, P.N.; Tai, B.H.; Huong, T.T.; Cuong, T.D.; Hai, H.V.; Luong, N.X.; Kim, Y.H.; Cuong, N.M. Terpenoids from The Leaves and Stems of Dysoxylum tpongense. Vietnam. J. Sci. Technol. 2019, 57, 139–145. [Google Scholar] [CrossRef]
- Vedernikov, D.N.; Roshchin, V.I. Extractive Compounds of Betulaceae Family Birch Buds (Betula pendula Roth.): V. Composition of Triterpene Seco-Acids. Russ. J. Bioorg Chem. 2012, 38, 762–768. [Google Scholar] [CrossRef]
- Seger, C.; Pointinger, S.; Greger, H.; Hofer, O. Isoeichlerianic Acid from Aglaia silvestris and Revision of the Stereochemistry of Foveolin B. Tetrahedron Lett. 2008, 49, 4313–4315. [Google Scholar] [CrossRef]
- Hidayat, A.T.; Farabi, K.; Harneti, D.; Maharani, R.; Darwati; Nurlelasari; Mayanti, T.; Setiawan, A.S.; Supratman, U.; Shiono, Y. Cytotoxicity and Structure Activity Relationship of Dammarane-Type Triterpenoids from the Bark of Aglaia elliptica against P-388 Murine Leukemia Cells. Nat. Prod. Sci. 2017, 23, 291–298. [Google Scholar] [CrossRef]
- Golodov, V.A. Synergistic Phenomena in Chemistry. Eurasian Chem. Technol. J. 2000, 2, 29–34. [Google Scholar] [CrossRef]
- Chan, H.C.S.; Pan, L.; Li, Y.; Yuan, S. Rationalization of Stereoselectivity in Enzyme Reactions. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2019, 9, e1403. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).