Effect of Pyrolysis Treatment on Phosphorus Migration and Transformation of Pig, Cow and Sheep Manure
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Preparation of Biochar Samples
2.3. Separation of Different P Fractionations
2.4. Olsen-P
2.5. P Determination
2.6. Residual Rate
2.7. Statistical Analysis
3. Results and Discussion
3.1. Mass Balance of P
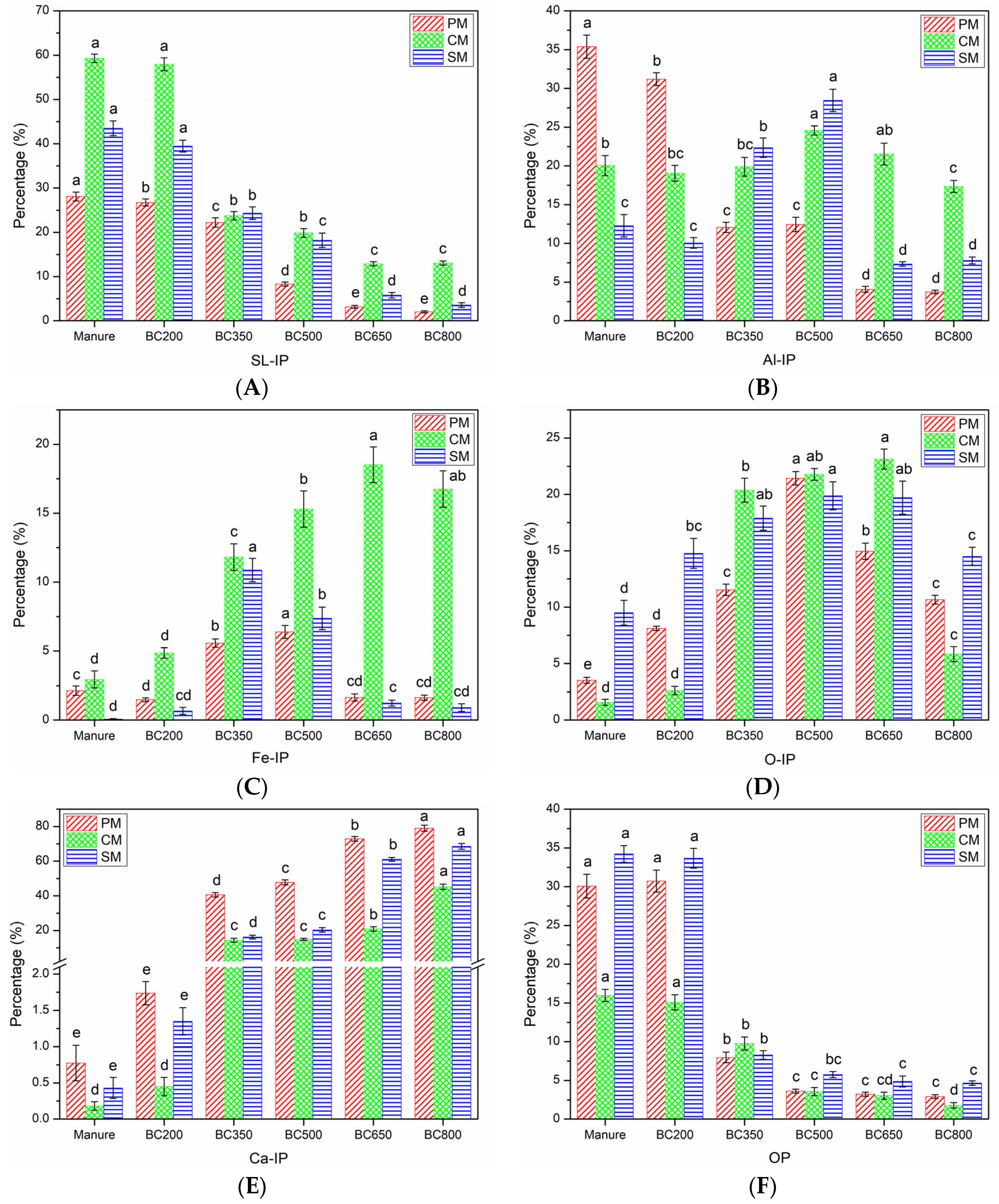
3.2. P Speciation
3.2.1. SL-IP
3.2.2. AL-IP
3.2.3. Fe-IP
3.2.4. O-IP
3.2.5. Ca-IP
3.2.6. OP
3.3. Olsen-P
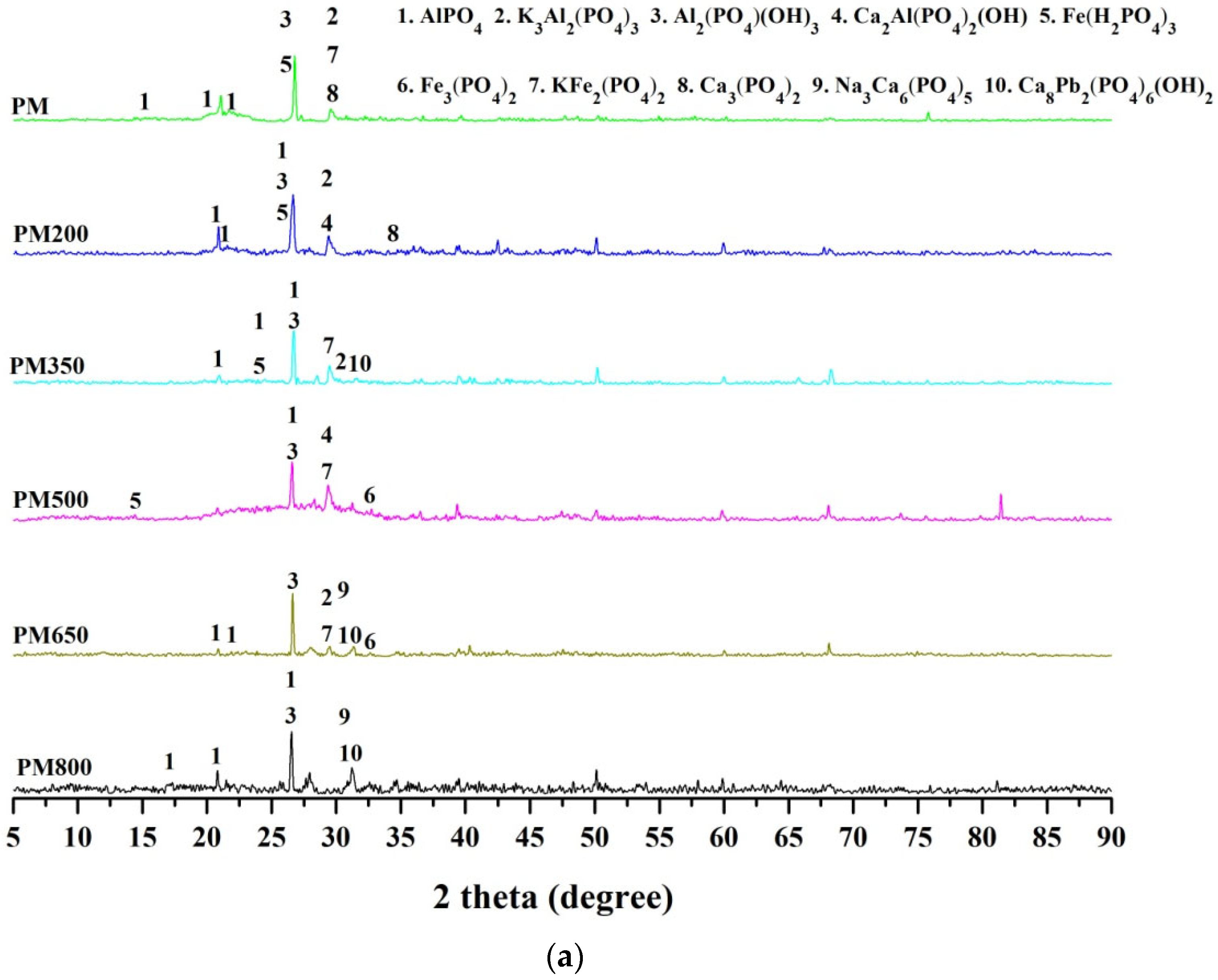
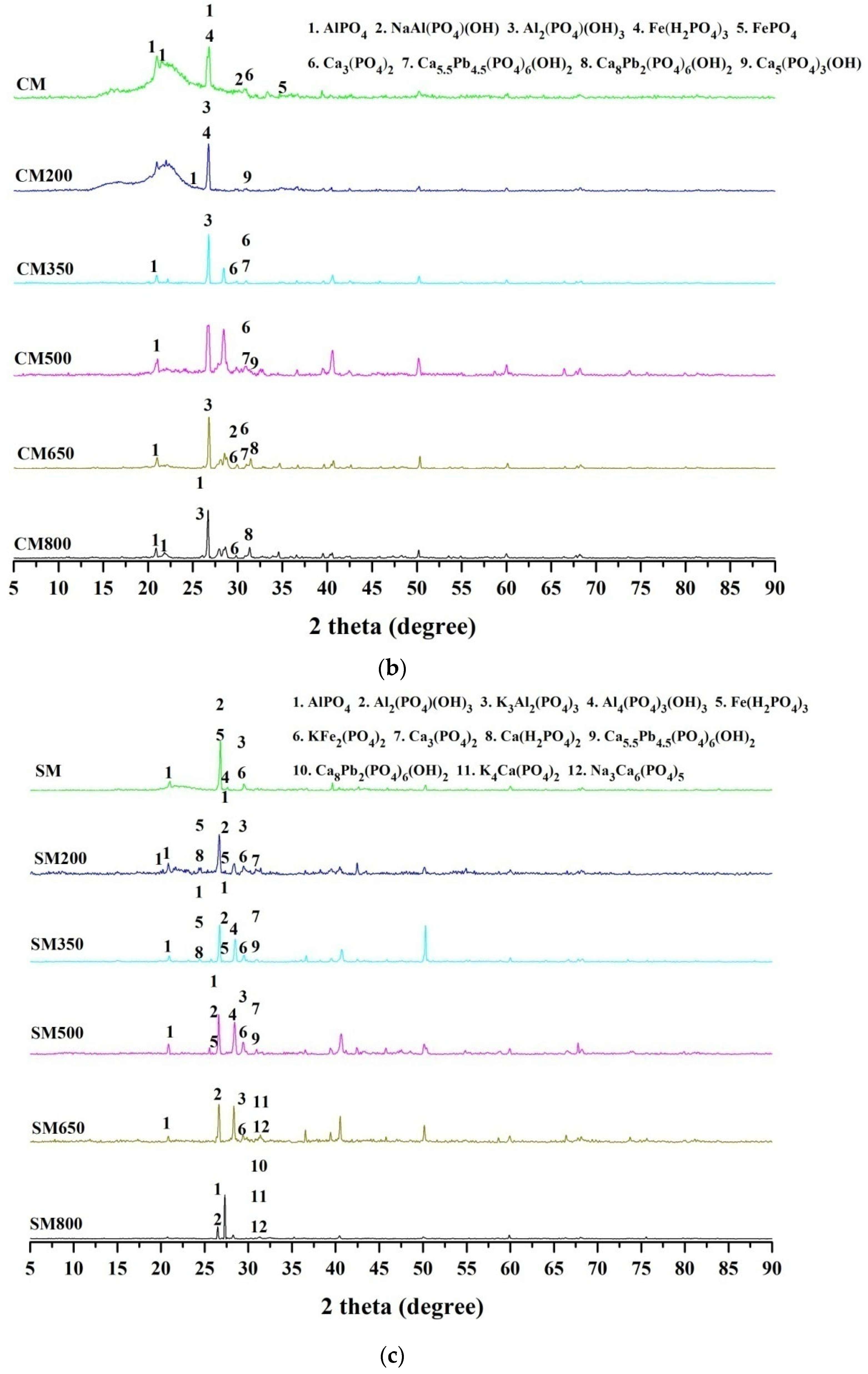
3.4. XRD of Raw Materials and the Derived Biochar of Olsen-P
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhang, Y.; Li, J.; Lin, J.-G.; Zhang, N.; Cao, W. Biogas Energy Generated from Livestock Manure in China: Current Situation and Future Trends. J. Environ. Manag. 2021, 297, 113324. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zou, D.; Xiao, Z.; Zeng, X.; Zhang, L.; Jiang, L.; Wang, A.; Ge, D.; Zhang, G.; Liu, F. Biochar as a Sorbent for Emerging Contaminants Enables Improvements in Waste Management and Sustainable Resource Use. J. Clean. Prod. 2019, 210, 1324–1342. [Google Scholar] [CrossRef]
- Zeng, X.; Zou, D.; Wang, A.; Zhou, Y.; Liu, Y.; Li, Z.; Liu, F.; Wang, H.; Zeng, Q.; Xiao, Z. Remediation of Cadmium-Contaminated Soils Using Brassica Napus: Effect of Nitrogen Fertilizers. J. Environ. Manag. 2020, 255, 109885. [Google Scholar] [CrossRef] [PubMed]
- Dalu, T.; Wasserman, R.J.; Magoro, M.L.; Froneman, P.W.; Weyl, O.L.F. River Nutrient Water and Sediment Measurements Inform on Nutrient Retention, with Implications for Eutrophication. Sci. Total Environ. 2019, 684, 296–302. [Google Scholar] [CrossRef]
- Wan, W.; Wang, Y.; Tan, J.; Qin, Y.; Zuo, W.; Wu, H.; He, H.; He, D. Alkaline Phosphatase-Harboring Bacterial Community and Multiple Enzyme Activity Contribute to Phosphorus Transformation during Vegetable Waste and Chicken Manure Composting. Bioresour. Technol. 2020, 297, 122406. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ma, H.; Liang, J.; Okopi, S.I.; Yang, S.; Cao, L.; Sun, W. Effects of Different Conditions Tested “in Vitro” on the Phosphorus Runoff Potential of Livestock Manure. Waste Manag. 