Development of Sustainable Radiation-Shielding Blend Using Natural Rubber/NBR, and Bismuth Filler
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composites
2.3. Material Characterizations
3. Theoretical Background
4. Results and Discussion
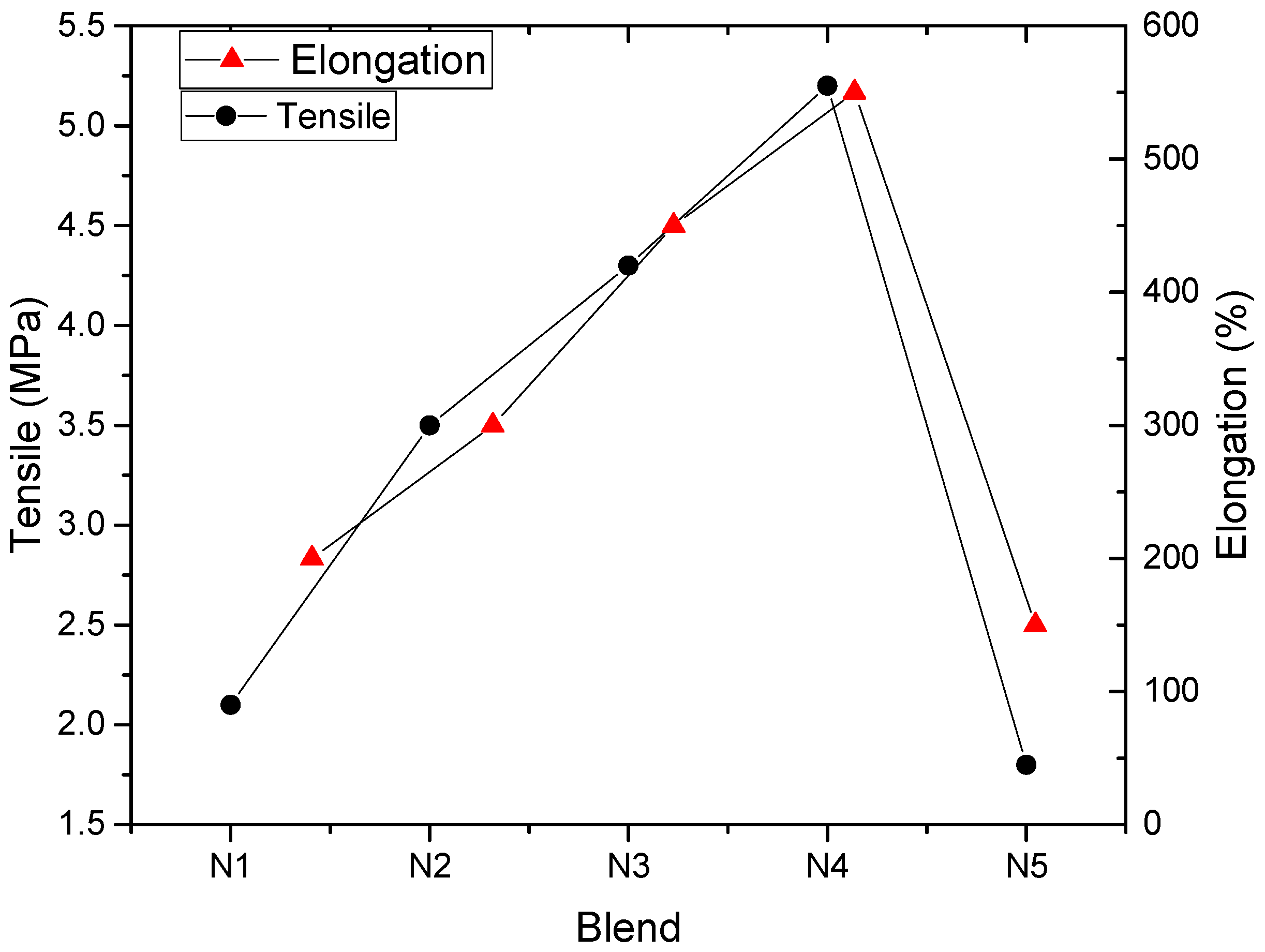
4.1. NR/NBR Blend
4.2. NR/NBR/Bi2O3 Matrix (Composites)
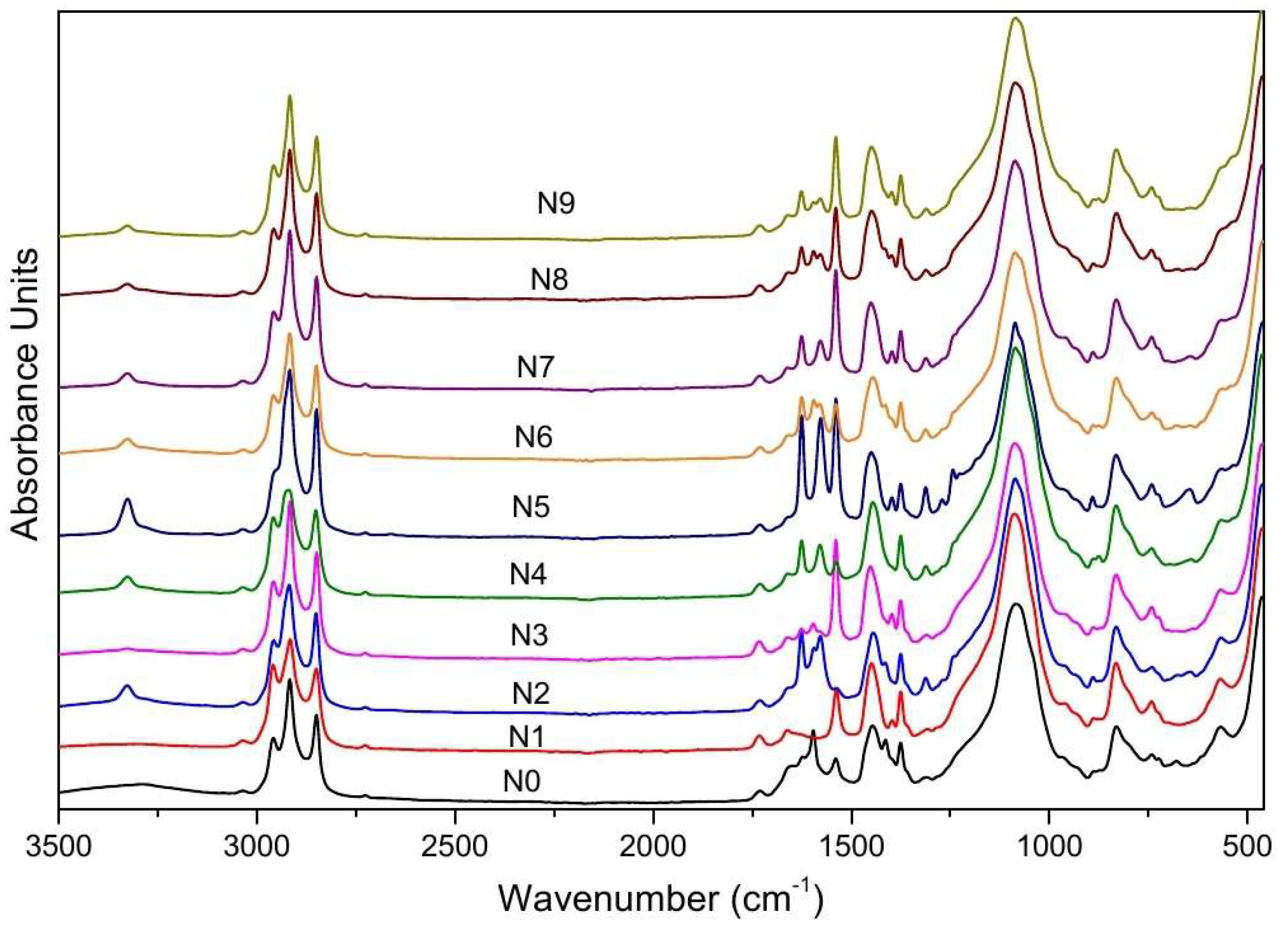
4.3. Total Reflectance Fourier Transform Infrared (ATR-FTIR)
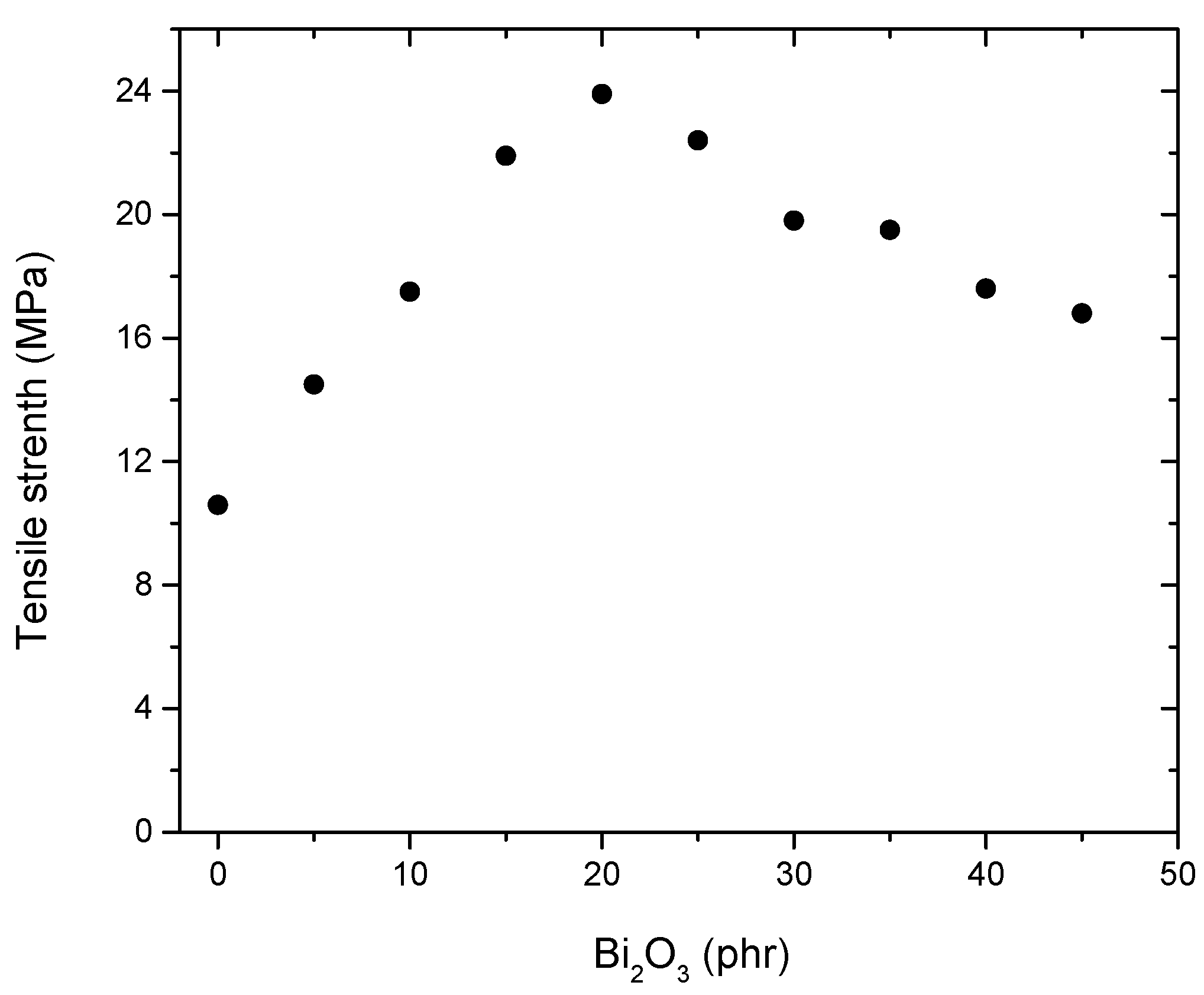
4.4. Mechanical Properties of NR/NBR/Bi2O3 Composites
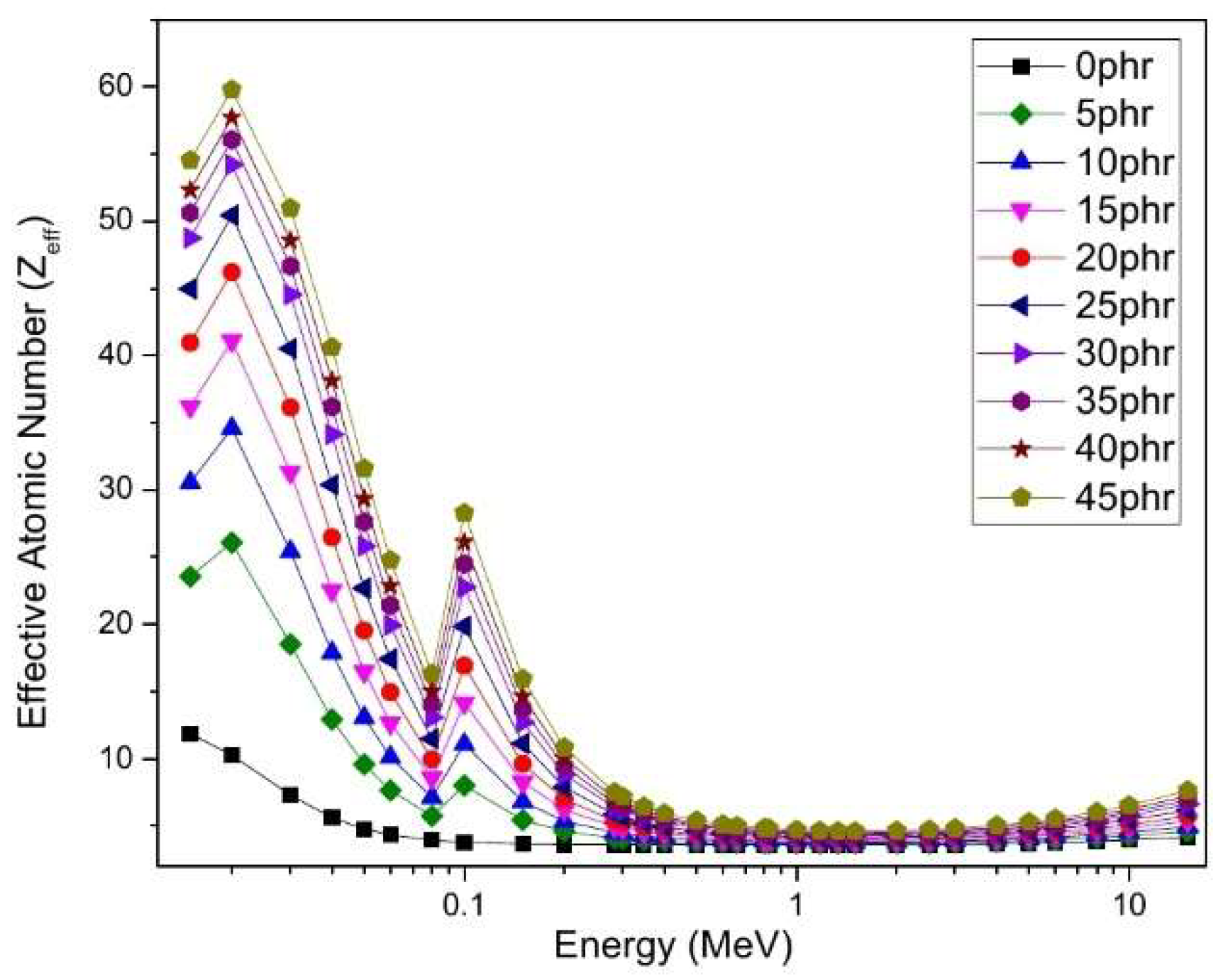
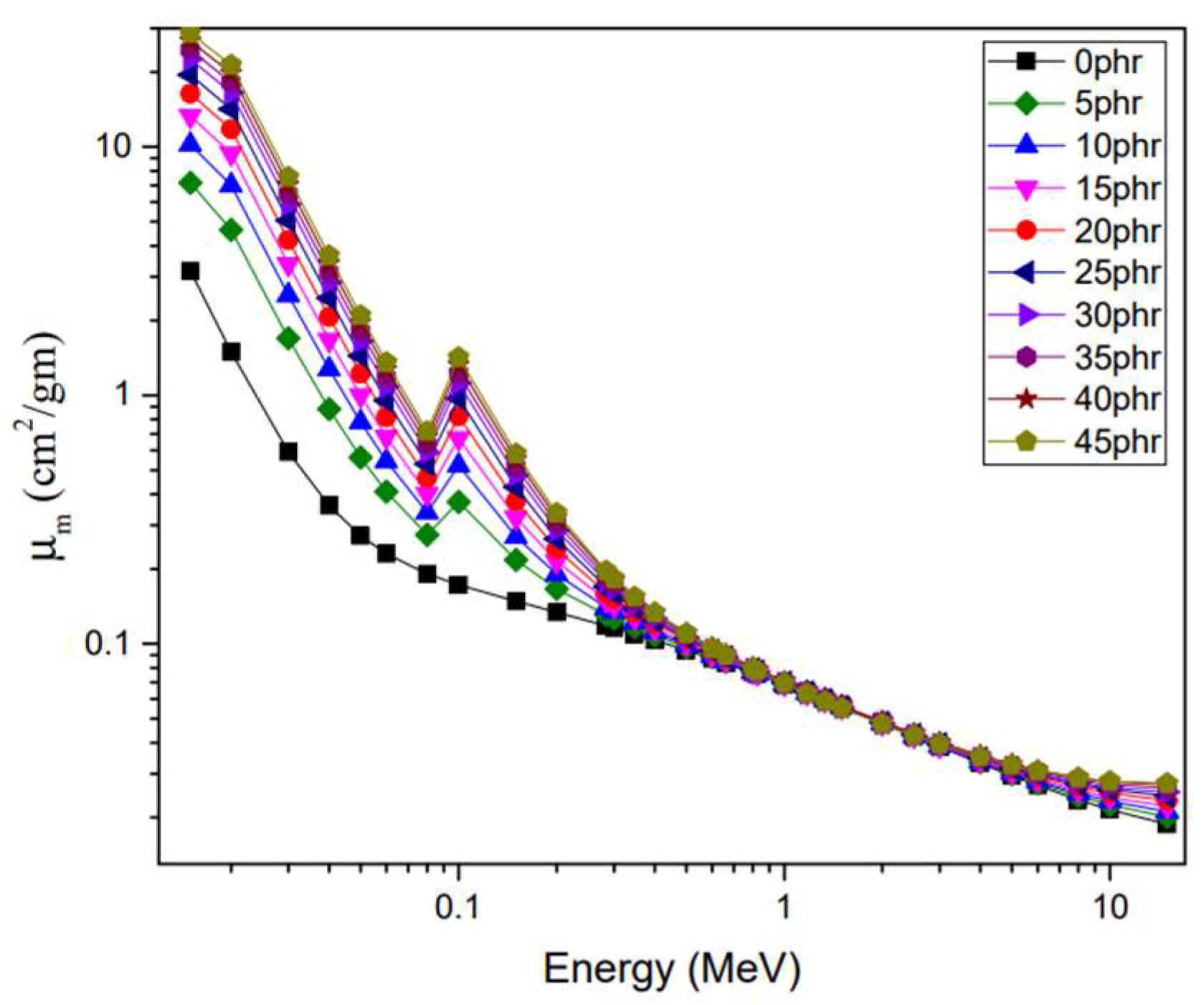
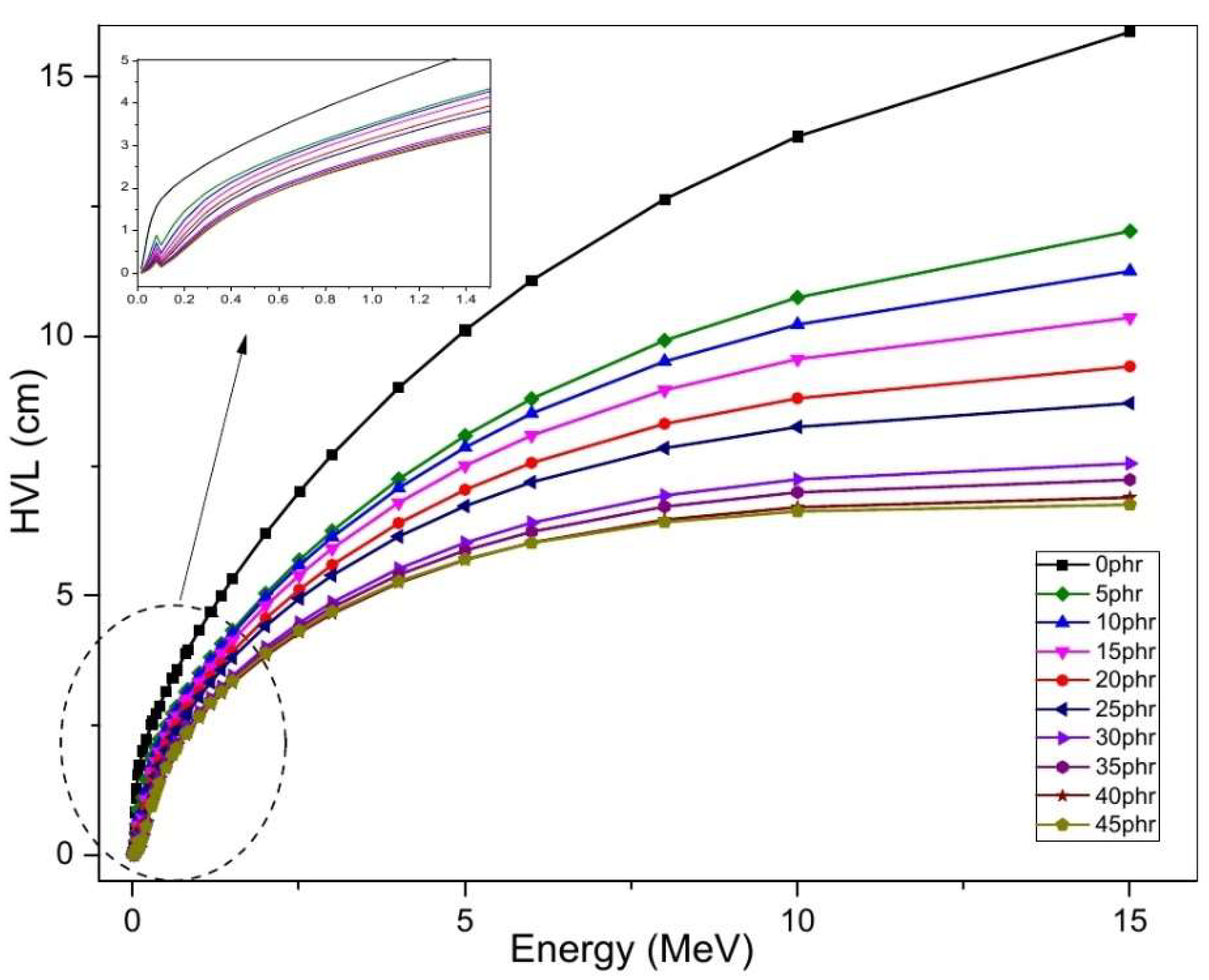
4.5. Gamma-Ray-Shielding Properties of NR/NBR/Bi2O3 Composites
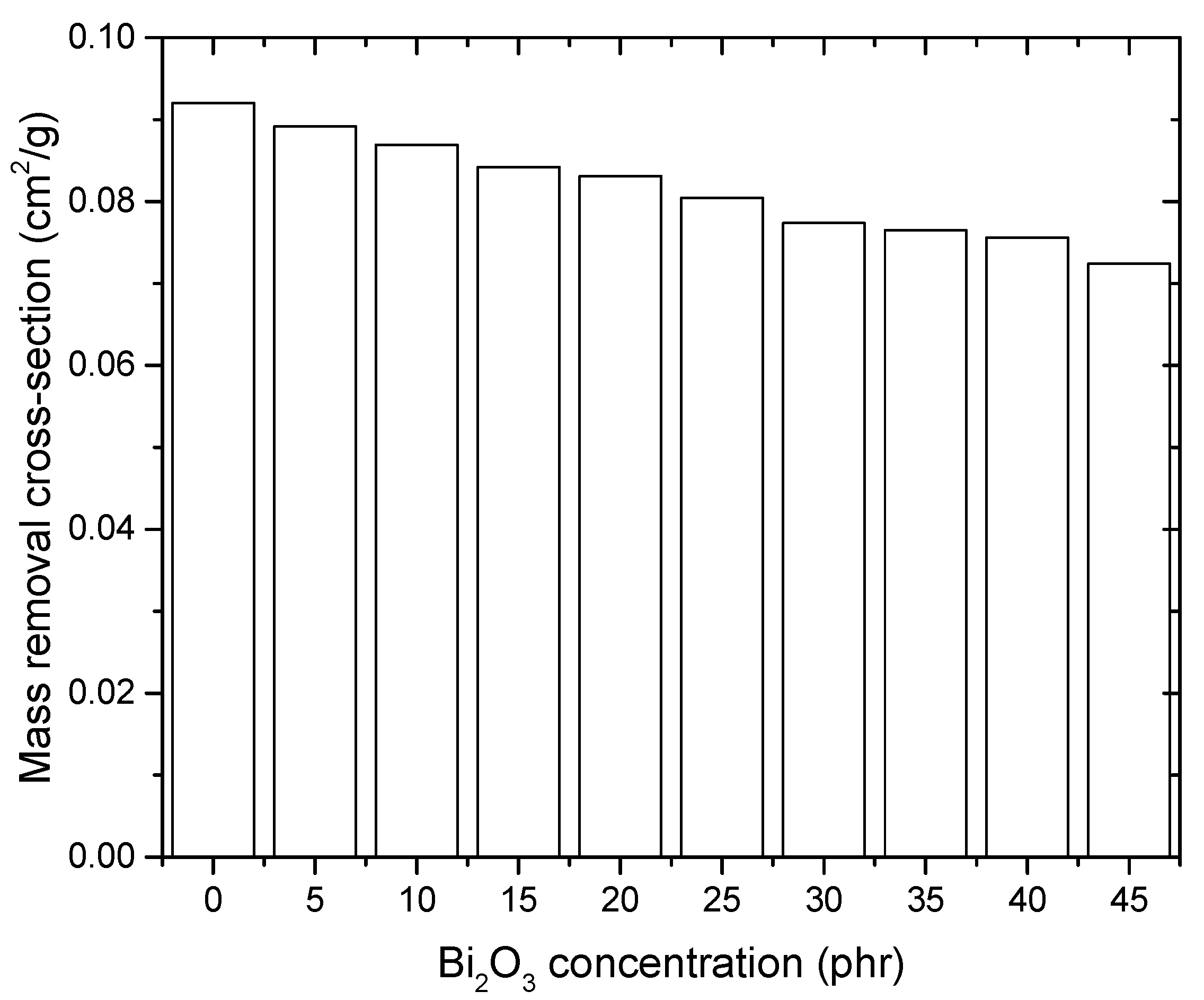
4.6. Neutron Attenuation
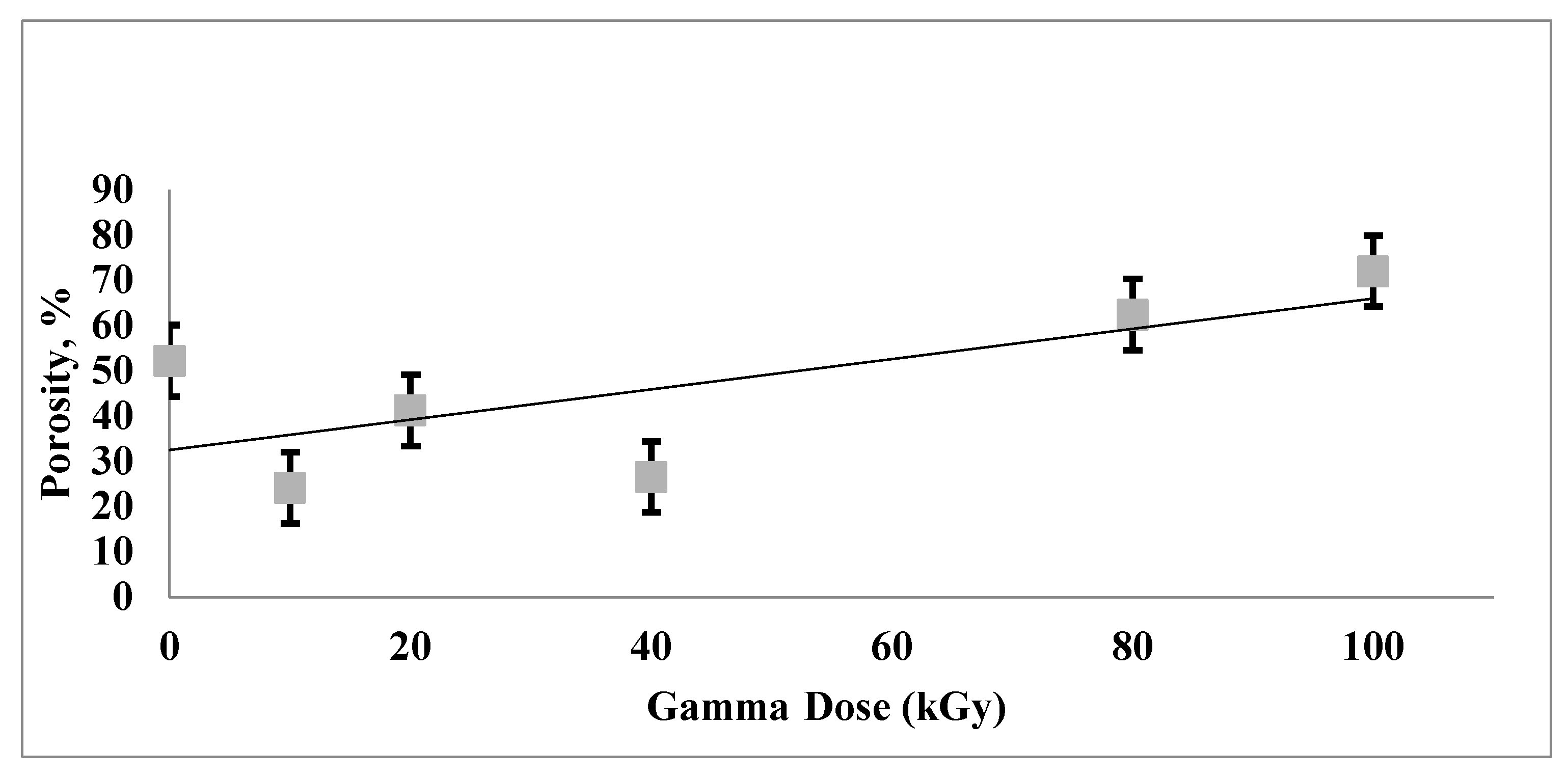
4.7. The Effect of Gamma Irradiation and Porosity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- More, C.V.; Alavian, H.; Pawar, P.P. Evaluation of gamma-ray attenuation characteristics of some thermoplastic polymers: Experimental, WinXCom and MCNPX studies. J. Non Cryst. Solids 2020, 546, 120277. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J.T.W. Polymer-composite materials for radiation protection. ACS Appl. Mater. Interfaces 2012, 4, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, M.I.; El-Mesady, I.A.; Abouhaswa, A.S.; Askin, A.; Rammah, Y.S. Comprehensive study on the structural, optical, physical and gamma photon shielding features of B2O3-Bi2O3-PbO-TiO2 glasses using WinXCOM and Geant4 code. J. Mol. Struct. 2019, 1197, 656–665. [Google Scholar] [CrossRef]
- Ambika, M.R.; Nagaiah, N.; Suman, S.K. Role of bismuth oxide as a reinforcer on gamma shielding ability of unsaturated polyester based polymer composites. J. Appl. Polym. Sci. 2017, 134, 44657. [Google Scholar] [CrossRef]
- Saleh, H.M.; Bondouk, I.I.; Salama, E.; Esawii, H.A. Consistency and shielding efficiency of cement-bitumen composite for use as gamma-radiation shielding material. Prog. Nucl. Energy 2021, 137, 103764. [Google Scholar] [CrossRef]
- Esawii, H.A.; Salama, E.; Sayed El-ahll, L.; Moustafa, M.; Saleh, H.M. High impact tungsten-doped borosilicate glass composite for gamma and neutron transparent radiation shielding. Prog. Nucl. Energy 2022, 150, 104321. [Google Scholar] [CrossRef]
