The Sorption Behaviors of Barium during Reinjection of Gas Field Produced Water into Sandstone Reservoir: An Experimental Water-Rock Interaction Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Rock Samples
2.2. Water-Rock Interactions Experiments
3. Experimental Result and Discussion
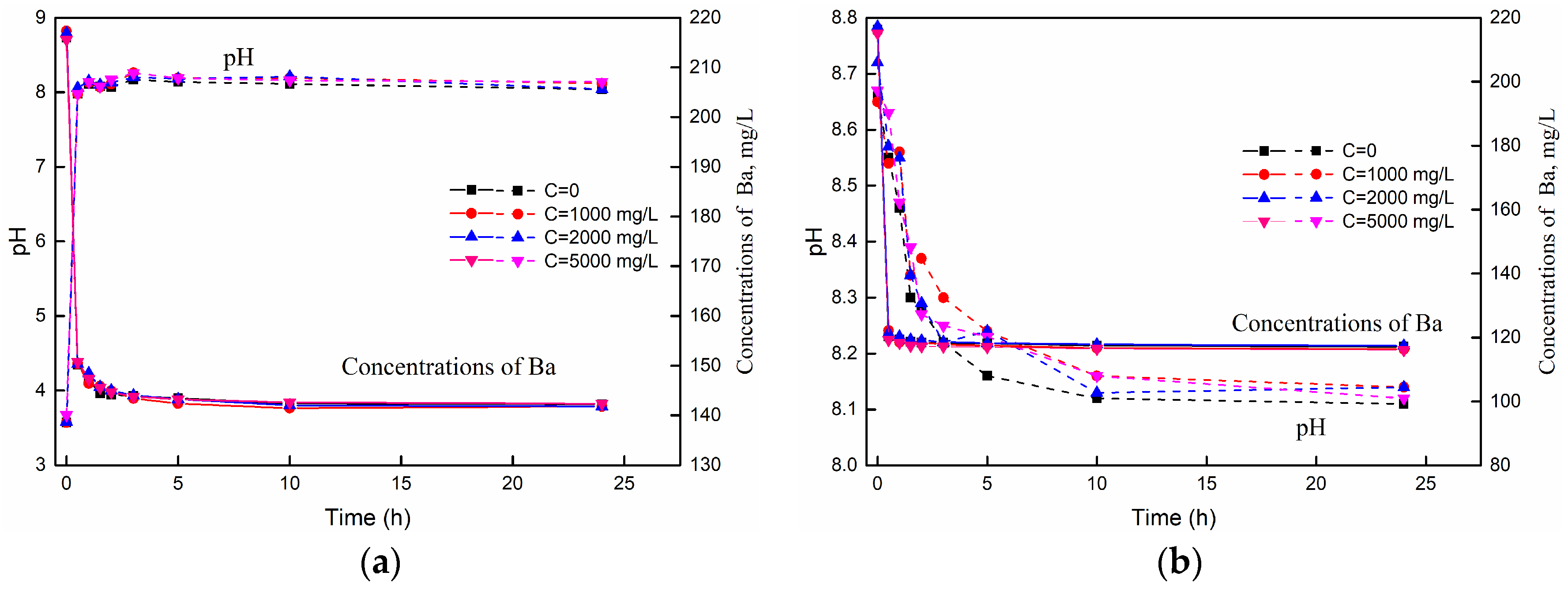
3.1. The Variations of pH and Barium Concentration with Time
3.2. Sorption Kinetics Fitting
3.3. Discussion
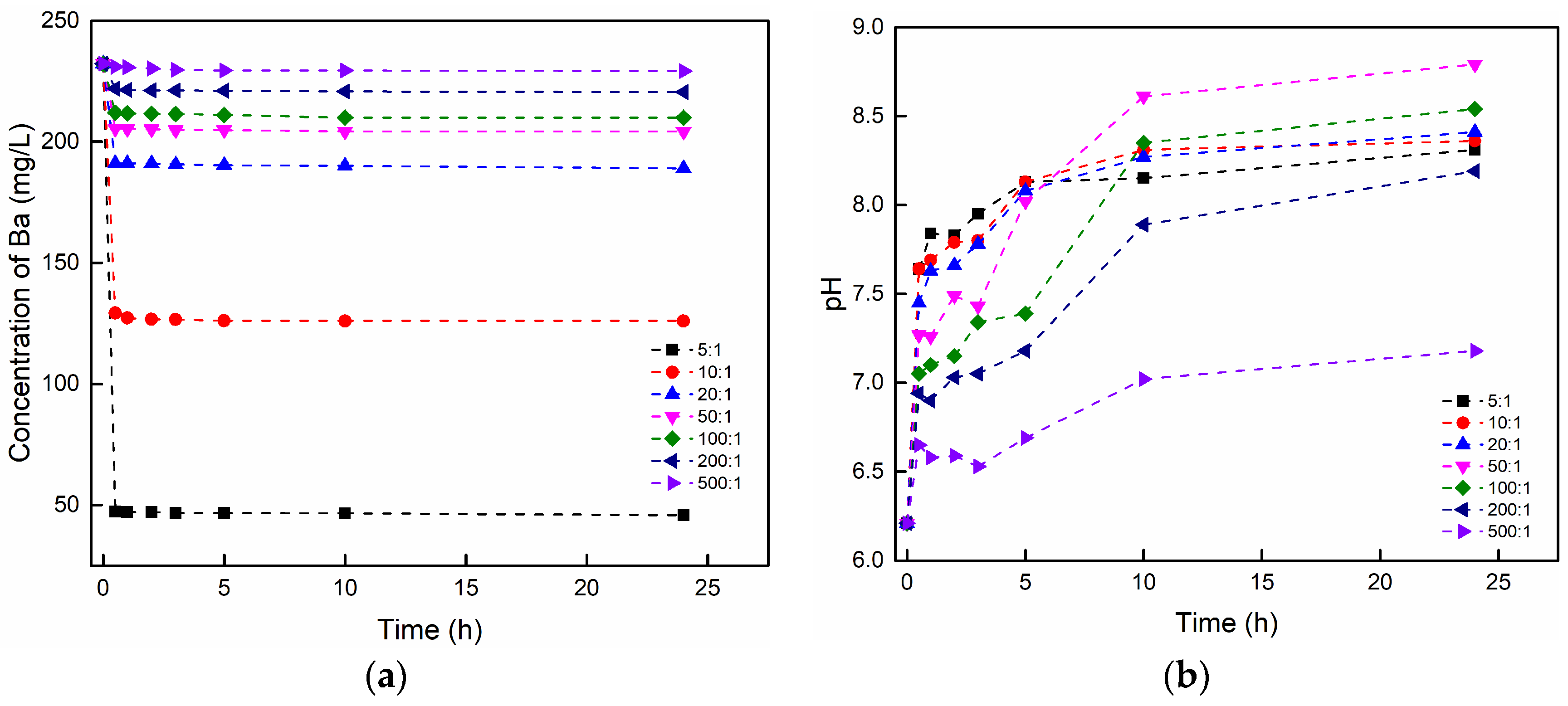
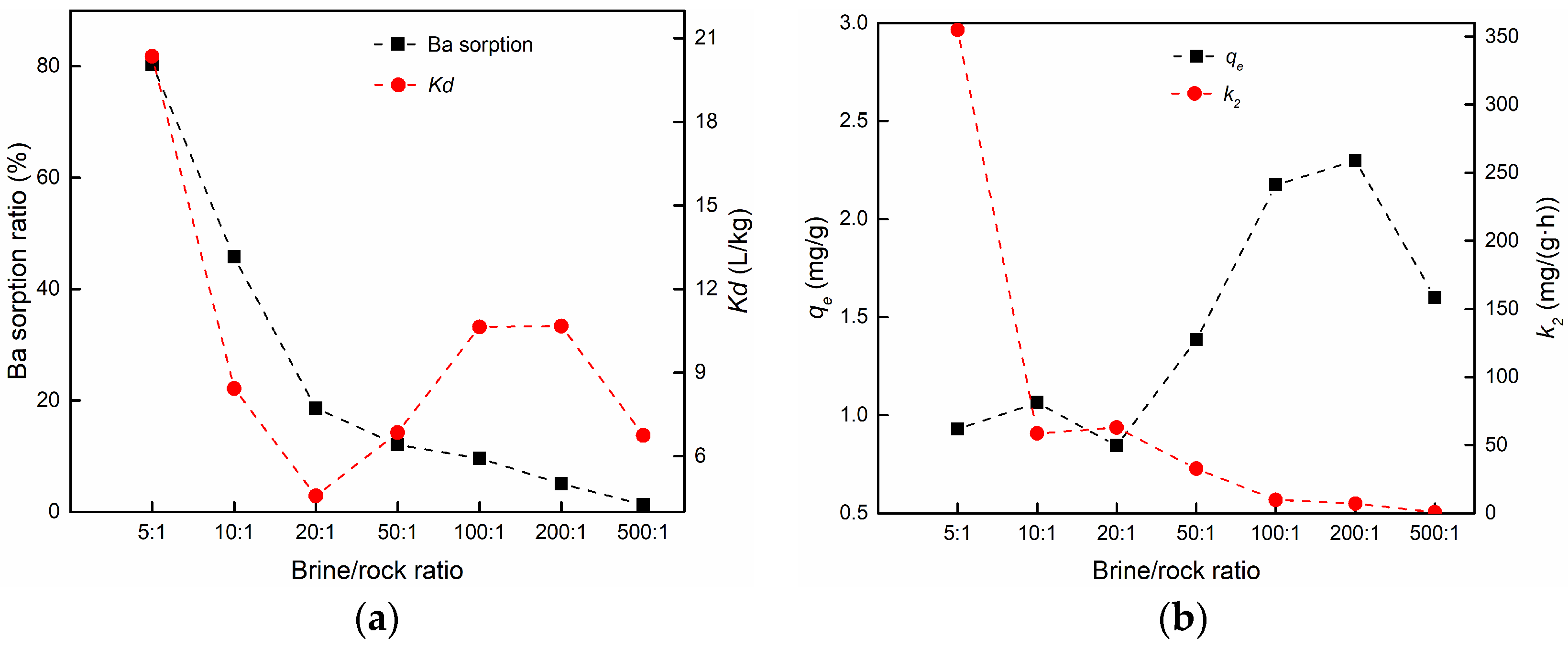
3.3.1. The Effect of the Brine/Rock Ratio
