Hydrochemical and Isotopic Explanations of the Interaction between Surface Water and Groundwater in a Typical-Desertified Steppe of Northern China
Abstract
:1. Introduction
2. Materials and Methods
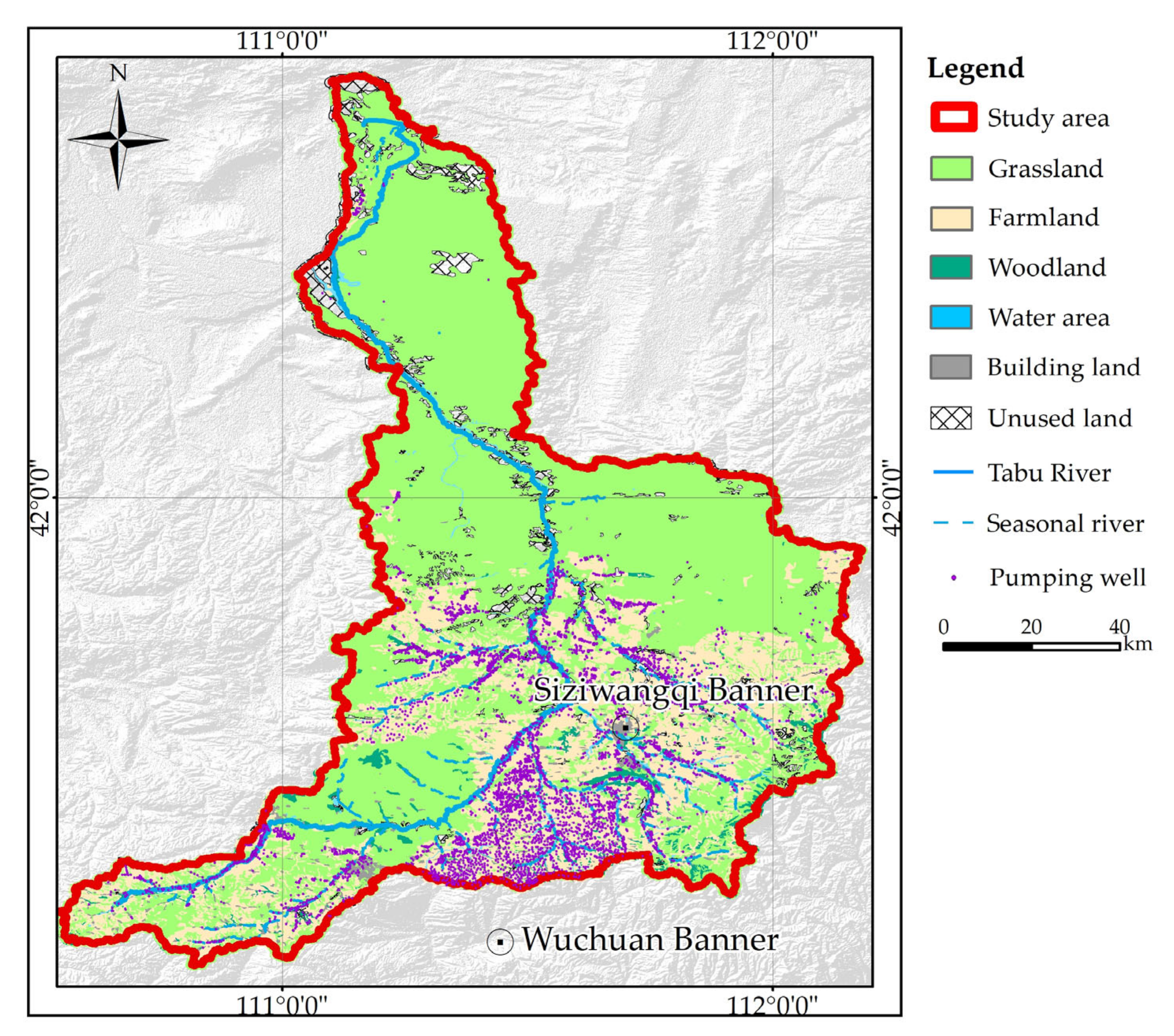
2.1. Study Area
2.2. Methods and Data Preparation
2.2.1. Methods
2.2.2. Data Preparation
3. Results
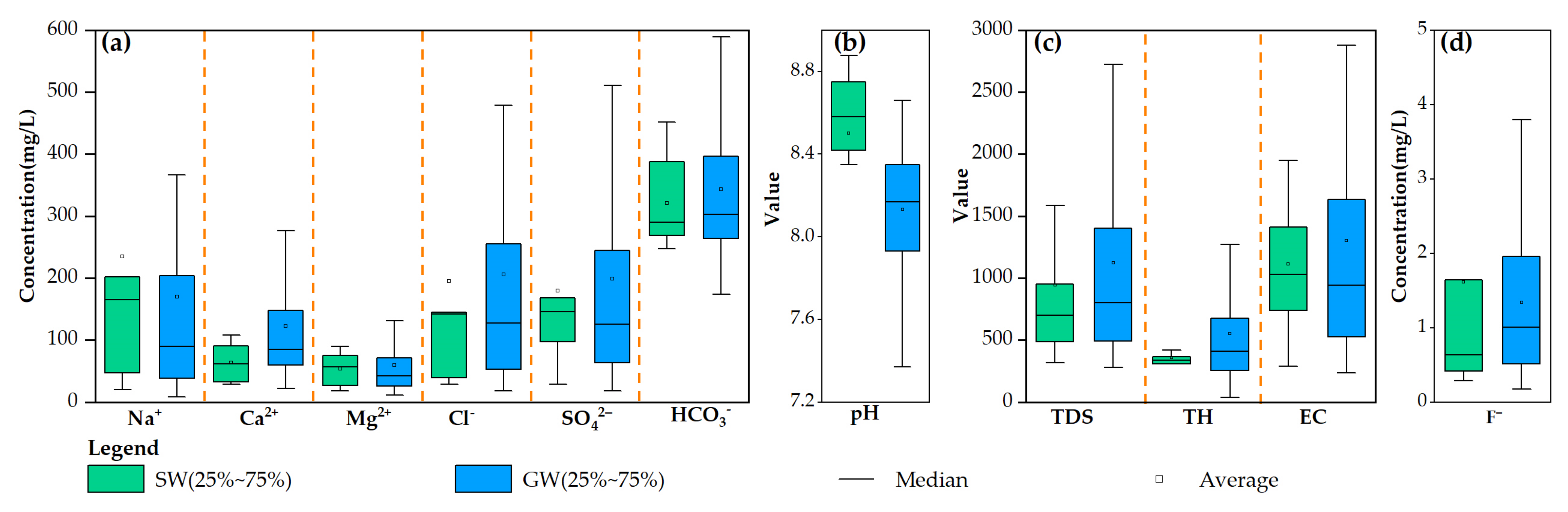
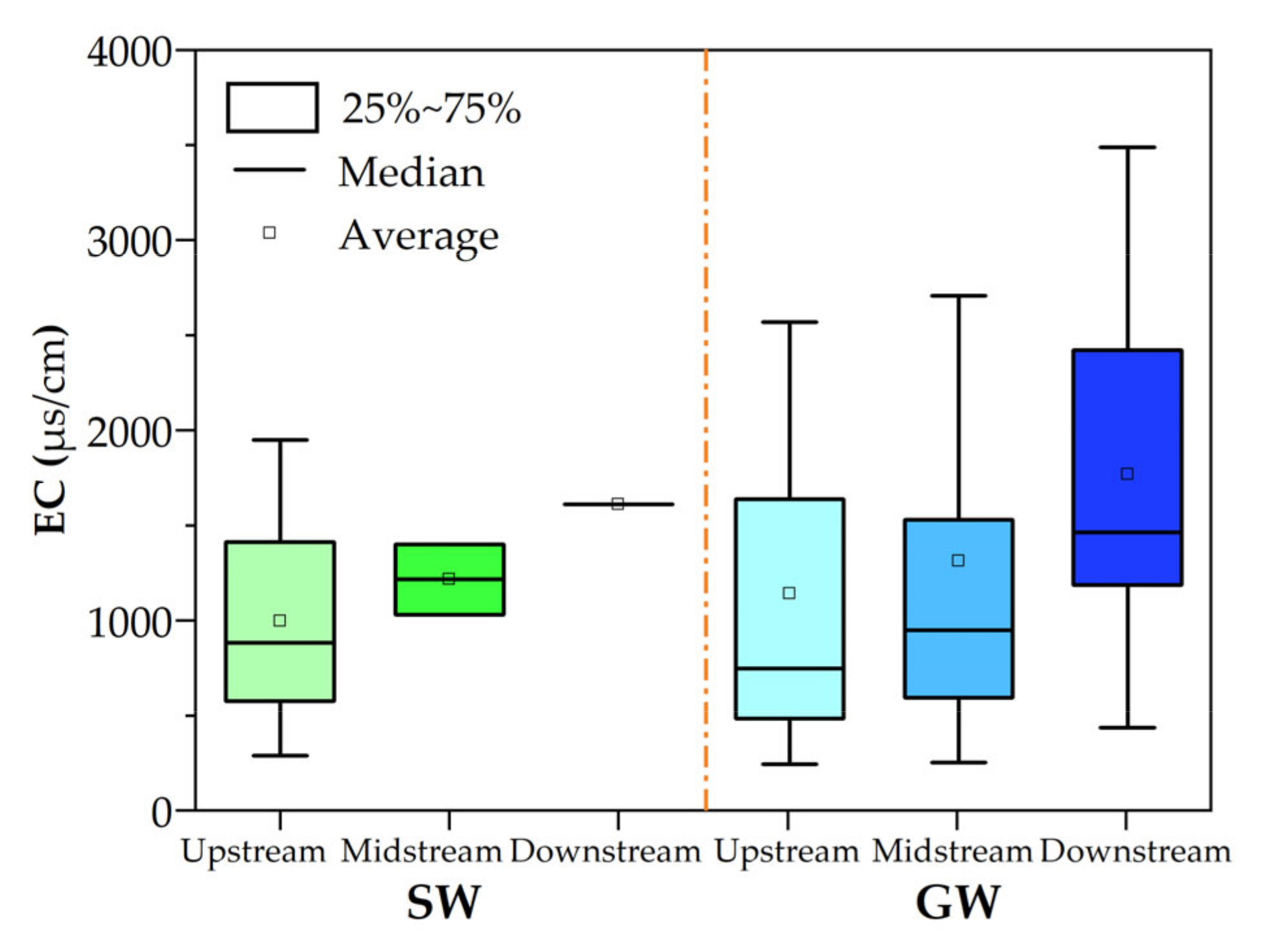
3.1. Hydrochemical Characteristics
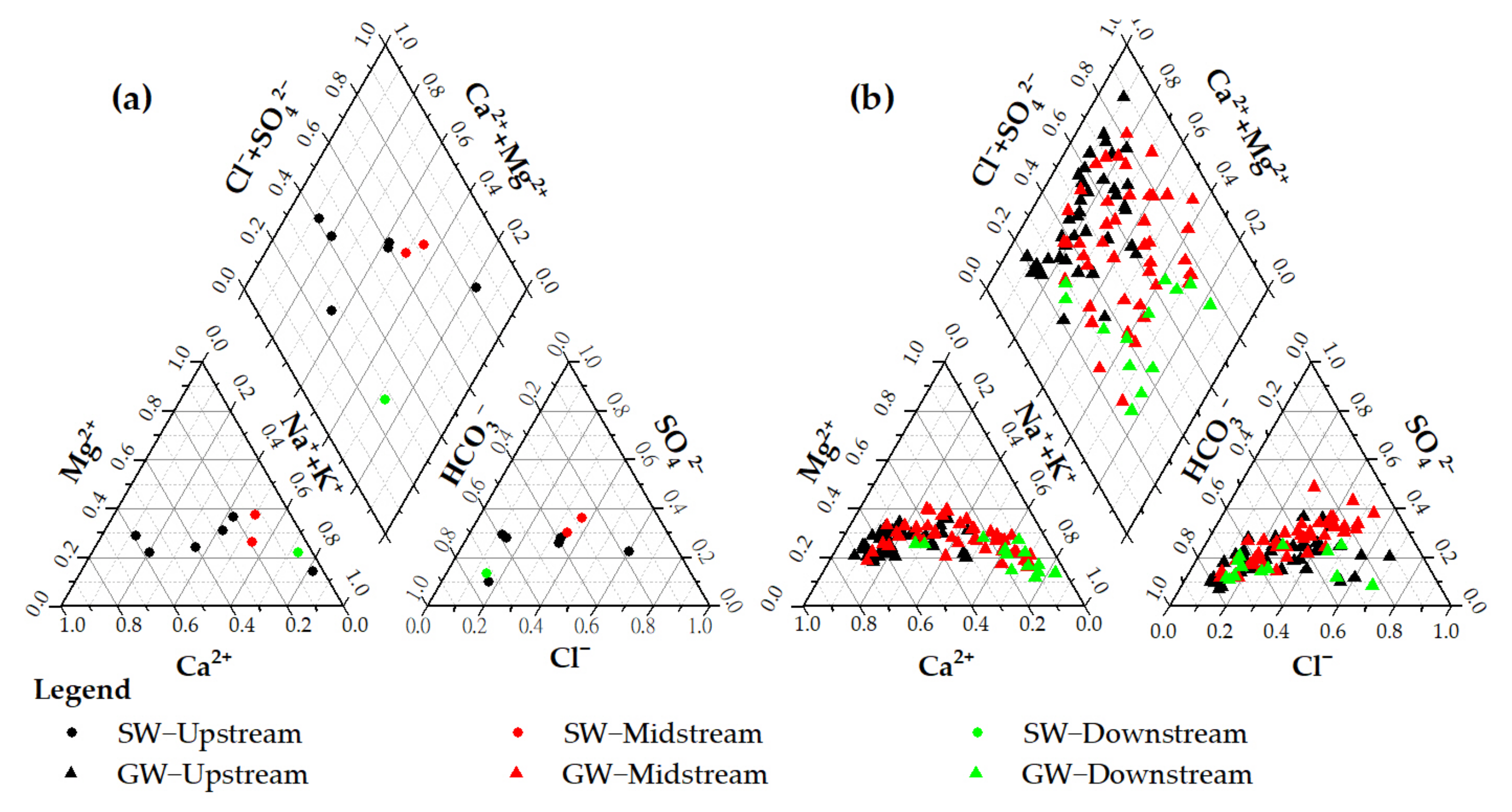
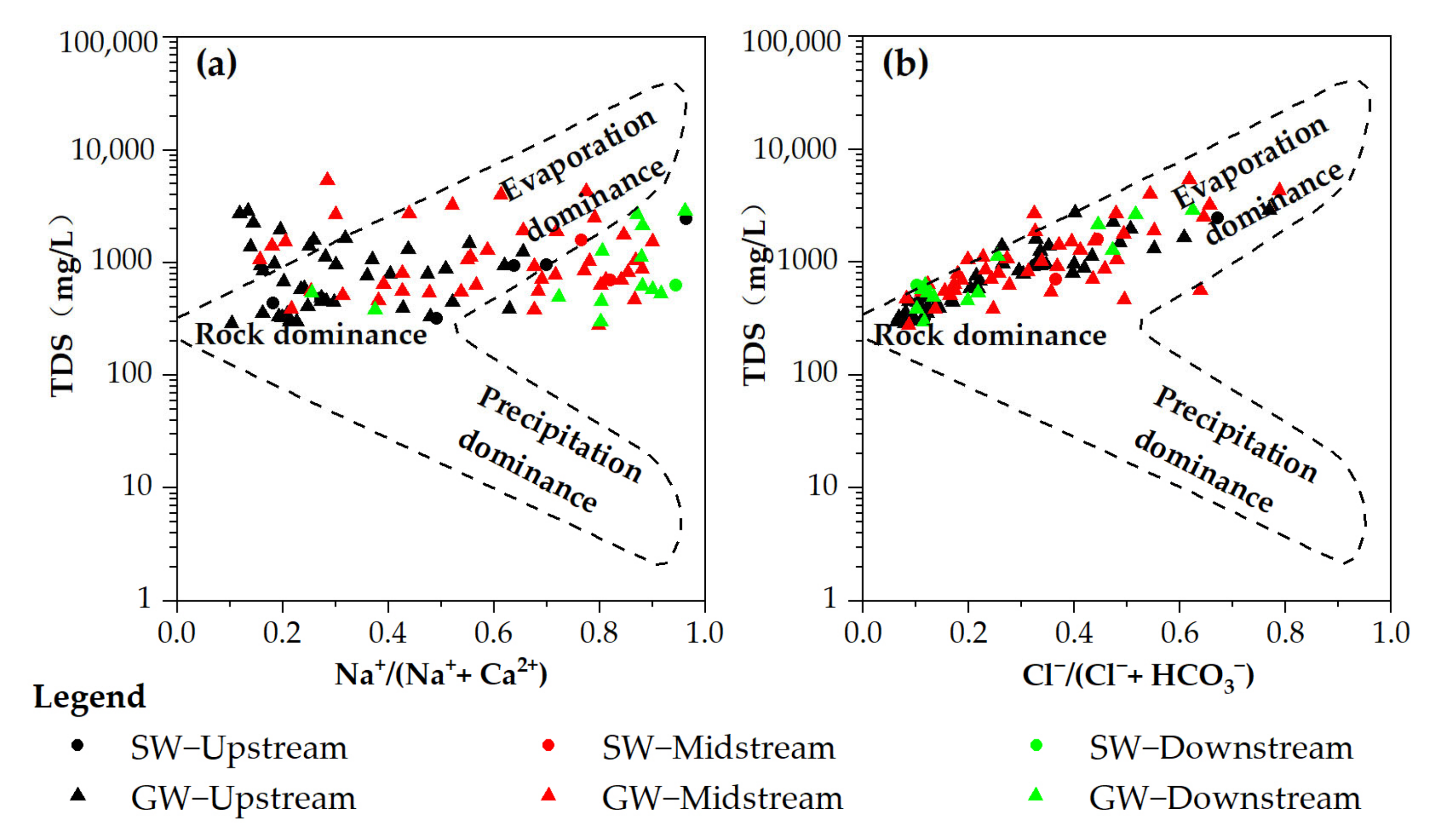
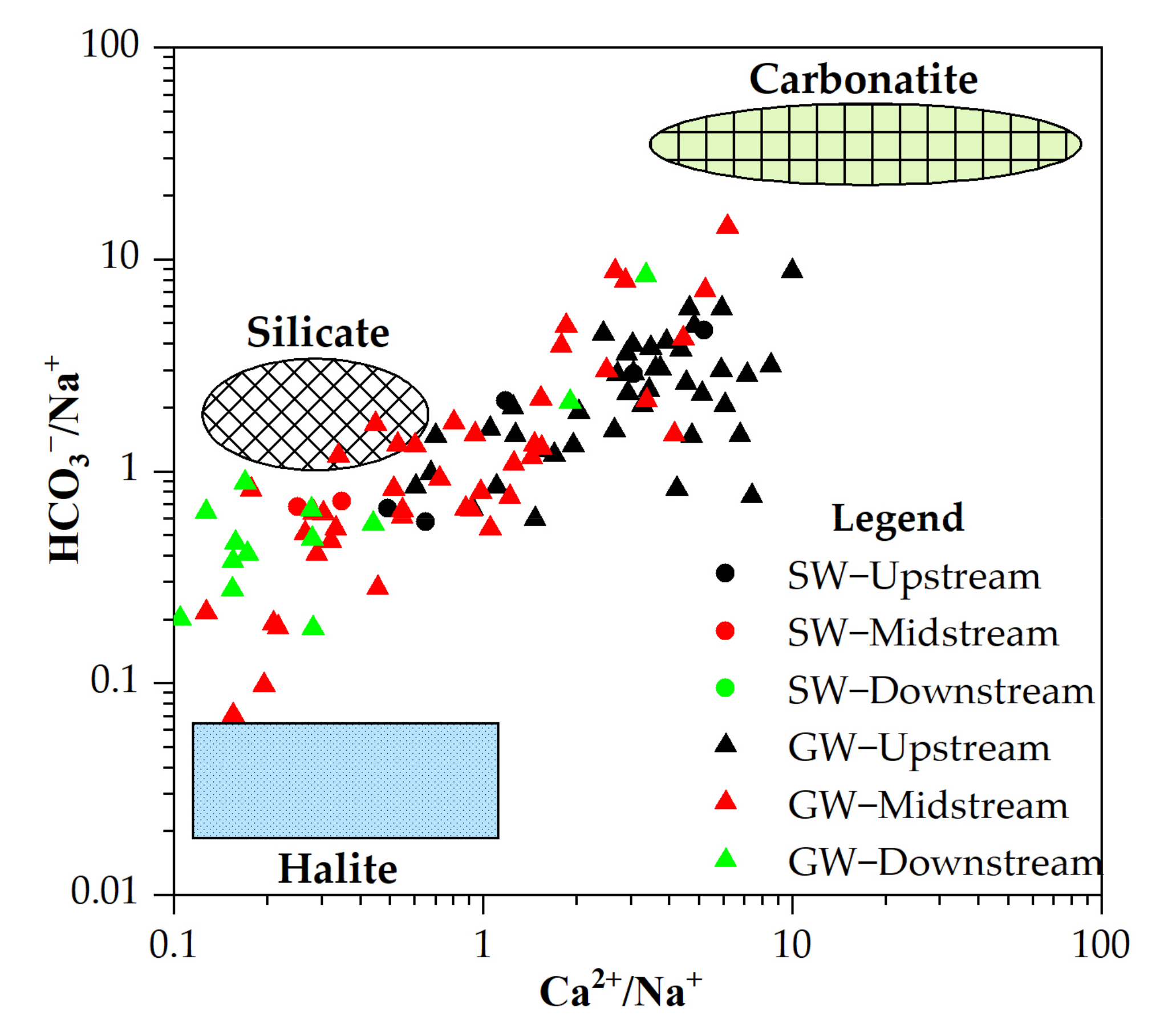
3.2. Hydrochemical Evolutionary Mechanism
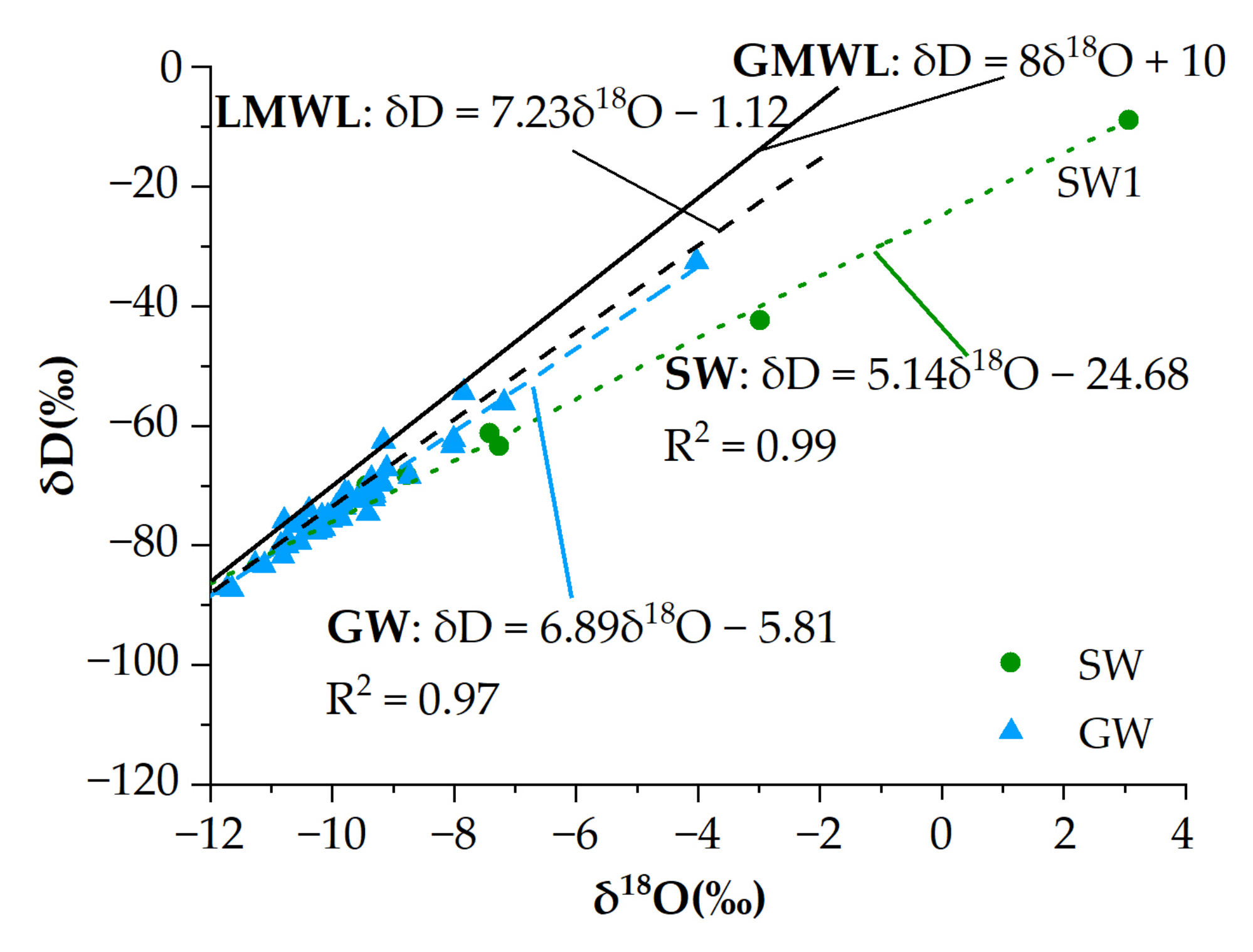
3.3. Isotopic Compositions
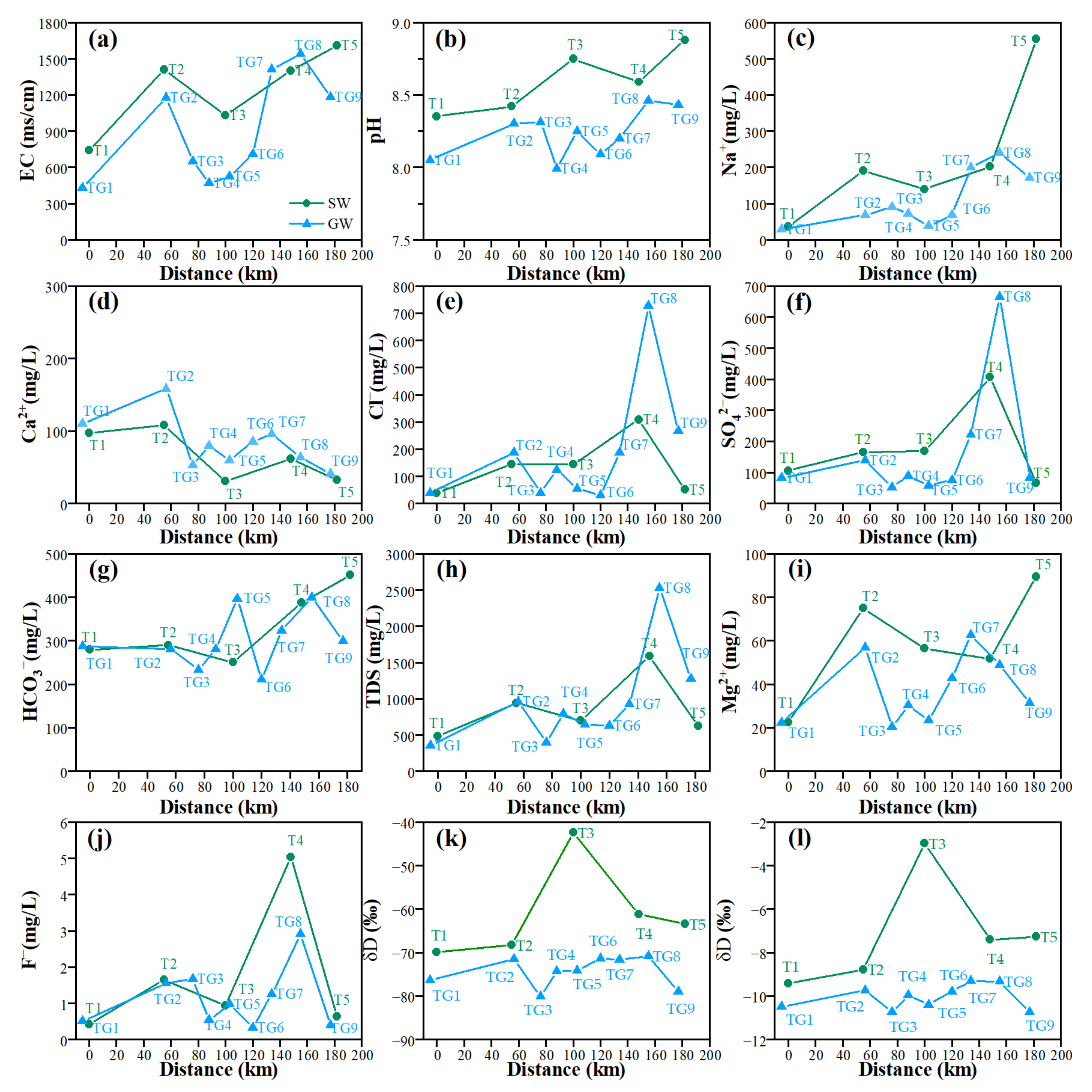
3.4. Analysis of Hydrochemical Indices and Isotopic Characteristics along a Typical Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, N.; Zhou, P.P.; Wang, G.C.; Zhang, B.; Shi, Z.M.; Liao, F.; Li, B.; Chen, X.L.; Guo, L.; Dang, X.Y.; et al. Hydrochemical and isotopic interpretation of interactions between surface water and groundwater in Delingha, Northwest China. J. Hydrol. 2021, 598, 126243. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Feng, Y.Q.; Jiang, X. Literature analysis of surface water and groundwater interaction based on Citespace. South–North Water Transf. Water Sci. Technol. 2022, 20, 218–229. (In Chinese) [Google Scholar] [CrossRef]
