Water Quality of the Odra (Oder) River before and during the Ecological Disaster in 2022: A Warning to Water Management
Abstract
1. Introduction
2. Methods
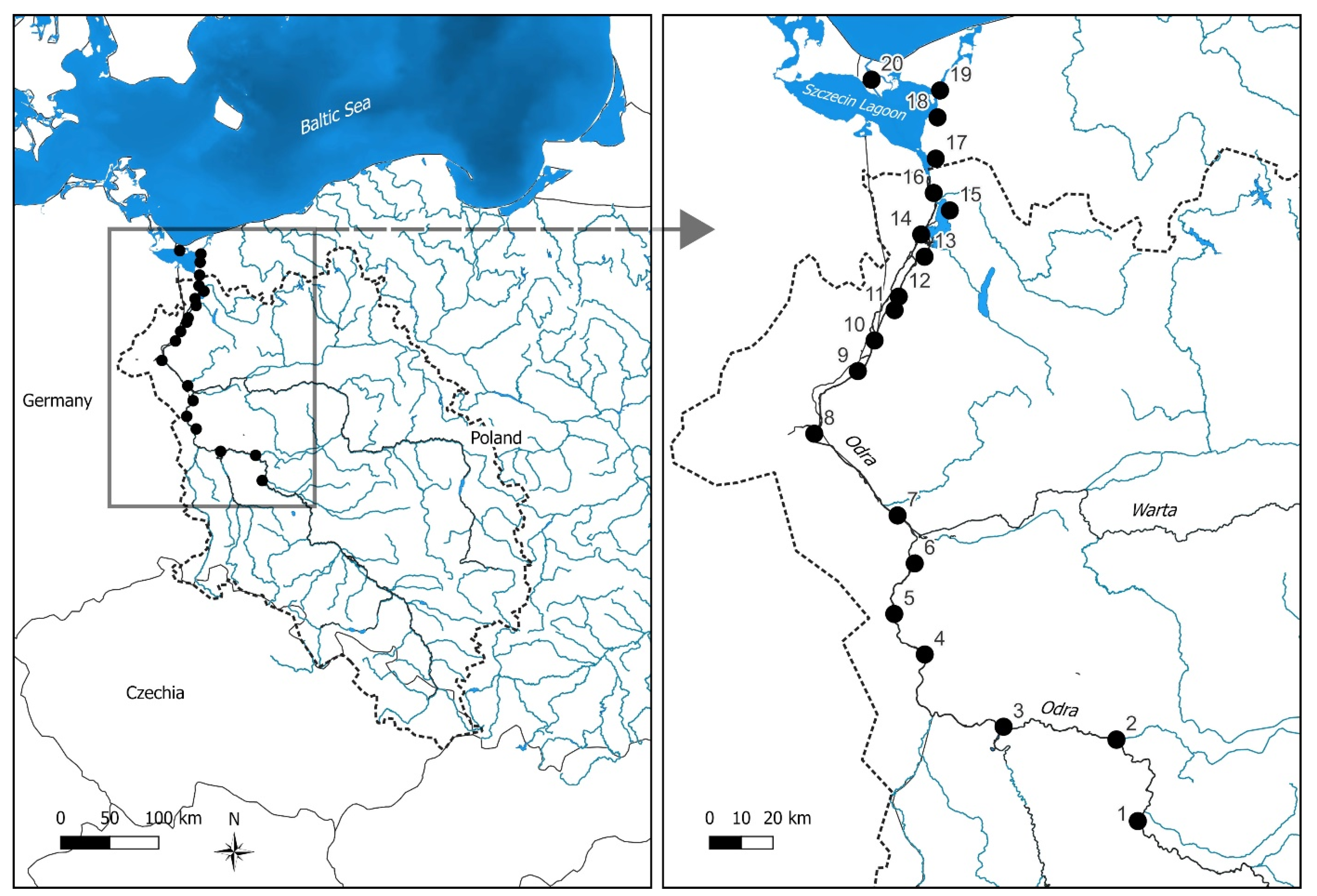
2.1. Study Area
2.2. Data Collection
2.3. Statistical Analysis
3. Results
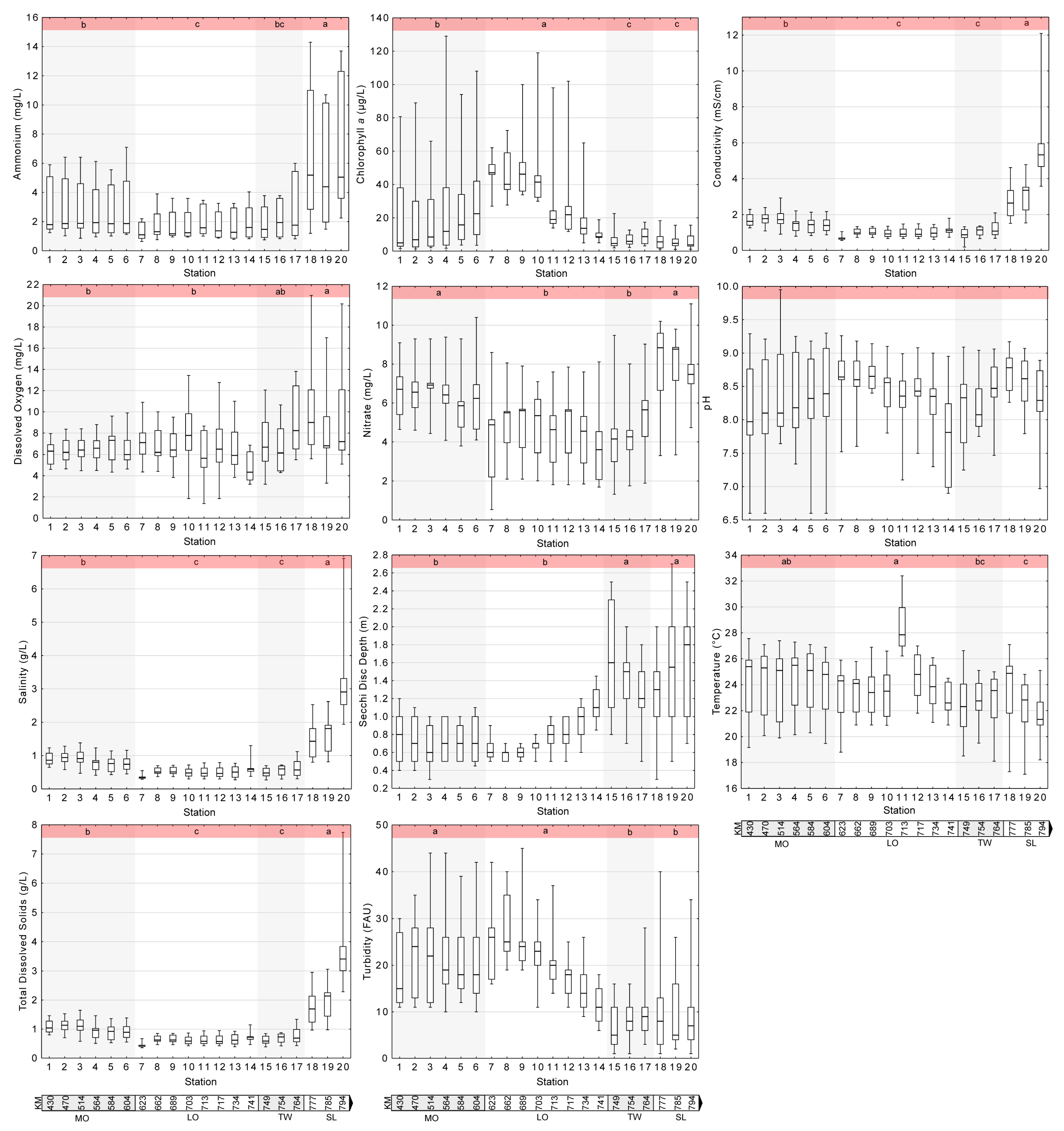
3.1. Water Quality Parameters
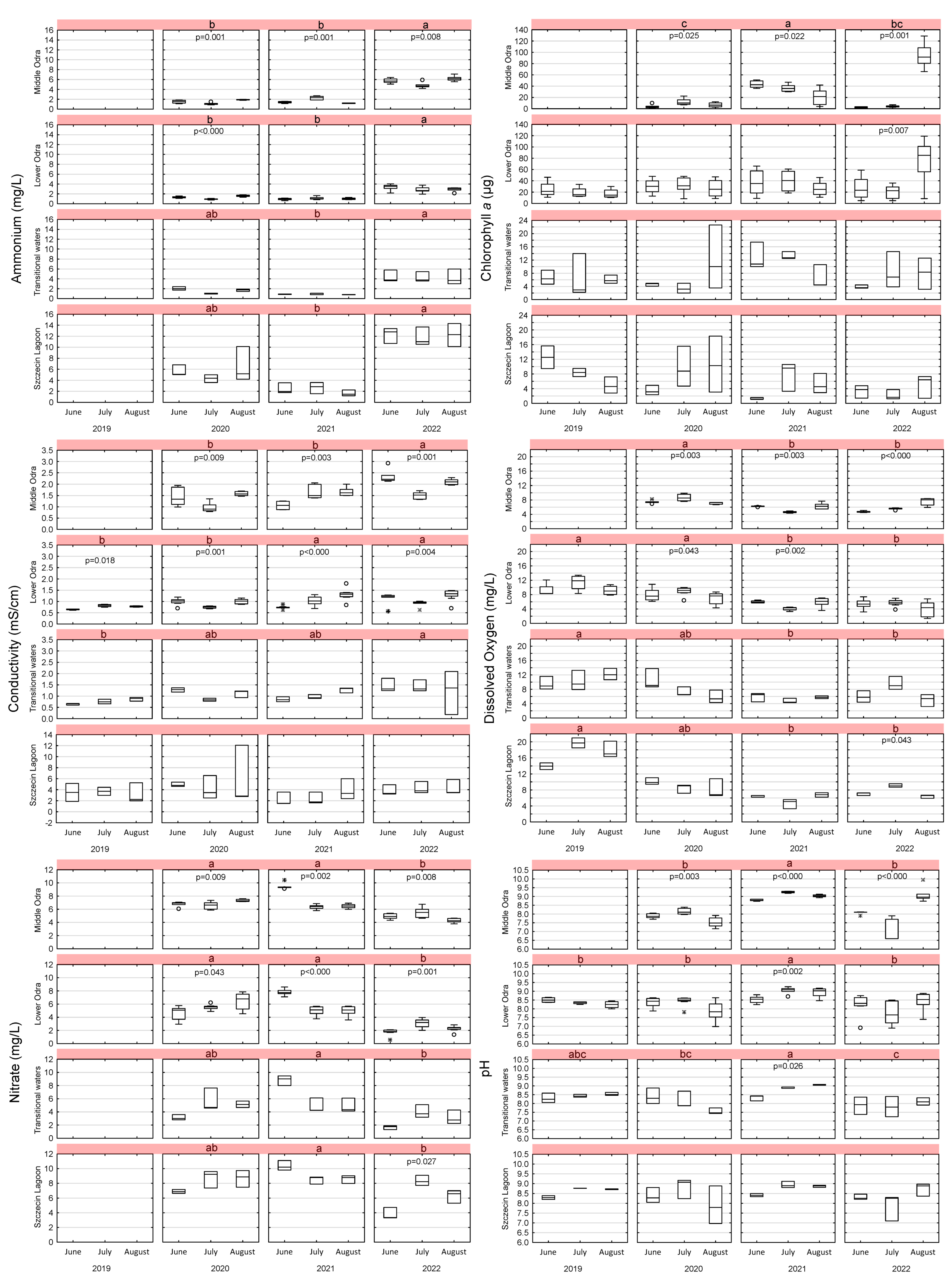
3.1.1. Ammonium and Nitrate
3.1.2. Chlorophyll a
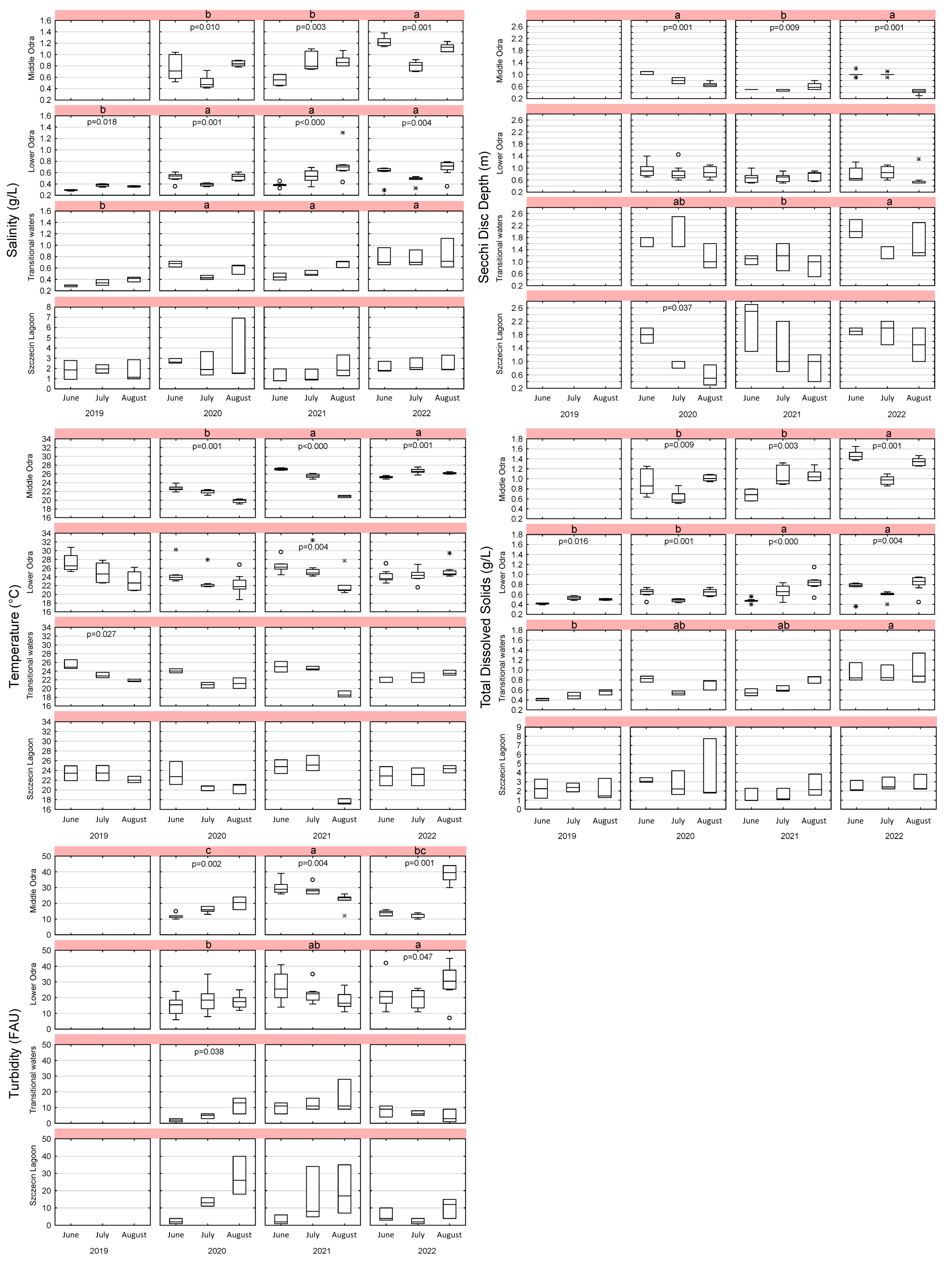
3.1.3. Conductivity, Salinity, and Total Dissolved Solids (TDS)
3.1.4. Dissolved Oxygen
3.1.5. pH
3.1.6. Secchi Disc Depth and Turbidity (Transparency Indicators)
3.1.7. Temperature
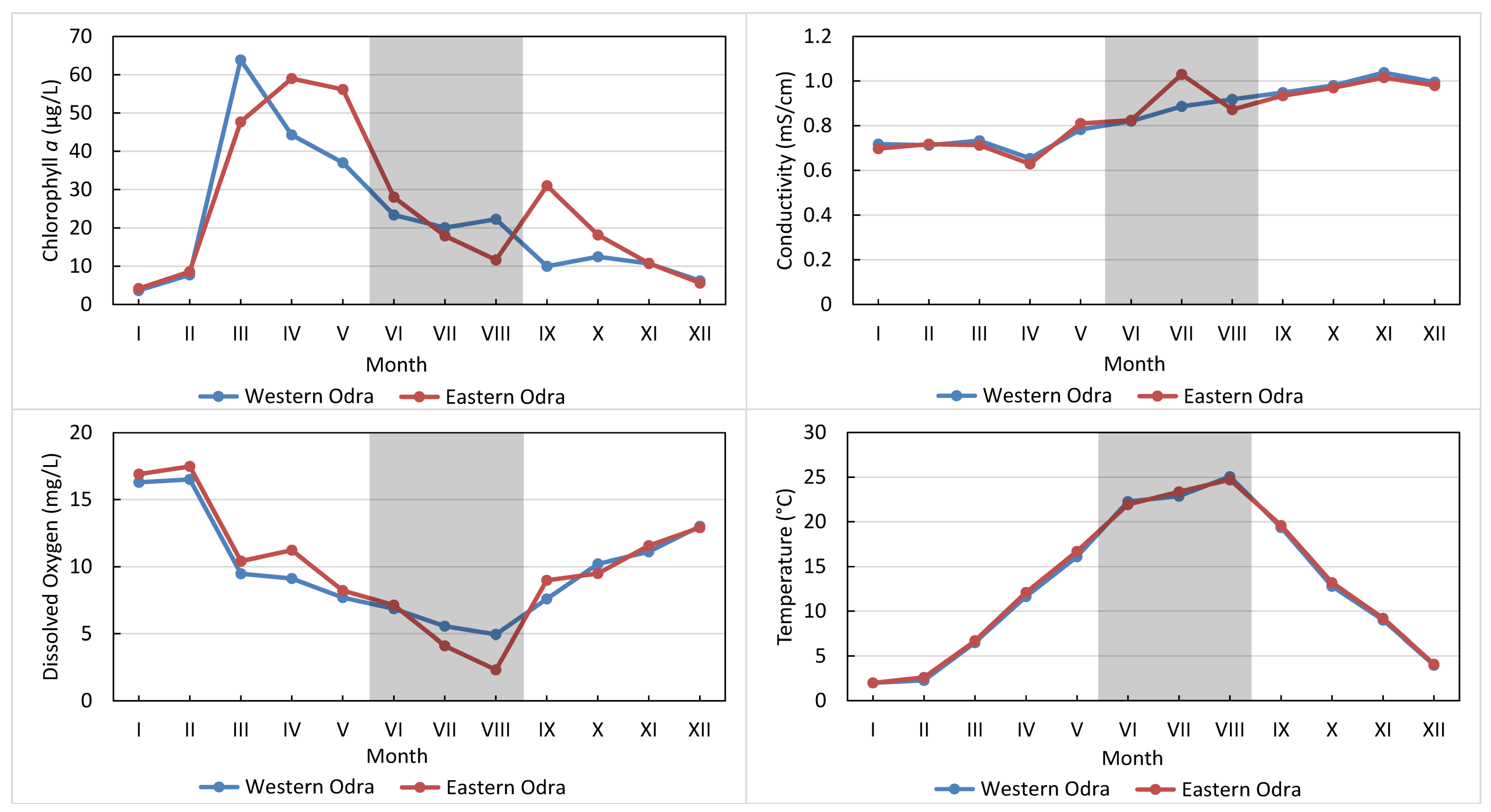
3.2. Water Quality Parameters along the River Continuum
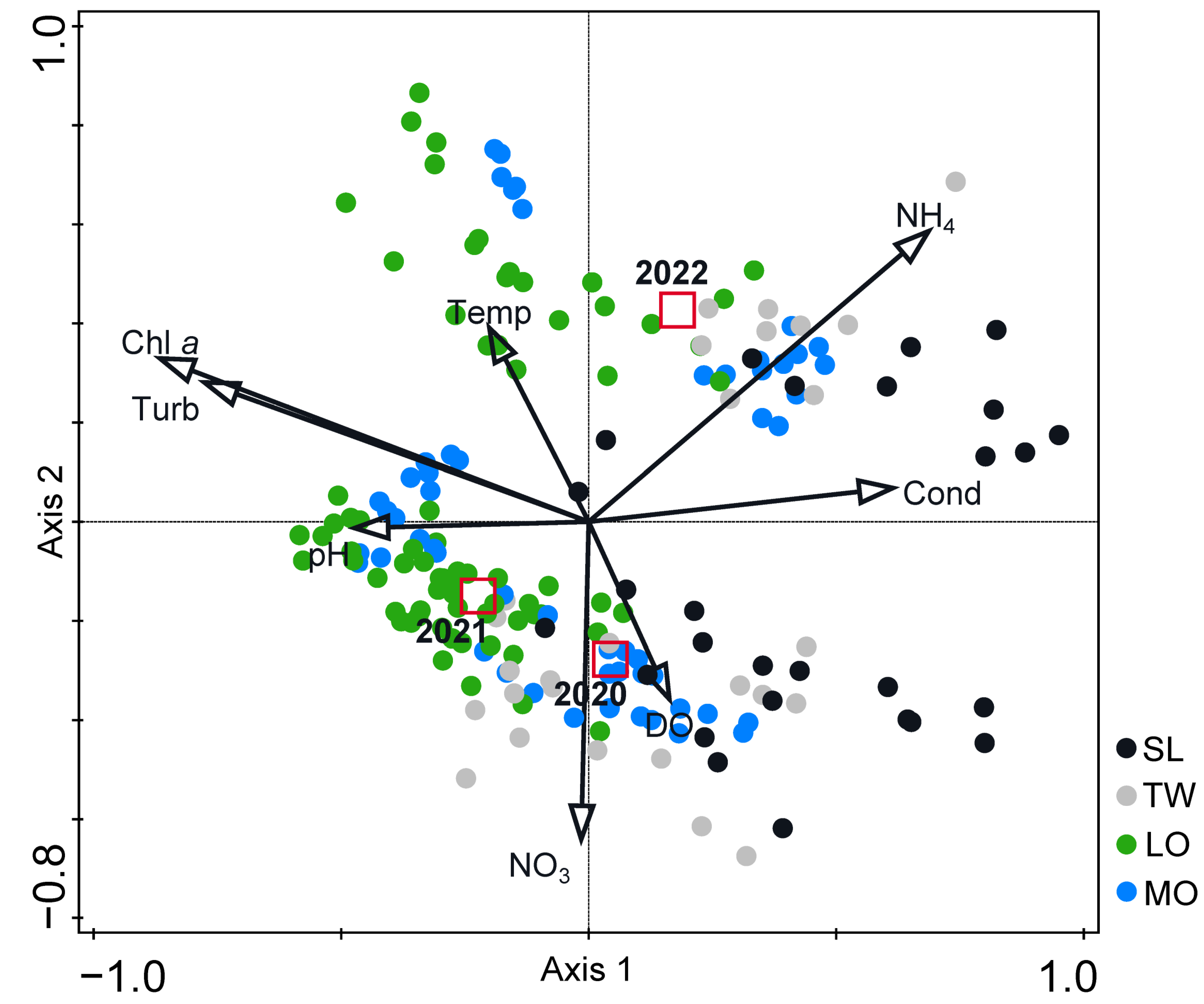
3.3. Multivariate Analyses
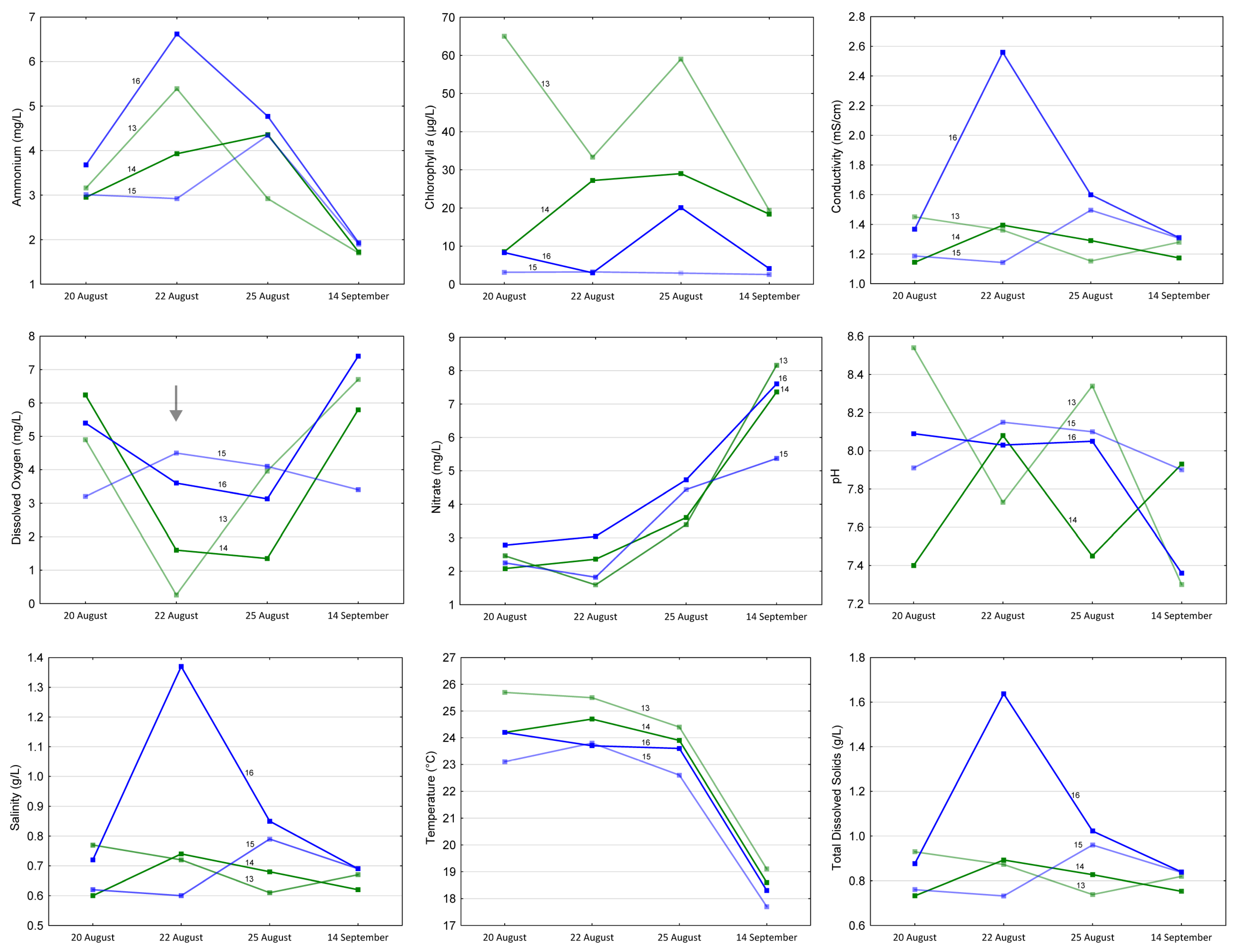
3.4. Hypoxia of the Lower Section of the Odra River
3.5. Phenological Variability in the Parameters
3.6. Other Remarks
4. Discussion
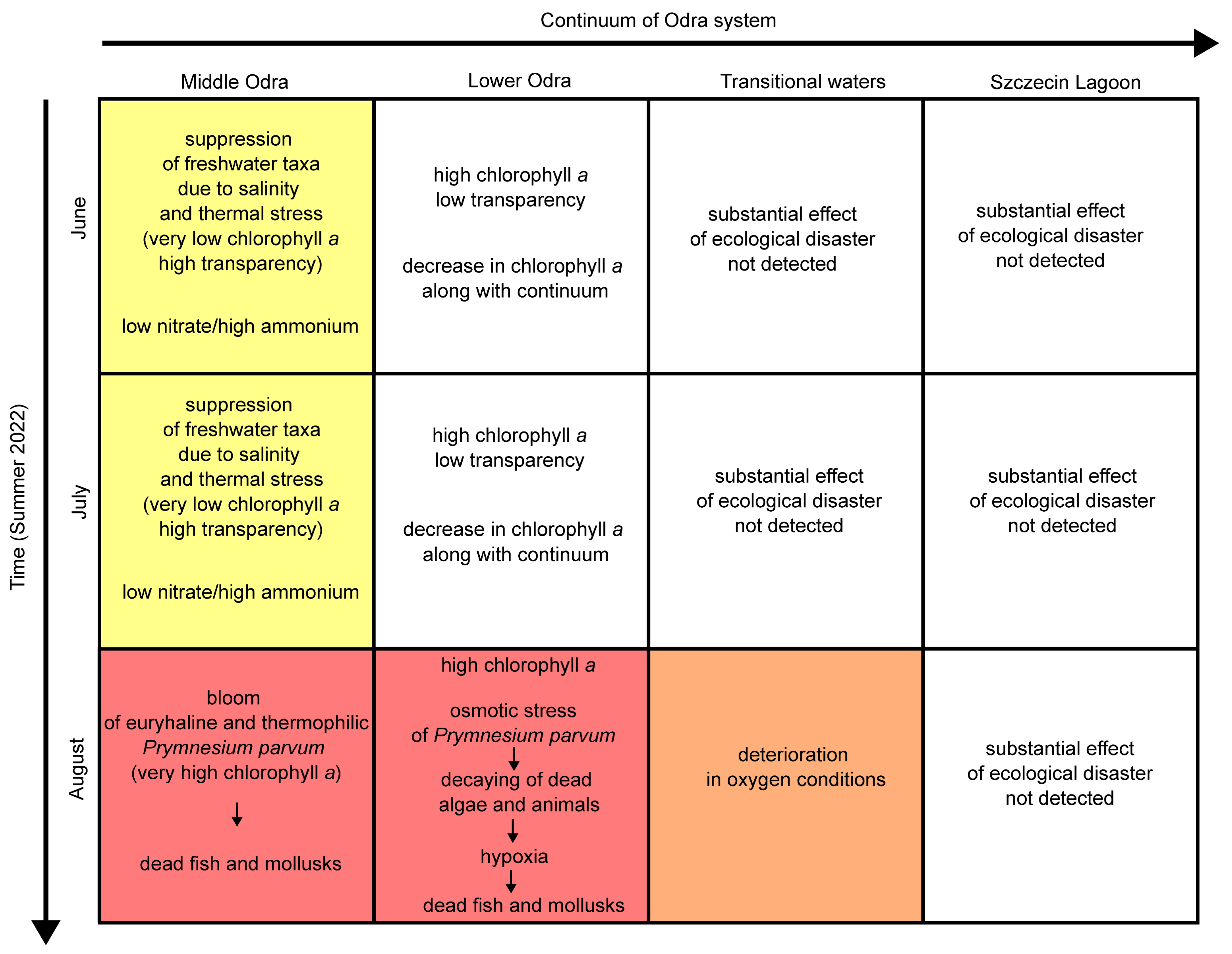
4.1. Zonation and Disruption of the River Continuum
4.2. Trophic State Indicators
4.3. Excessive Salinity
4.4. Temperature
4.5. Ecological Disaster and Consequences
4.6. Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zalewski, M. Ecohydrology—The scientific background to use ecosystem properties as management tools toward sustainability of water resources. Ecol. Eng. 2000, 16, 1–8. [Google Scholar] [CrossRef]
- Wolter, C.; Arlinghaus, R. Navigation impacts on freshwater fish assemblages: The ecological relevance of swimming performance. Rev. Fish Biol. Fish. 2003, 13, 63–89. [Google Scholar] [CrossRef]
- Belletti, B.; Garcia de Leaniz, C.; Jones, J.; Bizzi, S.; Börger, L.; Segura, G.; Castelletti, A.; Van de Bund, W.; Aarestrup, K.; Barry, J.; et al. More than one million barriers fragment Europe’s rivers. Nature 2020, 588, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Guse, B.; Kail, J.; Radinger, J.; Schröder, M.; Kiesel, J.; Hering, D.; Wolter, C.; Fohrer, N. Eco-hydrologic model cascades: Simulating land use and climate change impacts on hydrology, hydraulics and habitats for fish and macroinvertebrates. Sci. Total Environ. 2015, 533, 542–556. [Google Scholar] [CrossRef]