- Ehab, M.; Salama, E.; Ashour, A.; Attallah, M.; Saleh, H.M. Optical Properties and Gamma Radiation Shielding Capability of Transparent Barium Borosilicate Glass Composite. Sustainability 2022, 14, 13298. [Google Scholar] [CrossRef]
- Eid, M.S.; Bondouk, I.I.; Saleh, H.M.; Omar, K.M.; Sayyed, M.I.; El-Khatib, A.M.; Elsafi, M. Implementation of waste silicate glass into composition of ordinary cement for radiation shielding applications. Nucl. Eng. Technol. 2022, 54, 1456–1463. [Google Scholar] [CrossRef]
- Eid, M.S.; Bondouk, I.I.; Saleh, H.M.; Omar, K.M.; Diab, H.M. Investigating the Effect of Gamma and Neutron Irradiation on Portland Cement Provided with Waste Silicate Glass. Sustainability 2023, 15, 763. [Google Scholar] [CrossRef]
- Welborn, D.; Lockwood, P. Lead-rubber shielding effect on radiation dose to the gonads from a bilateral hand X-ray examination. Radiography 2022, 28, 360–365. [Google Scholar] [CrossRef]
- Intom, S.; Kalkornsurapranee, E.; Johns, J.; Kaewjaeng, S.; Kothan, S.; Hongtong, W.; Chaiphaksa, W.; Kaewkhao, J. Mechanical and radiation shielding properties of flexible material based on natural rubber/Bi2O3 composites. Radiat. Phys. Chem. 2020, 172, 108772. [Google Scholar] [CrossRef]
- Kalkornsurapranee, E.; Kothan, S.; Intom, S.; Johns, J.; Kaewjaeng, S.; Kedkaew, C.; Chaiphaksa, W.; Sareein, T.; Kaewkhao, J. Wearable and flexible radiation shielding natural rubber composites: Effect of different radiation shielding fillers. Radiat. Phys. Chem. 2021, 179, 109261. [Google Scholar] [CrossRef]
- Yılmaz, S.N.; Güngör, A.; Özdemir, T. The investigations of mechanical, thermal and rheological properties of polydimethylsiloxane/bismuth (III) oxide composite for X/Gamma ray shielding. Radiat. Phys. Chem. 2020, 170, 108649. [Google Scholar] [CrossRef]
- Özdemir, T.; Yılmaz, S.N. Mixed radiation shielding via 3-layered polydimethylsiloxane rubber composite containing hexagonal boron nitride, boron (III) oxide, bismuth (III) oxide for each layer. Radiat. Phys. Chem. 2018, 152, 17–22. [Google Scholar] [CrossRef]
