Nitrite-Oxidizing Bacterial Strains Isolated from Soils of Andean Ecosystems and Their Potential Use in Nitrogen Reduction
Abstract
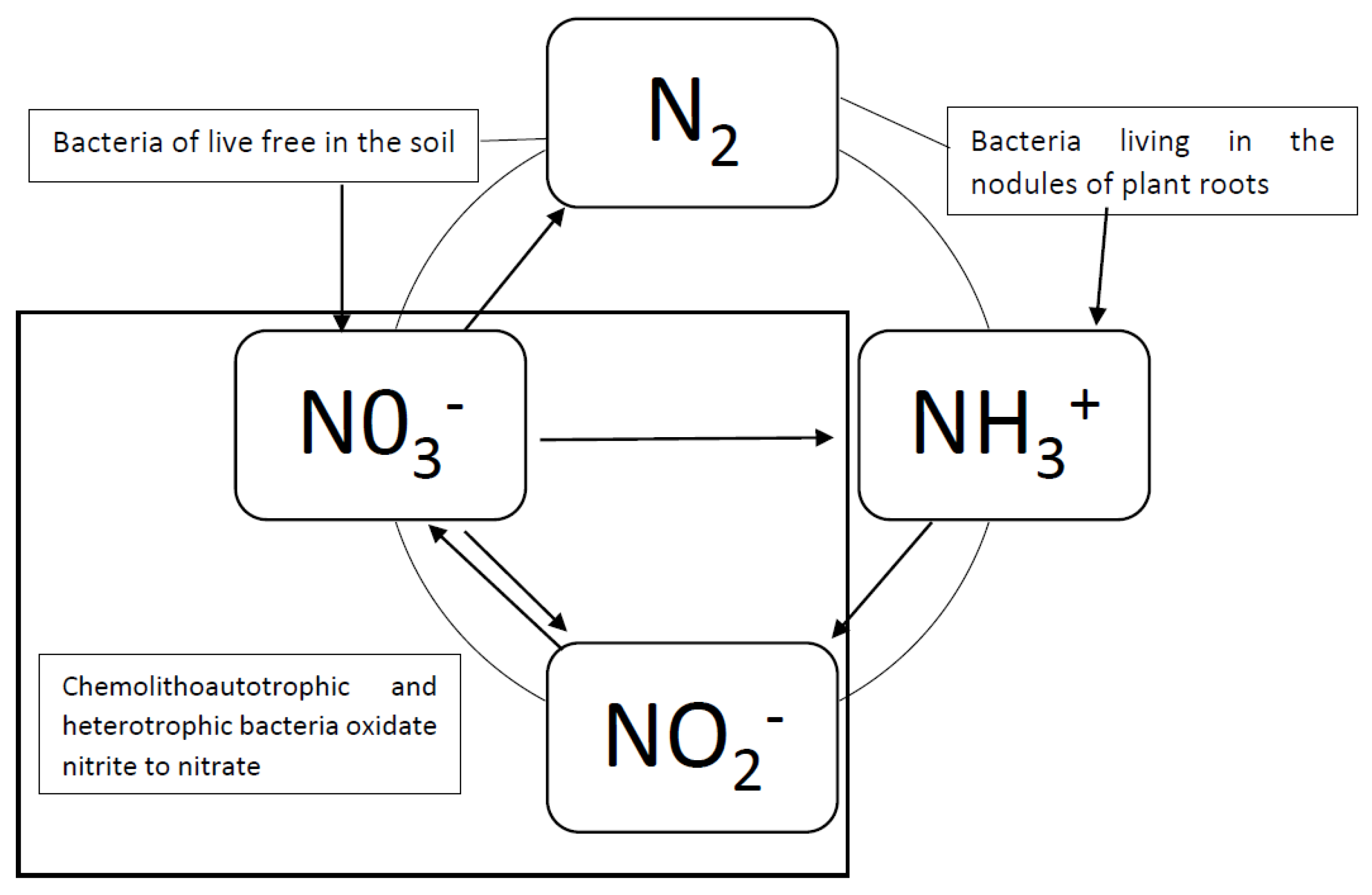
1. Introduction
2. Materials and Methods
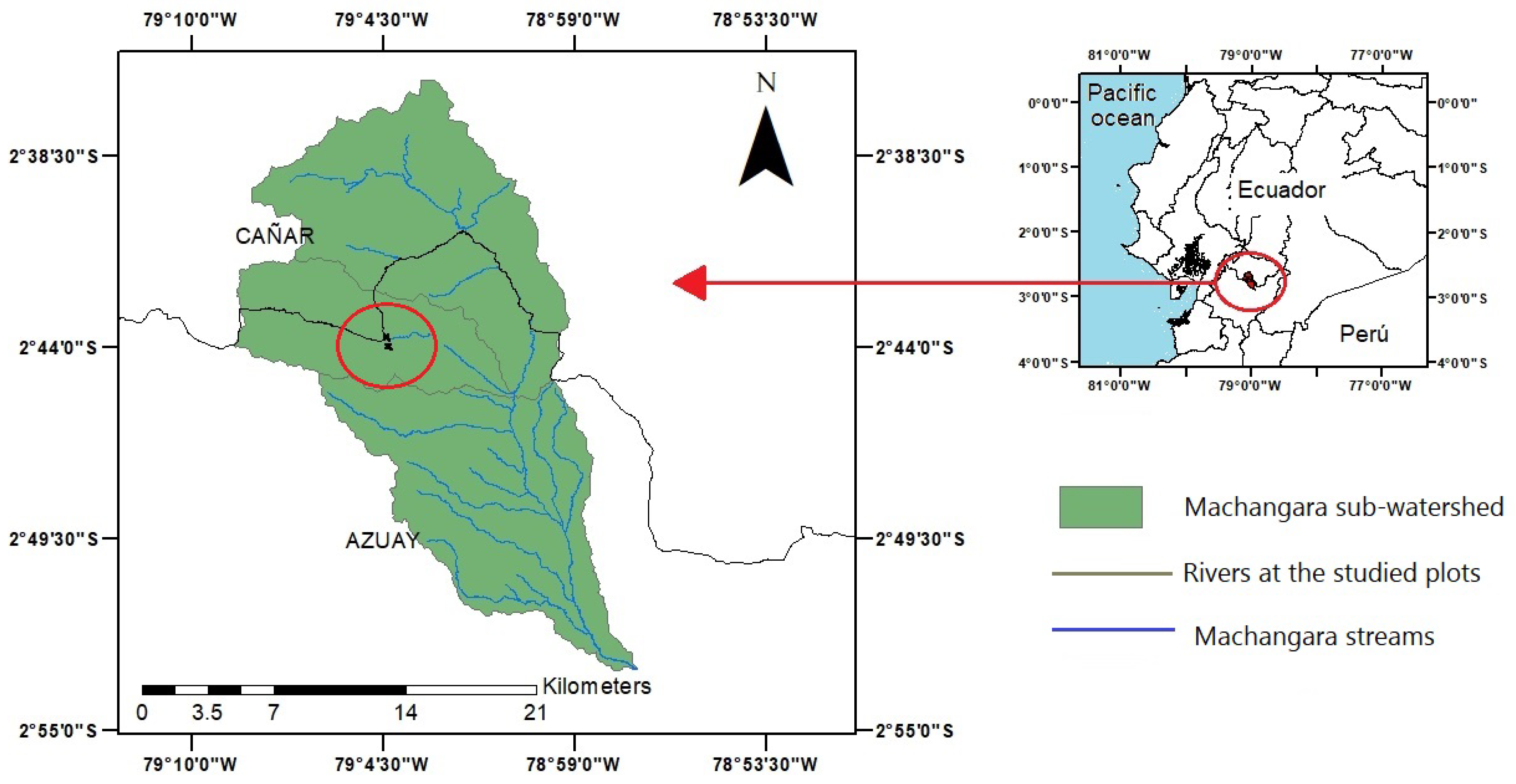
2.1. Study Site
2.2. Samples Preparation and pH Measurement
2.3. Isolation and Identification of Bacterial Strains
2.4. Bacterial Density by Most Probable Number (MPN)
2.5. Evaluation of the Nitrifying Potential
2.6. Detection of Oxidation Products (NO3−)
2.7. Application of Nitrifying Bacteria in a Synthetic Medium Rich in Nitrogenous Products
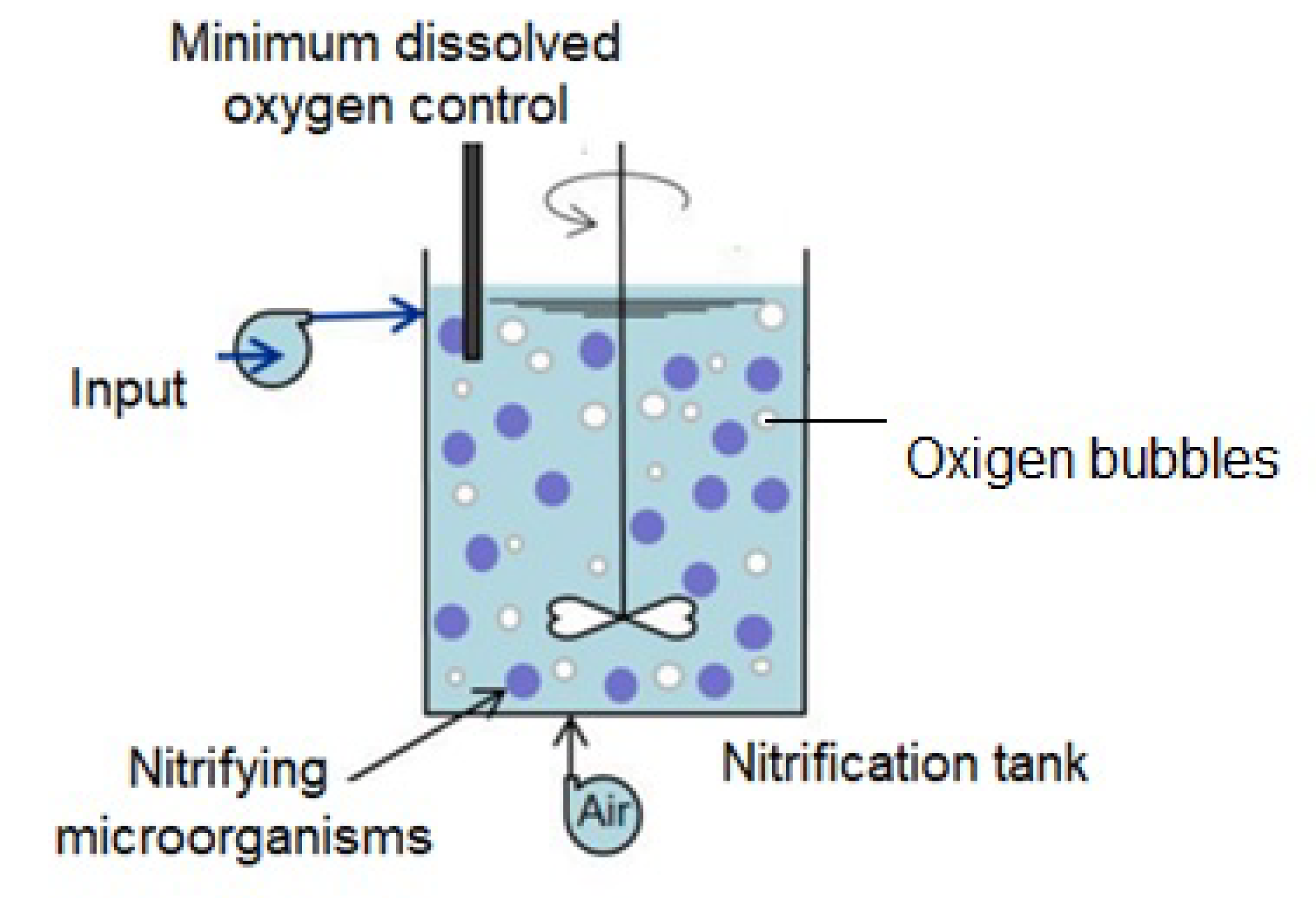
2.8. Experimental Setup
3. Results
3.1. Characterization of Strains and Bacterial Density
3.2. Determination of Nitrifying Potential and Detection of Oxidation Products
3.3. Application of Nitrifying Bacteria in Synthetic Medium Rich in Nitrogenous Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Botello, W.; Puerto, D.; Montes, D.; Rodas, E. Detection and Evaluation of Nitrifying Potential of Bacterial Isolates Associated to Rhizosphere of Three Macrophyte Species. Rev. Colomb. Cienc. Anim. 2014, 6, 358–362. [Google Scholar]
- Ward, B.B. Nitrification. Encycl. Ecol. 2018, 351–358. [Google Scholar]
- Madigan, M.T.; Martinko, J.; Bender, K.; Buckley, D.; Clark, D.P. Brock. Biología de Los Microorganismos; 14ª Edición Pearson: Madrid, Spain, 2015; pp. 105–107. [Google Scholar]
- Ruiz, A.K.; Quinteros, M.E.; González, B.A.; Arango, S. Bacterias nitrificantes y desnitrificantes asociadas a la rizosfera y a la biopelícula formada en humedales artificiales de flujo subsuperficial y flujo superficial. Activa 2019, 12, 21–39. [Google Scholar]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 1621. [Google Scholar] [CrossRef]
- Fiencke, C.; Spieck, E.; Bock, E. Nitrifying bacteria. In Nitrogen Fixation in Agriculture, Forestry, Ecology, and the Environment; Springer: Berlin/Heidelberg, Germany, 2005; Volume 4, pp. 255–270. [Google Scholar]