- Marx, A.; Kumar, R.; Thober, S.; Rakovec, O.; Wanders, N.; Zink, M.; Wood, E.F.; Pan, M.; Sheffield, J.; Samaniego, L. Climate change alters low flows in Europe under global warming of 1.5, 2, and 3 C. Hydrol. Earth Syst. Sci. 2018, 22, 1017–1032. [Google Scholar] [CrossRef]
- Mankiewicz, J.; Komárková, J.; Izydorczyk, K.; Jurczak, T.; Tarczynska, M.; Zalewski, M. Hepatotoxic cyanobacterial blooms in the lakes of northern Poland. Environ. Toxicol. Int. J. 2005, 20, 499–506. [Google Scholar] [CrossRef]
- Kobos, J.; Błaszczyk, A.; Hohlfeld, N.; Toruńska-Sitarz, A.; Krakowiak, A.; Hebel, A.; Sutryk, K.; Grabowska, M.; Toporowska, M.; Kokociński, M.; et al. Cyanobacteria and cyanotoxins in Polish freshwater bodies. Oceanol. Hydrobiol. Stud. 2013, 42, 358–378. [Google Scholar] [CrossRef]
- Mallin, M.A.; Johnson, V.L.; Ensign, S.H.; MacPherson, T.A. Factors contributing to hypoxia in rivers, lakes, and streams. Limnol. Oceanogr. 2006, 51, 690–701. [Google Scholar] [CrossRef]
- Haines-Young, R.; Potschin, M. The links between biodiversity, ecosystem services and human well-being. Ecosyst. Ecol. New Synth. 2010, 1, 110–139. [Google Scholar]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Patiño, R.; Dawson, D.; VanLandeghem, M.M. Retrospective analysis of associations between water quality and toxic blooms of golden alga (Prymnesium parvum) in Texas reservoirs: Implications for understanding dispersal mechanisms and impacts of climate change. Harmful Algae 2014, 33, 1–11. [Google Scholar] [CrossRef]
- Roelke, D.L.; Barkoh, A.; Brooks, B.W.; Grover, J.P.; Hambright, K.D.; LaClaire, J.W.; Moeller, P.D.R.; Patino, R. A chronicle of a killer alga in the west: Ecology, assessment, and management of Prymnesium parvum blooms. Hydrobiologia 2016, 764, 29–50. [Google Scholar] [CrossRef]
- IOŚ PIB. Raport Kończący Prace Zespołu ds. Sytuacji w Odrze; Instytut Ochrony Środowiska—Państwowy Instytut Badawczy: Warszawa, Poland, 2023; p. 191. Available online: https://ios.edu.pl/wp-content/uploads/2022/12/raport-konczacy-prace-zespolu-ds-sytuacji-w-odrze-2.pdf (accessed on 25 April 2023).
