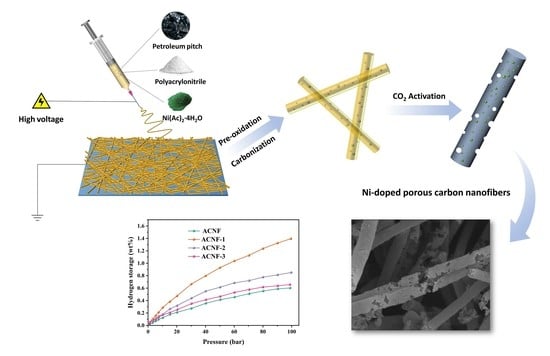
In Situ Ni-Doped Hierarchically Porous Carbon Nanofibers Derived from Polyacrylonitrile/Pitch for Hydrogen Storage at Ambient Temperature
Abstract
1. Introduction
2. Experiments
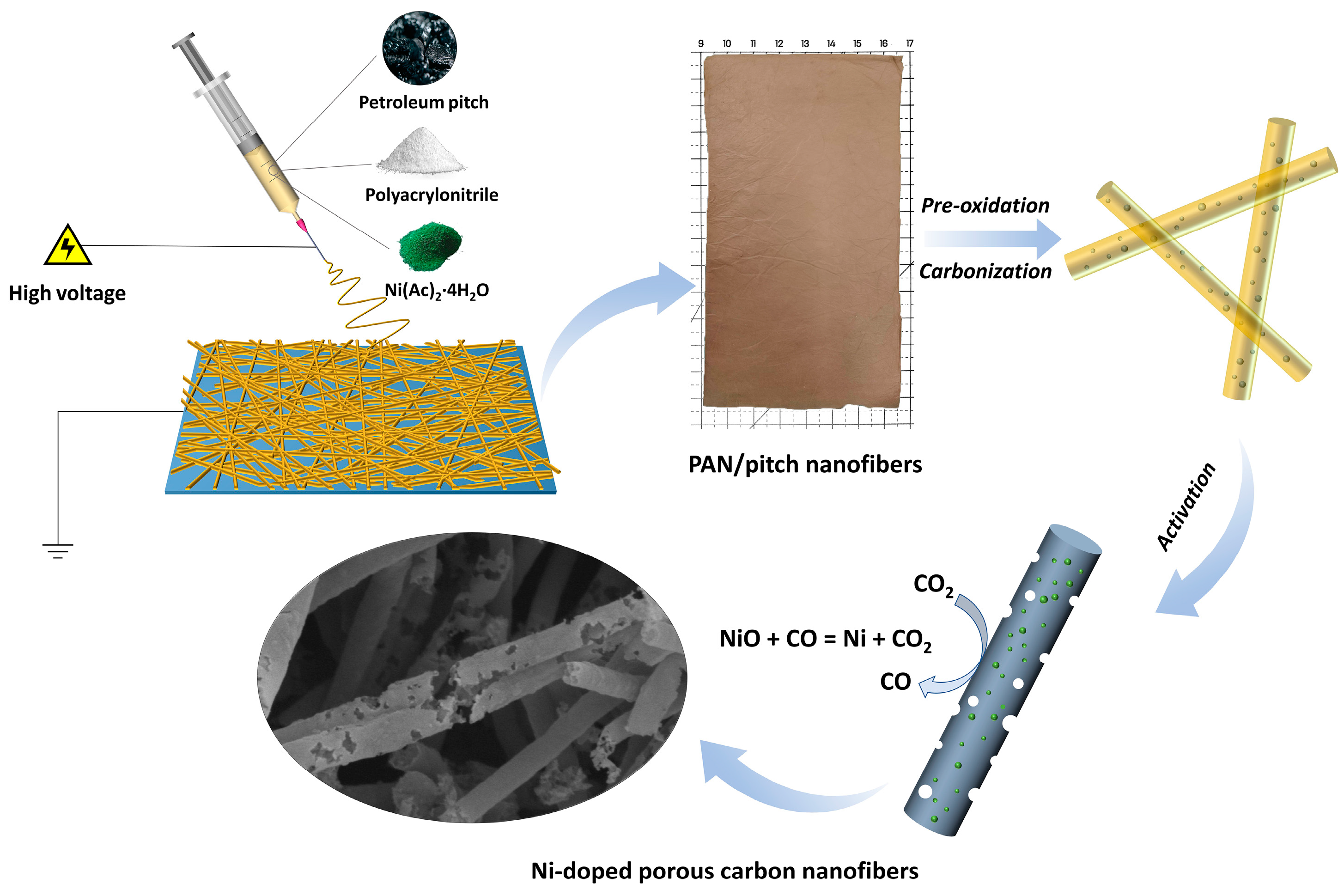
2.1. Materials and Fabrication
2.2. Characterizations
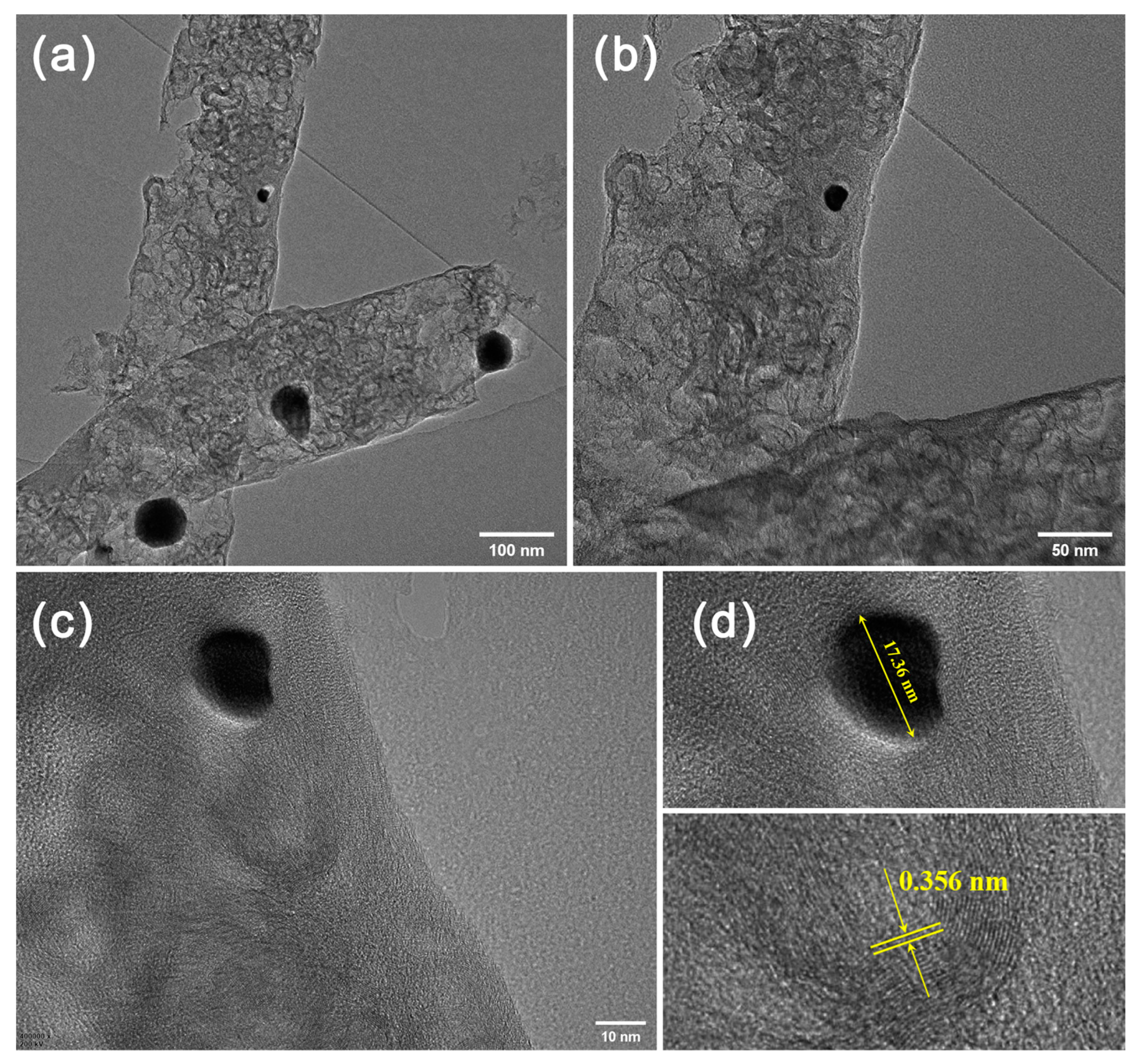
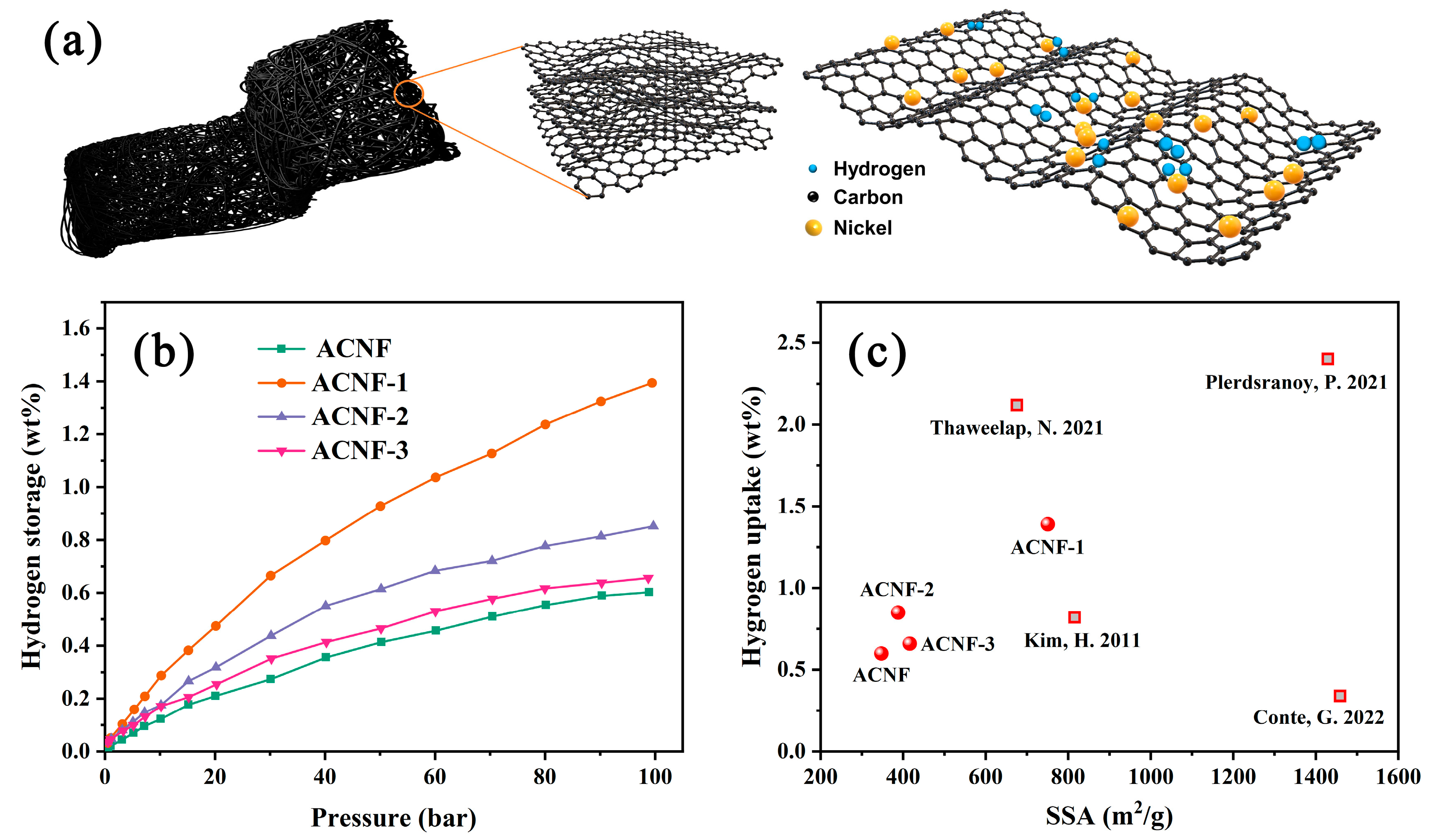
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cipriani, G.; Di Dio, V.; Genduso, F.; La Cascia, D.; Liga, R.; Miceli, R.; Galluzzo, G.R. Perspective on hydrogen energy carrier and its automotive applications. Int. J. Hydrogen Energy 2014, 39, 8482–8494. [Google Scholar] [CrossRef]
- Grochala, W. First there was hydrogen. Nat. Chem. 2015, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy. Int. J. Energy Res. 2020, 44, 4110–4131. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Kim, H.T.; Jung, S.; Roh, S.H.; Park, J.H.; Jung, H.Y. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review. Renew. Sustain. Energy Rev. 2017, 100, 523–534. [Google Scholar] [CrossRef]
- Pingkuo, L.; Xue, H. Comparative analysis on similarities and differences of hydrogen energy development in the World’s top 4 largest economies: A novel framework. Int. J. Hydrogen Energy 2022, 47, 9485–9503. [Google Scholar] [CrossRef]
- Demirocak, D.E. Hydrogen Storage Technologies in Nanostructured Materials for Next Generation Energy Storage and Conversion; University of Houston: Houston, TX, USA, 2017; pp. 117–142. [Google Scholar]
- Zhao, Y.; Gong, M.; Zhou, Y.; Dong, X.; Shen, J. Thermodynamics analysis of hydrogen storage based on compressed gaseous hydrogen, liquid hydrogen and cryo-compressed hydrogen. Int. J. Hydrogen Energy 2019, 44, 16833–16840. [Google Scholar]
