Ecological State of Haplic Chernozem after Pollution by Oil at Different Levels and Remediation by Biochar
Abstract
:1. Introduction
2. Materials and Methods
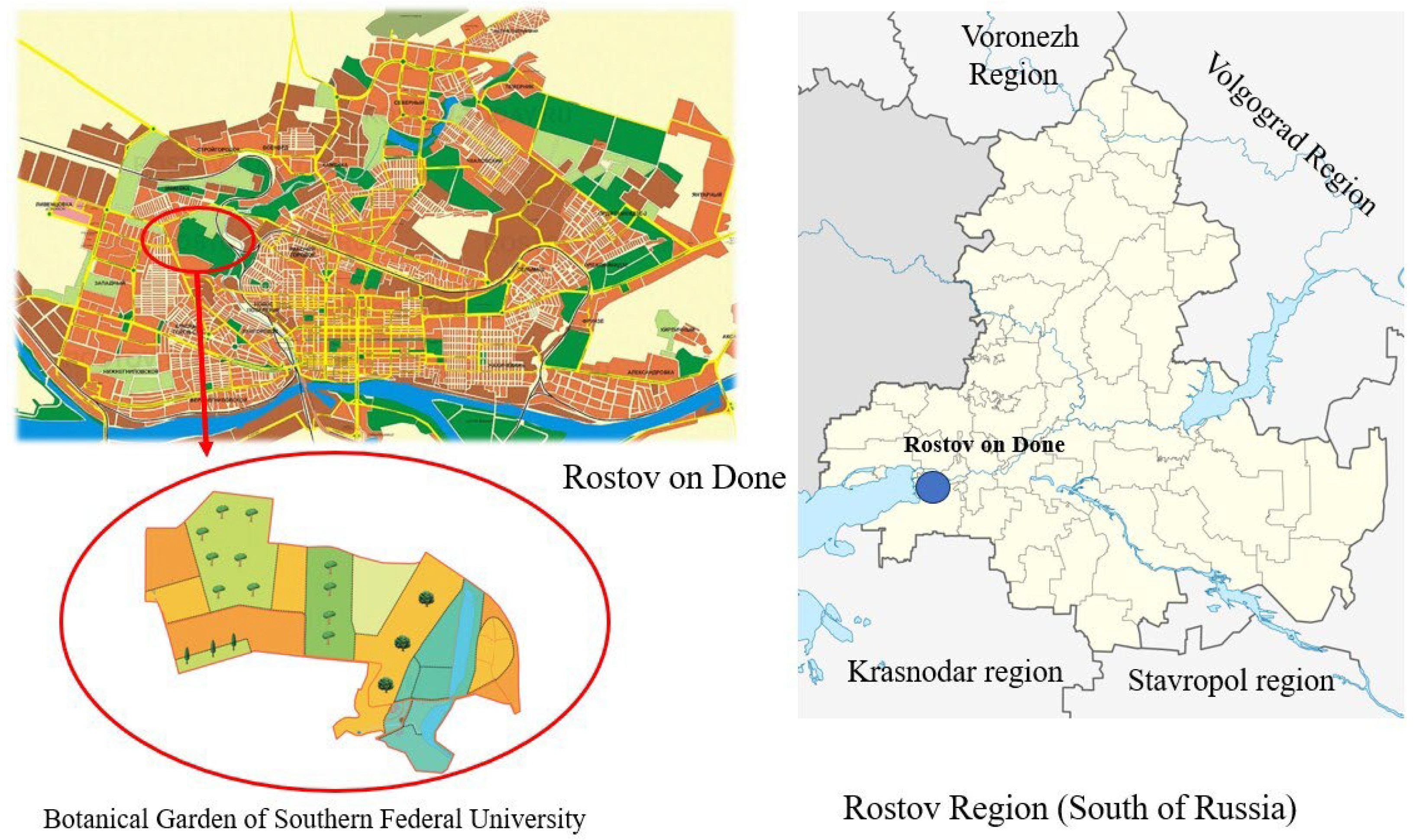
2.1. Study Object
2.2. Petroleum Hydrocarbons Characteristics
2.3. Biochar Characteristics
2.4. Simulation Experiment
2.5. Physical and Chemical Characteristics of Uncontaminated Soil
2.6. Biological Indicators
2.7. Statistical Processing
3. Results
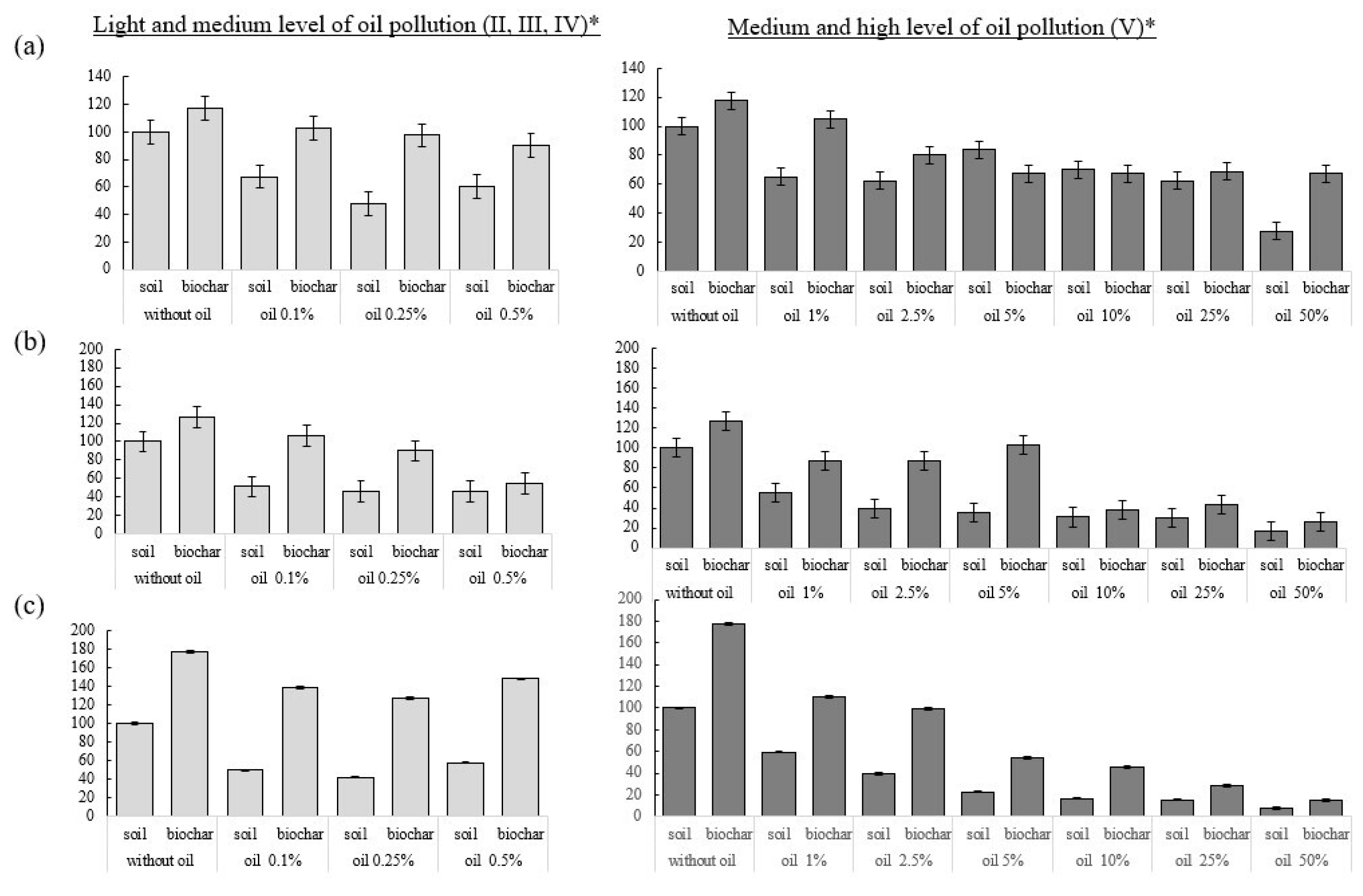