- Dworkin, M.; Falkow, S.; Rosenberg, E.; Schleifer, K.H.; Stackebrandt, E. Bacteria: Firmicutes, Cyanobacteria. In The Prokaryotes; Springer: New York, NY, USA, 2006; Volume 4, pp. 4–37. [Google Scholar]
- Sánchez, J.; Sanabria, J. Microbial metabolisms over advanced processes for Nitrogen removal, a prospective review. Rev. Colomb. De Biotecnol. 2009, 11, 114–124. [Google Scholar]
- Daniel, J.A.; Sayavedra-Soto, L.A.; Norman, G.H. Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea. Arch. Microbiol. 2002, 178, 250–255. [Google Scholar]
- Montoya, M. Strategies to Mitigate N2O Emissions and Improve Crop Quality. Effect of Zn-N Co-Fertilization and N-Enhanced Efficiency Fertilizers on Microbial Communities. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2020. [Google Scholar]
- Cañón-Cortázar, R.G.; Avellaneda-Torres, L.M.; Torres-Rojas, E. Microorganismos asociados al ciclo del nitrógeno en suelos bajo tres sistemas de uso: Cultivo de papá, ganadería y páramo, en el Parque Los Nevados en Colombia. Acta Agronómica 2012, 61, 371–379. [Google Scholar]
- Freitag, T.E.; Chang, L.; Clegg, C.D.; Prosser, J.I. Influence of inorganic nitrogen management regime on the diversity of nitrite-oxidizing bacteria in agricultural grassland soils. Appl. Environ. Microbiol. 2005, 71, 8323–8334. [Google Scholar] [CrossRef]
- Demergasso, C.; Dorador, C.; Meneses, D.; Blamey, J.; Cabrol, N.; Escudero, L.; Chong, G. Prokaryotic diversity pattern in high-altitude ecosystems of the Chilean Altiplano. J. Geophys. Res. Biogeosciences 2010, 115, 1–14. [Google Scholar] [CrossRef]
- Suaza Niño, E.V. Caracterización de los rasgos de historia de vida de Espeletia sp. y los grupos funcionales de bacterias solubilizadoras de fosfatos y fijadoras de nitrógeno, asociados al suelo de una zona conservada e intervenida del parque natural regional Siscunsí-Ocetá, Boyacá. Tesis obtención título de grado, Universidad El Bosque, Bogotá, Colombia, 2020. [Google Scholar]