- Singh, A. Conjunctive use of water resources for sustainable irrigated agriculture. J. Hydrol. 2014, 519, 1688–1697. [Google Scholar] [CrossRef]
- Khan, H.H.; Khan, A. Groundwater–Surface water interaction along river Kali, near Aligarh, India. HydroResearch 2019, 2, 119–128. [Google Scholar] [CrossRef]
- Thomas, C.W. Recent advances in understanding the interaction of groundwater and surface water. Rev. Geophys. 1995, 33, 985–994. [Google Scholar] [CrossRef]
- Tang, Q.C. Transformation of groundwater and surface water in Chinese arid lands. Geogr. Res. 1990, 9, 51–57. (In Chinese) [Google Scholar] [CrossRef]
- Elsawwaf, M.; Feyen, J.; Batelaan, O.; Bakr, M. Groundwater–surface water interaction in Lake Nasser, Southern Egypt. Hydrol. Process. 2014, 28, 414–430. [Google Scholar] [CrossRef]
- Delgado, M.I.; Carol, E.; Casco, M.A.; Casco, M.A. Chemical variation of surface water and groundwater in relation to water balance in the watershed of EI Pescado Creek (Central–Eastern Argentina). Environ. Earth Sci. 2020, 79, 399. [Google Scholar] [CrossRef]
