Insight into the Enzymatic Mechanism of Straw Carbon Source and Its Denitrification Availability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Straw Carbon Release under Different Enzyme Activity and Substrate Loading
2.3. Component Test Procedure and Related Parameter Settings
2.4. The Denitrifying Treatment of the Carbon Source
3. Results and Discussion
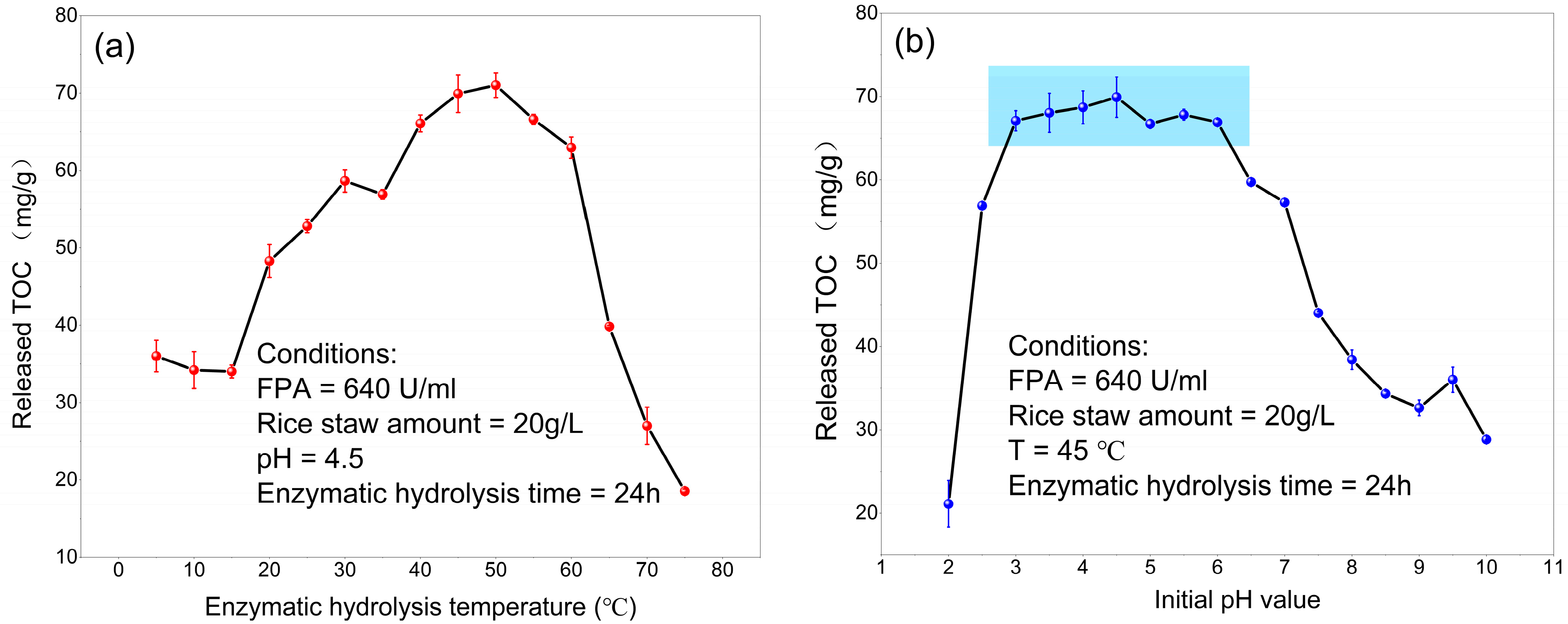
3.1. Carbon Release Kinetics via Cellulase Hydrolysis
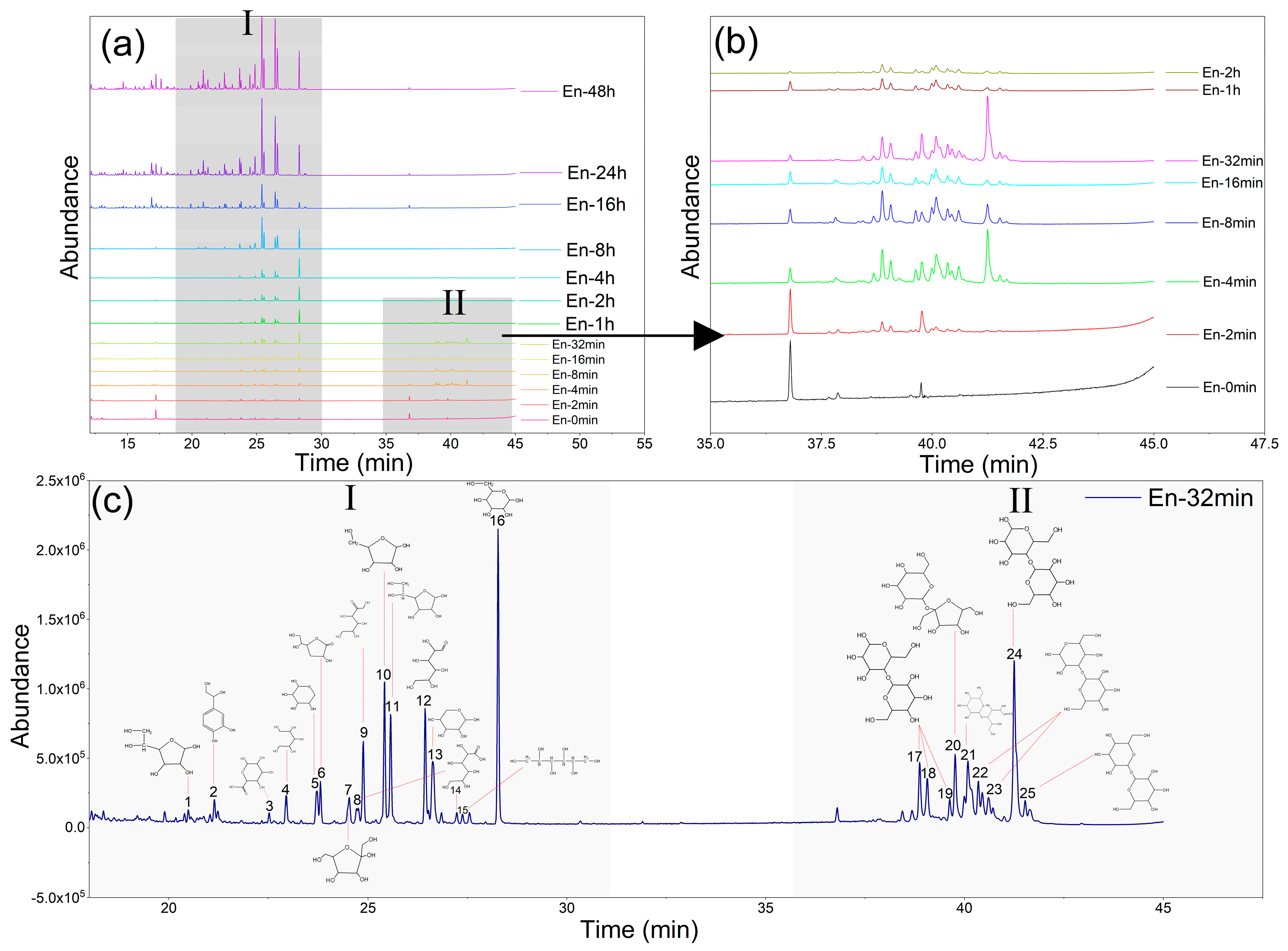
3.2. Component Analysis of the Enzymatic SCS
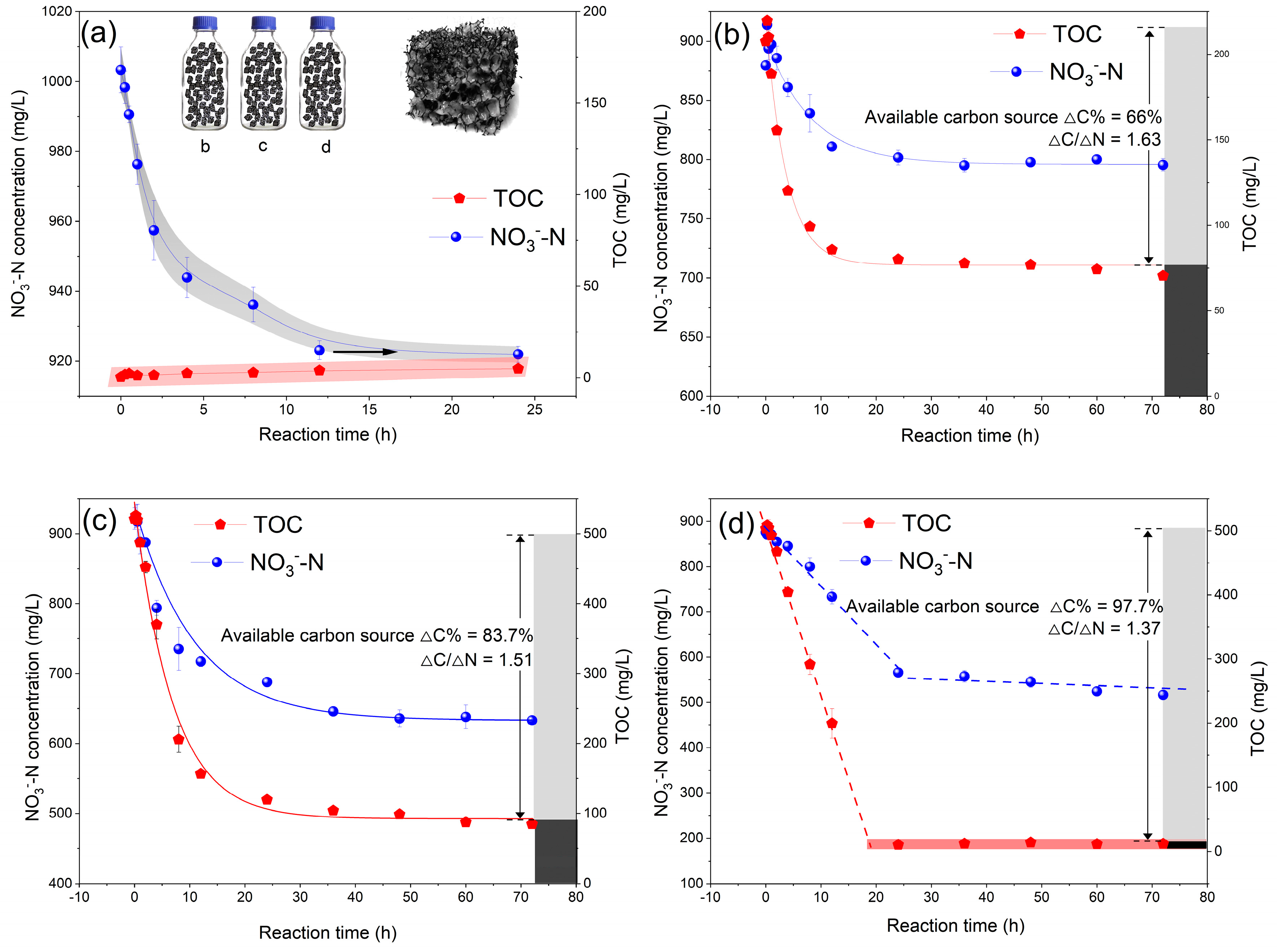
3.3. Carbon Source Availability and Component Change in Denitrification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, C.; Ji, L.; Chen, Z.; Zhou, X.; Fang, F. Analysis of China’s rice industry in 2018 and the outlook for 2019. China Rice 2019, 25, 1–9. Available online: http://www.zgdm.net/CN/10.3969/j.issn.1006-8082.2019.02.001 (accessed on 20 March 2019).
