Storage Time Affects the Viability, Longevity, and Germination of Eriochloa villosa (Thunb.) Kunth Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Germination Index Determination
2.2.2. Seed Viability Determination
2.2.3. Electrical Conductivity Determination
2.2.4. Effects of Storage Age on Physiological and Biochemical Indices of E. villosa Seeds
Malondialdehyde Determination
Soluble Sugar Determination
Soluble Protein Determination
Superoxide Dismutase Activity Determination
Peroxidase Activity Determination
Catalase Activity Determination
α-Amylase Activity Determination
2.2.5. Correlation of Germination Indices with Physiological and Biochemical Indices
2.3. Data Processing and Analysis
3. Results
3.1. Effects of Storage Years on Germination of E. villosa Seeds
3.2. Effects of Storage Years on the Viability of E. villosa Seeds
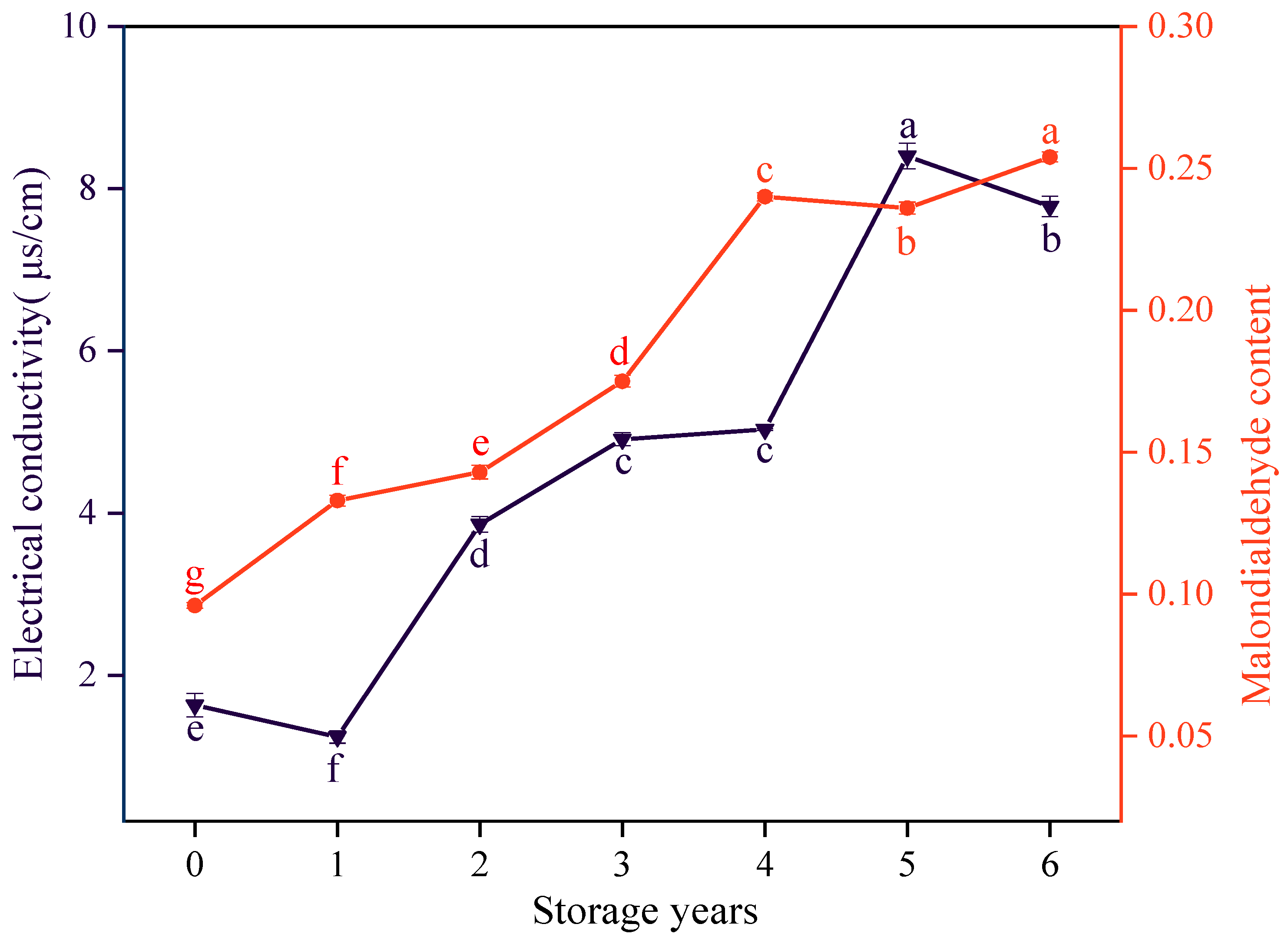
3.3. Effects of Storage Years on the Electrical Conductivity and MDA Content of E. villosa Seeds
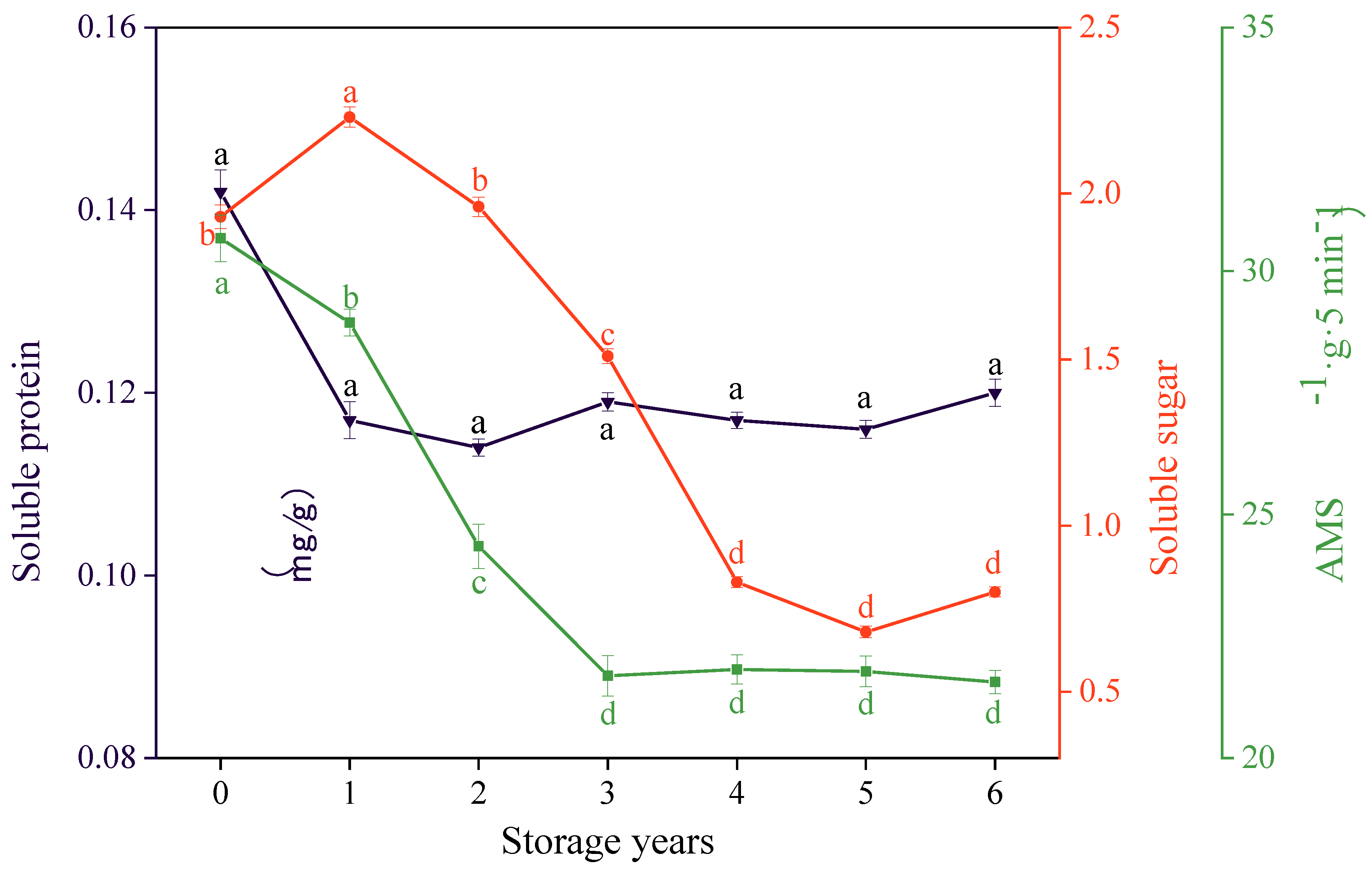
3.4. Effects of Storage Years on the Soluble Sugar Content, Soluble Protein Content, and AMS Activity of E. villosa Seeds
3.5. Effects of Storage Years on the SOD, POD, and CAT Activity of E. villosa Seeds
3.6. Correlation of the Seed Germination Indexes of E. villosa with Its Physiological and Biochemical Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y. Weed Journal of China; China Agriculture Press: Beijing, China, 1998. [Google Scholar]