- Gu, H.B.; Chi, B.M.; Wang, H.; Zhang, Y.W.; Wang, M.Y. Relationship between surface water and groundwater in the Liujiang basin–hydrochemical constrains. Adv. Earth Sci. 2017, 32, 789–799. (In Chinese) [Google Scholar] [CrossRef]
- Ochoa, C.G.; Sierra, A.M.; Vives, L. Spatio–temporal effect of climate change on water balance and interactions between groundwater and surface water in plains. Sci. Total Environ. 2020, 722, 137886. [Google Scholar] [CrossRef]
- Shu, L.C.; Yin, X.R.; Yuan, Y.J.; Lu, Y.; Lu, C.P.; Liu, B. Temporal and spatial variation of water quantity exchange between surface water and groundwater in typical district of Sanjiang Plain. J. Hydraul. Eng. 2021, 52, 1151–1162. (In Chinese) [Google Scholar] [CrossRef]
- Loh, Y.S.A.; Fynn, O.F.; Manu, E.; Afrifa, G.Y.; Addai, M.O.; Akurugu, B.A.; Yidana, S.M. Groundwater–surface water interactions: Application of hydrochemical and stable isotope tracers to the lake bosumtwi area in Ghana. Environ. Earth Sci. 2022, 81, 518. [Google Scholar] [CrossRef]
- Qiao, X.Y.; Jiang, M.; Ma, S.Y. Analysis of interaction between lake water and groundwater in beach of Maowusu Lake Basin. Bull. Geol. Sci. Technol. 2022, 41, 50–59. (In Chinese) [Google Scholar] [CrossRef]
- Wang, X.H.; Liu, C.L.; Hou, H.B.; Wang, X.Y. Identifying watershed–scale spatiotemporal groundwater and surface water mixing function in the Yiluo River, Middle of China. Environ. Sci. Pollut. Res. Int. 2020, 28, 11053–11065. [Google Scholar] [CrossRef]
