Biobased Natural Sapindus mukorossi–Carvacrol Emulsion for Sustainable Laundry Washing
Abstract
1. Introduction
2. Materials and Methods
2.1. Commercial Laundry Detergent, Soapnut Extract, and Soapnut Extract with Carvacrol
2.2. Standard Strains of Microorganisms
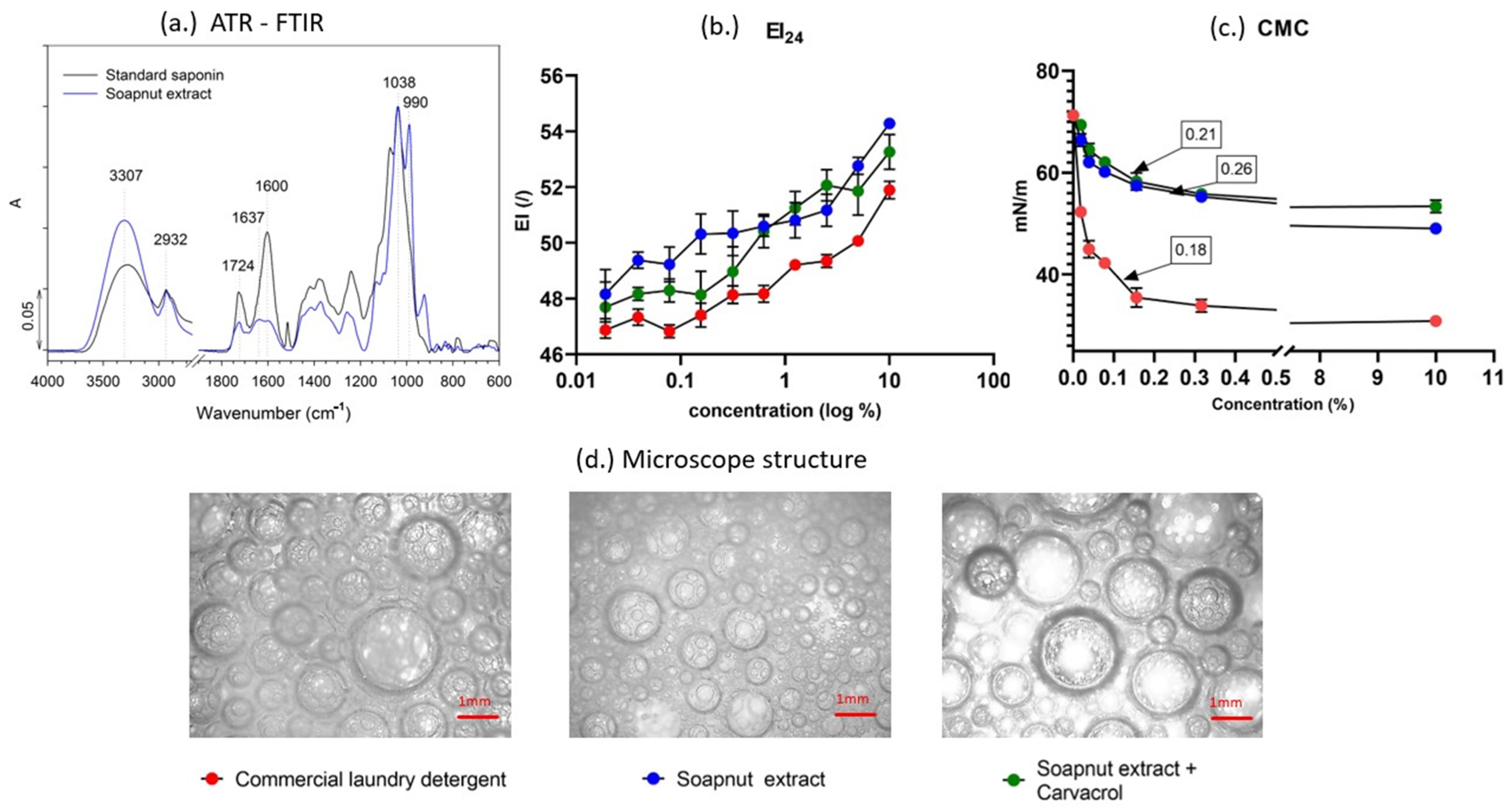
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Quantification of Saponins in Soapnut Extract
2.5. Laundry Detergent’s Characteristics
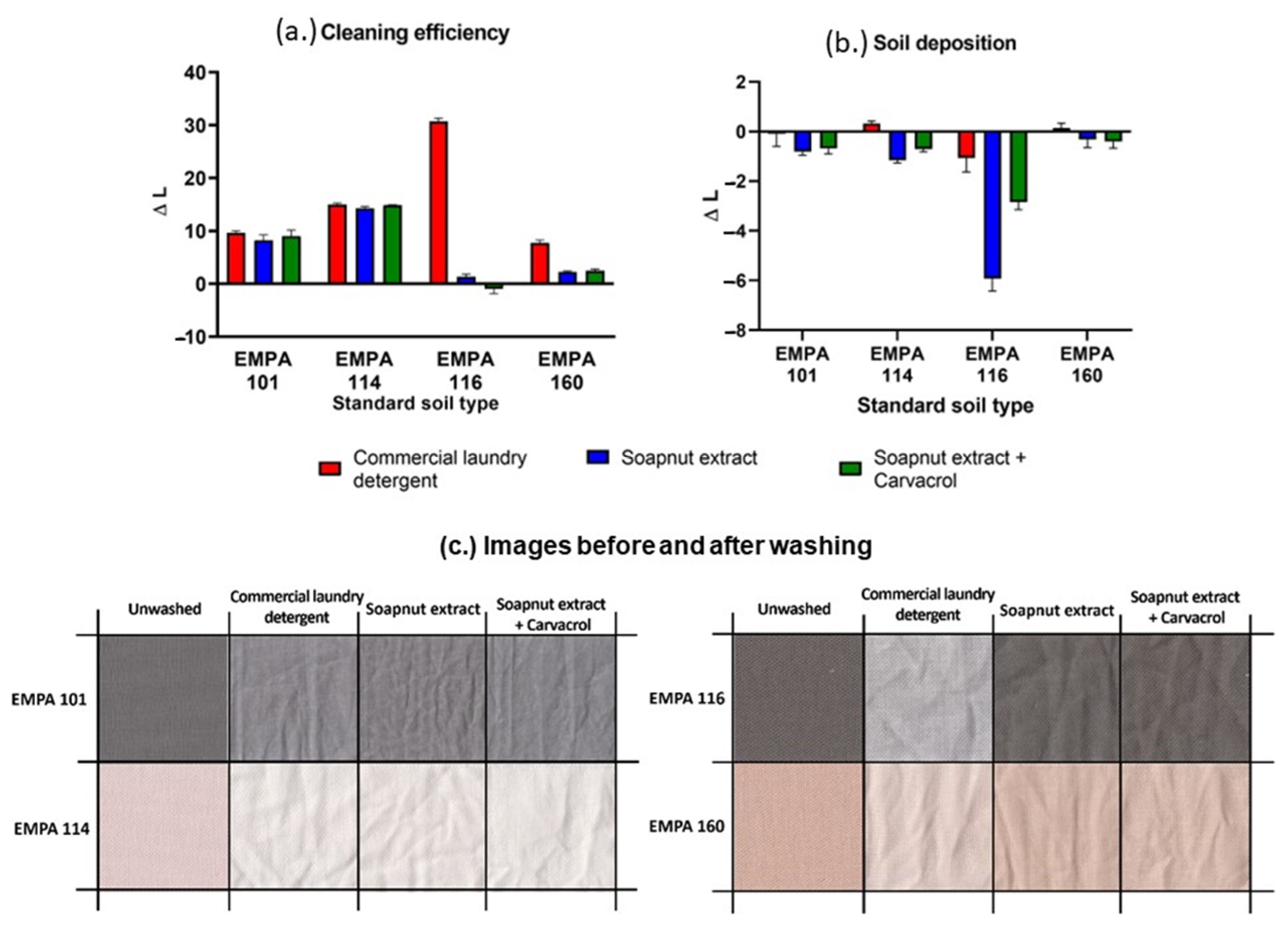
2.6. Standard Soil Removal
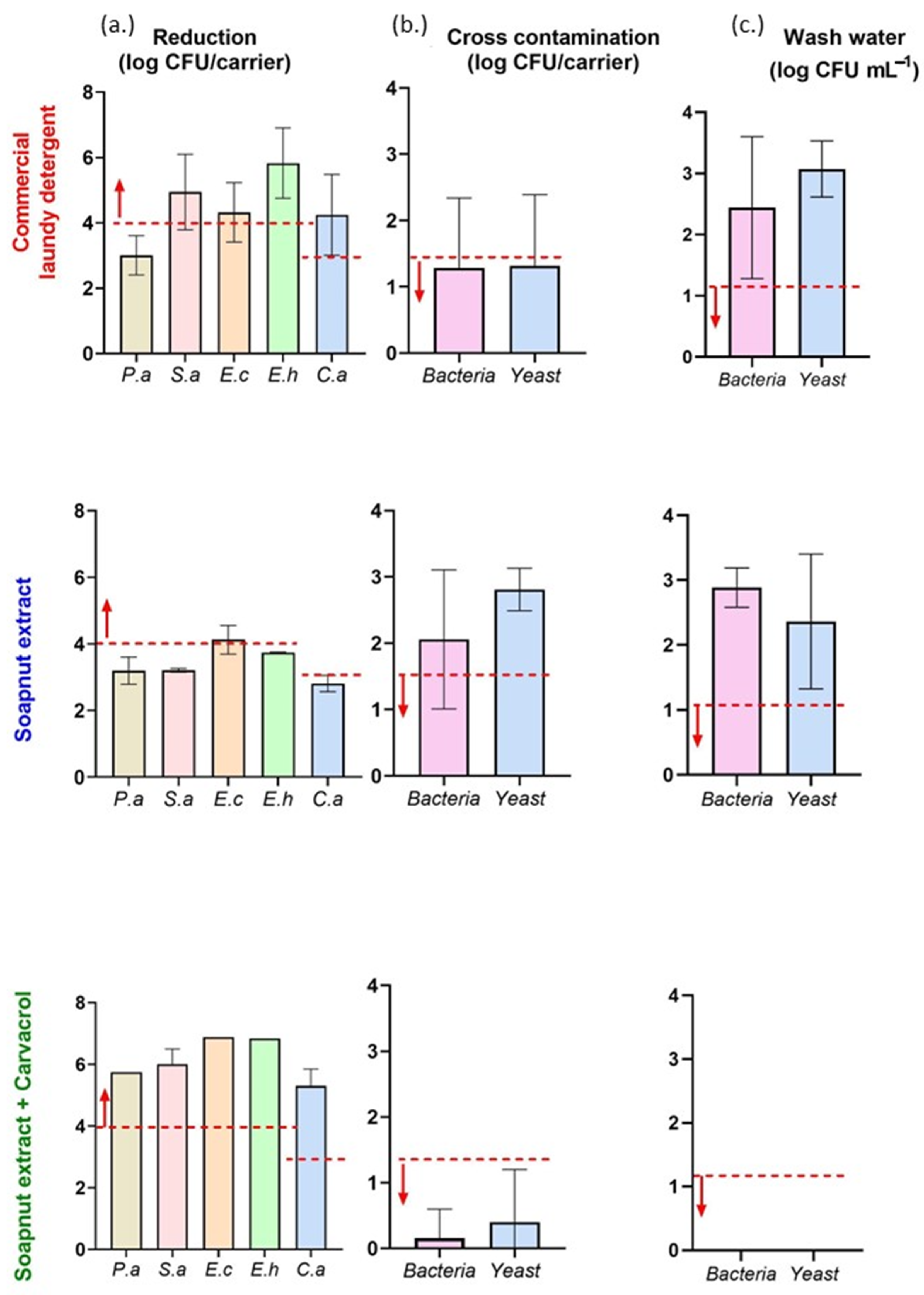
2.7. Laundry Disinfection
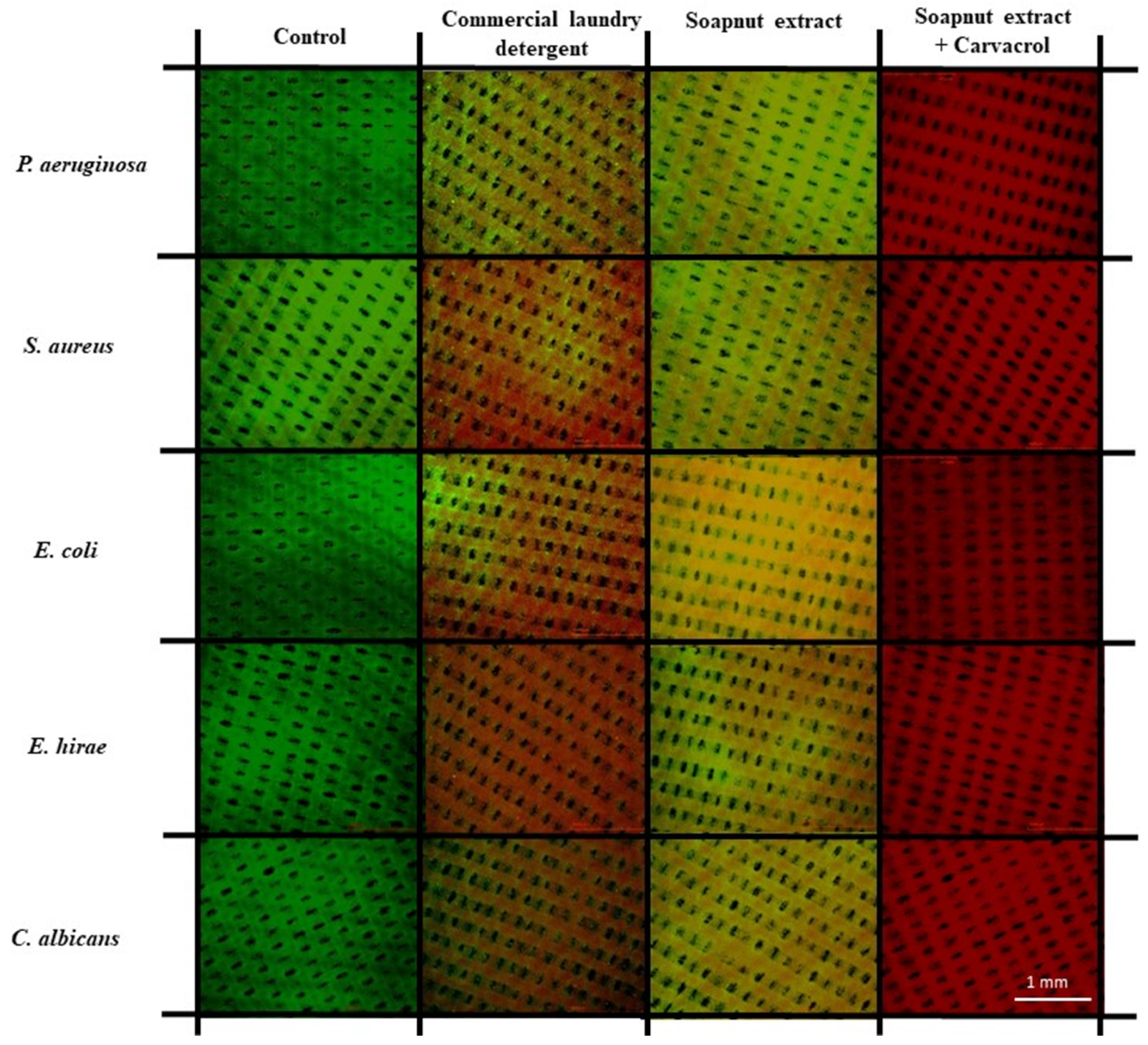
2.8. Microorganisms’ Membrane Integrity Assessment
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reinhart, V.; Fonte, C.C.; Hoffmann, P.; Bechtel, B.; Rechid, D.; Böhner, J. Comparison of ESA climate change initiative land cover to CORINE land cover over Eastern Europe and the Baltic States from a regional climate modeling perspective. Int. J. Appl. Earth Obs. Geoinf. 2021, 94, 102221. [Google Scholar] [CrossRef]
- Owen, G. What makes climate change adaptation effective? A systematic review of the literature. Glob. Environ. Chang. 2020, 62, 102071. [Google Scholar] [CrossRef]
- Sohn, J.; Nielsen, K.S.; Birkved, M.; Joanes, T.; Gwozdz, W. The environmental impacts of clothing: Evidence from United States and three European countries. Sustain. Prod. Consum. 2021, 27, 2153–2164. [Google Scholar] [CrossRef]