2022, 147, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Gascó, G.; Méndez, A.; Surapaneni, A.; Jegatheesan, V.; Shah, K.; Paz-Ferreiro, J. Influence of Pyrolysis Parameters on Phosphorus Fractions of Biosolids Derived Biochar. Sci. Total Environ. 2019, 695, 133846. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, X.; Feng, X.; Wang, E.; Li, H.; Shen, J.; Zhang, F. Management Strategies to Optimize Soil Phosphorus Utilization and Alleviate Environmental Risk in China. J. Environ. Qual. 2019, 48, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dinkler, K.; Zhao, N.; Sobhi, M.; Merkle, W.; Liu, S.; Dong, R.; Oechsner, H.; Guo, J. Influence of Anaerobic Digestion on the Labile Phosphorus in Pig, Chicken, and Dairy Manure. Sci. Total Environ. 2020, 737, 140234. [Google Scholar] [CrossRef]
- Yang, Y.; Meehan, B.; Shah, K.; Surapaneni, A.; Hughes, J.; Fouché, L.; Paz-Ferreiro, J. Physicochemical Properties of Biochars Produced from Biosolids in Victoria, Australia. Int. J. Environ. Res. Public Health 2018, 15, 1459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, X.; Deng, Y.; Tsang, D.C.W.; Yuan, H.; Shen, J.; Zhang, S. Swine Manure Valorization for Phosphorus and Nitrogen Recovery by Catalytic–Thermal Hydrolysis and Struvite Crystallization. Sci. Total Environ. 2020, 729, 138999. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ren, C.; Guo, H.; Guo, M.; Li, W. Speciation Transformation of Phosphorus in Poultry Litter during Pyrolysis: Insights from X-Ray Diffraction, Fourier Transform Infrared, and Solid-State NMR Spectroscopy. Environ. Sci. Technol. 2019, 53, 13841–13849. [Google Scholar] [CrossRef]
- Cui, X.; Yang, X.; Sheng, K.; He, Z.; Chen, G. Transformation of Phosphorus in Wetland Biomass during Pyrolysis and Hydrothermal Treatment. ACS Sustain. Chem. Eng. 2019, 7, 16520–16528. [Google Scholar] [CrossRef]
- Chen, X.; Gao, M.; Li, Y.; Zhang, X.; Zhang, F.; Hu, B. Effects of Freeze-Thaw Cycles on the Physicochemical Characteristics of Animal Manure and Its Phosphorus Forms. Waste Manag. 2019, 88, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, H.; Wei, W.; Hu, B. Electrocoagulation of Dairy Manure Using Low-Carbon Steel Electrodes for Phosphorus Removal. J. Environ. Eng. 2020, 146, 04020044. [Google Scholar] [CrossRef]