- Ren, J.; Yu, P.; Xu, X. Straw utilization in China—status and recommendations. Sustainability 2019, 11, 1762. [Google Scholar] [CrossRef]
- Singh, S.K. Biological treatment of plant biomass and factors affecting bioactivity. J. Clean. Prod. 2020, 279, 123546. [Google Scholar] [CrossRef]
- Li, C.; Yang, X.; Gao, S.; Chuh, A.H.; Lin, C.S.K. Hydrolysis of fruit and vegetable waste for efficient succinic acid production with engineered Yarrowia lipolytica. J. Clean. Prod. 2018, 179, 151–159. [Google Scholar] [CrossRef]
- Harding, K.; Dennis, J.; von Blottnitz, H.; Harrison, S. A life-cycle comparison between inorganic and biological catalysis for the production of biodiesel. J. Clean. Prod. 2008, 16, 1368–1378. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, Y.; Wang, J.; Zhang, L. Biological phosphorus and nitrogen removal in low C/N ratio domestic sewage treatment by A2/O-BAF combined system. Acta Sci. Circumstantiae 2010, 30, 1957–1963. Available online: https://www.oalib.com/paper/1590576 (accessed on 1 October 2010).
- Li, Y.; Wang, S.; Li, Y.; Kong, F.; Xi, H.; Liu, Y. Corn Straw as a Solid Carbon Source for the Treatment of Agricultural Drainage Water in Horizontal Subsurface Flow Constructed Wetlands. Water 2018, 10, 511. [Google Scholar] [CrossRef]
- Liu, G.Y.; Zhang, H.Z.; Li, W.; Zhang, X. Advances of External Carbon Source in Denitrification. Adv. Mater. Res. 2012, 518–523, 2319–2323. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, L.; Tan, X.; Yan, B. Influence of different carbon source and ratio of carbon and nitrogen for water denitrification. Environ. Prot. Sci. 2004, 30, 15–17. (In Chinese) [Google Scholar] [CrossRef]
- Her, J.-J.; Huang, J.-S. Influences of carbon source and C/N ratio on nitrate/nitrite denitrification and carbon breakthrough. Bioresour. Technol. 1995, 54, 45–51. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, X.; He, S.; Zhao, M. Nitrification, denitrification and anammox process coupled to iron redox in wetlands for domestic wastewater treatment. J. Clean. Prod. 2021, 300, 126953. [Google Scholar] [CrossRef]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total Environ. 2022, 817, 153061. [Google Scholar] [CrossRef]
- Aslan, Ş.; Türkman, A. Simultaneous biological removal of endosulfan (α+β) and nitrates from drinking waters using wheat straw as substrate. Environ. Int. 2004, 30, 449–455. [Google Scholar] [CrossRef]
- Liu, G.; Han, D.; Yang, S. Combinations of mild chemical and bacterial pretreatment for improving enzymatic saccharification of corn stover. Biotechnol. Biotechnol. Equip. 2022, 36, 598–608. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Huang, C.; Ling, Z.; Lai, C.; Yong, Q. Natural surfactant-aided dilute sulfuric acid pretreatment of waste wheat straw to enhance enzymatic hydrolysis efficiency. Bioresour. Technol. 2021, 324, 124651. [Google Scholar] [CrossRef]
- Li, P.; Cai, D.; Zhang, C.; Li, S.; Qin, P.; Chen, C.; Wang, Y.; Wang, Z. Comparison of two-stage acid-alkali and alkali-acid pretreatments on enzymatic saccharification ability of the sweet sorghum fiber and their physicochemical characterizations. Bioresour. Technol. 2016, 221, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Su, X.; Tan, L.; Liu, X.; Wu, A.; Su, D.; Tian, K.; Xiong, X. Effects of a Steam Explosion Pretreatment on Sugar Production by Enzymatic Hydrolysis and Structural Properties of Reed Straw. Biosci. Biotechnol. Biochem. 2013, 77, 2181–2187. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Lee, C.-K. Enhancement of enzymatic saccharification of cellulose by cellulose dissolution pretreatments. Carbohydr. Polym. 2009, 77, 41–46. [Google Scholar] [CrossRef]