- Juan, C. Screening and Evaluation of Herbicides to Eriochloa Villosa in Soybean Field. J. Northeast. Agric. Sci. 2021, 46, 72–74+119. [Google Scholar]
- Xi, Z.H.; Hu, Y.F.; Y.W. Control of E. villosa, a pernicious weed in soybean fields. Mod. Agric. 2002, 12, 9. [Google Scholar]
- Yulian, G.; Yan, H.C.; Yuanju, H.; Yu, W.; Dewan, P. Efficacy of 15 Herbicides on Eriochloa villosa (Thunb.) Kunth. J. Weed Sci. 2014, 32, 127–129. [Google Scholar]
- Simard, M.-J.; Nurse, R.E.; Darbyshire, S.J. Emergence and seed production of woolly cupgrass (Eriochloa villosa) in legume forage crops. Can. J. Plant Sci. 2015, 95, 539–548. [Google Scholar] [CrossRef]
- Wen, Y. Pernicious weed E. villosa (Thunb.) Kunth control technology. Mod. Agric. 2007, 6, 7. [Google Scholar]
- Li, W.; Cui, J.; Xu, W.; Shi, S. Effects of Eriochloa villosa (Thunb.) Kunth on the Growth and Development of Spring Soybean and Its Economic Threshold in Northeast China. Soybean Sci. 2019, 38, 584–588. [Google Scholar]
- Nadarajan, J.; Walters, C.; Pritchard, H.W.; Ballesteros, D.; Colville, L. Seed Longevity-The Evolution of Knowledge and a Conceptual Framework. Plants 2023, 12, 471. [Google Scholar] [CrossRef]