- Tian, B.Y.; Hu, H.Z.; Xu, L.P.; Zhao, K.; Ren, J.W.; Yu, R.H. Seasonal variations of river–groundwater interaction in a semiarid steppe. J. Arid Land Resour. Environ. 2021, 35, 118–125. (In Chinese) [Google Scholar]
- Fang, L.J.; Gao, R.Z.; Jia, D.B.; Yu, R.H.; Liu, X.Y.; Liu, T.X.; Wang, X.X. Characteristics and environmental driving factors of water transformation in the Balaguer river watershed of Inner Mongolia steppe. Chin. J. Appl. Ecol. 2021, 32, 860–868. (In Chinese) [Google Scholar] [CrossRef]
- Woessner, W.W. Stream and fluvial plain ground water interactions: Rescaling hydrogeologic thought. Groundwater 2000, 38, 423–429. [Google Scholar] [CrossRef]
- Li, F.F.; Liang, W.T.; Yin, H.; Dong, T.T.; Han, Y.J. Effects of climate change and human activities on runoff change in the Tabu river basin. Inn. Mong. Water Resour. 2021, 5, 12–15. (In Chinese) [Google Scholar]
- Irawan, D.E.; Silaen, H.; Sumintadireja, P.; Lubis, R.F.; Brahmantyo, B.; Puradimaja, D.J. Groundwater–surface water interactions of Ciliwung River streams, segment Bogor–Jakarta, Indonesia. Environ. Earth Sci. 2015, 73, 1295–1302. [Google Scholar] [CrossRef]