- Vallejo, V.E.; Gómez, M.M.; Cubillos, A.M.; Roldán, F. Effect of land use on the density of nitrifying and denitrifying bacteria in the Colombian Coffee Region. Agron. Colomb. 2011, 29, 455–464. [Google Scholar]
- Molina, V.; Dorador, C.; Fernández, C.; Bristow, L.; Eissler, Y.; Hengst, M.; Cornejo, M. The activity of nitrifying microorganisms in a high-altitude Andean wetland. FEMS Microbiol. Ecol. 2018, 94, fiy062. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Modin, O.; Mijakovic, I. Technologies for biological removal and recovery of nitrogen from wastewater. Biotechnol. Adv. 2020, 43, 107570. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Sharma, B.; Shukla, P. Integrated approaches in microbial degradation of plastics. Environ. Technol. Innov. 2020, 17, 100567. [Google Scholar] [CrossRef]
- Calva, J.A.; Barceló, O.G.; Martínez, S.G. Remoción de nitrógeno por vía autótrofa en biopelícula. In Proceedings of the 5th International Conference of Greening of Industry Network, Ciudad de Mexico, Mexico, 28–30 October 2019. [Google Scholar]
- Bovio, P. Taxonomía y función de organismos del filo Chloroflexi en sistemas de tratamiento de aguas residuales. Ph.D. Thesis, Universidad de la República, Montevideo, Uruguay, 2021. [Google Scholar]
- Cerón, L.E.; Aristizábal, F.A. Nitrogen and phosphorus cycles dynamics in soils. Rev. Colomb. Biotecnol. 2012, 14, 285–295. [Google Scholar]
- Montaño, N.M.; Sandoval-Perez, A.L.; Nava-Mendoza, M.; Sánchez-Yañez, J.M.; García-Oliva, F. Variación espacial y estacional de grupos funcionales de bacterias cultivables del suelo de un bosque tropical seco en México. Rev. De Biol. Trop. 2013, 61, 439–453. [Google Scholar] [CrossRef]
- Quichimbo, P.; Jiménez, L.; Veintimilla, D.; Potthast, K.; Tischer, A.; Günter, S.; Hamer, U. Nutrient dynamics in an Andean forest region: A case study of exotic and native species plantations in southern Ecuador. New For. 2020, 51, 313–334. [Google Scholar] [CrossRef]