- van der Walt, K.; Nadarajan, J. Seed Storage Physiology of Lophomyrtus and Neomyrtus, Two Threatened Myrtaceae Genera Endemic to New Zealand. Plants 2023, 12, 1067. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, E.V.; Camargo, M.B.P.d.; Teixeira, S.d.P.; Silva, E.A.A.d.; Faria, J.M.R.; Barbedo, C.J. Variations in desiccation tolerance in seeds ofEugenia pyriformis: Dispersal at different stages of maturation. Rev. CiÊncia AgronÔmica 2016, 47, 118–126. [Google Scholar] [CrossRef]
- Sanchez-Coronado, M.E.; Coates, R.; Castro-Colina, L. Improving seed germination and seedling growth of Omphalea oleifera (Euphorbiaceae) for restoration projects in tropical rain forests. For. Ecol. Manag. 2007, 243, 144–155. [Google Scholar] [CrossRef]
- Aragão, V.P.M.; Trindade, B.M.C.; Reis, R.S.; Silveira, V.; Santa-Catarina, C. Storage time affects the germination and proteomic profile of seeds of Cariniana legalis (Mart.) O. Kuntze (Lecythidaceae), an endangered tree species native to the Brazilian Atlantic Forest. Braz. J. Bot. 2019, 42, 407–419. [Google Scholar] [CrossRef]
- Yalu, Z.; Xiao, Y.X.; Chou, Y. Comparison of wheat seed vigor determination methods. Jiangsu Agric. Sci. 2017, 45, 61–64. [Google Scholar]
- Shichao, G.; Pi, W.W. An introduction to the determination of the viability of transgenic soybean seeds. Seed World 2011, 9, 20–21. [Google Scholar]
- Qingfu, D.; Xihai, J.; Baochun, L.; Huijie, G.; Jianhua, W. Study on the Fitting Vigor Testing Methods of Different Types Maize. Maize Sci. 2007, 15, 122–126. [Google Scholar]