- Bajpai, D. Laundry detergents: An overview. J. Oleo Sci. 2007, 56, 327–340. [Google Scholar] [CrossRef]
- Shahmohammadi, S.; Steinmann, Z.; Clavreul, J.; Hendrickx, H.; King, H.; Huijbregts, M.A. Quantifying drivers of variability in life cycle greenhouse gas emissions of consumer products—A case study on laundry washing in Europe. Int. J. Life Cycle Assess. 2018, 23, 1940–1949. [Google Scholar] [CrossRef]
- DeLeo, P.C.; Ciarlo, M.; Pacelli, C.; Greggs, W.; Williams, E.S.; Scott, W.C.; Brooks, B.W. Cleaning product ingredient safety: What is the current state of availability of information regarding ingredients in products and their function? ACS Sustain. Chem. Eng. 2018, 6, 2094–2102. [Google Scholar] [CrossRef]
- Yates, L.; Evans, D. Dirtying Linen: Re-evaluating the sustainability of domestic laundry. Environ. Policy Gov. 2016, 26, 101–115. [Google Scholar] [CrossRef]
- Eberle, U.; Lange, A.; Dewaele, J.; Schowanek, D. LCA study and environmental benefits for low temperature disinfection process in commercial laundry (12 pp). Int. J. Life Cycle Assess. 2007, 12, 127–138. [Google Scholar] [CrossRef]
- Chen, F.; Ji, X.; Chu, J.; Xu, P.; Wang, L. A review: Life cycle assessment of cotton textiles. Ind. Textila 2021, 72, 19–29. [Google Scholar] [CrossRef]
- Monleón-Getino, T.; Cavalleri, M. International ring trial to validate a new method for testing the antimicrobial efficacy of domestic laundry products. PLoS ONE 2022, 17, e0269556. [Google Scholar] [CrossRef]