- Li, L. Transformation relationship between surface water and groundwater in Tarim river basin. Bull. Soil Water Conserv. 2021, 41, 23–28. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.; Wu, H.W.; Zhou, Y.Q.; Zhao, Z.H.; Wang, X.L.; Cai, Y.J.; He, B.; Chen, W.; Sun, W. Variations of stable oxygen and deuterium isotopes in river and lake waters during flooding season along the middle and lower reaches of the Yangtze River regions. Environ. Sci. 2020, 41, 1176–1183. (In Chinese) [Google Scholar] [CrossRef]
- Wang, X.Y.; Chang, M.Y.; Gao, C.; Lu, Y.C.; Sha, J.Y. The role of seasonal river in the evolution of desertification in semi–arid region: Taking Tabu river basin of Inner Mongolia as an Example. Geogr. Res. 2004, 7, 440–446. (In Chinese) [Google Scholar]
- Liang, W.T.; Yin, H.; Han, Z.H.; Zhang, Y.F.; Zhang, P. Temporal and Spatial Variation Analysis of evapotranspiration in Tabu River Basin based on remote sensing. J. Inn. Mong. Agric. Univ. (Nat. Sci. Ed.) 2022, 43, 27–34. (In Chinese) [Google Scholar] [CrossRef]
- Hou, Q.Q.; Dong, S.G.; Zhang, M.W. Chemical characteristics and genesis of shallow groundwater in Siziwang Banner, Inner Mongolia. J. Arid Land Resour. Environ. 2020, 34, 116–121. (In Chinese) [Google Scholar]