- Seo, D.-J.; Sakoda, A. Assessment of the structural factors controlling the enzymatic saccharification of rice straw cellulose. Biomass Bioenergy 2014, 71, 47–57. [Google Scholar] [CrossRef]
- Deng, Y.; Wheatley, A. Mechanisms of Phosphorus Removal by Recycled Crushed Concrete. Int. J. Environ. Res. Public Health 2018, 15, 357. [Google Scholar] [CrossRef]
- Yadav, S.; Chandra, R. Biodegradation of organic compounds of molasses melanoidin (MM) from biomethanated distillery spent wash (BMDS) during the decolourisation by a potential bacterial consortium. Biodegradation 2012, 23, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Desvaux, M.; Guedon, E.; Petitdemange, H. Kinetics and Metabolism of Cellulose Degradation at High Substrate Concentrations in Steady-State Continuous Cultures of Clostridium cellulolyticum on a Chemically Defined Medium. Appl. Environ. Microbiol. 2001, 67, 3837–3845. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Yu, L. New insights into pseudo-second-order kinetic equation for adsorption. Colloids Surf. A. 2008, 320, 275–278. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Islam, M.M.; Das, R. Cellulase biocatalysis: Key influencing factors and mode of action. Cellulose 2015, 22, 2157–2182. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Can, M. Studies of the Kinetics for Rhodium Adsorption onto Gallic Acid Derived Polymer: The Application of Nonlinear Regression Analysis. Acta Phys. Pol. A 2015, 127, 1308–1310. [Google Scholar] [CrossRef]
- Chuah, L.F.; Klemeš, J.J.; Yusup, S.; Bokhari, A.; Akbar, M.M. A review of cleaner intensification technologies in biodiesel production. J. Clean. Prod. 2016, 146, 181–193. [Google Scholar] [CrossRef]
- Fromm, H.J. The Effect of Temperature and pH on Enzyme Activity; Springer: Berlin/Heidelberg, Germany, 1975; pp. 201–235. [Google Scholar] [CrossRef]
- Gou, C.; Wang, X.; Yu, Y.; Huang, J.; Wang, X.; Hui, M. One-step enzymatic hydrolysis of sweet potato residue after gelatinization for bioethanol production by Saccharomyces cerevisiae. Biomass Convers. Biorefinery 2023, 1–10. [Google Scholar] [CrossRef]
- Mokale, A.L.; Chio, C.; Khatiwada, J.R.; Shrestha, S.; Chen, X.; Han, S.; Li, H.; Jiang, Z.-H.; Xu, C.C.; Qin, B. Characterization of Cellulose-Degrading Bacteria Isolated from Soil and the Optimization of Their Culture Conditions for Cellulase Production. Appl. Biochem. Biotechnol. 2022, 194, 5060–5082. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Yin, L.; Ruan, X. The characteristics of carbon sources for denitrification in groundwater from ten kinds of agricultural wastes. Acta Sci. Circumstantiae 2017, 37, 1787–1797. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-HJXX201705021.htm (accessed on 1 May 2017).
- Qi, L.; Li, L.; Yin, L.; Zhang, W. Study on the properties of denitrifying carbon sources from cellulose plants and their nitrogen removal mechanisms. Water Sci. Technol. 2021, 85, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Dong, M.; Shim, W.J.; Kannan, N. Application of pressurized fluid extraction technique in the gas chromatography–mass spectrometry determination of sterols from marine sediment samples. J. Chromatogr. 2007, 1160, 64–70. [Google Scholar] [CrossRef]
- Li, D.; Park, J.; Oh, J.-R. Silyl Derivatization of Alkylphenols, Chlorophenols, and Bisphenol A for Simultaneous GC/MS Determination. Anal. Chem. 2001, 73, 3089–3095. [Google Scholar] [CrossRef] [PubMed]
- Ünver, H.; Polat, K.; Uçar, M.; Zengin, D. Synthesis and Keto-Enol Tautomerism in N-(2-Hydroxy-1-Naphthylidene) Anils. Spectrosc. Lett. 2003, 36, 287–301. [Google Scholar] [CrossRef]
- Taj-Aldeen, S.; Alkenany, K. Separation and partial purification of beta-glucosidase and two endoglucanases in Aspergillus niveus. Microbiol. 1996, 12, 91–98. Available online: https://pubmed.ncbi.nlm.nih.gov/9019140/ (accessed on 1 March 1996).