- Schulte, C.; Abbas, B.; Engelke, C.; Fischer, H.; Henneberg, S.; Hentschel, H.; Jekel, H.; Jeske, R.; Pietsch, K.; Scholl, F.; et al. Fischsterben in der Oder, August 2022. Nationale Expert*Innengruppe zum Fischsterben in der Oder unter Leitung des Umweltbundesamtes. 2022, p. 34. Available online: https://www.bmuv.de/fileadmin/Daten_BMU/Download_PDF/Naturschutz/Bericht_-_Fischsterben_in_der_Oder_20220929_bf.pdf (accessed on 25 April 2023).
- Absalon, D.; Matysik, M.; Woźnica, A.; Janczewska, N. Detection of changes in the hydrobiological parameters of the Oder River during the ecological disaster in July 2022 based on multi-parameter probe tests and remote sensing methods. Ecol. Indic. 2023, 148, 110103. [Google Scholar] [CrossRef]
- Molins-Legua, C.; Meseguer-Lloret, S.; Moliner-Martinez, Y.; Campíns-Falcó, P. A guide for selecting the most appropriate method for ammonium determination in water analysis. Trends Anal. Chem. 2006, 25, 282–290. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Y.; Liu, Y.; Zhang, R. Review of chloride ion detection technology in water. Appl. Sci. 2021, 11, 11137. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Krodkiewska, M.; Spyra, A.; Cieplok, A. Assessment of pollution, and ecological status in rivers located in the Vistula and Oder river basins impacted by the mining industry in Central Europe (Poland). Ecol. Indic. 2022, 144, 109505. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Radziejewska, T.; Schernewski, G. The Szczecin (Oder-) Lagoon. In Ecology of Baltic Coastal Waters Series; Schiewer, U., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 197, pp. 115–129. [Google Scholar]
- Lürling, M.; van Geest, G.; Scheffer, M. Importance of nutrient competition and allelopathic effects in suppression of the green alga Scenedesmus obliquus by the macrophytes Chara, Elodea and Myriophyllum. Hydrobiologia 2006, 556, 209–220. [Google Scholar] [CrossRef]