- Wei, Z.Y.; Tang, Q.Y. Interaction between surface water and groundwater resources and calculations of total amount of water resources in the arid zone of northwest China. J. Arid Land Resour. Environ. 1997, 12, 2–8. (In Chinese) [Google Scholar] [CrossRef]
- Yin, L.H.; Zhang, J.; Wang, Z.; Dong, J.Q.; Chang, L.; Li, C.Y.; Zhang, P.W.; Gu, X.F.; Nie, Z.L. Groundwater circulation patterns and its resources assessment of inland river catchments in northwestern China. Geol. China 2021, 48, 1094–1111. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.M.; Jiao, Y.F.; Hu, B.X.; Li, F.L.; Li, D. Study on interaction between surface water and groundwater in typical reach of Xiaoqing River based on WEP–L model. Water 2023, 15, 492. [Google Scholar] [CrossRef]
- Zhan, L.C.; Chen, J.S.; Zhang, S.Y.; Li, L.; Huang, D.W. Isotopic signatures of precipitation, surface water, and groundwater interactions, Poyang Lake Basin, China. Environ. Earth Sci. 2016, 75, 1307. [Google Scholar] [CrossRef]
- Kumar, M.; Ramanathan, A.; Keshari, A.K. Understanding the extent of interactions between groundwater and surface water through major ion chemistry and multivariate statistical techniques. Hydrol. Process. 2009, 23, 297–310. [Google Scholar] [CrossRef]