- Ferri, A.; Osset, M.; Abeliotis, K.; Amberg, C.; Candan, C.; Owens, J.; Stamminger, R. Laundry performance: Effect of detergent and additives on consumer satisfaction. Tenside Surfactants Deterg. 2016, 53, 375–386. [Google Scholar] [CrossRef]
- Honisch, M.; Brands, B.; Weide, M.; Speckmann, H.D.; Stamminger, R.; Bockmühl, D.P. Antimicrobial efficacy of laundry detergents with regard to time and temperature in domestic washing machines. Tenside Surfactants Deterg. 2016, 53, 547–552. [Google Scholar] [CrossRef]
- Schages, J.; Stamminger, R.; Bockmühl, D.P. A new method to evaluate the antimicrobial efficacy of domestic laundry detergents. J. Surfactants Deterg. 2020, 23, 629–639. [Google Scholar] [CrossRef]
- Bockmühl, D.P.; Schages, J.; Rehberg, L. Laundry and textile hygiene in healthcare and beyond. Microb. Cell 2019, 6, 299. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chiang, T.-H.; Chen, J.-H. Properties of soapnut (Sapindus mukorossi) oil biodiesel and its blends with diesel. Biomass Bioenergy 2013, 52, 15–21. [Google Scholar] [CrossRef]
- Kose, M.D.; Bayraktar, O. Extraction of Saponins from Soapnut (Sapindus mukorossi) and Their Antimicrobial Properties. World J. Res. Rev. 2016, 2, 262952. [Google Scholar]