- Liu, L.; Tan, Z.; Gong, H.; Huang, Q. Migration and Transformation Mechanisms of Nutrient Elements (N, P, K) within Biochar in Straw–Biochar–Soil–Plant Systems: A Review. ACS Sustain. Chem. Eng. 2019, 7, 22–32. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Cayuela, M.L.; Rasse, D.P.; Sánchez-Monedero, M.A. Biochars from Mediterranean Agroindustry Residues: Physicochemical Properties Relevant for C Sequestration and Soil Water Retention. ACS Sustain. Chem. Eng. 2019, 7, 4724–4733. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, F.; Li, Y.; Li, B.; Yang, T.; Li, R. Influence of Microwave-Assisted Pyrolysis Parameters and Additives on Phosphorus Speciation and Transformation in Phosphorus-Enriched Biochar Derived from Municipal Sewage Sludge. J. Clean. Prod. 2021, 287, 125550. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, B.; Zhang, X.; Xu, H.; Cheng, N.; Feng, Q.; Zhao, R.; Gao, Y.; Wei, M. Release Characteristics of Phosphate from Ball-Milled Biochar and Its Potential Effects on Plant Growth. Sci. Total Environ. 2022, 821, 153256. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Wang, W.; Zhao, J.; Zhou, D. Transformation of Phosphorus Species during Phosphoric Acid-Assisted Pyrolysis of Lignocellulose. Sci. Total Environ. 2023, 866, 161010. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Y.; Lu, X.-Y.; Zhang, Z.; Cao, Y. Phosphorus Speciation in Sewage Sludge and the Sludge-Derived Biochar by a Combination of Experimental Methods and Theoretical Simulation. Water Res. 2018, 140, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, Q.; Li, D.; Li, J.; Guo, W. Performance Comparison of Phosphorus Recovery from Different Sludges in Sewage Treatment Plants through Pyrolysis. J. Clean. Prod. 2022, 372, 133728. [Google Scholar] [CrossRef]
- Backnäs, S.; Laine-Kaulio, H.; Kløve, B. Phosphorus Forms and Related Soil Chemistry in Preferential Flowpaths and the Soil Matrix of a Forested Podzolic till Soil Profile. Geoderma 2012, 189–190, 50–64. [Google Scholar] [CrossRef]
- Müller, A.L.; Reichmann, W. Architecture, Materiality and Society; Palgrave Macmillan UK: London, UK, 2015; Volume 27, ISBN 978-1-349-69001-5. [Google Scholar]