- van Voss, O.H.; Aguirre, N.; Hofstede, R. Sistemas Forestales Integrales Para La Sierra Del Ecuador; University of New Mexico: Albuquerque, NM, USA, 2001. [Google Scholar]
- Evans, J. Planted Forests: Uses, Impacts and Sustainability; Cabi: Wallingford, UK, 2009. [Google Scholar]
- Navarrete, E.F.; Morante-Carballo, J.; Dueñas-Tovar, P.; Carrión-Mero, M.; Jaya-Montalvo, M.; Berrezueta, E. Assessment of Geosites within a Natural Protected Area: A Case Study of Cajas National Park. Sustainability 2022, 14, 3120. [Google Scholar] [CrossRef]
- Veintimilla-Reyes, J.; De Meyer, A.; Cattrysse, D.; Tacuri, E.; Vanegas, P.; Cisneros, F.; Van Orshoven, J. MILP for optimizing water allocation and reservoir location: A case study for the Machángara river basin, Ecuador. Water 2019, 11, 1011. [Google Scholar] [CrossRef]
- Papen, H.; Von Berg, R. A Most Probable Number method (MPN) for the estimation of cell numbers of heterotrophic nitrifying bacteria in soil. Plant Soil 1998, 199, 123–130. [Google Scholar] [CrossRef]
- Koubek, J.; Uhlik, O.; Jecna, K.; Junkova, P.; Vrkoslavova, J.; Lipov, J.; Mackova, M. Whole-cell MALDI-TOF: Rapid screening method in environmental microbiology. Int. Biodeterior. Biodegrad. 2012, 69, 82–86. [Google Scholar] [CrossRef]
- Elbanna, K.H.; El-Shahawy, R.M.; Atalla, K.M. A new simple method for the enumeration of nitrifying bacteria in different environments. Plant Soil Environ. 2012, 58, 49–53. [Google Scholar] [CrossRef]
- Mahecha, S. Comparación de la densidad y actividad bacteriana fijadora libre de nitrógeno entre tres usos de suelo (Cuenca del Otún, Risaralda). Master’s Thesis, Pontificia Universidad Javeriana, Bogotá, Colombia, 2011. [Google Scholar]
- Standard Methods 4500-NO3-B; Standard Methods for the examination of Water and Wastewater. American Public Health Association: Washington, DC, USA, 2012.