- Waran, A.; Chandran, P. Cradle to grave: The multifaceted soapnut-an update on the applications of Sapindus spp. Sustain. Chem. Pharm. 2021, 24, 100557. [Google Scholar] [CrossRef]
- Basu, A.; Basu, S.; Bandyopadhyay, S.; Chowdhury, R. Optimization of evaporative extraction of natural emulsifier cum surfactant from Sapindus mukorossi—Characterization and cost analysis. Ind. Crops Prod. 2015, 77, 920–931. [Google Scholar] [CrossRef]
- Chaudhary, S.K.; Mandal, A.B.; Bhar, R.; Rokade, J.J.; Jadhav, S.E.; Kannan, A.; Gopi, M. Assessment of phytochemical constituents, fatty acids profile and in vitro antioxidant activity in soapnut shell powder. Indian J. Anim. Sci. 2018, 88, 700–705. [Google Scholar] [CrossRef]
- Pradhan, M.; Bhargava, P. Defect and microstructural evolution during drying of soapnut-based alumina foams. J. Eur. Ceram. Soc. 2018, 28, 3049–3057. [Google Scholar] [CrossRef]
- Sochacki, M.; Vogt, O. Triterpenoid Saponins from Washnut (Sapindus mukorossi Gaertn)—A Source of Natural Surfactants and Other Active Components. Plants 2022, 11, 2355. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Kumar, A.; Mishra, S.; Mohapatra, S. Soapnut: A replacement of synthetic surfactant for cosmetic and biomedical applications. Sustain. Chem. Pharm. 2020, 17, 100297. [Google Scholar] [CrossRef]
- Hoque, M.S.; Chakraborty, S.; Hossain, M.F.; Alam, M.M. Knit fabric scouring with soapnut: A sustainable approach towards textile pre-treatment. Am. J. Environ. Prot. 2018, 7, 19–22. [Google Scholar]
- Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-derived saponins: A review of their surfactant properties and applications. Science 2021, 3, 44. [Google Scholar] [CrossRef]
- Du, M.; Huang, S.; Zhang, J.; Wang, J.; Hu, L.; Jiang, J. Toxicolological test of saponins from Sapindus mukorossi Gaerth. Open J. For. 2015, 5, 749. [Google Scholar]
- Bera, I.; Tyagi, P.K.; Mir, N.A.; Begum, J.; Dev, K.; Tyagi, P.K.; Biswas, A.; Sharma, D.; Mandal, A.B. Effect of dietary saponin rich soapnut (Sapindus mukorossi) shell powder on growth performance, immunity, serum biochemistry and gut health of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1800–1809. [Google Scholar] [CrossRef]
- Sedaghat Doost, A.; Devlieghere, F.; Dirckx, A.; Van der Meeren, P. Fabrication of Origanum compactum essential oil nanoemulsions stabilized using Quillaja Saponin biosurfactant. J. Food Process. Preserv. 2018, 42, e13668. [Google Scholar] [CrossRef]
- Ahmadi, O.; Jafarizadeh-Malmiri, H. Green approach in food nanotechnology based on subcritical water: Effects of thyme oil and saponin on characteristics of the prepared oil in water nanoemulsions. Food Sci. Biotechnol. 2020, 29, 783–792. [Google Scholar] [CrossRef]
- Kumari, S.; Kumaraswamy, R.; Choudhary, R.C.; Sharma, S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Thymol nanoemulsion exhibits potential antibacterial activity against bacterial pustule disease and growth promotory effect on soybean. Sci. Rep. 2018, 8, 6650. [Google Scholar] [CrossRef]