- Shen, J.; Li, R.; Zhang, F.; Fan, J.; Tang, C.; Rengel, Z. Crop Yields, Soil Fertility and Phosphorus Fractions in Response to Long-Term Fertilization under the Rice Monoculture System on a Calcareous Soil. Field Crops Res. 2004, 86, 225–238. [Google Scholar] [CrossRef]
- Szögi, A.A.; Vanotti, M.B.; Hunt, P.G. Phosphorus Recovery from Pig Manure Solids Prior to Land Application. J. Environ. Manag. 2015, 157, 1–7. [Google Scholar] [CrossRef]
- Huang, R.; Fang, C.; Zhang, B.; Tang, Y. Transformations of Phosphorus Speciation during (Hydro)Thermal Treatments of Animal Manures. Environ. Sci. Technol. 2018, 52, 3016–3026. [Google Scholar] [CrossRef]
- Mickdam, E.; Khiaosa-ard, R.; Metzler-Zebeli, B.U.; Humer, E.; Harder, H.; Khol-Parisini, A.; Zebeli, Q. Modulation of Ruminal Fermentation Profile and Microbial Abundance in Cows Fed Diets Treated with Lactic Acid, without or with Inorganic Phosphorus Supplementation. Anim. Feed Sci. Technol. 2017, 230, 1–12. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, J.; Hou, F.; Zhang, Y.; Wang, G.; Liu, B.; Zhai, Z. Understanding Effect of Phosphorus-Based Additive on Ash Deposition Characteristics during High-Sodium and High-Calcium Zhundong Coal Combustion in Drop-Tube Furnace. Fuel 2021, 287, 119462. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Y.; Tu, Y.; Zhang, N.; Bai, Z.; Chadwick, D.; Dou, Z.; Ma, L. A Higher Water-Soluble Phosphorus Supplement in Pig Diet Improves the Whole System Phosphorus Use Efficiency. J. Clean. Prod. 2020, 272, 122586. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring Phosphorus Fertilizers and Fertilization Strategies for Improved Human and Environmental Health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Hedley, M.; McLaughlin, M. Reactions of Phosphate Fertilizers and By-Products in Soils. Phosphorus Agric. Environ. 2015, 46, 181–252. [Google Scholar]
- Chahine, S.; Garau, G.; Castaldi, P.; Vittoria, M.; Sara, P.; Seddaiu, G.; Roggero, P.P. Stabilising Fluoride in Contaminated Soils with Monocalcium Phosphate and Municipal Solid Waste Compost: Microbial, Biochemical and Plant Growth Impact. Environ. Sci. Pollut. Res. 2022, 29, 41820–41833. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.E. How Effective Are Existing Phosphorus Management Strategies in Mitigating Surface Water Quality Problems in the U.S.? Sustainability 2021, 13, 6565. [Google Scholar] [CrossRef]