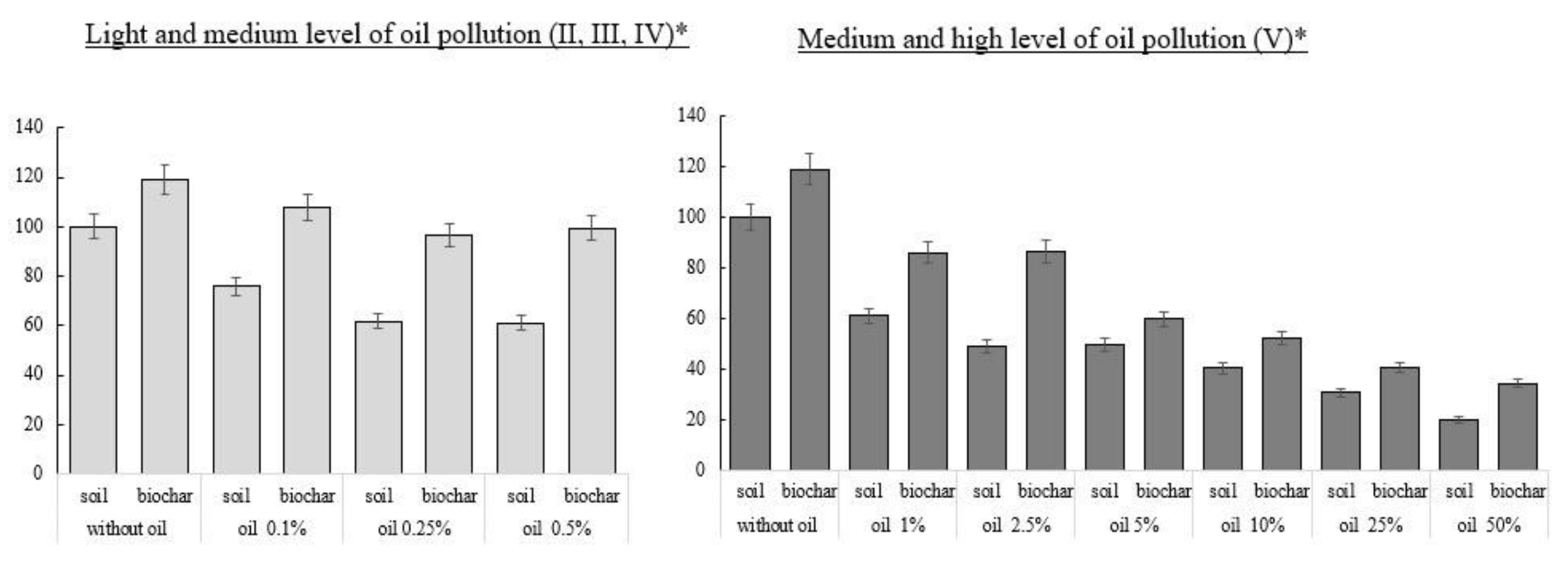
3.1. Indicators of the Intensity of Initial Growth and Germination of Radish Seeds
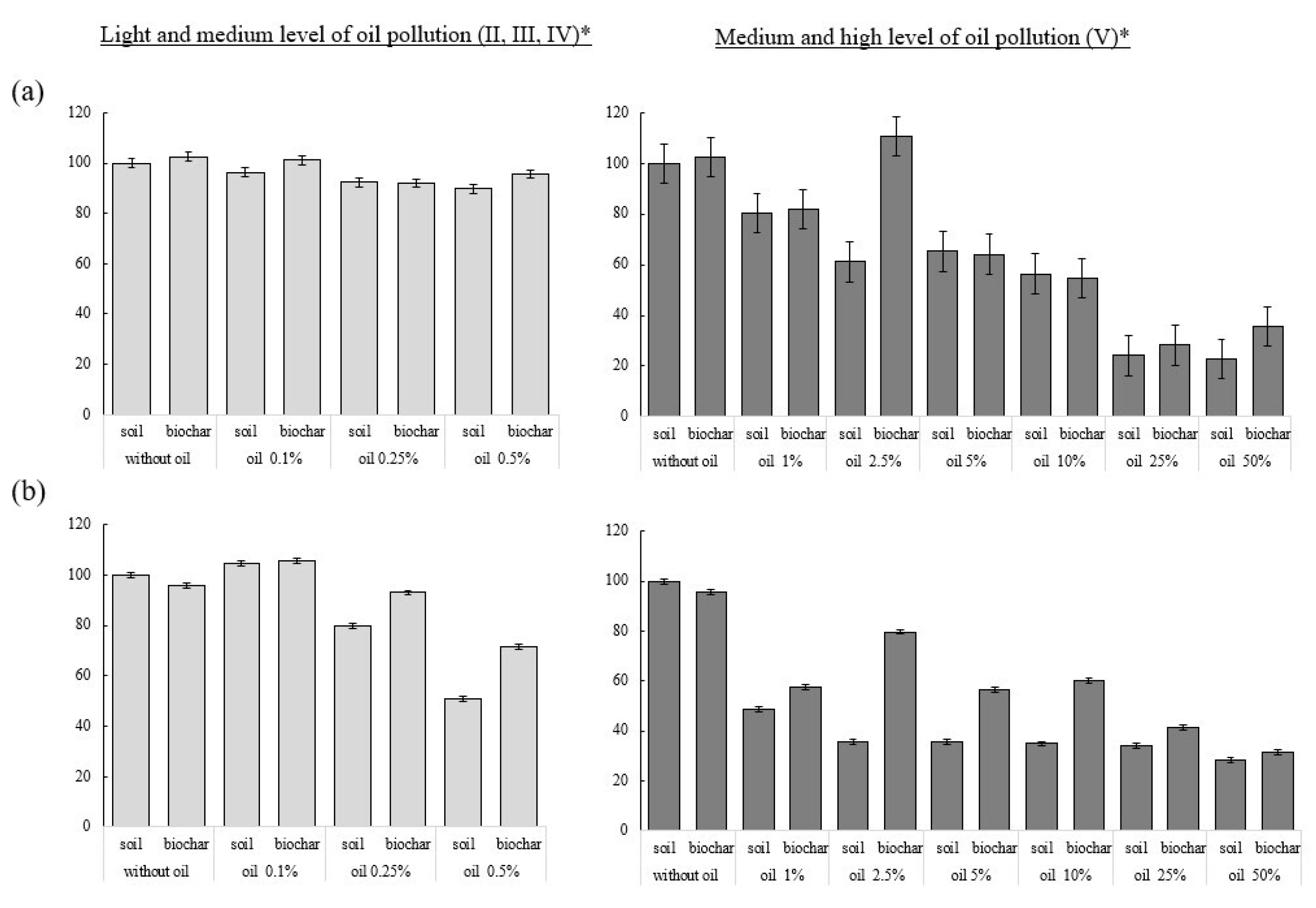
3.2. Activity of Soil Enzymes
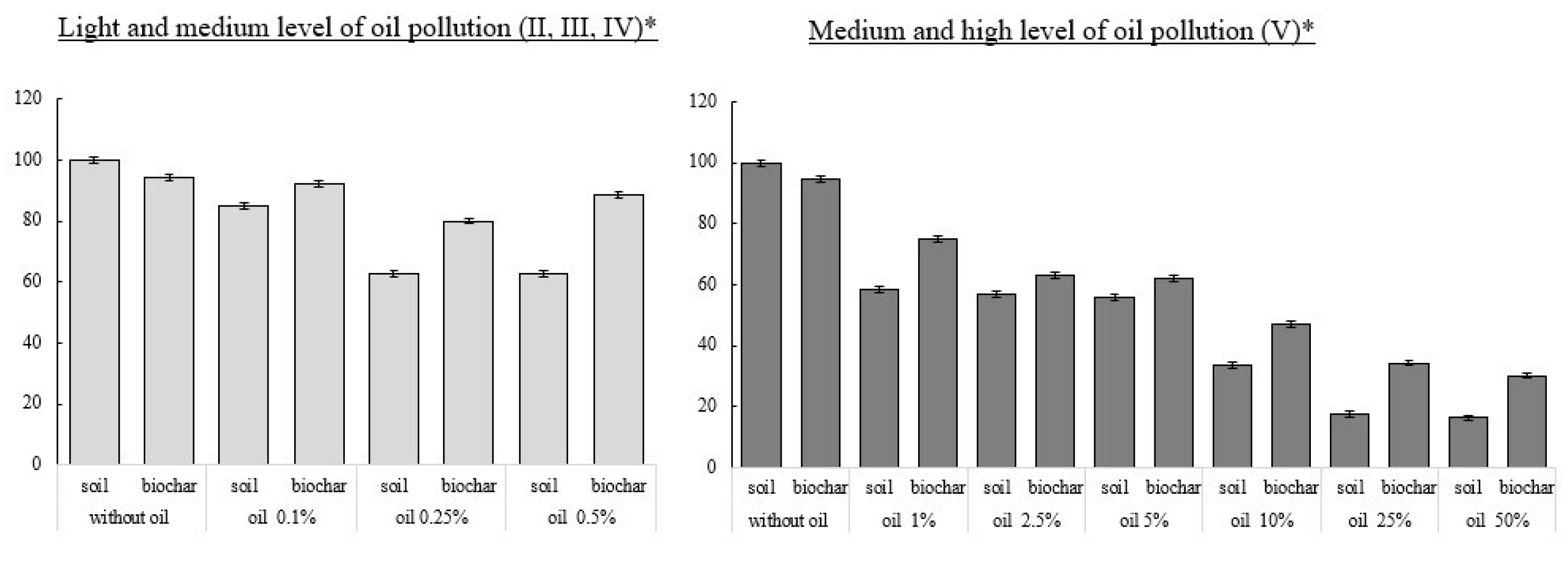
3.3. Total Number of Bacteria
3.4. Integral Indicator of the Biological State of Soils
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.; Yan, C.; Li, Z. Microalgal cultivation with biogas slurry for biofuel production. Bioresour. Technol. 2016, 220, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Lv, Y.; Liu, C.; Li, S.; Yin, Z.; Yu, Y.; Zhu, L. Performance evaluation of rhamnolipids addition for the biodegradation and bioutilization of petroleum pollutants during the composting of organic wastes with waste heavy oil. iScience 2022, 25, 104403. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Zhang, C.; Zhou, X.; Yin, Z.; Hu, T.; Hu, D.; Liu, C.; Zhu, L. Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 714, 136767. [Google Scholar] [CrossRef]
- Lv, Y.; Bao, J.; Dang, Y.; Liu, D.; Li, T.; Li, S.; Yu, Y.; Zhu, L. Biochar aerogel enhanced remediation performances for heavy oil-contaminated soil through biostimulation strategy. J. Hazard. Mater. 2023, 443, 130209. [Google Scholar] [CrossRef]
- British Petroleum, 2020. BP Statistical Review of World Energy 2020. 69 Ed. London, UK. Available online: https://www.bp.com/content/dam/bp/businesssites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-statsreview-2020-oil.pdf (accessed on 20 April 2023).