- Siwek, H.; Wybieralski, J.; Galczyńska, M. Zawartość chlorofilu a i jego feopochodnych jako element monitoringu rzek. Zesz. Probl. Postępów Nauk. Rol. 2001, 476, 497–502. [Google Scholar]
- Siwek, H.; Wybieralski, J.; Gałczyńska, M. Porównanie stanu troficznego rzek Przymorza z ilościowym opisem eutrofizacji jezior. Chem. Inżynieria Ekol. 1999, 6, 181–187. [Google Scholar]
- Mądrecka, B.; Szeląg-Wasielewska, E. Mass development of phytoplankton in the River Warta in Poznań (Poland) in the 21 century. Limnol. Rev. 2017, 17, 79–88. [Google Scholar] [CrossRef]
- Chakraborty, P.; Acharyya, T.; Babu, P.R.; Bandyopadhyay, D. Impact of salinity and pH on phytoplankton communities in a tropical freshwater system: An investigation with pigment analysis by HPLC. J. Environ. Monit. 2011, 13, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Czerniawski, R.; Sługocki, Ł. Analysis of zooplankton assemblages from man-made ditches in relation to current velocity. Oceanol. Hydrobiol. Stud. 2017, 46, 199–211. [Google Scholar] [CrossRef]
- Sługocki, Ł.; Goździk, I.; Pilecka-Rapacz, M.; Czerniawski, R.; Domagała, J. The effect of environmental factors on the structure of phytoplankton in the lower Odra River. Pol. J. Natur. Sc 2014, 29, 137–152. [Google Scholar]
- Domingues, R.B.; Barbosa, A.B.; Sommer, U.; Galvão, H.M. Ammonium, nitrate and phytoplankton interactions in a freshwater tidal estuarine zone: Potential effects of cultural eutrophication. Aquat. Sci. 2011, 73, 331–343. [Google Scholar] [CrossRef]
- Mischke, U.; Venohr, M.; Behrendt, H. Using phytoplankton to assess the trophic status of German rivers. Int. Rev. Hydrobiol. 2011, 96, 578–598. [Google Scholar] [CrossRef]
- Bąk, M.; Halabowski, D.; Kryk, A.; Lewin, I.; Sowa, A. Mining salinisation of rivers: Its impact on diatom (Bacillariophyta) assemblages. Fottea 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Noges, T. Relationships between morphometry, geographic location and water quality parameters of European lakes. Hydrobiologia 2009, 633, 33–43. [Google Scholar] [CrossRef]
- Schulz, C.J.; Cañedo-Argüelles, M. Lost in translation: The German literature on freshwater salinization. Philos. Trans. R. Soc. B 2019, 374, 20180007. [Google Scholar] [CrossRef]