- Rahayu, F.; Tajima, T.; Kato, J.; Kato, S.; Nakashimada, Y. Ethanol yield and sugar usability in thermophilic ethanol production from lignocellulose hydrolysate by genetically engineered Moorella thermoacetica. J. Biosci. Bioeng. 2020, 129, 160–164. [Google Scholar] [CrossRef]
- Sharma, A.; Tewari, R.; Rana, S.S.; Soni, R.; Soni, S.K. Cellulases: Classification, Methods of Determination and Industrial Applications. Appl. Biochem. Biotechnol. 2016, 179, 1346–1380. [Google Scholar] [CrossRef]
- Kipper, K.; Väljamäe, P.; Johansson, G. Processive action of cellobiohydrolase Cel7A from Trichoderma reesei is revealed as ‘burst’ kinetics on fluorescent polymeric model substrates. Biochem. J. 2005, 385 Pt 2, 527–535. [Google Scholar] [CrossRef]
- Ohno, E.; Miyafuji, H. Production of disaccharides from glucose by treatment with an ionic liquid, 1-ethyl-3-methylimidozolium chloride. J. Wood Sci. 2014, 61, 165–170. [Google Scholar] [CrossRef]
- Glasser, W.G.; Atalla, R.H.; Blackwell, J.; Malcolm Brown, R.; Burchard, W.; French, A.D.; Klemm, D.O.; Nishiyama, Y.J.C. About the structure of cellulose: Debating the Lindman hypothesis. Cellulose 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Jele, T.B.; Lekha, P.; Sithole, B. Role of cellulose nanofibrils in improving the strength properties of paper: A review. Cellulose 2021, 29, 55–81. [Google Scholar] [CrossRef]
- Ellis, L.B.; Gao, J.; Fenner, K.; Wackett, L.P. The University of Minnesota pathway prediction system: Predicting metabolic logic. Nucleic Acids Res. 2008, 36, W427–W432. [Google Scholar] [CrossRef] [PubMed]
- DeLorenzo, R.J.; Ruddle, F.H. Genetic control of two electrophoretic variants of glucosephosphate isomerase in the mouse (Mus musculus). Biochem. Genet. 1969, 3, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Dauenhauer, P.J.; Huber, G.W.; Auerbach, S.M. Ab initio dynamics of cellulose pyrolysis: Nascent decomposition pathways at 327 and 600 °C. J. Am. Chem. Soc. 2012, 134, 14958–14972. [Google Scholar] [CrossRef]
- Li, R.; Lin, Q.; Wang, Y.; Yang, W.; Liu, X.; Li, W.; Wang, X.; Wang, X.; Liu, C.; Ren, J. Brønsted acid-driven conversion of glucose to xylose, arabinose and formic acid via selective C–C cleavage. Appl. Catal. B 2021, 286, 119862. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Kelly, S.D.; Williams, D.M.; Nothof, J.T.; Kim, T.; Lowary, T.L.; Kimber, M.S.; Whitfield, C. The biosynthetic origin of ribofuranose in bacterial polysaccharides. Nat. Chem. Biol. 2022, 18, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Bernat, K.; Wojnowska-Baryła, I.; Dobrzyńska, A. Denitrification with endogenous carbon source at low C/N and its effect on P(3HB) accumulation. Bioresour. Technol. 2008, 99, 2410–2418. [Google Scholar] [CrossRef]