- Dike, C.C.; Shahsavari, E.; Surapaneni, A.; Shah, K.; Ball, A.S. Can biochar be an effective and reliable biostimulating agent for the remediation of hydrocarbon contaminated soils? Environ. Int. 2021, 154, 106553. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Bao, J.; Zhu, L.A. Comprehensive review of recent and perspective technologies and challenges for the remediation of oil-contaminated sites. Energy Rep. 2022, 8, 7976–7988. [Google Scholar] [CrossRef]
- Hou, D.; Al-Tabbaa, A. Sustainability: A new imperative in contaminated land remediation. Environ. Sci. Policy 2014, 39, 25–34. [Google Scholar] [CrossRef]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Sun, S.; Tang, F.; Chen, H.; Chen, S.; Zhao, C.; Li, L. A novel chitosan-biochar immobilized microorganism strategy to enhance bioremediation of crude oil in soil. Chemosphere 2023, 313, 137367. [Google Scholar] [CrossRef]
- Lomza, P.; Poszytek, K.; Sklodowska, A.; Drewniak, L. Evaluation of bioremediation of soil highly contaminated by petroleum hydrocarbons. New Biotechnol. 2016, 33, 141. [Google Scholar] [CrossRef]
- Ren, H.Y.; Wei, Z.J.; Wang, Y.; Deng, Y.P.; Li, M.Y.; Wang, B. Effects of biochar properties on the bioremediation of the petroleum-contaminated soil from a shale-gas field. Environ. Sci. Pollut. Res. 2020, 27, 36427–36438. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.; Azzi, E.S.; Enell, A.; Sundberg, C. Biochar produced from wood waste for soil remediation in Sweden: Carbon sequestration and other environmental impacts. Sci. Total Environ. 2021, 776, 145953. [Google Scholar] [CrossRef] [PubMed]
- Minnikova, T.; Kolesnikov, S.; Ruseva, A.; Kazeev, K.; Minkina, T.; Mandzhieva, S.; Sushkova, S. Influence of the Biochar on Petroleum Hydrocarbon Degradation Intensity and Ecological Condition of Haplic Chernozem. Eurasian J. Soil. Sci. 2022, 11, 157–166. [Google Scholar] [CrossRef]
- Vasilyeva, G.; Mikhedova, E.; Zinnatshina, L.; Strijakova, E.; Akhmetov, L.; Sushkova, S.; Ortega-Calvo, J.-J. Use of natural sorbents for accelerated bioremediation of grey forest soil contaminated with crude oil. Sci. Total Environ. 2022, 850, 157952. [Google Scholar] [CrossRef] [PubMed]
- Blenis, N.; Hue, N.; Maaz, T.M.; Kantar, M. Biochar Production, Modification, and Its Uses in Soil Remediation: A Review. Sustainability 2023, 15, 3442. [Google Scholar] [CrossRef]
- Ji, M.; Wang, X.; Usman, M.; Liu, F.; Dan, Y.; Zhou, L.; Campanaro, S.; Luo, G.; Sang, W. Effects of different feedstocks-based biochar on soil remediation: A review. Environ. Pollut. 2022, 294, 118655. [Google Scholar] [CrossRef] [PubMed]
- Meynet, P.; Moliterni, E.; Davenport, R.J.; Sloan, W.T.; Camacho, J.V.; Werner, D. Predicting the effects of biochar on volatile petroleum hydrocarbon biodegradation and emanation from soil: A bacterial community finger-print analysis inferred modelling approach. Soil. Biol. Biochem. 2014, 68, 20–30. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Tang, W.; Tang, L.; Dong, H.; Huang, B.; et al. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Mosa, A.; El-Naggar, A.; Hossain, M.F.; Abdelrahman, H.; Niazi, N.K.; Shaheed, M.; Zhang, T.; Tsany. Y.F.; Trakal, L.; et al. Manganese oxide-modified biochar: Production, characterization and applications for the removal of pollutants from aqueous environments-a review. Bioresour. Technol. 2022, 346, 126581. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, Y.; Li, J.; Fang, Z.; Bolan, N.; Bhatnagar, A.; Gao, B.; Hou, D.; Wang, S.; Song, H.; et al. Engineered biochar for environmental decontamination in aquatic and soil systems: A review. Carbon. Res. 2022, 1, 4. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil. Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Q.; Wang, X.; Chen, X.; Wang, Y.; Mao, Z. Effects of biochar on replant disease by amendment soil environment. Commun. Soil. Sci. Plant Anal. 2021, 52, 673–685. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biolo. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Bruun, S.; El-Zehery, T. Biochar effect on the mineralization of soil organic matter. Pesqui. Agropecuária Bras. 2012, 47, 665–671. [Google Scholar] [CrossRef]