- Sorensen, B.; Spazzafumo, G. Hydrogen and Fuel Cells: Emerging Technologies and Applications; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Zhang, H.; Zhu, Y.; Liu, Q.; Li, X. Preparation of porous carbon materials from biomass pyrolysis vapors for hydrogen storage. Appl. Energy 2022, 306, 118131. [Google Scholar] [CrossRef]
- Bicil, Z.; Dogan, M. Characterization of activated carbons prepared from almond shells and their hydrogen storage properties. Energy Fuels 2021, 35, 10227–10240. [Google Scholar] [CrossRef]
- Isinkaralar, K.; Gullu, G.; Turkyilmaz, A.; Dogan, M.; Turhan, O. Activated carbon production from horse chestnut shells for hydrogen storage. Int. J. Glob. Warm. 2022, 26, 361–373. [Google Scholar] [CrossRef]
- Hwang, S.I.; Sopher, E.M.; Zeng, Z.; Schulte, Z.M.; White, D.L.; Rosi, N.L. Metal-Organic Frameworks on Palladium Nanoparticle-Functionalized Carbon Nanotubes for Monitoring Hydrogen Storage. ACS Appl. Nano Mater. 2022, 5, 13779–13786. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, N.; Liu, P. Theoretical Study on Hydrogen Storage Properties of Carbon Aerogels. Chin. J. Comput. Phys. 2019, 36, 749–756. [Google Scholar]
- Meng, Q.; Huang, Y.; Ye, J.; Xia, G.; Wang, G.; Dong, L.; Yang, Z.; Yu, X. Electrospun carbon nanofibers with in-situ encapsulated Ni nanoparticles as catalyst for enhanced hydrogen storage of MgH2. J. Alloys Compd. 2021, 851, 156874. [Google Scholar] [CrossRef]
- Chen, X.; Xue, Z.; Niu, K.; Liu, X.; Lv, W.; Zhang, B.; Li, Z.; Zeng, H.; Ren, Y.; Wu, Y.; et al. Li–fluorine codoped electrospun carbon nanofibers for enhanced hydrogen storage. RSC Adv. 2021, 11, 4053–4061. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhu, W.; Zhang, Q.; Lu, C.; Sun, F.; Liu, X.; Zou, J. MgH2 confinement in MOF-derived N-doped porous carbon nanofibers for enhanced hydrogen storage. Chem. Eng. J. 2022, 434, 134701. [Google Scholar] [CrossRef]
- Yadav, A.; Faisal, M.; Subramaniam, A.; Verma, N. Nickel nanoparticle-doped and steam-modified multiscale structure of carbon micro-nanofibers for hydrogen storage: Effects of metal, surface texture and operating conditions. Int. J. Hydrogen Energy 2017, 42, 6104–6117. [Google Scholar] [CrossRef]