- Saleh, H.M.; Bondouk, I.I.; Salama, E.; Mahmoud, H.H.; Omar, K.; Esawii, H.A. Asphaltene or Polyvinylchloride Waste Blended with Cement to Produce a Sustainable Material Used in Nuclear Safety. Sustainabilitiy 2022, 14, 3525. [Google Scholar] [CrossRef]
- Luan, W.; Wang, Q.; Sun, Q.; Lu, Y. Preparation of CF/Ni-Fe/CNT/silicone layered rubber for aircraft sealing and electromagnetic interference shielding applications. Chin. J. Aeronaut. 2021, 34, 91–102. [Google Scholar] [CrossRef]
- Wei, H.; Lou, L.; Yang, Z.; He, R.; Fan, J.; Zhang, K.; Yang, W. Multifunctional composites silicone rubber/paraffin@lead tungstate with different core/shell ratio for thermal regulation and gamma shielding. J. Energy Storage 2021, 36, 102363. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Gao, Y.; Xie, Y.; Zhang, J.; Hu, Y.; Wang, D.; You, Z.; Wang, S.; Li, H.; et al. The characterization of silicone-tungsten-based composites as flexible gamma-ray shields. Materials 2021, 14, 5970. [Google Scholar] [CrossRef]
- Poltabtim, W.; Wimolmala, E.; Saenboonruang, K. Properties of lead-free gamma-ray shielding materials from metal oxide/EPDM rubber composites. Radiat. Phys. Chem. 2018, 153, 1–9. [Google Scholar] [CrossRef]