- Karim, M.R.; Halim, M.A.; Gale, N.V.; Thomas, S.C. Biochar effects on soil physiochemical properties in degraded managed ecosystems in northeastern Bangladesh. Soil. Syst. 2020, 4, 69. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Tian, J. Biochar-facilitated soil remediation: Mechanisms and efficacy variations. Front. Environ. Sci. 2020, 8, 183. [Google Scholar] [CrossRef]
- Limwikran, T.; Kheoruenromne, I.; Suddhiprakarn, A.; Prakongkep, N.; Gilkes, R.J. Most plant nutrient elements are retained by biochar in soil. Soil. Syst. 2019, 3, 75. [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Kazeev, K.S.; Denisova, T.V.; Dadenko, E.V.; Mazanko, M.S.; Rotina, E.N. Methodology to evaluate the feasibility and effectiveness of remediation of soils contaminated by oil and oil products, the biological indicators. Eng. Bull. Don. 2013, 3, 51–55. [Google Scholar]
- Letter of the Ministry of Environmental Protection and Natural Resources of the Russian Federation dated December 27, 1993, No. 04-25-61-5678(D), “Procedure for determining the extent of damage from land pollution by chemicals” 1993, 21. Available online: https://docs.cntd.ru/document/556185926 (accessed on 4 September 2023). (In Russian).
- Okolelova, A.; Zheltobryukhov, V.; Nefedieva, E.; Egorova, G.; Kaplya, V.; Merzlyakova, A. Peculiarities of assessment of content of oil products in soils. E3S Web Conf. 2020, 161, 01100. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; Volume 234. [Google Scholar]
- GOST 7657-84 Charcoal. Specifications, 8 p. Available online: https://data.1000gost.ru/catalog/Data/209/20941.pdf (accessed on 1 July 2023). (In Russian).
- Wylie, E.M.; Colletti, L.M.; Walker, L.F.; Lujan, E.J.; Garduno, K.; Mathew, K.J. Comparison of the Davies and Gray titrimetric method with potassium dichromate and ceric titrants. J. Radioanal. Nucl. Chem. 2018, 318, 227–233. [Google Scholar] [CrossRef]
- Takamoto, A.; Takahashi, T.; Togami, K. Estimation models from soil pH with a solid-to-liquid ratio of 1:2.5 to pH measured by other methods using soils in Japan. Soil. Sci. Plant Nutr. 2023, 69, 190–198. [Google Scholar] [CrossRef]
- Bado, S.; Forster, B.P.; Ghanim, A.M.A.; Jankowicz-Cieslak, J.; Berthold, G.; Luxiang, L. Protocol for measuring soil salinity. In Protocols for Pre-Field Screening of Mutants for Salt Tolerance in Rice, Wheat and Barley; Springer: Cham, Switzerland, 2016; Volume 44. [Google Scholar] [CrossRef]
- An, Y.; Yamin, W.; Ngin, T.S.; Yusof, M.; Lokman, M.D.; Subhadip, G.; Zhong, C. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Dikarev, A.V.; Dikarev, V.G.; Dikareva, N.S. Study of lead phytotoxicity for radish and lettuce plants grown on different soil types. Agrochemistry 2021, 3, 71–81. [Google Scholar] [CrossRef]
- Myasnikova, I.B.; Pavlova, S.M. Determination of the joint effect of heavy metals and petroleum products on soil phytotoxicity. Russ. J. Water Transp. 2022, 72, 2260–2266. [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Kazeev, K.S.; Akimenko, Y.V. Development of regional standards for pollutants in the soil using biological parameters. Environ. Monit. Assess. 2019, 191, 544. [Google Scholar] [CrossRef] [PubMed]
- Micuți, M.-M.; Bădulescu, L.; Burlacu, A.; Israel-Roming, F. Activity of peroxidase and catalase in soils as influenced by some insecticides and fungicides. AgroLife Sci. J. 2018, 7, 2. [Google Scholar]
- Mikayilov, F. Assessment of the main parameters of catalase kinetcs in soil. Living Bio-Inert. Syst. 2018, 25, 1–7. [Google Scholar]