- Bailly, C.; Kranner, I. Analyses of reactive oxygen species and antioxidants in relation to seed longevity and germination. Methods Mol. Biol. 2011, 773, 343–367. [Google Scholar] [PubMed]
- Qian, J.; Han, J.; Xiaoqin, N. A Study on Physiological and Biochemical Changes in Storing Zoysiagrass Seed. Acta Agrestia Sin. 2000, 8, 177–185. [Google Scholar]
- Basavarajappa, B.S.; Shetty, H.S.; Prakash, H.S. Membrane deterioration and other biochemical changes associated with accelerated ageing of maize seeds. Seed Sci. Technol. 1991, 19, 279–286. [Google Scholar]
- Zhang, H.; Meng, S.; Xianghui, K. Study on the Physiologica-l biochemical Characteristics of Welsh Onion (Allium f istulosum L.) Seed Under Ultra-low Moisture Content. Acta Agric. Boreali-Sin. 2001, 16, 47. [Google Scholar]
- Wei, Z.; Zhiqing, M.; Chunping, W.; Zhenghong, W.; Xiupu, G. Effect of Storage Time on Germination Characteristic of Breeder Seed Wheat. Seed 2007, 26, 67–69. [Google Scholar]
- Ji, J.; Meng, C.; Qingfei, H. Influence of Different Aging Times on Physiological and Biochemical Characteristics of Cotton. Seed 2017, 36, 14–17. [Google Scholar]
- Sui, X.; Xing, L.-W.; Yu, H.-J.; Yu, Y.; Lu, H.-K.; Jing, G. Effects of the Artificial Aging on Physiological and Biochemical Indexes of American Ginseng Seeds with Different Water Content. Spec. Wild Econ. Anim. Plant Res. 2022, 44, 95–100. [Google Scholar]
- Effect of Soluble Sugars and Gibberellic Acid in Breaking Dormancy of Excised Wild Oat (Avena fatua) Embryos. Weed Sci. 1992, 40, 2.