- Güngör, A.; Akbay, I.K.; Özdemir, T. EPDM Rubber with hexagonal Boron Nitride: A Thermal Neutron Shielding Composite. Radiat. Phys. Chem. 2019, 165, 108391. [Google Scholar] [CrossRef]
- White, J.L.; Kim, K.J. Thermoplastic and Rubber Compounds: Technology and Physical Chemistry; Hanser: Munich, Germany, 2008; ISBN 9783446409804. [Google Scholar]
- Girard, S.; Alessi, A.; Richard, N.; Martin-Samos, L.; De Michele, V.; Giacomazzi, L.; Agnello, S.; Di Francesca, D.; Morana, A.; Winkler, B.; et al. Overview of radiation induced point defects in silica-based optical fibers. Rev. Phys. 2019, 4, 100032. [Google Scholar] [CrossRef]
- Şen, M.; Aksüt, D.; Karaağaç, B. The effect of ionizing radiation on the mechanical properties of NBR elastomers reinforced by lignin. Radiat. Phys. Chem. 2020, 168, 108626. [Google Scholar] [CrossRef]
- ASTM D412-06a; Vulcanized Rubber and Thermoplastic Elastomers–Tension. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C20-00; ASTM Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water. ASTM International: West Conshohocken, PA, USA, 2015.