- Dadenko, E.V.; Denisova, T.V.; Kazeev, K.S.; Kolesnikov, S.I. Applicability of enzyme activity indices for soil bioindication and monitoring. Volga Ecol. J. 2013, 4, 385–393. (In Russian) [Google Scholar]
- Małachowska-Jutsz, A.; Matyja, K. Discussion on methods of soil dehydrogenase determination. Int. J. Environ. Sci. Technol. 2019, 16, 7777–7790. [Google Scholar] [CrossRef]
- Myszura, M.; Żukowska, G.; Kobyłka, A.; Mazurkiewicz, J. Enzymatic Activity of Soils Forming on an Afforested Heap from an Opencast Sulphur Mine. Forests 2021, 12, 1469. [Google Scholar] [CrossRef]
- Wolińska, A.; Zapasek, M.; Stępniewska, Z. The optimal TTC dose and its chemical reduction level during soil dehydrogenase activity assay. Acta Agrophysica 2016, 23, 303–314. [Google Scholar]
- Klauth, P.; Wilhelm, R.; Klumpp, E.; Poschen, L.; Groeneweg, J. Enumeration of soil bacteria with the green fluorescent nucleic acid dye Sytox green in the presence of soil particles. J. Microbiol. Methods 2004, 59, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Polyanskaya, L.M.; Pinchuk, I.P.; Stepanov, A.L. Comparative analysis of the luminescence microscopy and cascade filtration methods for estimating bacterial abundance and biomass in the soil: Role of soil suspension dilution. Eurasian Soil. Sci. 2017, 50, 1173–1176. [Google Scholar] [CrossRef]
- Wu, M.; Dick, W.A.; Li, W.; Wang, X.; Yang, Q.; Wang, T.; Xu, L.; Zhang, M.; Chen, L. Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. Int. Biodeterior. Biodegrad. 2016, 107, 158–164. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Bakina, L.G.; Chugunova, M.V.; Mayachkina, N.V.; Gerasimov, A.O.; Bure, V.M. Effect of remediation strategies on biological activity of oil-contaminated soil-A field study. Int. Biodeterior. Biodegrad. 2018, 26, 57–68. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Sukharevich, V.I. Soil enzymes and soil pollution: Biodegradation, bioremediation, bioindication. Agrochemistry 2020, 3, 83–93. [Google Scholar] [CrossRef]
- Chakravarty, P.; Chowdhury, D.; Deka, H. Ecological risk assessment of priority PAHs pollutants in crude oil contaminated soil and its impacts on soil biological properties. J. Hazard. Mater. 2022, 437, 129325. [Google Scholar] [CrossRef] [PubMed]
- Cherdakova, A.S.; Galchenko, S.V. Change of phytotoxicity of soils contaminated with oil products in the process of their microbiological remediation during the application of humic preparations. Bull. Peoples′ Friendsh. Univ. Russia. Ser. Ecol. Life Saf. 2020, 28, 336–348. [Google Scholar] [CrossRef]
- Adelfinskaya, E.A.; Myazin, V.A. The use of activated peat for the re-cultivation of soils contaminated with oil products. Bull. RUFP. Ser. Ecol. Life Saf. 2020, 28, 160–171. [Google Scholar] [CrossRef]
- Ovsyannikova, I.V.; Pryanichnikova, V.V.; Kadyrov, R.R.; Kichigin, I.A.; Shugaepov, I.R.; Yapparov, B.A. Study of the toxic properties of oil-contaminated soil using biotesting methods. Environ. Control Syst. 2022, 1, 81–85. [Google Scholar] [CrossRef]
- Panchenko, L.; Muratova, A.; Dubrovskaya, E.; Golubev, S.; Turkovskaya, O. Natural and Technical Phytoremediation of Oil-Contaminated Soil. Life 2023, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Masakorala, K.; Yao, J.; Guo, H.; Chandankere, R.; Wang, J.; Cai, M.; Liu, H.; Choi, M.M. Phytotoxicity of Long-Term Total Petroleum Hydrocarbon-Contaminated Soil—A Comparative and Combined Approach. Water Air Soil Pollut. 2013, 224, 1153. [Google Scholar] [CrossRef]
- Minnikova, T.V.; Ruseva, A.S.; Kolesnikov, S.I. Evaluation of the enzymatic activity of haplic chernozem contaminated by petroleum hydrocarbons after bioremediation. Izv. Timiryazev Agric. Acad. 2022, 5, 5–20. [Google Scholar] [CrossRef]