- Kaskun, S.; Kayfeci, M. The synthesized nickel-doped multi-walled carbon nanotubes for hydrogen storage under moderate pressures. Int. J. Hydrogen Energy 2018, 43, 10773–10778. [Google Scholar] [CrossRef]
- Browning, D.J.; Gerrard, M.L.; Lakeman, J.B.; Mellor, I.M.; Mortimer, R.J.; Turpin, M.C. Studies into the storage of hydrogen in carbon nanofibers: Proposal of a possible reaction mechanism. Nano Lett. 2002, 2, 201–205. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Wdowin, M.; Rambau, K.M.; Franus, W.; Panek, R.; Madej, J.; Czarna-Juszkiewicz, D. Synthesis of activated carbon from high-carbon coal fly ash and its hydrogen storage application. Renew. Energy 2020, 155, 1264–1271. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R.; Fuertes, A.B. Ultrahigh surface area polypyrrole-based carbons with superior performance for hydrogen storage. Energy Environ. Sci. 2011, 4, 2930–2936. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.; Moon, J. Co-electrospun Pd-coated porous carbon nanofibers for hydrogen storage applications. Int. J. Hydrogen Energy 2011, 36, 3566–3573. [Google Scholar] [CrossRef]
- Yodsin, N.; Sakagami, H.; Udagawa, T.; Ishimoto, T.; Jungsuttiwong, S.; Tachikawa, M. Metal-doped carbon nanocones as highly efficient catalysts for hydrogen storage: Nuclear quantum effect on hydrogen spillover mechanism. Mol. Catal. 2021, 504, 111486. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, B.H. Low-cost effective photocatalytic activity under visible light of pitch-based porous carbon nanofiber composites aided by zinc oxide. Synth. Met. 2019, 247, 163–169. [Google Scholar] [CrossRef]