- Qian, T.; Jiang, H. Migration of Phosphorus in Sewage Sludge during Different Thermal Treatment Processes. Sustain. Chem. Eng. 2014, 2, 1411–1419. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef] [PubMed]
- Eduah, J.O.; Nartey, E.K.; Abekoe, M.K.; Breuning-madsen, H.; Andersen, M.N. Geoderma Phosphorus Retention and Availability in Three Contrasting Soils Amended with Rice Husk and Corn Cob Biochar at Varying Pyrolysis Temperatures. Geoderma 2019, 341, 10–17. [Google Scholar] [CrossRef]
- Yuan, H.; Tai, Z.; Li, Q.; Liu, E. In-situ, high-resolution evidence from water-sediment interface for significant role of iron bound phosphorus in eutrophic lake. Sci. Total Environ. 2019, 706, 136040. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fang, C.; Lu, X.; Jiang, R.; Tang, Y. Transformation of Phosphorus during (Hydro)Thermal Treatments of Solid Biowastes: Reaction Mechanisms and Implications for P Reclamation and Recycling. Environ. Sci. Technol. 2017, 51, 10284–10298. [Google Scholar] [CrossRef] [PubMed]
- Bruun, S.; Harmer, S.L.; Bekiaris, G.; Christel, W.; Zuin, L.; Hu, Y.; Jensen, L.S.; Lombi, E. The Effect of Different Pyrolysis Temperatures on the Speciation and Availability in Soil of P in Biochar Produced from the Solid Fraction of Manure. Chemosphere 2017, 169, 377–386. [Google Scholar] [CrossRef]
- Uchimiya, M.; Hiradate, S. Pyrolysis Temperature-Dependent Changes in Dissolved Phosphorus Speciation of Plant and Manure Biochars. J. Agric. Food Chem. 2014, 62, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, Y.; Shao, H.; Sun, J. Pyrolysis Temperature Affects Phosphorus Transformation in Biochar: Chemical Fractionation and 31P NMR Analysis. Sci. Total Environ. 2016, 569–570, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, S.; Feng, G.; Zhu, P.; Huang, S.; Wang, B.; Xu, M. Determining the Optimum Range of Soil Olsen P for High P Use Efficiency, Crop Yield, and Soil Fertility in Three Typical Cropland Soils. Pedosphere 2020, 30, 832–843. [Google Scholar] [CrossRef]