- Chen, M.; Wang, W.; Feng, Y.; Zhu, X.; Zhou, H.; Tan, Z.; Li, X. Impact resistance of different factors on ammonia removal by heterotrophic nitrification-aerobic denitrification bacterium Aeromonas sp. HN-02. Bioresour. Technol. 2014, 167, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, T.; Liu, C.; Gao, J.; Zhang, J.; Li, Y. Ammonium removal characteristics of heterotrophic nitrifying bacterium Pseudomonas stutzeri GEP-01 with potential for treatment of ammonium-rich wastewater. Bioprocess Biosyst. Eng. 2020, 43, 959–969. [Google Scholar] [CrossRef]
- Farazaki, M.; Gikas, P. Nitrification-denitrification of municipal wastewater without recirculation, using encapsulated microorganisms. J. Environ. Manag. 2019, 242, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, L.M. Estudio de la población de bacterias nitrificantes y su relación con los parámetros físico-químicos, biológicos y operacionales en una EDAR con sistema convencional de Fangos Activos. Master’s Thesis, Universidad Politécnica de Valencia, Valencia, Spain, 2011. [Google Scholar]
- Kouki, S.; Saidi, N.; M’hiri, F.; Nars, H.; Cherif, H.; Ouzari, H.; Hassen, A. Isolation and characterization of facultative mixotrophic ammonia-oxidizing bacteria from constructed wetlands. J. Environ. Sci. 2011, 23, 1699–1708. [Google Scholar] [CrossRef]
- Faeflen, S.J.; Li, S.; Xin, X.; Wright, A.L.; Jiang, X. Autotrophic and Heterotrophic Nitrification in a Highly Acidic Subtropical Pine Forest Soil. Pedosphere 2016, 26, 904–910. [Google Scholar] [CrossRef]
- Taylor, A.E.; Zeglin, L.H.; Wanzek, T.A.; Myrold, D.D.; Bottomley, P.J. Dynamics of ammonia-oxidizing archaea and bacteria populations and contributions to soil nitrification potentials. ISME J. 2012, 6, 2024–2032. [Google Scholar] [CrossRef]
- Díaz-Granados, M.A.; Arévalo, A.; Chaparro-Giraldo, A.; Escobar-Núñez, L.A. Soil microbial community structure and function in different types of tropical forests and agroecosystems in the Colombian Andes. Soil Biol. Biochem. 2018, 123, 112–120. [Google Scholar]
- Lambais, M.R.; de Oliveira, C.R.; Wainwright, M.; Krüger, R.H. Soil microbial communities in native forest and exotic Pinus plantations in the Brazilian Atlantic rainforest. Appl. Soil Ecol. 2021, 163, 103930. [Google Scholar]
- Hamel, C. Impact of arbuscular mycorrhizal fungi on N and P cycling in the root zone. Can. J. Soil Sci. 2004, 84, 383–395. [Google Scholar] [CrossRef]
- Sakai, K.; Nakamura, K.; Wakayama, M.; Moriguchi, M. Change in nitrite conversion direction from oxidation to reduction in heterotrophic bacteria depending on the aeration conditions. J. Ferment. Bioeng. 1997, 84, 47–52. [Google Scholar] [CrossRef]