- Ge, J.C.; Wu, G.; Yoon, S.K.; Kim, M.S.; Choi, N.J. Study on the Preparation and Lipophilic Properties of Polyvinyl Alcohol (PVA) Nanofiber Membranes via Green Electrospinning. Nanomaterials 2021, 11, 2514. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Lee, J.S. Electrospinning-Based Carbon Nanofibers for Energy and Sensor Applications. Appl. Sci. 2022, 12, 6048. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Thaweelap, N.; Poo-arporn, Y.; Khajondetchairit, P.; Suthirakun, S.; Fongkaew, I.; Chanlek, N.; Utke, O.; Pangon, A.; Utke, R. Hydrogen adsorption of O/N-rich hierarchical carbon scaffold decorated with Ni nanoparticles: Experimental and computational studies. Int. J. Hydrogen Energy 2021, 46, 5427–5440. [Google Scholar] [CrossRef]
- Kuang, M.; Guan, A.; Gu, Z.; Han, P.; Qian, L.; Zheng, G. Enhanced N-doping in mesoporous carbon for efficient electrocatalytic CO2 conversion. Nano Res. 2019, 12, 2324–2329. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, B.H. Influence of boron content on the structure and capacitive properties of electrospun polyacrylonitrile/pitch-based carbon nanofiber composites. Synth. Met. 2018, 242, 1–7. [Google Scholar] [CrossRef]
- Thaweelap, N.; Plerdsranoy, P.; Poo-arporn, Y.; Khajondetchairit, P.; Suthirakun, S.; Fongkaew, I.; Hirunsit, P.; Chanlek, N.; Utke, O.; Pangon, A.; et al. Ni-doped activated carbon nanofibers for storing hydrogen at ambient temperature: Experiments and computations. Fuel 2021, 288, 119608. [Google Scholar] [CrossRef]
- Gao, Z.; Ding, C.; Wang, J.; Ding, G.; Xue, Y.; Zhang, Y.; Zhang, K.; Liu, P.; Gao, X. Cobalt nanoparticles packaged into nitrogen-doped porous carbon derived from metal-organic framework nanocrystals for hydrogen production by hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 8365–8375. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, H.; Zhang, C.; Wang, Y.; Wei, Q.; Zhang, R. Interfacial Electronic Effects in Co@N-Doped Carbon Shells Heterojunction Catalyst for Semi-Hydrogenation of Phenylacetylene. Nanomaterials 2021, 11, 2776. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Du, X.; Zhou, S.; Yang, P.; Li, M.; Kang, Z.; Guo, H.; Fan, W.; Kang, W.; Zhang, L.; et al. Efficient platinum harvesting of MOF-derived N-doped carbon through cathodic cyclic voltammetry for hydrogen evolution. Electrochim. Acta 2019, 317, 173–181. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, Y.; Jia, Y.; Ye, B.C. Synthesis of MOF-derived Ni@C materials for the electrochemical detection of histamine. Talanta 2020, 219, 121360. [Google Scholar] [CrossRef]
- Guo, L.; Hao, L.; Zhang, Y.; Yang, X.; Wang, Q.; Wang, Z.; Wang, C. Metal-organic framework precursors derived Ni-doping porous carbon spheres for sensitive electrochemical detection of acetaminophen. Talanta 2021, 228, 122228. [Google Scholar] [CrossRef]
- Yadav, A.; Verma, N. Enhanced hydrogen storage in graphitic carbon micro-nanofibers at moderate temperature and pressure: Synergistic interaction of asymmetrically-dispersed nickel-ceria nanoparticles. Int. J. Hydrogen Energy 2017, 42, 27139–27153. [Google Scholar] [CrossRef]
- Weng, Q.; Zeng, L.; Chen, Z.; Han, Y.; Jiang, K.; Bando, Y.; Golberg, D. Hydrogen Storage in Carbon and Oxygen Co-Doped Porous Boron Nitrides. Adv. Funct. Mater. 2021, 31, 2007381. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Lin, B.; Sun, Y.; Zhang, X.; Yang, H. Hydrothermal synthesis of Ni-doped hierarchically porous carbon monoliths for hydrogen storage. J. Porous Mater. 2015, 22, 1417–1422. [Google Scholar] [CrossRef]
- Wei, W.; Wang, F.; Yang, J.; Zou, J.; Li, J.; Shi, K. A superior potassium-ion anode material from pitch-based activated carbon fibers with hierarchical pore structure prepared by metal catalytic activation. ACS Appl. Mater. Interfaces 2021, 13, 6557–6565. [Google Scholar] [CrossRef]
- Yang, C.R.; Tseng, S.F.; Chen, Y.T. Characteristics of graphene oxide films reduced by using an atmospheric plasma system. Nanomaterials 2018, 8, 802. [Google Scholar]