- Huandi, Y. Approach to Physiological Mechanism of Vegetable Seed Longevity. Master’s Thesis, Southwestern University, Georgetown, NJ, USA, 2008. [Google Scholar]
- Hourston, J.E.; Perez, M.; Gawthrop, F.; Richards, M.; Steinbrecher, T.; Leubner-Metzger, G. The effects of high oxygen partial pressure on vegetable Allium seeds with a short shelf-life. Planta 2020, 251, 9. [Google Scholar] [CrossRef] [PubMed]
- Company, T.; Soriano, P.; Estrelles, E.; Mayoral, O. Seed bank longevity and germination ecology of invasive and native grass species from Mediterranean wetlands. Folia Geobot. 2019, 54, 151–161. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, H.; Zhang, Y.; Zheng, Y.; Wang, S.; Peishen, M. Study on Viability and physiological Characteristics of Aohan Alfalfa Seeds with Different Storage Time. Seed 2017, 36, 23–27+32. [Google Scholar]
- Chen, J.; Li, J.; Li, R. Physiological and Biochemical Characteristics of Waxy Maize Seeds during Artificial Aging. Acat Agric. Boreali Occident. Sinica 2016, 25, 857–862. [Google Scholar]
- Pawłat, J.; Starek-Wójcicka, A.; Kopacki, M. Germination Energy, Germination Capacity and Microflora of Allium cepa L. Seeds after RF Plasma Conditioning. Energies 2022, 15, 7687. [Google Scholar] [CrossRef]
- Hong, W. Optimization of TTC method for seed viability determination of Isatis indigotica. J. Zhejiang Agric. Sci. 2022, 63, 1465–1468. [Google Scholar]
- Lei, Z. Population Characteristics and Seed’s Biology of Paeonia ludlowii. Master’s Thesis, Beijing Forestry University, Beijing, China, 2008. [Google Scholar]
- Xuemei, R.; Wente, W.; Hongyun, T.; Chuanjing, Z.; Haihong, Z. Determination of Malondiadehyde Content in Duck Oil by Colorimetry. Shandong Agric. Sci. 2014, 46, 117–119. [Google Scholar]
- Yue, S.Y.; Zhou, R.R.; Nan, T.G.; Huang, L.Q.Y.Y. Comparison of major chemical components in Puerariae Thomsonii Radix and Puerariae Lobatae Radix. Zhongguo Zhong Yao Za Zhi 2022, 47, 2689–2697. [Google Scholar]
- Grintzalis, K.; Georgiou, C.D.; Schneider, Y.-J. An accurate and sensitive Coomassie Brilliant Blue G-250-based assay for protein determination. Anal. Biochem. 2015, 480, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Quanping, S. High Temperature and Its Duration: Effect on SOD and POD Activity of Larix principis-rupprechtii. Chin. Agric. Sci. Bull. 2017, 34, 33–38. [Google Scholar]
- Li, Z.; Xiong, D.; Dan, H. Study on the enzymatic properties of wheat germinating seed amylase. Sci. Technol. Innov. 2017, 13, 64–65. [Google Scholar]
- Mira, S.; Estrelles, E.; Gonzalez-Benito, M.E. Effect of water content and temperature on seed longevity of seven Brassicaceae species after 5 years of storage. Plant Biol. 2015, 17, 153–162. [Google Scholar] [CrossRef]
- Yan, H.; Xia, F.; Mao, P. Research Progress of Seed Aging and Vigor Repair. Chin. Agric. Sci. Bull. 2014, 30, 20–26. [Google Scholar]
- Liava, V.; Ntatsi, G.; Karkanis, A. Seed Germination of Three Milk Thistle (Silybum marianum (L.) Gaertn.) Populations of Greek Origin: Temperature, Duration, and Storage Conditions Effects. Plants 2023, 12, 1025. [Google Scholar] [CrossRef]
- Hidalgo, M.J.; Saavedra, M.; Garcíatorres, L. Germination of Phalaris species as affected by temperature and light. In Proceedings of the 1993 Congress of the Spanish Weed Science Society, Lugo, Spain, 1–3 December 1993. [Google Scholar]