- Mekuto, L.; Ntwampe, S.K.O.; Jackson, V.A. Biodegradation of free cyanide and subsequent utilisation of biodegradation by-products by Bacillus consortia: Optimisation using response surface methodology. Environ. Sci. Pollut. Res. 2015, 22, 10434–10443. [Google Scholar] [CrossRef] [PubMed]
- Mpongwana, N.; Ntwampe, S.K.O.; Mekuto, L.; Akinpelu, E.A.; Dyantyi, S.; Mpentshu, Y. Isolation of high-salinity-tolerant bacterial strains, Enterobacter sp., Serratia sp., Yersinia sp., for nitrification and aerobic denitrification under cyanogenic conditions. Water Sci. Technol. 2016, 73, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Martínez, A.M. Microorganisms in Concrete; Universidad de los Andes: Bogotá, Colombia, 2011. [Google Scholar]
- Sánchez, F.; Viedma, A.; Kaiser, A.S. Hydraulic characterization of an activated sludge reactor with recycling system by tracer experiment and analytical models. Water Res. 2016, 101, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Uemoto, H.; Watanabe, A. Nitrogen-removal bioreactor capable of simultaneous nitrification and denitrification for application to industrial wastewater treatment. Biochem. Eng. J. 2008, 41, 59–66. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Z.; Jin, Y.; Lu, J.; Cheng, X.; Li, J.; Chen, D. Nitrification characteristics of nitrobacteria immobilized in waterborne Polyurethane in wastewater of corn-based ethanol fuel production. J. Environ. Sci. 2012, 24, 999–1005. [Google Scholar] [CrossRef]
- De Oliveira-Freitas, B.; Daniel, L.A. A new anaerobic, aerobic, nitrification, anoxic reactor (AANAR) with overlaid biological zones: Sulfide removal from wastewater and biogas. Chem. Eng. J. 2023, 452, 139255. [Google Scholar] [CrossRef]
- Cho, K.W.; Song, K.G.; Cho, J.W.; Kim, T.G.; Ahn, K.H. Removal of nitrogen by a layered soil infiltration system during intermittent storm events. Chemosphere 2009, 76, 690–696. [Google Scholar] [CrossRef]
- Prodanovic, V.; Zhang, K.; Zheng, M.; Hu, S.; Hong, P.Y.; Yuan, Z.; Deletic, A. Nitrification potential of daily-watered biofiltration designs for high ammonium wastewater treatment. Sci. Total Environ. 2023, 863, 160989. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Xu, X.; Zhang, C.; Wang, D.; Yang, F. Achieving mainstream nitrogen removal through simultaneous partial nitrification, anammox and denitrification process in an integrated fixed film activated sludge reactor. Chemosphere 2018, 203, 457–466. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Yang, F.; Xue, Y.; Wang, T. The development of simultaneous partial nitrification, ANAMMOX and denitrification (SNAD) process in a single reactor for nitrogen removal. Bioresour. Technol. 2009, 100, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Matovelle, C. Analysis of a high andean river’s behavior at loads of organic matter through the use of mathematical models with experimentally determined kinetic rates. Int. J. Sustain. Dev. Plan. 2021, 16, 675–682. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mazumder, D. Kinetic study on nitrification of ammonium nitrogen-enriched synthetic wastewater using activated sludge. Water Sci. Technol. 2020, 81, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Mazumder, D. Evaluation of nitrification kinetics for treating ammonium nitrogen enriched wastewater in moving bed hybrid bioreactor. J. Environ. Chem. Eng. 2021, 9, 104589. [Google Scholar] [CrossRef]
- Mehrani, M.J.; Lu, X.; Kowal, P.; Sobotka, D.; Mąkinia, J. Incorporation of the complete ammonia oxidation (comammox) process for modeling nitrification in suspended growth wastewater treatment systems. J. Environ. Manag. 2021, 297, 11322. [Google Scholar] [CrossRef]
- Graham, D.W.; Knapp, C.W.; Van Vleck, E.S.; KBloor, K.; Lane, T.B.; Graham, C.E. Experimental demonstration of chaotic instability in biological nitrification. ISME J. 2007, 1, 385–393. [Google Scholar] [CrossRef]

| Composition | Culture Media | |||
|---|---|---|---|---|
| OAL | OAH | ONL | ONH | |
| MgSO4·7H2O | 0.3 | 0.3 | 0.05 | 0.05 |
| CaCO3 | 7.5 | 7.5 | 0.03 | 0.03 |
| KH2PO4 | 1 | 1 | 0.15 | 0.15 |
| FeSO4·7H2O | 0.03 | 0.03 | 0.00015 | 0.00015 |
| NaCl | 0.3 | 0.3 | 0.5 | - |
| (NH4)2SO4 | 0.5 | - | - | |
| NaNO2 | 0.2 | 0.2 | ||
| (NH4)2Mo7O24 4H2O | 50 (µg) | 50 (µg) | ||
| Sodium pyruvate | 0.55 | |||
| Yeast extract | 1.5 | 1.5 | ||
| Peptone | 1.5 | 1.5 | ||
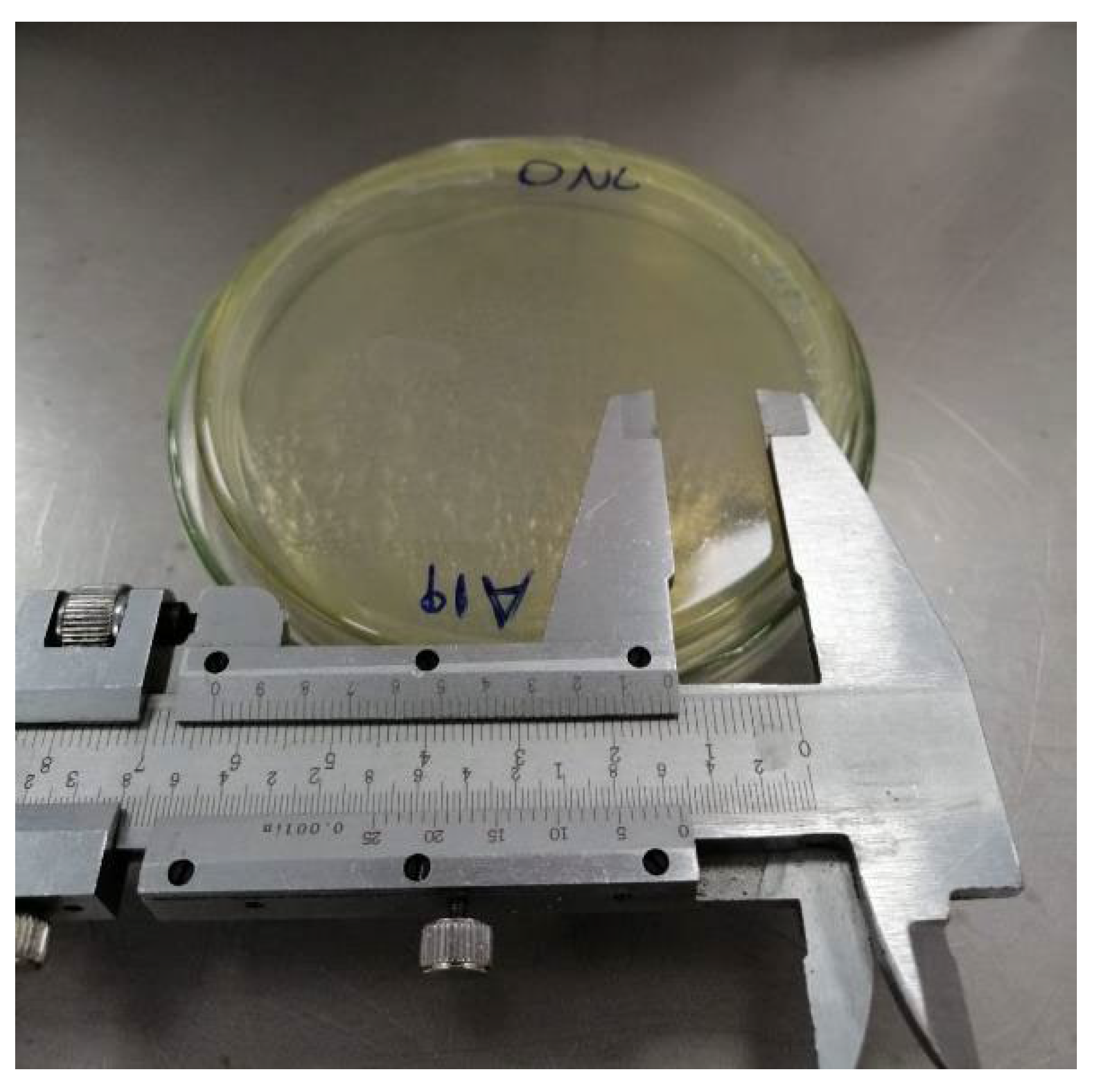
| Strain | Diameter (cm) | Genus | Plot | |
|---|---|---|---|---|
| ONL | BP 1 | BN 2 | ||
| b3R | 1.1 | Bacillus pumilus | x | |
| b2R9 | 1.2 | Yersinia kristensenii | x | |
| b1R14 | - | Serratia proteamaculans | x | |
| G23 | 1.3 | Yersinia enterocolítica | x | |
| A1 | 1.0 | Aeromonas encheleia | x | x |
| C2(2) | - | Paenibacillus stefellifer | x | |
| b5R | 1.1 | Aeromonas molluscorum | x | |
| C2-1 | 1.6 | Serratia plymuthica | x | |
| A19 | 1.3 | Buttiauxella gaviniae | x | |
| b17R | 1.1 | Bacillus muralis | x | |
| A6 | 1.1 | Buttiauxella ferraguliae | x | |