- Wu, L.; Wei, C.; Zhang, S.; Wang, Y.; Kuzyakov, Y.; Ding, X. MgO-Modified Biochar Increases Phosphate Retention and Rice Yields in Saline-Alkaline Soil. J. Clean. Prod. 2019, 235, 901–909. [Google Scholar] [CrossRef]
- Fang, J.; Liu, Z.; Luan, H.; Liu, F.; Yuan, X.; Long, S.; Wang, A.; Ma, Y.; Xiao, Z. Thermochemical Liquefaction of Cattle Manure Using Ethanol as Solvent: Effects of Temperature on Bio-Oil Yields and Chemical Compositions. Renew. Energy 2021, 167, 32–41. [Google Scholar] [CrossRef]
- Luan, H.; Liu, F.; Long, S.; Liu, Z.; Qi, Y.; Xiao, Z.; Fang, J. The Migration, Transformation, and Risk Assessment of Heavy Metals in Residue and Bio-Oil Obtained by the Liquefaction of Pig Manure. Environ. Sci. Pollut. Res. 2020, 28, 15055–15069. [Google Scholar] [CrossRef] [PubMed]
| Properties | Pig Manure | Cattle Manure | Sheep Manure |
|---|---|---|---|
| Elemental analysis a (wt %) | |||
| C | 33.51 ± 0.44 | 37.96 ± 0.20 | 38.74 ± 0.27 |
| H | 5.72 ± 0.04 | 5.65 ± 0.05 | 5.24 ± 0.07 |
| O | 57.48 ± 0.42 | 54.14 ± 0.17 | 53.65 ± 0.28 |
| N | 3.06 ± 0.07 | 2.17 ± 0.06 | 2.31 ± 0.06 |
| S | 0.23 ± 0.02 | 0.08 ± 0.01 | 0.06 ± 0.01 |
| P | 2.08 ± 0.09 | 1.37 ± 0.04 | 0.80 ± 0.03 |
| Proximate analysis b (wt %) | |||
| Moisture | 6.61 ± 0.05 | 7.49 ± 0.08 | 6.34 ± 0.10 |
| Ash | 32.43 ± 0.25 | 22.63 ± 0.19 | 27.39 ± 0.20 |
| Volatile matter | 54.52 ± 0.28 | 54.95 ± 0.43 | 55.48 ± 0.36 |
| Fixed carbon | 6.44 ± 0.14 | 14.93 ± 0.18 | 10.79 ± 0.11 |
| Fiber analysis a (wt %) | |||
| Hemicelluloses | 20.23 ± 0.11 | 21.51 ± 0.14 | 16.87 ± 0.05 |
| Cellulose | 8.03 ± 0.04 | 18.05 ± 0.11 | 11.57 ± 0.08 |
| Lignin | 9.09 ± 0.06 | 14.48 ± 0.07 | 21.88 ± 0.18 |
| Temperature | Pig Manure | Cow Manure | Sheep Manure | |||
|---|---|---|---|---|---|---|
| Contents (mg g−1) | Ratios (%) | Contents (mg g−1) | Ratios (%) | Contents (mg g−1) | Ratios (%) | |
| Material | 20.76 ± 0.87 d | 13.72 ± 0.44 d | 7.96 ± 0.33 d | |||
| Biochar 200 | 23.34 ± 0.39 c | 98.44 | 15.58 ± 0.61 c | 97.54 | 8.88 ± 0.54 d | 96.94 |
| Biochar 350 | 37.48 ± 0.52 b | 98.71 | 26.49 ± 0.56 b | 98.26 | 13.89 ± 0.38 c | 97.92 |
| Biochar 500 | 43.12 ± 0.94 a | 97.20 | 29.41 ± 0.73 b | 96.02 | 15.97 ± 0.64 b | 97.67 |