- Kattan, Z. Chemical and isotopic characteristics of the Euphrates River water, Syria: Factors controlling its geochemistry. Environ. Earth Sci. 2015, 73, 4763–4778. [Google Scholar] [CrossRef]
- Prabhat, S.; Suhas, K.; Kumar, J.S.; Sudhir, K. Variation in δ18O and δ2H values of rainfall, surface water, and groundwater in the Sukhna Lake basin in northwest India. Environ. Earth Sci. 2020, 79, 535. [Google Scholar] [CrossRef]
- Lei, M.; Zhou, J.L.; Zhang, J.; Chen, Y.P.; Teng, J.; Wu, T.; Xu, D.S.; Sun, Y.; Ji, Y.Y. Hydrochemical characteristics and transformation relationship of surface water and groundwater in the plain area of Bortala River Basin, Xinjiang. Environ. Sci. 2022, 43, 1873–1884. (In Chinese) [Google Scholar] [CrossRef]
- Gil-M’arquez, J.M.; Andreo, B.; Mudarra, M. Combining hydrodynamics, hydrochemistry, and environmental isotopes to understand the hydrogeological functioning of evaporite–karst springs: An example from southern Spain. J. Hydrol. 2020, 576, 299–314. [Google Scholar] [CrossRef]
- GB3838–2002; Environmental Quality Standards for Surface Water. Standards Press of China: Beijing, China, 2017. (In Chinese)
- GB/T14848–2017; Standard for Groundwater Quality. Standards Press of China: Beijing, China, 2017. (In Chinese)
- China Geological Survey. Handbook of Hydrogeology, 2nd ed.; Geological Publishing House: Beijing, China, 2018.