- Dobbeleers, T.; D’aes, J.; Miele, S.; Caluwé, M.; Akkermans, V.; Daens, D.; Geuens, L.; Dries, J. Aeration control strategies to stimulate simultaneous nitrification-denitrification via nitrite during the formation of aerobic granular sludge. Appl. Microbiol. Biotechnol. 2017, 101, 6829–6839. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, P.; Xie, C.; Zhang, W.; Sun, J.; Qian, W.-J.; Yang, B. Biodegradation of alkaline lignin by Bacillus ligniniphilus L1. Biotechnol. Biofuels 2017, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.E. Comments on “Demonstration of a Process for the Conversion of Kraft Lignin into Vanillin and Methyl Vanillate by Acidic Oxidation in Aqueous Methanol”. Ind. Eng. Chem. Res. 2010, 49, 3500. [Google Scholar] [CrossRef]
- Song, S.; Zhang, J.; Gözaydın, G.; Yan, N. Production of terephthalic acid from corn stover lignin. Angew. Chem. Int. Ed. 2019, 58, 4934–4937. [Google Scholar] [CrossRef] [PubMed]






| Moisture Content | Porosity * | Carbon | Nitrogen | Cellulose | Hemicellulose | Lignin | |
|---|---|---|---|---|---|---|---|
| (% wet weight) | (%) | (% dry mass) | |||||
| Rice Straw | 22.7 ± 1.9 | 37.6 ± 1.8 | 43.4 ± 0.02 | 0.48 ± 0.01 | 30.5 ± 0.2 | 25.1 ± 0.36 | 16.6 ± 0.28 |
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| FPA (U/mL) | 0, 40, 160, 640, 1000 | 640 | 640 | 640 |
| Straw amount (g/L) | 20 | 1, 5, 20, 50 | 20 | 20 |
| Temperature (°C) | 45 | 45 | 5–75 | 45 |
| Initial pH value | 4.5 | 4.5 | 4.5 | 2–10 |
| Enzyme Activity | Pseudo-1st-Order | Pseudo-2st-Order | Elovich Model | Internal Diffusion Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe mg/g | k1 1/h | R2 | Qe mg/g | k2 g/mg·h | R2 | α mg/g·h | β g/mg | R2 | Kint mg/(g·h0.5) | C | R2 | |
| 0 U/mL | 21.91 | 0.33 | 0.984 | 22.19 | 0.49 | 0.9997 | 13.78 | 0.92 | 0.9952 | 0.64 | 19.75 | 0.6204 |
| 40 U/mL | 39.58 | 0.37 | 0.9803 | 39.17 | 0.74 | 0.9922 | 29.18 | 0.23 | 0.9392 | 4.66 | 19.98 | 0.8 |
| 160 U/mL | 49.77 | 0.42 | 0.9944 | 49.33 | 1.01 | 0.9952 | 60.07 | 0.16 | 0.9764 | 5.72 | 26.41 | 0.4002 |
| 640 U/mL | 62.1 | 0.5 | 0.7307 | 63.45 | 1.39 | 0.9891 | 60.06 | 0.1 | 0.946 | 8.84 | 27.4 | 0.4821 |
| 1000 U/mL | 64.31 | 0.56 | 0.8832 | 66.71 | 1.47 | 0.9885 | 69.17 | 0.09 | 0.956 | 13.46 | 15.23 | 0.9171 |
| SCS | Enzymatic SCS | ||||
|---|---|---|---|---|---|
| Peak | Components | Peak | Components | Peak | Components |
| 1 | Terephthalic acid | 1 | Oxalic acid | 29 | Pinacol |
| 2 | Methyl syringate | 2 | Gentisic acid | 30 | 2-Acetonaphthone |
| 3 | Benzoic acid | 3 | 3-Hydroxypropionic acid | 31 | Dihydroxyacetophenone |
| 4 | Glycerinum | 4 | Methyl vanillate | 32 | Phthalimide |
| 5 | 2-Isopropylphenol | 5 | Lactic acid | 33 | Adipic acid |
| 6 | Resacetophenone | 6 | Glycolic acid | 34 | 1,3-propanediol |
| 7 | Methyl 2,4-dihydroxybenzoate | 7 | 3-Hydroxybenzoic Acid | 35 | Glycidol |
| 8 | 3-Ethylphenol | 8 * | D-Ribofuranose | 36 | 2,6-Ditert-butylphenol |
| 9 | 2-Methylphenol | 9 | Benzoic acid | 37 | 4-n-Propylphenol |