- Peng, Z.; Xu, Y.; Luo, W.; Wang, C.; Ma, L. Conversion of biomass wastes into activated carbons by chemical activation for hydrogen storage. ChemistrySelect 2020, 5, 11221–11228. [Google Scholar] [CrossRef]
- Hernández-Vázquez, E.E.; Munoz, F.; López-Moreno, S.; Morán-López, J.L. First-principles study of Ni adatom migration on graphene with vacancies. RSC Adv. 2019, 9, 18823–18834. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Y.; Liu, Y.; Pang, H. Exposing {001} crystal plane on hexagonal Ni-MOF with surface-grown cross-linked mesh-structures for electrochemical energy storage. Small 2019, 15, 1902463. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, X.; Chen, X.; Jiang, L.; Zheng, Y. Synthesis of Mg-doped ordered mesoporous Pd–Al2O3 with different basicity for CO, NO, and HC elimination. Ind. Eng. Chem. Res. 2017, 56, 1687–1695. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, N.; Li, H.; Dong, Q.; Wang, Y.; Li, H.; Wang, S.; Zhang, X.; Qiu, J. Nitrogen-doped soft carbon frameworks built of well-interconnected nanocapsules enabling a superior potassium-ion batteries anode. Chem. Eng. J. 2020, 382, 121759. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Dong, H.; Hu, H.; Yu, P.; Peng, L.; Zheng, M.; Xiao, Y.; Liu, Y. Revealing contribution of pore size to high hydrogen storage capacity. Int. J. Hydrogen Energy 2018, 43, 18077–18082. [Google Scholar] [CrossRef]
- Lee, H.M.; Heo, Y.J.; An, K.H.; Jung, S.C.; Chung, D.C.; Park, S.J.; Kim, B.J. A study on optimal pore range for high pressure hydrogen storage behaviors by porous hard carbon materials prepared from a polymeric precursor. Int. J. Hydrogen Energy 2018, 43, 5894–5902. [Google Scholar] [CrossRef]
- Im, J.S.; Park, S.J.; Kim, T.J.; Kim, Y.H.; Lee, Y.S. The study of controlling pore size on electrospun carbon nanofibers for hydrogen adsorption. J. Colloid Interface Sci. 2008, 318, 42–49. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, L.; Xu, F.; Zhou, H.; Peng, X.; Sun, D.; Wang, J.; Du, Y. Nitrogen-doped porous carbons with high performance for hydrogen storage. Int. J. Hydrogen Energy 2016, 41, 8489–8497. [Google Scholar] [CrossRef]