- Woźnica, A.; Absalon, D.; Matysik, M.; Bąk, M.; Cieplok, A.; Halabowski, D.; Koczorowska, A.; Krodkiewska, M.; Libera, M.; Sierka, E.; et al. Analysis of the Salinity of the Vistula River Based on Patrol Monitoring and State Environmental Monitoring. Water 2023, 15, 838. [Google Scholar] [CrossRef]
- Szklarek, S.; Górecka, A.; Wojtal-Frankiewicz, A. The effects of road salt on freshwater ecosystems and solutions for mitigating chloride pollution—A review. Sci. Total Environ. 2022, 805, 150289. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.S.; Canedo-Argüelles, M.; Hintz, W.D.; Dyack, B.; Birk, S.; Relyea, R.A. Regulations are needed to protect freshwater ecosystems from salinization. Philos. Trans. R. Soc. B 2019, 374, 20180019. [Google Scholar] [CrossRef] [PubMed]
- Cunillera-Montcusí, D.; Beklioğlu, M.; Cañedo-Argüelles, M.; Jeppesen, E.; Ptacnik, R.; Amorim, C.A.; Arnott, S.E.; Berger, S.A.; Brucet, S.; Dugan, H.A.; et al. Freshwater salinisation: A research agenda for a saltier world. Trends Ecol. Evol. 2022, 37, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.E.; Downey, K.; Smith Colby, R.; Freire, C.A.; Nichols, S.; Burgess, M.N.; Judy, K.J. Recognizing salinity threats in the climate crisis. Integr. Comp. Biol. 2022, 62, 441–460. [Google Scholar] [CrossRef]
- Herbert, E.R.; Boon, P.; Burgin, A.J.; Neubauer, S.C.; Franklin, R.B.; Ardón, M.; Hopfensperger, K.N.; Lamers, L.P.M.; Gell, P. A global perspective on wetland salinization: Ecological consequences of a growing threat to freshwater wetlands. Ecosphere 2015, 6, 1–43. [Google Scholar] [CrossRef]
- Grabowski, M.; Bacela, K.; Konopacka, A.; Jazdzewski, K. Salinity-related distribution of alien amphipods in rivers provides refugia for native species. Biol. Invasions 2009, 11, 2107–2117. [Google Scholar] [CrossRef]
- Sługocki, Ł.; Rymaszewska, A.; Kirczuk, L. To fit or to belong: Characterization of the non-native invader Eurytemora carolleeae (Copepoda: Calanoida) in the Oder River system (Central Europe). Aquat. Invasions 2021, 16, 443–460. [Google Scholar] [CrossRef]