- Singh, V.P.; Badiger, N.M.; Kaewkhao, J. Radiation shielding competence of silicate and borate heavy metal oxide glasses: Comparative study. J. Non Cryst. Solids 2014, 404, 167–173. [Google Scholar] [CrossRef]
- Singh, N.; Singh, K.J.; Singh, K.; Singh, H. Comparative study of lead borate and bismuth lead borate glass systems as gamma-radiation shielding materials. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2004, 225, 305–309. [Google Scholar] [CrossRef]
- Bashter, I.I. Calculation of radiation attenuation coefficients for shielding concretes. Ann. Nucl. Energy 1997, 24, 1389–1401. [Google Scholar] [CrossRef]
- Gaafar, I.; El-Shershaby, A.; Zeidan, I.; El-Ahll, L.S. Natural radioactivity and radiation hazard assessment of phosphate mining, Quseir-Safaga area, Central Eastern Desert, Egypt. NRIAG J. Astron. Geophys. 2016, 5, 160–172. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Sayyed, M.I.; Abohaswa, A.S.; Tekin, H.O. FTIR, electronic polarizability and shielding parameters of B2O3 glasses doped with SnO2. Appl. Phys. A Mater. Sci. Process. 2018, 124, 650. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Qashou, S.I.; Khattari, Z.Y. Radiation shielding competence of newly developed TeO2-WO3 glasses. J. Alloys Compd. 2017, 696, 632–638. [Google Scholar] [CrossRef]
- Umar, S.A.; Halimah, M.K.; Chan, K.T.; Amirah, A.A.; Azlan, M.N.; Grema, L.U.; Hamza, A.M.; Ibrahim, G.G. Optical and structural properties of rice husk silicate incorporated borotellurite glasses doped with erbium oxide nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 18606–18616. [Google Scholar] [CrossRef]
- Tijani, S.A.; Kamal, S.M.; Al-Hadeethi, Y.; Arib, M.; Hussein, M.A.; Wageh, S.; Dim, L.A. Radiation shielding properties of transparent erbium zinc tellurite glass system determined at medical diagnostic energies. J. Alloys Compd. 2018, 741, 293–299. [Google Scholar] [CrossRef]
- Sathiyaraj, P.; Samuel, E.J.J.; Valeriano, C.C.S.; Kurudirek, M. Effective atomic number and buildup factor calculations for metal nano particle doped polymer gel. Vacuum 2017, 143, 138–149. [Google Scholar] [CrossRef]
- Kavaz, E.; Yorgun, N.Y. Gamma ray buildup factors of lithium borate glasses doped with minerals. J. Alloys Compd. 2018, 752, 61–67. [Google Scholar] [CrossRef]
- El-Kameesy, S.U.; Youssef, G.M.; El-Zaiat, S.Y.; Saudi, H.A.; Abd El-Kawy, F.S. Gamma Rays Attenuation Properties and the Associated Optical and Mechanical Behavior of Development (70-x) B2O3-10Al2O3-10Na2O-10ZnO-x PbO Glasses. Silicon 2018, 10, 1881–1886. [Google Scholar] [CrossRef]
- Oto, B.; Gür, A.; Kavaz, E.; Çakır, T.; Yaltay, N. Determination of gamma and fast neutron shielding parameters of magnetite concretes. Prog. Nucl. Energy 2016, 92, 71–80. [Google Scholar] [CrossRef]
- Harima, Y. An historical review and current status of buildup factor calculations and applications. Radiat. Phys. Chem. 1993, 41, 631–672. [Google Scholar] [CrossRef]
- Kaplan, M.F. Concrete Radiation Shielding: Nuclear Physics, Concrete Properties, Design and Construction; Longman Scientific & Technical: Harlow, UK, 1989; ISBN 0470213388. [Google Scholar]
- Singh, V.P.; Badiger, N.M. Gamma ray and neutron shielding properties of some alloy materials. Ann. Nucl. Energy 2014, 64, 301–310. [Google Scholar] [CrossRef]
- ANSI/ANS-6.4.3; Gamma-Ray Attenuation Coefficients and Buildup Factors for Engineering Materials. American Nuclear Society: La Grange Park, IL, USA, 1991.
- White, J.; De, S.K. Rubber Technologist’s Handbook; Rapra Technology Ltd.: Shawbury, UK, 2001; ISBN 1859572626. [Google Scholar]
- El-Fiki, S.; El Kameesy, S.U.; Nashar, D.E.; Abou-Leila, M.A.; El-Mansy, M.K.; Ahmed, M. Influence of Bismuth Contents on Mechanical and Gamma Ray Attenuation Properties of Silicone Rubber Composite. Int. J. Adv. Res. 2015, 3, 1035–1041. [Google Scholar]
- Marković, G.; Marinović-Cincović, M.S.; Jovanović, V.; Samaržija-Jovanović, S.; Budinski-Simendić, J. Gamma irradiation aging of NBR/CSM rubber nanocomposites. Compos. Part B Eng. 2012, 43, 609–615. [Google Scholar] [CrossRef]
- Elabbasy, M.T.; Abd El-Kader, M.F.H.; Ismail, A.M.; Menazea, A.A. Regulating the function of bismuth (III) oxide nanoparticles scattered in Chitosan/Poly (Vinyl Pyrrolidone) by laser ablation on electrical conductivity characterization and antimicrobial activity. J. Mater. Res. Technol. 2021, 10, 1348–1354. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: New York, NY, USA, 2005; ISBN 0-471-39362-2. [Google Scholar]
- Herzberg, G.; Crawford, B.L. Infrared and Raman Spectra of Polyatomic Molecules. J. Phys. Chem. 1946. [Google Scholar] [CrossRef]
- Smith, B. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Atef, S.; El-Nashar, D.E.; Ashour, A.H.; El-Fiki, S.; El-Kameesy, S.U.; Medhat, M. Effect of gamma irradiation and lead content on the physical and shielding properties of PVC/NBR polymer blends. Polym. Bull. 2020, 77, 5423–5438. [Google Scholar] [CrossRef]
- Samir, A.; El-Nashar, D.E.; Ashour, A.H.; Medhat, M.; El-Kameesy, S.U. Polyvinyl chloride/styrene butadiene rubber polymeric blend filled with bismuth subcarbonate (BiO)2CO3 as a shielding material for gamma rays. Polym. Compos. 2020, 41, 535–543. [Google Scholar] [CrossRef]
- Ao, W.; Li, J.; Yang, H.; Zeng, X.; Ma, X. Mechanochemical synthesis of zinc oxide nanocrystalline. Powder Technol. 2006, 168, 148–151. [Google Scholar] [CrossRef]
- Iqbal, T.; Sohaib, M. Synthesis of novel lanthanum-doped zinc oxide nanoparticles and their application for wastewater treatment. Appl. Nanosci. 2021, 11, 2599–2609. [Google Scholar] [CrossRef]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2014; ISBN 9789400760646. [Google Scholar]
- Riaz, U.; Ashraf, S.M. Characterization of Polymer Blends with FTIR Spectroscopy. In Characterization of Polymer Blends: Miscibility, Morphology and Interfaces; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; ISBN 9783527645602. [Google Scholar]
- Singh, V.P.; Badiger, N.M. Investigation on radiation shielding parameters of ordinary, heavy and super heavy concretes. Nucl. Technol. Radiat. Prot. 2014, 29, 149–156. [Google Scholar] [CrossRef]
- Berger, M.J.; Photon Cross Sections Database. NIST Standard Reference Database 8 (XGAM). 1998. Available online: http://physics.nist.gov/PhysRefData/Xcom/Text/XCOM.html (accessed on 11 June 2023).