- Conte, G.; Policicchio, A.; De Luca, O.; Rudolf, P.; Desiderio, G.; Agostino, R.G. Copper-doped activated carbon from amorphous cellulose for hydrogen, methane and carbon dioxide storage. Int. J. Hydrogen Energy 2022, 47, 18384–18395. [Google Scholar] [CrossRef]
- Singh, S.; Bhatnagar, A.; Dixit, V.; Shukla, V.; Shaz, M.A.; Sinha, A.S.K.; Srivastava, O.N.; Sekkar, V. Synthesis, characterization and hydrogen storage characteristics of ambient pressure dried carbon aerogel. Int. J. Hydrogen Energy 2016, 41, 3561–3570. [Google Scholar] [CrossRef]
- Balderas-Xicohtencatl, R.; Schlichtenmayer, M.; Hirscher, M. Volumetric hydrogen storage capacity in metal–organic frameworks. Energy Technol. 2018, 6, 578–582. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, Y.K.; Seo, H.J.; Jeong, S.M.; Kim, J.; Lim, S.K. The enhanced hydrogen storage capacity of carbon fibers: The effect of hollow porous structure and surface modification. Nanomaterials 2021, 11, 1830. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.V.; Banerjee, S.; Patwardhan, A.W.; Joshi, J.B.; Dasgupta, K. Synthesis of boron and nitrogen co-doped carbon nanotubes and their application in hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 13406–13413. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, Y.S.; Park, S.J. A study on the hydrogen storage capacity of Ni-plated porous carbon nanofibers. Int. J. Hydrogen Energy 2008, 33, 4112–4115. [Google Scholar] [CrossRef]



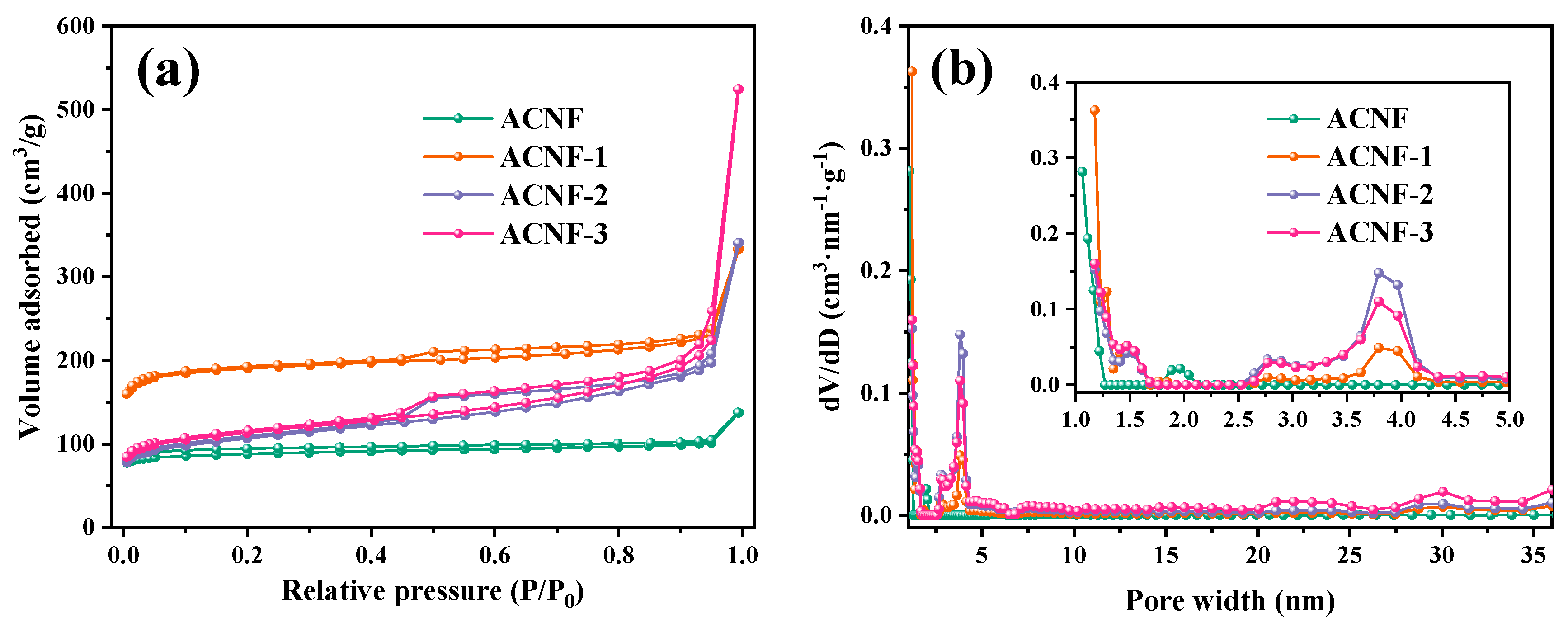
| Sample | SBET a (m2/g) | Vtotal b (cm3/g) | Smicro c (m2/g) | Vmicro d (cm3/g) | D e (nm) | H2 Uptake f (wt%) |
|---|---|---|---|---|---|---|
| ACNF | 347.1 | 0.213 | 308.8 | 0.120 | 2.5 | 0.60 |