- Richardson, E.T.; Patiño, R. Growth of the harmful alga, Prymnesium parvum (Prymnesiophyceae), after gradual and abrupt increases in salinity. J. Phycol. 2021, 57, 1335–1344. [Google Scholar] [CrossRef]
- Graf, R.; Wrzesiński, D. Detecting patterns of changes in river water temperature in Poland. Water 2020, 12, 1327. [Google Scholar] [CrossRef]
- Grygoruk, M.; Mirosław-Świątek, D.; Chrzanowska, W.; Ignar, S. How much for water? Economic assessment and mapping of floodplain water storage as a catchment-scale ecosystem service of wetlands. Water 2013, 5, 1760–1779. [Google Scholar] [CrossRef]
- Labecka, A.M.; Czarnoleski, M. Patterns of growth, brooding and offspring size in the invasive mussel Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae) from an anthropogenic heat island. Hydrobiologia 2021, 848, 3093–3113. [Google Scholar] [CrossRef]
- Czerniawski, R.; Pilecka-Rapacz, M.; Domagała, J. Zooplankton communities of inter-connected sections of lower River Oder (NW Poland). Open Life Sci. 2013, 8, 18–29. [Google Scholar] [CrossRef]
- Nowakowski, K.; Sługocki, Ł. Short-term heat shock perturbation affects populations of Daphnia magna and Eurytemora carolleeae: A warning to the water thermal pollution. Sci. Rep. 2021, 11, 16909. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Pambudi, D.S.A.; Ahmad, M.M.; Alfanda, B.D.; Imron, M.F.; Abdullah, S.R.S. Ecological impacts of ballast water loading and discharge: Insight into the toxicity and accumulation of disinfection by-products. Heliyon 2022, 8, e09107. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.; Beszteri, S.; Vogel, H.; John, U. Effects of physiological shock treatments on toxicity and polyketide synthase gene expression in Prymnesium parvum (Prymnesiophyceae). Eur. J. Phycol. 2011, 46, 193–201. [Google Scholar] [CrossRef]
- Hargis, W.J., Jr.; Roberts, M.H., Jr.; Zwerner, D.E. Effects of contaminated sediments and sediment-exposed effluent water on an estuarine fish: Acute toxicity. Mar. Environ. Res. 1984, 14, 337–354. [Google Scholar] [CrossRef]