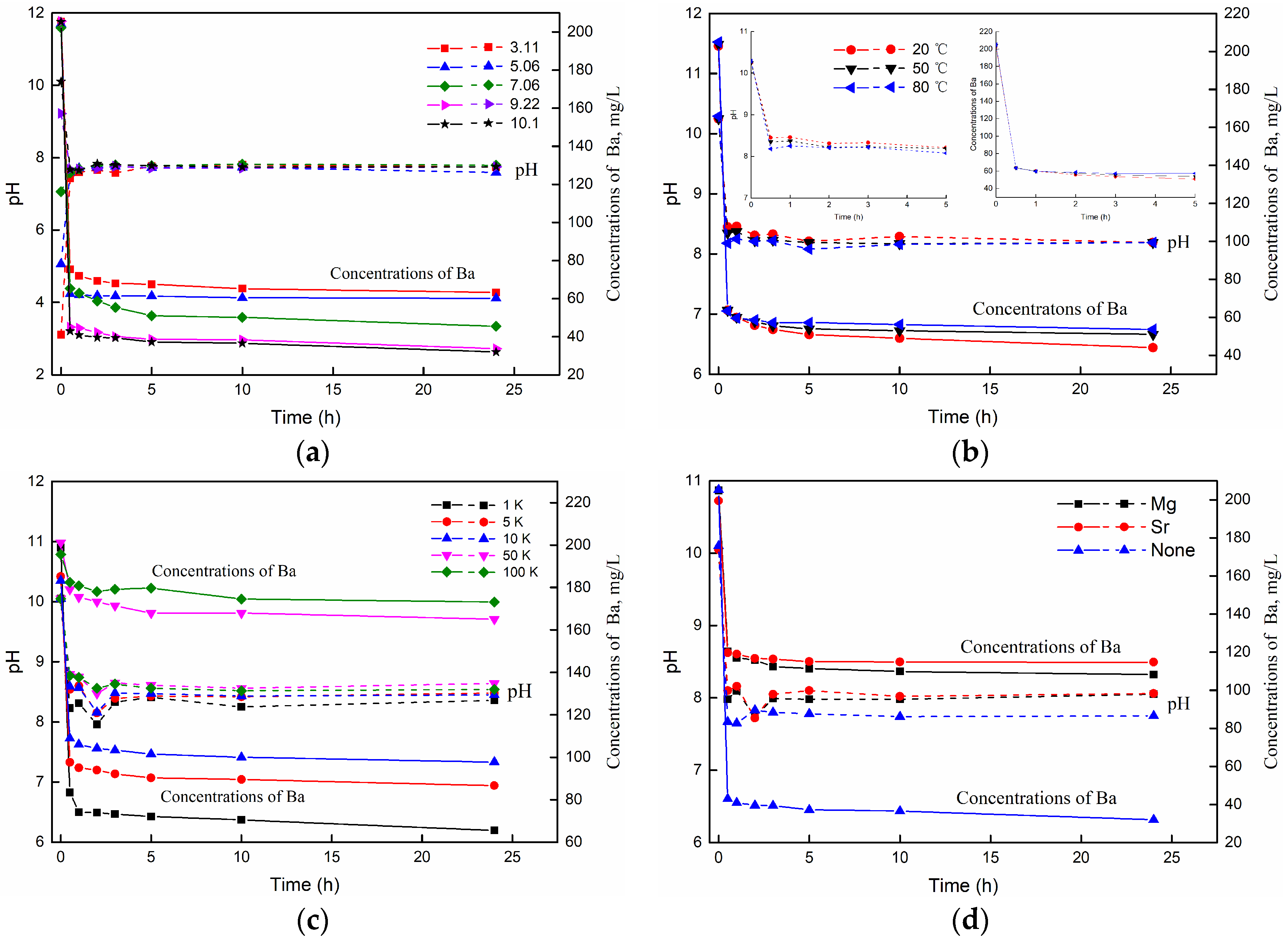
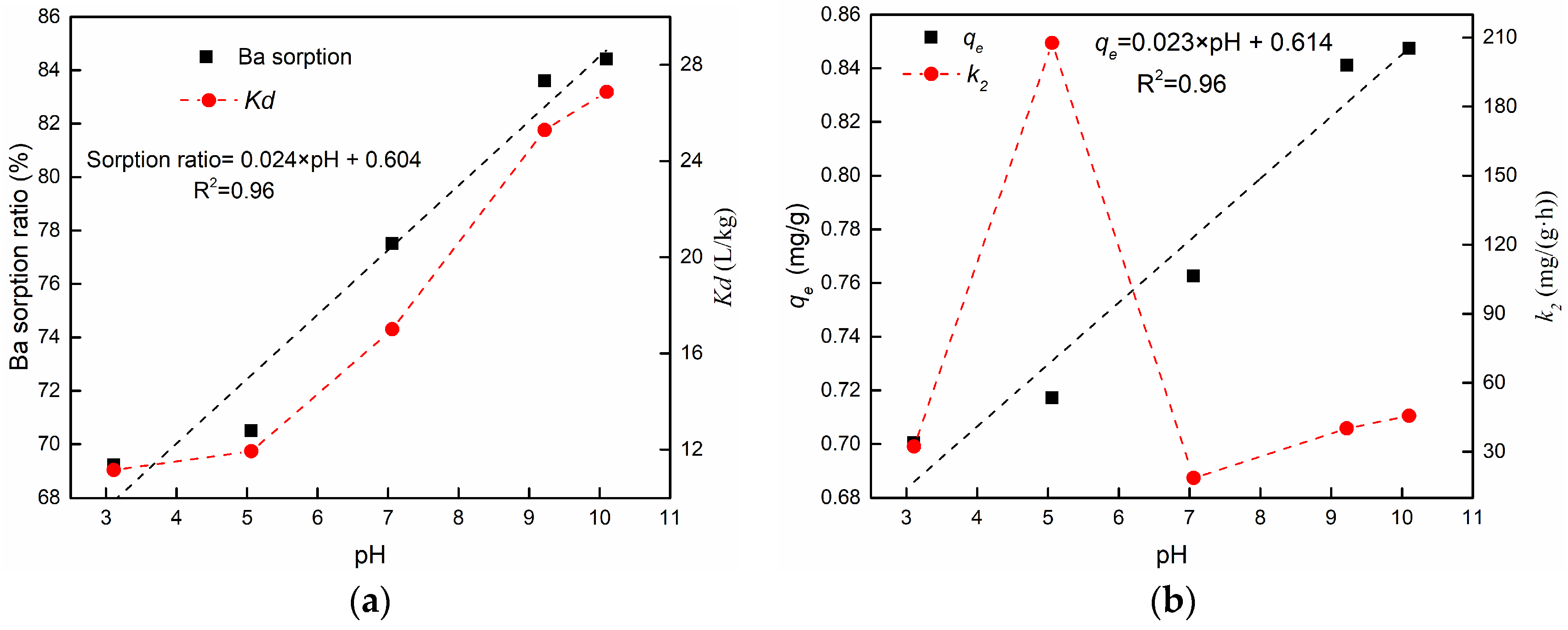
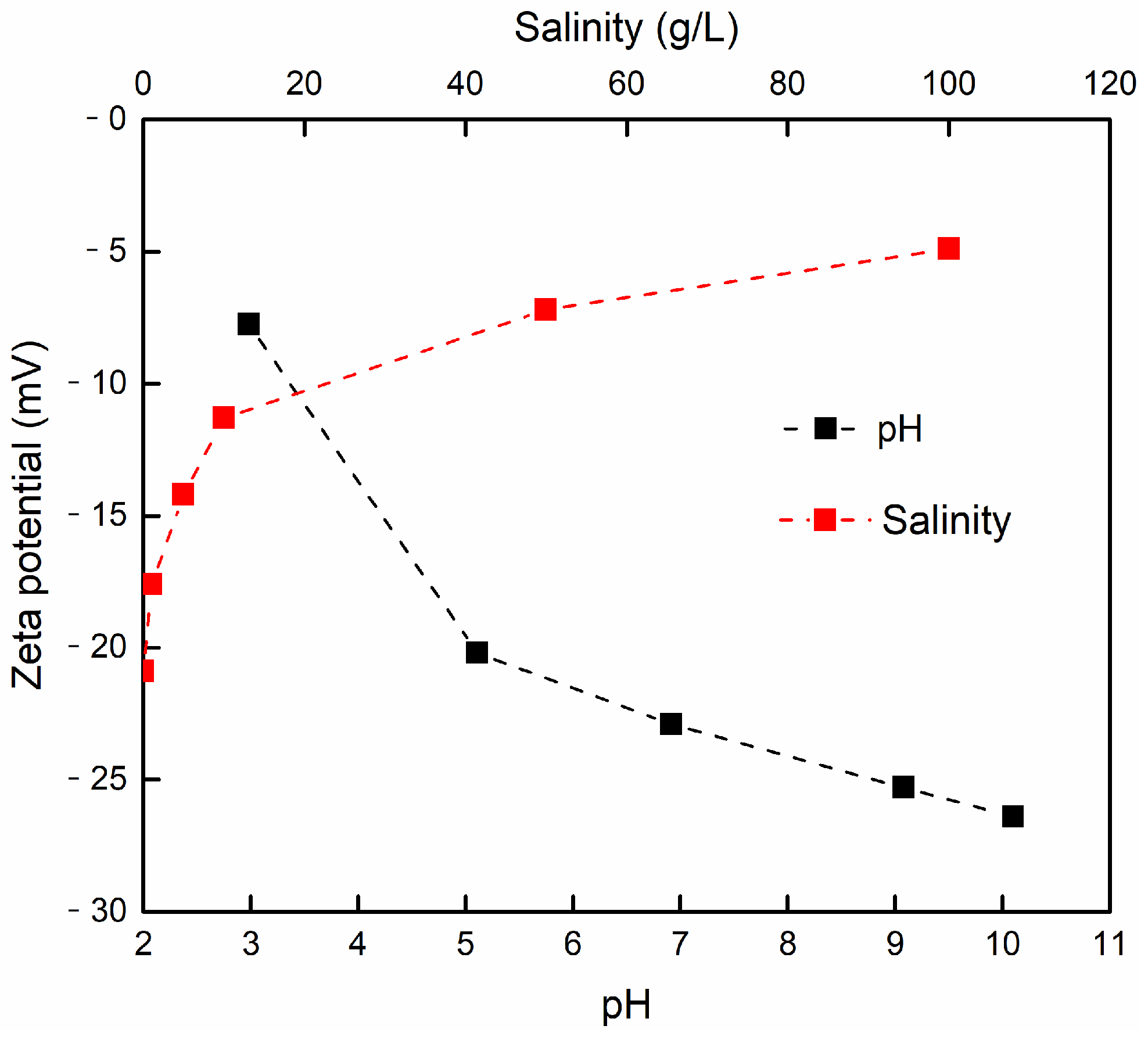
3.3.2. The Effect of pH
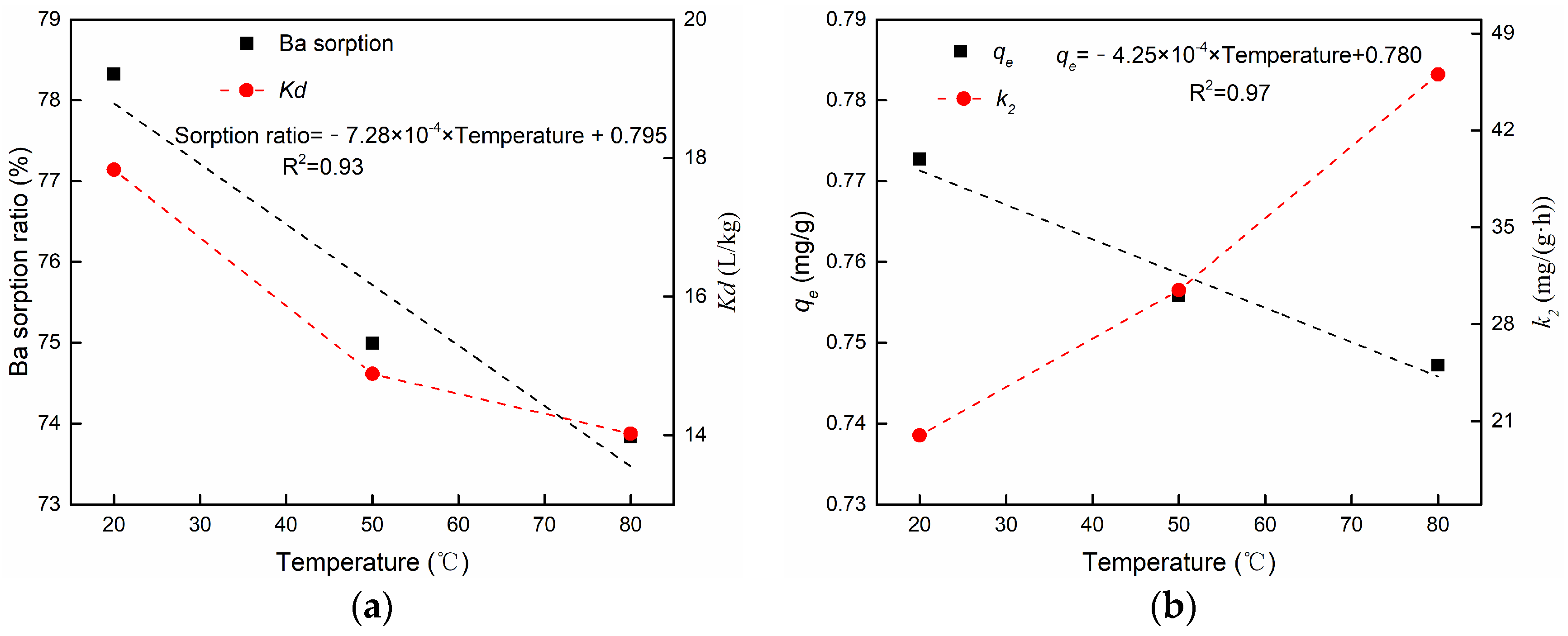
3.3.3. The Effect of Temperature
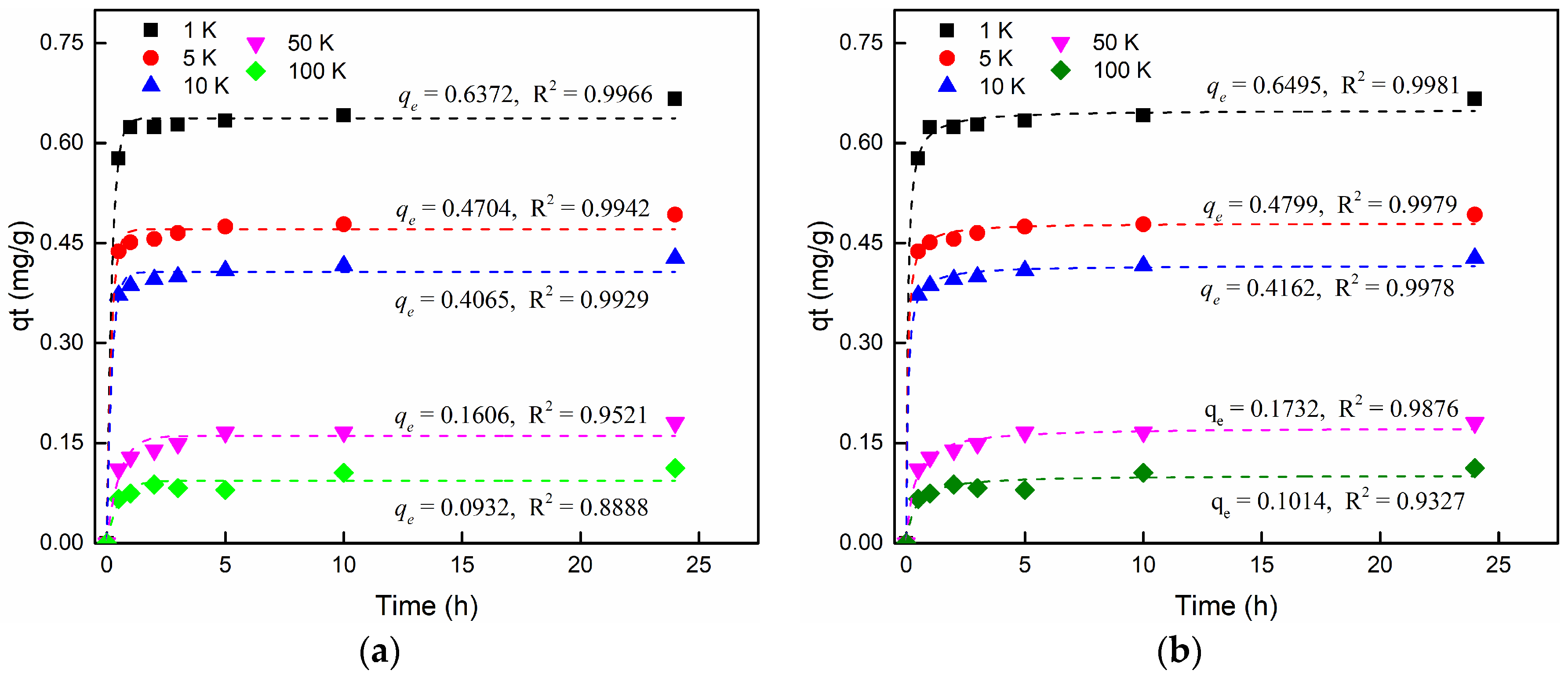
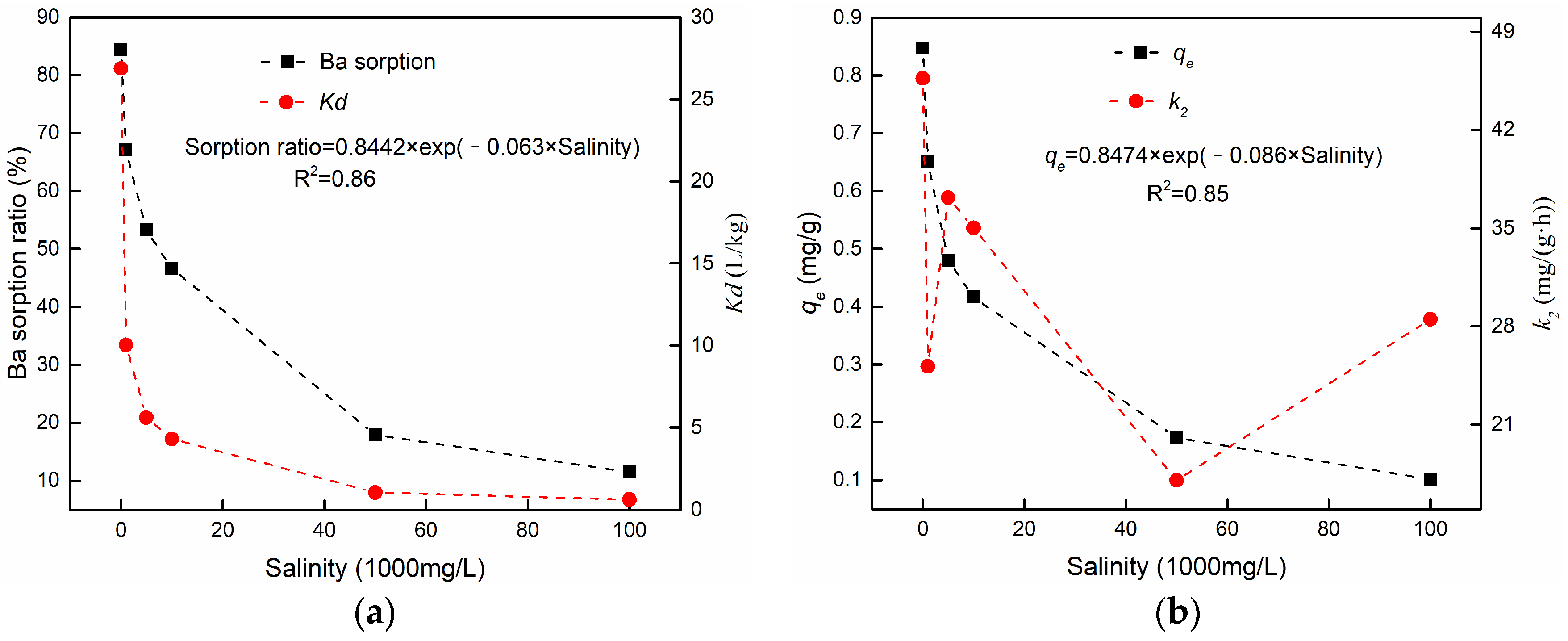
3.3.4. The Effect of Salinity
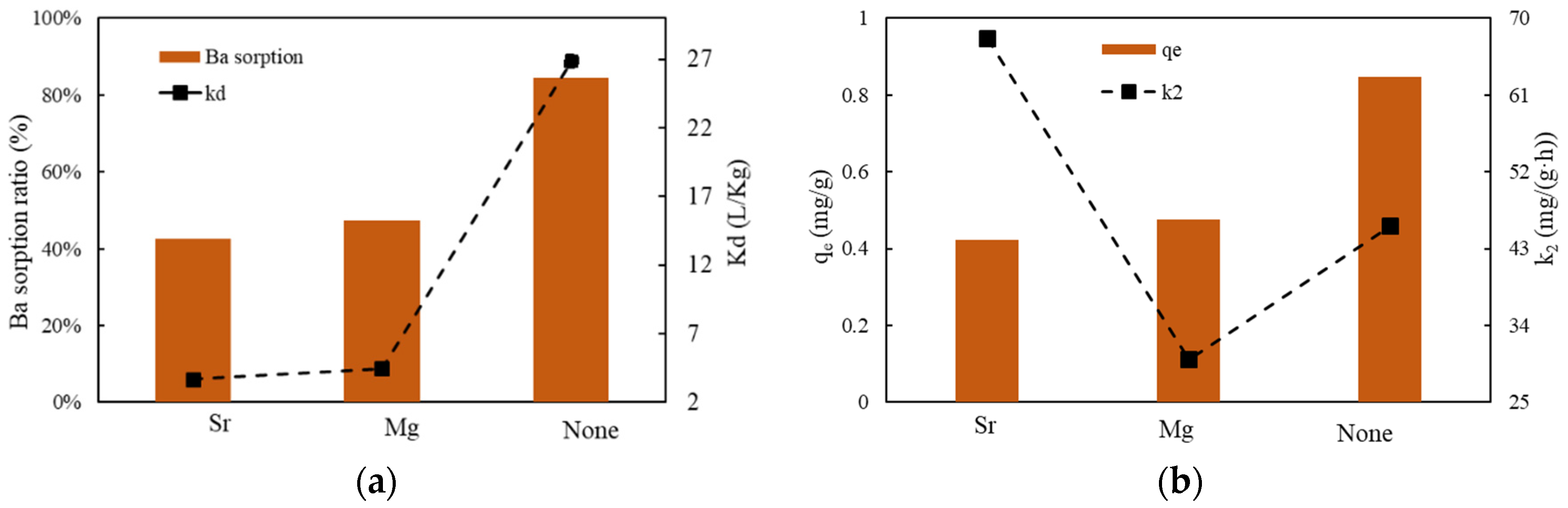
3.3.5. The Effect of Cations Competition
3.3.6. The Effect of Methanol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Zou, C.; Li, W.; Ni, Y.; Liao, F.; Yao, L.; Sui, J.; Vengosh, A. Hydrochemistry of flowback water from