| Biochar 650 | 44.81 ± 0.85 a | 97.35 | 32.26 ± 0.97 a | 97.26 | 17.42 ± 0.52 a | 94.76 |
| Biochar 800 | 44.54 ± 1.02 a | 92.14 | 32.48 ± 0.69 a | 94.90 | 17.91 ± 0.81 a | 92.46 |
| Biochar | SL-IP | Al-IP | Fe-IP | O-IP | Ca-IP | OP | Total | Recovery (%) |
|---|---|---|---|---|---|---|---|---|
| PM | 5.88 ± 0.21 b | 7.40 ± 0.31 a | 0.45 ± 0.07 d | 0.74 ± 0.05 f | 0.16 ± 0.05 e | 6.25 ± 0.32 b | 20.76 ± 0.87 d | 100.53 |
| PM 200 | 5.72 ± 0.17 b | 6.68 ± 0.18 b | 0.32 ± 0.03 d | 1.74 ± 0.04 e | 0.37 ± 0.03 e | 7.17 ± 0.33 a | 23.34 ± 0.39 c | 94.26 |
| PM 350 | 7.68 ± 0.37 a | 4.17 ± 0.23 d | 1.93 ± 0.10 b | 3.99 ± 0.18 d | 14.04 ± 0.44 d | 2.99 ± 0.26 c | 37.48 ± 0.52 b | 92.83 |
| PM 500 | 3.41 ± 0.19 c | 5.10 ± 0.38 c | 2.62 ± 0.19 a | 8.79 ± 0.25 a | 19.60 ± 0.61 c | 1.56 ± 0.11 d | 43.12 ± 0.94 a | 95.26 |
| PM 650 | 1.40 ± 0.15 d | 1.79 ± 0.17 e | 0.73 ± 0.11 c | 6.58 ± 0.32 b | 32.09 ± 0.59 b | 1.44 ± 0.12 d | 44.81 ± 0.85 a | 98.27 |
| PM 800 | 0.92 ± 0.11 d | 1.70 ± 0.11 e | 0.74 ± 0.09 c | 4.84 ± 0.18 c | 35.85 ± 0.81 a | 1.30 ± 0.11 d | 44.54 ± 1.02 a | 101.83 |
| CM | 7.92 ± 0.12 a | 2.68 ± 0.17 c | 0.40 ± 0.08 d | 0.21 ± 0.04 e | 0.02 ± 0.01 d | 2.19 ± 0.11 b | 13.72 ± 0.44 d | 97.82 |
| CM 200 | 8.32 ± 0.21 a | 2.73 ± 0.15 c | 0.70 ± 0.05 d | 0.38 ± 0.05 e | 0.06 ± 0.02 d | 2.35 ± 0.15 ab | 15.58 ± 0.61 c | 93.35 |
| CM 350 | 5.80 ± 0.23 b | 4.85 ± 0.29 b | 2.88 ± 0.24 c | 4.97 ± 0.26 c | 3.51 ± 0.29 c | 2.59 ± 0.22 a | 26.49 ± 0.56 b | 92.83 |
| CM 500 | 5.44 ± 0.27 b | 6.72 ± 0.16 a | 4.19 ± 0.36 b | 5.96 ± 0.15 b | 4.08 ± 0.18 c | 1.05 ± 0.15 c | 29.41 ± 0.73 b | 93.28 |
| CM 650 | 3.90 ± 0.14 c | 6.51 ± 0.43 a | 5.60 ± 0.39 a | 7.00 ± 0.27 a | 6.32 ± 0.40 b | 0.98 ± 0.15 c | 32.26 ± 0.97 a | 93.93 |
| CM 800 | 3.90 ± 0.14 c | 5.17 ± 0.23 b | 4.99 ± 0.40 a | 1.74 ± 0.20 d | 13.47 ± 0.46 a | 0.58 ± 0.12 d | 32.48 ± 0.69 a | 91.89 |
| SM | 3.65 ± 0.14 a | 1.03 ± 0.12 c | 0.01 ± 0.01 c | 0.80 ± 0.09 d | 0.04 ± 0.01 e | 2.72 ± 0.09 b | 7.96 ± 0.33 d | 103.54 |
| SM 200 | 3.85 ± 0.13 a | 0.98 ± 0.07 c | 0.07 ± 0.03 c | 1.44 ± 0.13 c | 0.13 ± 0.02 e | 2.99 ± 0.11 a | 8.88 ± 0.54 d | 106.54 |