- Heaton, T.H.E. Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: A review. Chem. Geol. Isot. Geosci. Sect. 1986, 59, 87–102. [Google Scholar] [CrossRef]
- Fuoco, I.; Marini, L.; De Rosa, R.; Figoli, A.; Gabriele, B.; Apollaro, C. Use of reaction path modelling to investigate the evolution of water chemistry in shallow to deep crystalline aquifers with a special focus on fluoride. Sci. Total Environ. 2022, 830, 154566. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Z.H.; Zhao, Y.P.; Ding, H.J.; Zhang, J. Delineation of hydrochemical characteristics and tracing nitrate contamination of groundwater based on hydrochemical methods and isotope techniques in the northern Huangqihai Basin, China. Water 2022, 14, 3168. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Hu, C.P.; Liu, Z.Q.; Xiong, K.N.; Liu, X.X.; Li, Y.; Zhang, R.K. Characteristics of and influencing factors of hydrochemistry and carbon/nitrogen variation in the Huangzhouhe river basin, a World Natural Heritage Site. Int. J. Environ. Res. Public Health 2021, 18, 13169. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wei, Y.F.; Liao, Z.L.; Xu, X.M.; Han, Z.H.; Liang, W.T.; Long, Y.H.; Guo, J.Y. Determination of soil-groundwater systems recharge mechanism in the middle Inner Mongolia Plateau by isotopic tracers. Environ. Earth Sci. 2020, 79, 473. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Claus, K.; Rolf, F.R.; Fernando, R.B.; Iñaki, V. Vapor source and spatiotemporal variation of precipitation isotopes in southwest Spain. Hydrol. Process. 2021, 35, e14445. [Google Scholar] [CrossRef]
- Mayo, A.L.; Tingey, D.G. Shallow groundwater chemical evolution, isotopic hyperfiltration, and salt pan formation in a hypersaline endorheic basin: Pilot Valley, Great Basin, USA. Hydrogeol. J. 2021, 29, 2219–2243. [Google Scholar] [CrossRef]
- Delgado, M.I.; Carol, E.; Casco, M.A. Land–use changes in the peri urban interface: Hydrologic consequences on a flat land watershed scale. Sci. Total Environ. 2020, 722, 137836. [Google Scholar] [CrossRef]
- Geris, J.; Comte, J.C.; Franchi, F.; Petros, A.K.; Tirivarombo, S.; Selepeng, Ame, T.; Villholth, K.G. Surface water-groundwater interactions and local land use control water quality impacts of extreme rainfall and flooding in a vulnerable semi-arid region of Sub-Saharan Africa. J. Hydrol. 2022, 609, 127834. [Google Scholar] [CrossRef]
- Geological Institute of Inner Mongolia. Report of Groundwater Resources Exploration and Utilization Zoning; Geological Institute of Inner Mongolia: Hohhot, China, 2017. [Google Scholar]


| Location | SW | GW | ||
|---|---|---|---|---|
| Sample | d-excess | Sample | d-excess | |
| Upstream | T1 | +5.48 | n = 56 | +6.21 |
| T2 | +1.93 | |||
| Midstream | T3 | −18.57 | n = 103 | +5.46 |
| T4 | −1.94 | |||
| Downstream | T5 | −5.35 | n = 13 | +2.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Liu, T.; Wang, M.; Liao, Z.; Zhang, J. Hydrochemical and Isotopic Explanations of the Interaction between Surface Water and Groundwater in a Typical-Desertified Steppe of Northern China. Sustainability 2023, 15, 11034. https://doi.org/10.3390/su151411034
Jin J, Liu T, Wang M, Liao Z, Zhang J. Hydrochemical and Isotopic Explanations of the Interaction between Surface Water and Groundwater in a Typical-Desertified Steppe of Northern China. Sustainability. 2023; 15(14):11034. https://doi.org/10.3390/su151411034
Chicago/Turabian StyleJin, Jing, Tiejun Liu, Mingxin Wang, Zilong Liao, and Jing Zhang. 2023. "Hydrochemical and Isotopic Explanations of the Interaction between Surface Water and Groundwater in a Typical-Desertified Steppe of Northern China" Sustainability 15, no. 14: 11034. https://doi.org/10.3390/su151411034
APA StyleJin, J., Liu, T., Wang, M., Liao, Z., & Zhang, J. (2023). Hydrochemical and Isotopic Explanations of the Interaction between Surface Water and Groundwater in a Typical-Desertified Steppe of Northern China. Sustainability, 15(14), 11034. https://doi.org/10.3390/su151411034