Changning shale gas field and associated shallow groundwater in Southern Sichuan Basin, China: Implications for the possible impact of shale gas development on groundwater quality. Sci. Total Environ. 2020, 713, 136591. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, R.; Akhavan, A.; Pakseresht, S.; Wood, D.A. Global natural gas demand to 2025: A learning scenario development model. Energy 2021, 224, 120167. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Q. The coupling effects of pore structure and rock mineralogy on the pre-Darcy behaviors in tight sandstone and shale. J. Petrol. Sci. Eng. 2022, 218, 110945. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Q. Experimental investigation on the movability of water in shale nanopores: A case study of Carboniferous shale from the Qaidam Basin, China. Water Resour. Res. 2020, 56, e2019WR026973. [Google Scholar] [CrossRef]
- Gregory, K.B.; Vidic, R.D.; Dzombak, D.A. Water Management Challenges Associated with the Production of Shale Gas by Hydraulic Fracturing. Elements 2011, 7, 181–186. [Google Scholar] [CrossRef]
- Wang, C.; Su, Y.; Wang, W.; Li, L.; Zhang, L.; Wang, T. Water blocking damage evaluation and mitigation method in tight gas reservoirs. Energy Fuels 2022, 36, 10934–10944. [Google Scholar] [CrossRef]
- Kang, M.; Jackson, R.B. Salinity of deep groundwater in California: Water quantity, quality, and protection. Proc. Natl. Acad. Sci. USA 2016, 113, 7768–7773. [Google Scholar] [CrossRef]
- Lutz, B.D.; Lewis, A.N.; Doyle, M.W. Generation, transport, and disposal of wastewater associated with Marcellus shale gas development. Water Resour. Res. 2013, 49, 647–656. [Google Scholar] [CrossRef]