- Minnikova, T.; Ruseva, A.; Kolesnikov, S. Assessment of ecological state of soils in southern Russia by petroleum hydrocarbons pollution after bioremediation. Environ. Process. 2022, 9, 49. [Google Scholar] [CrossRef]
- Ruseva, A.S.; Minnikova, T.V.; Kolesnikov, S.I.; Revina, S.Y.; Evstegneeva, N.A.; Trufanov, D.A. Effects of Baikal EM-1 on the Ecological State of Haplic Chernozem with Oil, Crude Oil, and Gasoline Pollution. Russ. Agric. Sci. 2022, 48, 369–377. [Google Scholar] [CrossRef]
- Kireeva, H.A.; Vodopyanov, V.V.; Miftakhova, A.M. Biological Activity of Oil-Contaminated Soils; Gilem: Ufa, Russia, 2016; Volume 376. [Google Scholar]
- Wyszkowska, J.; Wyszkowski, M. Activity of soil dehydrogenases, urease, and acid and alkaline phosphatases in soil polluted with petroleum. Toxicol. Environ. Health 2010, 73, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Polyak, Y.; Shigaeva, T.; Gubelit, Y.; Bakina, L.; Kudryavtseva, V.; Polyak, M. Sediment microbial activity and its relation to environmental variables along the eastern Gulf of Finland coastline. J. Mar. Sys. 2017, 171, 101–110. [Google Scholar] [CrossRef]
- Saeed, M.; Ilyas, N.; Jayachandran, K.; Gaffar, S.; Arshad, M.; Ahmad, M.S.; Bibi, F.; Jeddi, K.; Hessini, K. Biostimulation potential of biochar for remediating the crude oil contaminated soil and plant growth. Saudi J. Biol. Sci. 2021, 28, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. 2019, 42, 2495–2518. [Google Scholar] [CrossRef] [PubMed]
- Bykova, M.V. The problem of rationing in assessing the level of soil pollution with oil products. Eurasian Sci. J. 2019, 6, 1–7. [Google Scholar]
| Physical and Chemical Properties | Content |
|---|---|
| Density, g/m3 | 0.818 |
| Mass fraction of water, % | 0.270 |
| Mass fraction of sulfur, % | 0.430 |
| Mass fraction of mechanical impurities, % | 0.0028 |
| Level | Concentrations, % of Oil of the Soil Mass | Conversion of Oil, in mg of PHC in 1 kg of Soil | Level of Pollution of Soil |
|---|---|---|---|
| I | <0.10 | <1000 | acceptable |
| II | 0.10 | 1000 | light pollution |
| III | 0.25 | 2500 | light pollution |
| IV | 0.50 | 5000 | medium pollution |
| V | 1.00 | 10,000 | medium pollution |
| V | 2.50 | 25,000 | medium pollution |
| V | 5.00 | 50,000 | heavy pollution |
| V | 10.00 | 100,000 | heavy pollution |
| V | 25.00 | 250,000 | heavy pollution |
| V | 50.00 | 500,000 | heavy pollution |
| Biological Indicators | Introduction of Biochar | |
|---|---|---|
| Before | After | |
| Length of roots | −0.79 * | −0.95 * |
| Length of shoots | −0.82 * | −0.93 * |
| Germination | −0.49 | −0.86 * |
| Activity of catalase | −0.98 * | −0.79 * |
| Activity of dehydrogenases | −0.98 * | −0.90 * |
| The number of bacteria | −0.99 * | −0.98 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruseva, A.; Minnikova, T.; Kolesnikov, S.; Revina, S.; Trushkov, A. Ecological State of Haplic Chernozem after Pollution by Oil at Different Levels and Remediation by Biochar. Sustainability 2023, 15, 13375. https://doi.org/10.3390/su151813375
Ruseva A, Minnikova T, Kolesnikov S, Revina S, Trushkov A. Ecological State of Haplic Chernozem after Pollution by Oil at Different Levels and Remediation by Biochar. Sustainability. 2023; 15(18):13375. https://doi.org/10.3390/su151813375
Chicago/Turabian StyleRuseva, Anna, Tatyana Minnikova, Sergey Kolesnikov, Sofia Revina, and Anatoly Trushkov. 2023. "Ecological State of Haplic Chernozem after Pollution by Oil at Different Levels and Remediation by Biochar" Sustainability 15, no. 18: 13375. https://doi.org/10.3390/su151813375
APA StyleRuseva, A., Minnikova, T., Kolesnikov, S., Revina, S., & Trushkov, A. (2023). Ecological State of Haplic Chernozem after Pollution by Oil at Different Levels and Remediation by Biochar. Sustainability, 15(18), 13375. https://doi.org/10.3390/su151813375