- Nádudvari, Á.; Czajka, A.; Wyżga, B.; Zygmunt, M.; Wdowikowski, M. Patterns of Recent Changes in Channel Morphology and Flows in the Upper and Middle Odra River. Water 2023, 15, 370. [Google Scholar] [CrossRef]
- Miller, D.; Poucher, S.; Coiro, L. Determination of lethal dissolved oxygen levels for selected marine and estuarine fishes, crustaceans, and a bivalve. Mar. Biol. 2002, 140, 287–296. [Google Scholar]
- Sinha, B.; Annachhatre, A.P. Partial nitrification—Operational parameters and microorganisms involved. Rev. Environ. Sci. Bio/Technol. 2007, 6, 285–313. [Google Scholar] [CrossRef]
- Elshout, P.M.F.; Dionisio Pires, L.M.; Leuven, R.S.E.W.; Wendelaar Bonga, S.E.; Hendriks, A.J. Low oxygen tolerance of different life stages of temperate freshwater fish species. J. Fish Biol. 2013, 83, 190–206. [Google Scholar] [CrossRef]
- Karpowicz, M.; Ejsmont-Karabin, J.; Kozłowska, J.; Feniova, I.; Dzialowski, A.R. Zooplankton community responses to oxygen stress. Water 2020, 12, 706. [Google Scholar] [CrossRef]
- Czerniejewski, P.; Rybczyk, A.; Tański, A.; Keszka, S.; Antoszek, A. Growth rate and condition of vimba, Vimba vimba (Actinopterygii: Cypriniformes: Cyprinidae), a species under restitution in the Odra River estuary. Acta Ichthyol. Piscat. 2011, 41, 215–222. [Google Scholar] [CrossRef]
- Wolter, C. Temperature influence on the fish assemblage structure in a large lowland river, the lower Oder River, Germany. Ecol. Freshw. Fish 2007, 16, 493–503. [Google Scholar] [CrossRef]
- Gliwicz, M.Z. On the different nature of top-down and bottom-up effects in pelagic food webs. Freshw. Biol. 2002, 47, 2296–2312. [Google Scholar] [CrossRef]
- Czerniawski, R.; Domagała, J.; Dębowski, P.; Bernaś, R.; Pilecka-Rapacz, M.; Gancarczyk, J.; Krepski, T.; Sługocki, Ł.; Kraczek, G.; Bilski, P. Ichtiofauna wód płynących dorzecza Drawy. Rocz. Nauk. PZW 2016, 29, 43–87. [Google Scholar]
- Boys, C.A.; Baldwin, D.S.; Ellis, I.; Pera, J.; Cheshire, K. Review of options for creating and maintaining oxygen refuges for fish during destratification-driven hypoxia in rivers. Mar. Freshw. Res. 2021, 73, 200–210. [Google Scholar] [CrossRef]