- Sayyed, M.I.; Lakshminarayana, G.; Kityk, I.V.; Mahdi, M.A. Evaluation of shielding parameters for heavy metal fluoride based tellurite-rich glasses for gamma ray shielding applications. Radiat. Phys. Chem. 2017, 139, 33–39. [Google Scholar] [CrossRef]
- El Abd, A.; Mesbah, G.; Mohammed, N.M.A.; Ellithi, A. A simple Method for Determining the Effective Removal Cross Section for Fast Neutrons. J. Radiat. Nucl. Appl. 2017, 2, 53–58. [Google Scholar] [CrossRef]
- Otaguro, H.; de Lima, L.F.C.P.; Parra, D.F.; Lugão, A.B.; Chinelatto, M.A.; Canevarolo, S.V. High-energy radiation forming chain scission and branching in polypropylene. Radiat. Phys. Chem. 2010, 79, 318–324. [Google Scholar] [CrossRef]
- Khonakdar, H.A.; Jafari, S.H.; Wagenknecht, U.; Jehnichen, D. Effect of electron-irradiation on cross-link density and crystalline structure of low- and high-density polyethylene. Radiat. Phys. Chem. 2006, 75, 78–86. [Google Scholar] [CrossRef]





| Ingredients | Amounts (phr) * | Function |
|---|---|---|
| Natural rubber (NR) | 75 | Polymer |
| Acrylonitrile butadiene rubber (NBR) | 25 | Polymer |
| Stearic acid | 1 | Activators |
| Zinc oxide (ZnO) | 3 | Activators |
| Tetramethyl thiuram disulphide (TMTD) | 0.5 | Accelerators |
| N-cyclohexyl-2-benzothiazole sulphenamide (CBS) | 1 | Accelerators |
| Polymerized 2,2,4-trimethyl-1,2dihydroquinoline (TMQ) | 1 | Antioxidant |
| Sulfur (S) | 2 | Vulcanizing agent |
| Dioctyl phthalate (DOP) | 3 | Plasticizer |
| Silica (SiO2) | 20 | Filler |
| Bismuth oxide (Bi2O3) | Variable | Gamma attenuator filler |
| N1 | N2 | N3 | N4 | N5 | |
|---|---|---|---|---|---|
| NR (phr) | - | 25 | 50 | 75 | 100 |
| NBR (phr) | 100 | 75 | 50 | 25 | - |
| Peroxide(phr) | 3 | 3 | 3 | 3 | 3 |
| Rheometric Characteristics | N1 | N2 | N3 | N4 | N5 |
|---|---|---|---|---|---|
| ML (dN.m) | 0.62 | 0.61 | 0.45 | 0.53 | 0.78 |
| MH (dN.m) | 10.42 | 9.32 | 8.5 | 7.95 | 7.35 |
| Tc90 (min) | 16.15 | 16.5 | 16.53 | 16.6 | 16.31 |
| Ts2 (min) | 2.47 | 3.22 | 3.4 | 3.67 | 3.56 |
| CIR (min−1) | 7.31 | 7.53 | 7.62 | 7.73 | 7.84 |
| Sample No. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N0 | N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 | |
| Bi2O3 (phr) | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 |
| Density (g/cm3) | 2.326 | 2.864 | 2.911 | 3.012 | 3.142 | 3.251 | 3.624 | 3.653 | 3.709 | 3.741 |
| Rheometric Characteristics | Samples No. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N0 | N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 | |
| ML (dN.m) | 1.14 | 0.93 | 0.85 | 0.82 | 0.77 | 0.79 | 0.83 | 0.89 | 0.89 | 0.89 |
| MH (dN.m) | 10.22 | 12.33 | 14.48 | 15.76 | 18.01 | 17.58 | 17.2 | 16.8 | 16.3 | 16.2 |
| Tc90 (min) | 3.56 | 3.58 | 3.87 | 4.46 | 5.17 | 6.29 | 6.44 | 6.86 | 6.97 | 7.01 |
| Ts2 (min) | 1.94 | 1.83 | 1.74 | 1.70 | 1.65 | 1.54 | 1.51 | 1.45 | 1.41 | 1.33 |
| CIR (min−1) | 61.73 | 57.14 | 46.94 | 36.23 | 28.41 | 21.05 | 20.28 | 18.48 | 17.98 | 17.60 |
| Peak Position (cm−1) | Assignment | Reference Range |
|---|---|---|
| 3320 | OH stretching vibrations | 3330–3353 [43,44,45] |
| 2958 | C-H3 symmetric stretching | 2950–2960 [46] |
| 2917 | C-H3 asymmetric stretching vibration | 2910–2920 [46] |
| 2850 | Asymmetric C-H2 stretching vibration modes | 2850–2860 [46] |
| 2726 | Stretching vibration of C-H groups | 3000–2500 [47] |
| 1731 | C=O stretching vibration carbonyl group | 1725–1740 [46,47,48] |
| 1625 | Stretching vibration of C=C groups | 1600–1580 [46] |
| 1539 | N=O stretching vibration | 1585–1539 [46] |
| 1450 | C-H3 bending vibration | 1470–1410 [46] |
| 1396 | Stretching vibration of C-N groups | 1400–1300 [32] |
| 1375 | Stretching vibration of C-H groups | 1460–1340 [32] |
| 1311 | Stretching vibration of C-N groups | 1400–1200 [46] |
| 1086 | C-O chain stretching | 1050–1300 [46,49,50] |
| 900 | Si−H stretching | 800–950 [46] |
| 832 | Bending vibration of C-H groups | 900–625 [47] |
| 742 | Bending vibration of C-H groups | 900–625 [47] |
| 643 | Bending vibration of C-H groups | 900–625 [47] |
| 566 | Out-of-plane bending of the C-H bond in the CH2 group | 600–500 [45] |
| 462 | Metal–ligand stretching vibration such as Bi-O, Zn-O and Zn-CH3 stretching vibrations | 400–600 [51,52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, O.; Salama, E.; E. El-Nashar, D.; Bakry, A. Development of Sustainable Radiation-Shielding Blend Using Natural Rubber/NBR, and Bismuth Filler. Sustainability 2023, 15, 9679. https://doi.org/10.3390/su15129679
Aziz O, Salama E, E. El-Nashar D, Bakry A. Development of Sustainable Radiation-Shielding Blend Using Natural Rubber/NBR, and Bismuth Filler. Sustainability. 2023; 15(12):9679. https://doi.org/10.3390/su15129679
Chicago/Turabian StyleAziz, Ola, E. Salama, Doaa E. El-Nashar, and Assem Bakry. 2023. "Development of Sustainable Radiation-Shielding Blend Using Natural Rubber/NBR, and Bismuth Filler" Sustainability 15, no. 12: 9679. https://doi.org/10.3390/su15129679
APA StyleAziz, O., Salama, E., E. El-Nashar, D., & Bakry, A. (2023). Development of Sustainable Radiation-Shielding Blend Using Natural Rubber/NBR, and Bismuth Filler. Sustainability, 15(12), 9679. https://doi.org/10.3390/su15129679