- Ma, X.; He, C.; Luo, F.; Xu, W.; Duan, X. Effects of Hydro-priming on the Vigor of Setaria sphacelatacv. Narok’s Seeds in Different Storage Period. Chin. J. Grassl. 2017, 39, 16–23. [Google Scholar]
- Jianhua, L. Vitality of tomato seeds during storage. Beijing Agric. Sci. 1999, 17, 24–26. [Google Scholar]
- Jia, J.; Chengyi, M.A.; Hong, M. Dormancy characteristics of Eriochloa villosa seeds and methods to break them. Jiangsu Agric. Sci. 2017, 45, 88–91. [Google Scholar]
- Del Carmen Rodriguez, M.; Orozco-Segovia, A.; Sanchez-Coronado, M.E.; Sanchez-Coronado, C.V.-Y. Seed germination of six mature neotropical rain forest species in response to dehydration. Tree Physiol. 2000, 20, 693–699. [Google Scholar] [CrossRef]
- Leon-Gonzalez, R. Genetic and physiological characterization of seed dormancy regulation in common waterhemp [Amaranthus tuberculatus (Moq.) Sauer.]. Master’s Thesis, Iowa State University, Ames, IA, USA, 2005. [Google Scholar]
- Debieu, M.; Tang, C.; Stich, B.; Sikosek, T.; Effgen, S.; Josephs, E.; Schmitt, J.; Nordborg, M.; Koornneef, M.; de Meaux, J. Co-Variation between Seed Dormancy, Growth Rate and Flowering Time Changes with Latitude in Arabidopsis thaliana. PloS ONE 2013, 8, e61075. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Huang, X.; Xu, Q. Effects of Artificial Aging on Physiological and Biochemical Characteristics of Brassicaalboglabra Bailey Seeds. Seed 2020, 39, 24–29. [Google Scholar]
- Fang, J.; Zhu, Y.; Wang, C.; Ye, K.K.; Gao, W.-D.; Zhang, H.-H.; Yan, J.-J.; Li, Q.-M. Physiological and Biochemical Changes of Toona sinensis Seeds During Artificial Aging. For. Res. 2020, 33, 163–169. [Google Scholar]
- Hebing, W.; Zhimin, W.; Qinglin, T.; Ming, S.; Zijian, S. Study on the Correlation between Physiological Indexes and Vigor of Artificially Aged Mustard Seed. Journal of Southwest University. Nat. Sci. Ed. 2009, 31, 53–57. [Google Scholar]
- Long, J.; Zheng, Q.; Yang, Z.; Zhongren, A.E. Effect of Seed Longevity and Storage Material in Pugionium Gaertn at Different Storage Years. Seed 2017, 36, 15–20. [Google Scholar]
- Cai, Q.-H.; Huy, U.-Y.; Zhang, J.-F.; Xie, H.-A. Preliminary Study on Physiological Characteristics for Rice Seed After Aging. Fujian J. Agric. Sci. 2012, 27, 1061–1066. [Google Scholar]
- Cui, H.; Fei, W. A Study on the Physiological and Biochemical Regularities During Artificial Aging of Cucumber Seeds. J. Northwest A F Univ. 1992, 20, 51–54. [Google Scholar]
- Yuehui, L.; Denghua, W.; Hailong, H.; Xiumin, Y.; Aiqing, S. Physiological and Biochemical Analysis of Artificially Aged Pepper Seed. Seed 2003, 2, 51–52+87. [Google Scholar]
- Zhang, T. Physiological and Biochemical Changes During Artificial Aging of Rapeseed. J. Henan Norm. Univ. 1995, 23, 59–62. [Google Scholar]
- Dobiesz, M.; Piotrowicz-Cieślak, A.I.; Michalczyk, D.J. Physiological and Biochemical Parameters of Lupin Seed Subjected to 29 Years of Storage. Crop Sci. 2017, 57, 2149–2159. [Google Scholar] [CrossRef]