| f2 | 1.2 | Mycobacterium sp. | x | |
| Mean | 1.15 | |||
| SD | 0.17 | |||
| CV | 14.7% | |||
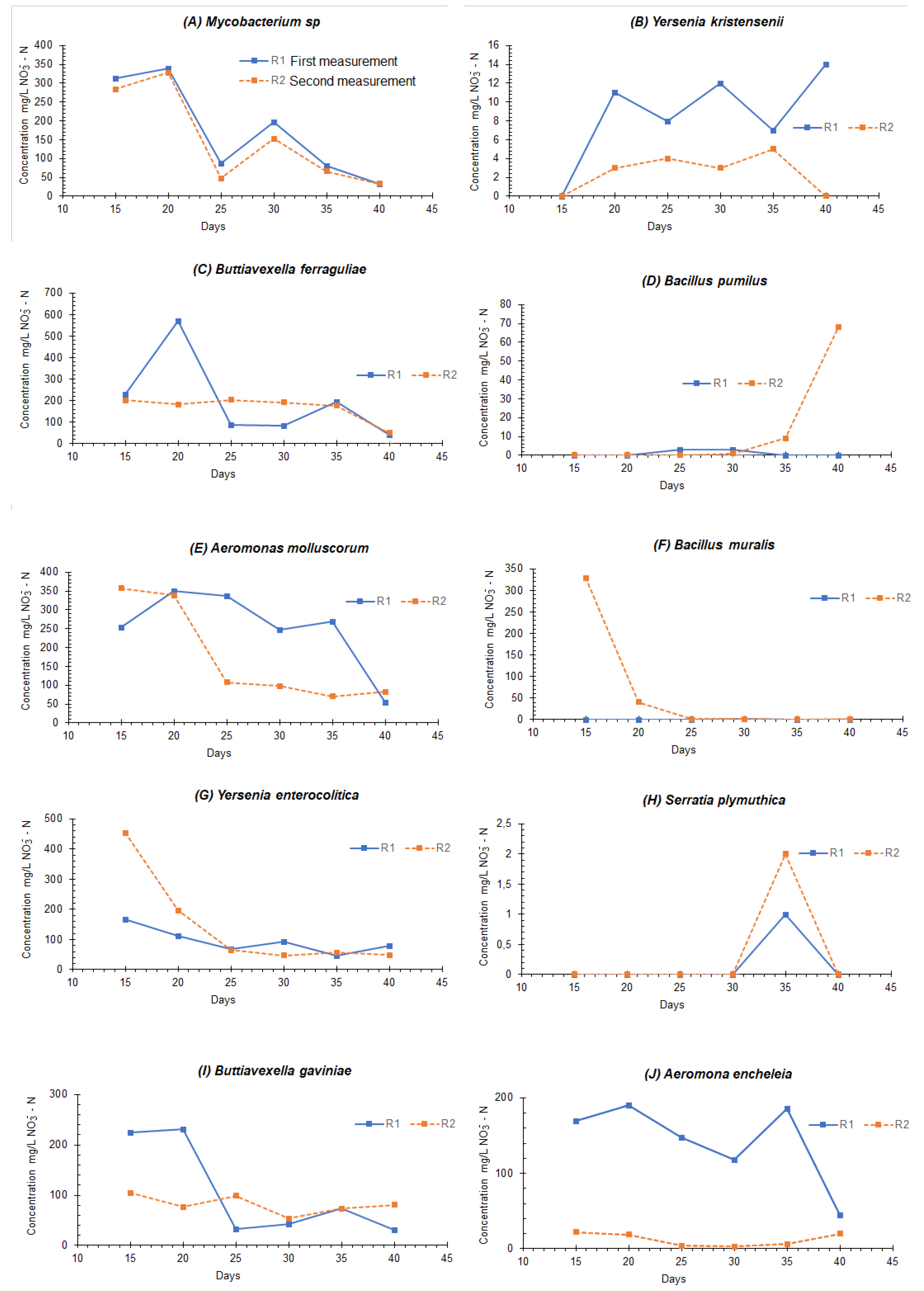
| Bacteria | Optimal TCR for Nitrification (Days)—Maximum Concentration of NO3−—N (mg/L) | Particular Considerations |
|---|---|---|
| Mycobacterium sp. | 20–350 | After day 20, there was a denitrification process; no oxygen was supplied to consider anoxia |
| Yersenia kristensenii | 40–14 | There was no denitrification process |
| Buttiavexella ferraguliae | 40–80 | There was no denitrification process |
| Bacillus pumilus | - | No clear nitrification processes were evident within the times proposed in the experiment |
| Aeromonas molluscorum | 20–350 | After day 20, there was a denitrification process; no oxygen was supplied to consider anoxia |
| Bacillus muralis | - | There was no concentration of nitrates, and no nitrification processes were observed |
| Yersenia enterocolítica | 15–450 | There was an increase in nitrates for up to 15 days and, subsequently, an anoxic process of denitrification |
| Serratia plymuthica | 35–2 | On day 35, a slight increase in the concentration of nitrates was seen, indicating a nitrification process |
| Buttiavexella gaviniae | 20–220 | After day 20, there was a denitrification process; no oxygen was supplied to consider anoxia |
| Aeromona encheleia | After day 20, there was a denitrification process; no oxygen was supplied to consider anoxia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, J.M.; Calle, J.; Pereira, S.; Cordero, P.; Matovelle, C. Nitrite-Oxidizing Bacterial Strains Isolated from Soils of Andean Ecosystems and Their Potential Use in Nitrogen Reduction. Sustainability 2023, 15, 9277. https://doi.org/10.3390/su15129277
Salazar JM, Calle J, Pereira S, Cordero P, Matovelle C. Nitrite-Oxidizing Bacterial Strains Isolated from Soils of Andean Ecosystems and Their Potential Use in Nitrogen Reduction. Sustainability. 2023; 15(12):9277. https://doi.org/10.3390/su15129277
Chicago/Turabian StyleSalazar, Jazmin M., Jessica Calle, Steeven Pereira, Paula Cordero, and Carlos Matovelle. 2023. "Nitrite-Oxidizing Bacterial Strains Isolated from Soils of Andean Ecosystems and Their Potential Use in Nitrogen Reduction" Sustainability 15, no. 12: 9277. https://doi.org/10.3390/su15129277
APA StyleSalazar, J. M., Calle, J., Pereira, S., Cordero, P., & Matovelle, C. (2023). Nitrite-Oxidizing Bacterial Strains Isolated from Soils of Andean Ecosystems and Their Potential Use in Nitrogen Reduction. Sustainability, 15(12), 9277. https://doi.org/10.3390/su15129277