| ACNF-1 | 750.7 | 0.515 | 666.8 | 0.258 | 2.7 | 1.39 |
| ACNF-2 | 387.7 | 0.527 | 218.3 | 0.092 | 5.3 | 0.85 |
| ACNF-3 | 415.8 | 0.811 | 245.4 | 0.101 | 7.8 | 0.66 |
| Absorbents | SBET (m2/g) | Condition | H2 Uptake (wt%) | References | |
|---|---|---|---|---|---|
| Temp. (K) | Pres. (bar) | ||||
| Cu-impregnated AC | 1459 | 298 | 80 | 0.34 | [53] |
| Ni-doped MWNCT | - | 298 | 20 | 0.298 | [20] |
| B,N-CNTs | - | 77 | 16 | 1.96 | [57] |
| Ni-ACF | 774 | 298 | 50 | 0.75 | [19] |
| N-doped PC | 2919 | 77 | 1 | 2.71 | [52] |
| Pd-loaded CF | 815.6 | 298 | 1 | 0.82 | [24] |
| Pt-doped CA | 379 | 77 | 22 | 5.15 | [54] |
| PC | 2564.6 | 77 | 1 | 2.67 | [55] |
| Ni-plated PCNF | 1310 | 298 | 100 | 2.2 | [58] |
| Ni-loaded ACNF | 763.4 | 298 | 100 | 2.12 | [32] |
| Ni-doped ACNF | 750.7 | 298 | 100 | 1.39 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, F.; Huang, L.; Ding, H.; Zhang, S.; Yu, J. In Situ Ni-Doped Hierarchically Porous Carbon Nanofibers Derived from Polyacrylonitrile/Pitch for Hydrogen Storage at Ambient Temperature. Sustainability 2023, 15, 8722. https://doi.org/10.3390/su15118722
Song F, Huang L, Ding H, Zhang S, Yu J. In Situ Ni-Doped Hierarchically Porous Carbon Nanofibers Derived from Polyacrylonitrile/Pitch for Hydrogen Storage at Ambient Temperature. Sustainability. 2023; 15(11):8722. https://doi.org/10.3390/su15118722
Chicago/Turabian StyleSong, Fuquan, Lintao Huang, Heying Ding, Shiming Zhang, and Jinbiao Yu. 2023. "In Situ Ni-Doped Hierarchically Porous Carbon Nanofibers Derived from Polyacrylonitrile/Pitch for Hydrogen Storage at Ambient Temperature" Sustainability 15, no. 11: 8722. https://doi.org/10.3390/su15118722
APA StyleSong, F., Huang, L., Ding, H., Zhang, S., & Yu, J. (2023). In Situ Ni-Doped Hierarchically Porous Carbon Nanofibers Derived from Polyacrylonitrile/Pitch for Hydrogen Storage at Ambient Temperature. Sustainability, 15(11), 8722. https://doi.org/10.3390/su15118722