- Chen, C.; Li, R.; Li, D.; Shen, F.; Xiao, G.; Zhou, J. Extraction and purification of saponins from Sapindus mukorossi. New J. Chem. 2021, 45, 952–960. [Google Scholar] [CrossRef]
- Almutairi, M.S.; Ali, M. Direct detection of saponins in crude extracts of soapnuts by FTIR. Nat. Prod. Res. 2015, 29, 1271–1275. [Google Scholar] [CrossRef]
- Li, R.; Wu, Z.L.; Wang, Y.J.; Li, L.L. Separation of total saponins from the pericarp of Sapindus mukorossi Gaerten. by foam fractionation. Ind. Crops Prod. 2013, 51, 163–170. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Bezerraa, K.; Durvala, I.; Silvab, I.A.; Fabiola, C. Emulsifying capacity of biosurfactants from Chenopodium quinoa and Pseudomonas aeruginosa UCP 0992 with focus of application in the cosmetic Industry. Chem. Eng. 2020, 79, 211–216. [Google Scholar]
- Kazemi, K.; Zhang, B.; Lye, L. Production of biosurfactant by Rhodococcus erythropolis sp. cultivated in a novel fish waste compost extract substrate. In Proceedings of the Conference CSCE Annual Conference, London, ON, Canada, 1–4 June 2016. [Google Scholar]
- SIST EN 17658:2021; Chemical Disinfectants and Antiseptics. Chemical Textile Disinfection for the Domestic Area. Test Method and Requirements (Phase 2, Step 2). Slovenski inštitut za Standardizacijo: Ljubljana, Slovenia, 2021; 44p.