| SM 350 | 3.28 ± 0.19 b | 3.01 ± 0.17 b | 1.47 ± 0.11 a | 2.41 ± 0.14 b | 2.19 ± 0.14 d | 1.15 ± 0.08 c | 13.89 ± 0.38 c | 97.30 |
| SM 500 | 2.95 ± 0.26 b | 4.62 ± 0.24 a | 1.20 ± 0.13 b | 3.23 ± 0.20 a | 3.30 ± 0.21 c | 0.92 ± 0.06 d | 15.97 ± 0.64 b | 101.58 |
| SM 650 | 0.94 ± 0.10 c | 1.19 ± 0.05 c | 0.20 ± 0.03 c | 3.21 ± 0.24 a | 9.93 ± 0.18 b | 0.85 ± 0.12 d | 17.42 ± 0.52 a | 93.72 |
| SM 800 | 0.61 ± 0.09 c | 1.32 ± 0.07 c | 0.16 ± 0.05 c | 2.46 ± 0.14 b | 11.64 ± 0.28 a | 0.84 ± 0.05 d | 17.91 ± 0.81 a | 95.02 |
| Manure | Biochar 200 | Biochar 350 | Biochar 500 | Biochar 650 | Biochar 800 | |
| PM | 5.73 ± 0.25 a | 4.94 ± 0.12 b | 4.45 ± 0.23 c | 5.02 ± 0.14 b | 1.53 ± 0.13 d | 1.38 ± 0.12 d |
| CM | 7.93 ± 0.14 b | 6.52 ± 0.24 c | 4.89 ± 0.31 e | 5.75 ± 0.10 d | 7.71 ± 0.19 b | 12.36 ± 0.37 a |
| SM | 3.95 ± 0.14 b | 3.43 ± 0.11 c | 5.16 ± 0.13 a | 5.03 ± 0.10 a | 2.15 ± 0.11 d | 1.73 ± 0.09 e |
| Proportion (%) | Manure | Biochar 200 | Biochar 350 | Biochar 500 | Biochar 650 | Biochar 800 |
| PM | 27.62 ± 1.83 a | 21.19 ± 0.87 b | 11.87 ± 0.78 c | 11.64 ± 0.39 c | 3.40 ± 0.23 d | 3.10 ± 0.22 d |
| CM | 57.78 ± 1.06 a | 41.84 ± 0.98 b | 18.46 ± 1.29 e | 19.54 ± 0.59 e | 23.90 ± 1.32 d | 38.05 ± 1.56 c |
| SM | 49.67 ± 1.42 a | 38.67 ± 1.10 b | 37.16 ± 1.70 b | 31.49 ± 0.83 c | 12.36 ± 0.98 d | 9.65 ± 0.65 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Xiao, Z.; Fang, J.; Li, H. Effect of Pyrolysis Treatment on Phosphorus Migration and Transformation of Pig, Cow and Sheep Manure. Sustainability 2023, 15, 9215. https://doi.org/10.3390/su15129215
Liu F, Xiao Z, Fang J, Li H. Effect of Pyrolysis Treatment on Phosphorus Migration and Transformation of Pig, Cow and Sheep Manure. Sustainability. 2023; 15(12):9215. https://doi.org/10.3390/su15129215
Chicago/Turabian StyleLiu, Fen, Zhihua Xiao, Jun Fang, and Hao Li. 2023. "Effect of Pyrolysis Treatment on Phosphorus Migration and Transformation of Pig, Cow and Sheep Manure" Sustainability 15, no. 12: 9215. https://doi.org/10.3390/su15129215
APA StyleLiu, F., Xiao, Z., Fang, J., & Li, H. (2023). Effect of Pyrolysis Treatment on Phosphorus Migration and Transformation of Pig, Cow and Sheep Manure. Sustainability, 15(12), 9215. https://doi.org/10.3390/su15129215