| 10 | Methyl vanillate | 10 | Glycerol | 38 | Erythritol |
| 11 | Meso-Erythritol | 11 | Urea | 39 | Pyrogallol |
| 12 | 4-tert-Butyl phenol | 12 | Gluconic acid | 40 | 4,4-Bibenzoic acid |
| 13 | Pyrogallol | 13 | Propionic acid | 41 | Mercaptoacetone |
| 14 | Oxalic acid | 14 * | D-fructofuranose | 43 | DL-Arabinose |
| 15 | D-glucofuranose | 15 | 2-Isopropylphenol | 44 | Ribonolactone |
| 16 | 4-Hydroxysalicylic acid | 16 | Oxaluric acid | 46 * | Glucofuranose |
| 17 * | Xylose | 17 | Glyceric acid | 48 * | Glucopyranose |
| 18 * | Arabinofuranose | 18 | Succinic acid | 50 | 4-Hydroxybenzoic acid |
| 19 | Xylitol | 19 | Quinoline | 51 * | Xylose |
| 20 | Vanillic acid | 20 | Syringol | 52 | Phthalic acid |
| 21 * | Open-chain glucose | 21 | Cuminic acid | 53 | 2-Hydroxy-Heptanoic acid |
| 22 | 4-n-Propylphenol | 22 | 1,4-Butanediol | 54 | D-Arabinitol |
| 23 * | Glucopyranose | 23 | 2,4-Dihydroxybenzoic acid methyl ester | 55 | Isophthalic acid |
| 25 | Fructofuranose | 24 | 2,3-Dihydroxy-acrylic acid | 57 | Terephthalic acid |
| 26 | Isopropyl naphthalene | 25 | Glutaric acid | 60 | L-Fructose |
| 29 | Mannitol | 26 | Pimelic acid | 65 | Glucuronic acid |
| 31 | Sucrose | 27 | 2-Hydroxy-2-Pentaenoic acid | 68 | Open-chain glucose |
| 28 | D-Glucosamine | ||||
| SCS | Enzymatic SCS | ||||
| Category | Species | Proportion | Category | Species | Proportion |
| Saccharides | 7 | 59.5% | Saccharides | 12 | 66.4% |
| VFAs | 5 | 14.4% | VFAs | 23 | 20.4% |
| Aromatics | 15 | 26.1% | Aromatics | 20 | 13.2% |
| Carbon | ΔC/ΔN | Remove 1g N Requirement (g) | Availability | Saccharides and VFAs | COD/N | |
|---|---|---|---|---|---|---|
| Effective Carbon Source | Total Carbon Source | |||||
| SCS | 1.63 | 1.63 | 2.47 | 66% | 73.9% | 4.4 |
| Enzymatic SCS | 1.51 | 1.51 | 1.8 | 83.7% | 86.8% | 4.08 |
| Glucose | 1.37 | 1.37 | 1.4 | 97.7% | / | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, C.; Wu, K.; Zhou, S.; Hu, W.; Qin, J.; Ye, Z. Insight into the Enzymatic Mechanism of Straw Carbon Source and Its Denitrification Availability. Sustainability 2023, 15, 8818. https://doi.org/10.3390/su15118818
Li L, Li C, Wu K, Zhou S, Hu W, Qin J, Ye Z. Insight into the Enzymatic Mechanism of Straw Carbon Source and Its Denitrification Availability. Sustainability. 2023; 15(11):8818. https://doi.org/10.3390/su15118818
Chicago/Turabian StyleLi, Lei, Chenxi Li, Kun Wu, Shuting Zhou, Wei Hu, Jiangzhou Qin, and Zhengfang Ye. 2023. "Insight into the Enzymatic Mechanism of Straw Carbon Source and Its Denitrification Availability" Sustainability 15, no. 11: 8818. https://doi.org/10.3390/su15118818
APA StyleLi, L., Li, C., Wu, K., Zhou, S., Hu, W., Qin, J., & Ye, Z. (2023). Insight into the Enzymatic Mechanism of Straw Carbon Source and Its Denitrification Availability. Sustainability, 15(11), 8818. https://doi.org/10.3390/su15118818