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef]
- Inès, M.; Mouna, B.; Marwa, E.; Dhouha, G. Biosurfactants as emerging substitutes of their synthetic counterpart in detergent formula: Efficiency and environmental friendly. J. Polym. Environ. 2023, 31, 2779–2791. [Google Scholar] [CrossRef]
- Lu, Z.; Lei, Z.; Zafar, M.N. Synthesis and performance characterization of an efficient environmental-friendly Sapindus mukorossi saponins based hybrid coal dust suppressant. J. Clean. Prod. 2021, 306, 127261. [Google Scholar] [CrossRef]
- Yekeen, N.; Malik, A.A.; Idris, A.K.; Reepei, N.I.; Ganie, K. Foaming properties, wettability alteration and interfacial tension reduction by saponin extracted from soapnut (Sapindus mukorossi) at room and reservoir conditions. J. Pet. Sci. Eng. 2020, 195, 107591. [Google Scholar] [CrossRef]
- Wojtoń, P.; Szaniawska, M.; Hołysz, L.; Miller, R.; Szcześ, A. Surface activity of natural surfactants extracted from Sapindus mukorossi and Sapindus trifoliatus soapnuts. Colloids Interfaces 2021, 5, 7. [Google Scholar] [CrossRef]
- Ghagi, R.; Satpute, S.K.; Chopade, B.A.; Banpurkar, A.G. Study of functional properties of Sapindus mukorossi as a potential bio-surfactant. Indian J. Sci. Technol. 2011, 4, 530–533. [Google Scholar] [CrossRef]
- Tmáková, L.; Sekretár, S.; Schmidt, Š. Plant-derived surfactants as an alternative to synthetic surfactants: Surface and antioxidant activities. Chem. Pap. 2016, 70, 188–196. [Google Scholar] [CrossRef]
- Laitala, K.; Jensen, H.M. Cleaning effect of household laundry detergents at low temperatures. Tenside Surfactants Deterg. 2010, 47, 413–420. [Google Scholar] [CrossRef]
- Kruschwitz, A.; Augsburg, A.; Stamminger, R. How effective are alternative ways of laundry washing? Tenside Surfactants Deterg. 2013, 50, 263–269. [Google Scholar] [CrossRef]
- Johansson, I.; Somasundaran, P. Handbook for Cleaning/Decontamination of Surfaces; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Ryu, V.; McClements, D.J.; Corradini, M.G.; Yang, J.S.; McLandsborough, L. Natural antimicrobial delivery systems: Formulation, antimicrobial activity, and mechanism of action of quillaja saponin-stabilized carvacrol nanoemulsions. Food Hydrocoll. 2018, 82, 442–450. [Google Scholar] [CrossRef]
- Felici, M.; Tugnoli, B.; Ghiselli, F.; Massi, P.; Tosi, G.; Fiorentini, L.; Piva, A.; Grilli, E. In Vitro anticoccidial activity of thymol, carvacrol, and saponins. Poult. Sci. 2020, 99, 5350–5355. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunder, M.; Tomšič, B.; Fink, R. Biobased Natural Sapindus mukorossi–Carvacrol Emulsion for Sustainable Laundry Washing. Sustainability 2023, 15, 11029. https://doi.org/10.3390/su151411029
Lunder M, Tomšič B, Fink R. Biobased Natural Sapindus mukorossi–Carvacrol Emulsion for Sustainable Laundry Washing. Sustainability. 2023; 15(14):11029. https://doi.org/10.3390/su151411029
Chicago/Turabian StyleLunder, Manca, Brigita Tomšič, and Rok Fink. 2023. "Biobased Natural Sapindus mukorossi–Carvacrol Emulsion for Sustainable Laundry Washing" Sustainability 15, no. 14: 11029. https://doi.org/10.3390/su151411029
APA StyleLunder, M., Tomšič, B., & Fink, R. (2023). Biobased Natural Sapindus mukorossi–Carvacrol Emulsion for Sustainable Laundry Washing. Sustainability, 15(14), 11029. https://doi.org/10.3390/su151411029