- Zarfl, C.; Berlekamp, J.; He, F.; Jähnig, S.C.; Darwall, W.; Tockner, K. Future large hydropower dams impact global freshwater megafauna. Sci. Rep. 2019, 9, 18531. [Google Scholar] [CrossRef]

| All Sections | MO + LO | MO | MO 2020 | MO 2022 | LO | LO 2019 | LO 2020 | LO 2021 | LO 2022 | |
|---|---|---|---|---|---|---|---|---|---|---|
| n = 208/180 | n = 138/126 | n = 54 | n = 18 | n = 18 | n = 84/72 | n = 12 | n = 24 | n = 24 | n = 24 | |
| Ammonium | −0.25 ** | x | 0.44 * | |||||||
| Chlorophyll a | −0.39 *** | 0.72 ** | −0.76 *** | −0.89 ** | −0.82 *** | −0.88 *** | −0.54 * | |||
| Conductivity | 0.16 * | −0.50 *** | −0.28 * | 0.24 * | 0.51 * | |||||
| Dissolved Oxygen | 0.20 * | 0.54 * | −0.25 * | −0.67 ** | −0.50 * | −0.42 * | ||||
| Nitrate | −0.43 *** | −0.47 * | x | |||||||
| pH | −0.47 *** | −0.41 * | −0.58 * | −0.76 *** | ||||||
| Salinity | 0.15 * | −0.51 *** | −0.28 * | 0.51 * | ||||||
| Secchi Disc Depth | 0.56 *** | 0.30 ** | 0.73 *** | x | 0.84 *** | 0.87 *** | 0.63 ** | |||
| Temperature | −0.22 * | |||||||||
| TDS | 0.16 * | −0.51 *** | −0.28 * | 0.23 * | 0.51 * | |||||
| Turbidity | −0.51 *** | −0.19 * | −0.66 *** | x | −0.64 ** | −0.73 *** | −0.69 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sługocki, Ł.; Czerniawski, R. Water Quality of the Odra (Oder) River before and during the Ecological Disaster in 2022: A Warning to Water Management. Sustainability 2023, 15, 8594. https://doi.org/10.3390/su15118594
Sługocki Ł, Czerniawski R. Water Quality of the Odra (Oder) River before and during the Ecological Disaster in 2022: A Warning to Water Management. Sustainability. 2023; 15(11):8594. https://doi.org/10.3390/su15118594
Chicago/Turabian StyleSługocki, Łukasz, and Robert Czerniawski. 2023. "Water Quality of the Odra (Oder) River before and during the Ecological Disaster in 2022: A Warning to Water Management" Sustainability 15, no. 11: 8594. https://doi.org/10.3390/su15118594
APA StyleSługocki, Ł., & Czerniawski, R. (2023). Water Quality of the Odra (Oder) River before and during the Ecological Disaster in 2022: A Warning to Water Management. Sustainability, 15(11), 8594. https://doi.org/10.3390/su15118594