- Cui, K.; Wang, H.; Li, K.; Liao, S.; Li, L.; Zhang, C. Physiological and Biochemical Effects of Ultra-Dry Storage on Barbados Nut Seeds. Crop Sci. 2014, 54, 1748–1755. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Rui, H. Study on the relationship between malondialdehyde content and stress resistance in different alfalfa varieties. Heilongjiang Anim. Sci. Vet. Med. 2008, 8, 53–54. [Google Scholar]
- Lie, G.; Ye, L.; Xue, L. Effects of ozone stress on major plant physiological functions. Acta Ecol. Sin. 2014, 34, 294–306. [Google Scholar]
- Guanghua, Z. A brief discussion on the key issues of seed storage. Seed 1984, 4, 46–48. [Google Scholar]
- Yangchun, H. The Effect of Storage Time on Characteristics of pinus tabulaeformis Seed Germination and Physiological Changes. Prot. For. Sci. Technol. 2016, 3, 34–35. [Google Scholar]
- Chang, H.; Zhang, F.; Yang, Z.; Kong, D.; Zheng, Q.; Hao, L. Physiological and Biochemical Responses of Allium mongolicum Seeds to Storage Aging. Plant Physiol. J. 2015, 51, 1075–1081. [Google Scholar]
- Han, Y.; Jin, H.; Jia, Z.; Mi, F.; Guihua, W. Effects of seed artificial accelerated aging on physiological and biochemical characteristics of Elymus Sibiricus. J. Inn. Mong. Agric. Univ. 2017, 38, 22–29. [Google Scholar]
- Zacheo, G.; Cappello, A.R.; Perrone, L.M. Analysis of Factors Influencing Lipid Oxidation of Almond Seeds during Accelerated Ageing. LWT 1998, 31, 6–9. [Google Scholar] [CrossRef]
- Li, S.; Han, W.; Zhang, K.; Yi, Y. Effect of Different Tillage Methods on Soil Structure and Maize Root Distribution in Cinnamon Soil Area in Western Liaoning. J. Maize Sci. 2020, 28, 101–106. [Google Scholar]


| Storage Years | (1) Grain Number | (2) Grain Number | (3) Grain Number | Vigorous (%) |
|---|---|---|---|---|
| 0 | 27 | 23 | 25 | 83.88 ± 1.73 b |
| 1 | 30 | 26 | 28 | 93.34 ± 2.12 a |
| 2 | 22 | 23 | 17 | 70.00 ± 1.56 cd |
| 3 | 22 | 22 | 19 | 70.00 ± 1.69 cd |
| 4 | 18 | 20 | 23 | 66.67 ± 3.44 de |
| 5 | 17 | 14 | 16 | 53.33 ± 2.58 f |
| 6 | 18 | 21 | 19 | 63.33 ± 3.05 e |
| 8 | 11 | 12 | 9 | 36.67 ± 2.81 g |
| Index | MDA | Soluble Sugar | Soluble Protein | POD | CAT | AMS |
|---|---|---|---|---|---|---|
| Germination rate | −0.991 ** | 0.910 * | 0.044 | 0.851 | 0.843 | 0.851 * |
| Germination potential | −0.984 ** | 0.944 * | −0.003 | 0.882 ** | 0.872 | 0.902 * |
| Germination index | −0.941 * | 0.483 | −0.645 | 0.886 ** | 0.875 | 0.972 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Gao, H.; Wang, Y.; Zhang, L.; Jia, J.; Ma, H. Storage Time Affects the Viability, Longevity, and Germination of Eriochloa villosa (Thunb.) Kunth Seeds. Sustainability 2023, 15, 8576. https://doi.org/10.3390/su15118576
Han Y, Gao H, Wang Y, Zhang L, Jia J, Ma H. Storage Time Affects the Viability, Longevity, and Germination of Eriochloa villosa (Thunb.) Kunth Seeds. Sustainability. 2023; 15(11):8576. https://doi.org/10.3390/su15118576
Chicago/Turabian StyleHan, Yujun, Hong Gao, Yuechao Wang, Liguo Zhang, Jinrong Jia, and Hong Ma. 2023. "Storage Time Affects the Viability, Longevity, and Germination of Eriochloa villosa (Thunb.) Kunth Seeds" Sustainability 15, no. 11: 8576. https://doi.org/10.3390/su15118576
APA StyleHan, Y., Gao, H., Wang, Y., Zhang, L., Jia, J., & Ma, H. (2023). Storage Time Affects the Viability, Longevity, and Germination of Eriochloa villosa (Thunb.) Kunth Seeds. Sustainability, 15(11), 8576. https://doi.org/10.3390/su15118576