- Abdelrazeq, H.; Khraisheh, M.; Ashraf, H.M.; Ebrahimi, P.; Kunju, A. Sustainable innovation in membrane technologies for produced water treatment: Challenges and limitations. Sustainability 2021, 13, 6759. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Reedy, R.C.; Wolaver, B.D. Assessing cumulative water impacts from shale oil and gas production: Permian Basin case study. Sci. Total Environ. 2022, 811, 152306. [Google Scholar] [CrossRef]
- Cui, X.; Zheng, Z.; Zhang, H.; Zhang, C.; Li, X.; Zhu, P.; Chen, Z. Impact of water-rock interactions on indicators of hydraulic fracturing flowback fluids produced from the Jurassic shale of Qaidam Basin, NW China. J. Hydrol. 2020, 590, 125541. [Google Scholar] [CrossRef]
- Grant, F. Deep injection of waste water in the western Canada sedimentary basin. Groundwater 2014, 53, 187–194. [Google Scholar] [CrossRef]
- Waly, M.M.; Mickovski, S.B.; Thomson, C. Application of circular economy in oil and gas producedwater treatment. Sustainability 2023, 15, 2132. [Google Scholar] [CrossRef]
- Yin, W.; Hu, L.; Yao, L.; Teng, Y. Numerical investigation of the influence of underground water injection on the groundwater system in a shale gas reservoir in Southwestern China. Environ. Earth Sci. 2016, 75, 1092. [Google Scholar] [CrossRef]
- Huang, T.; Pang, Z.; Li, Z. A framework to determine sensitive inorganic monitoring indicators for tracing groundwater contamination by produced formation water from shale gas development in the Fuling Gasfield, SW China. J. Hydrol. 2020, 581, 124403. [Google Scholar] [CrossRef]
- Rahm, B.G.; Riha, S.J. Toward strategic management of shale gas development: Regional, collective impacts on water resources. Environ. Sci. Policy 2012, 17, 12–23. [Google Scholar] [CrossRef]
- Pearce, J.K.; Khan, C.; Golding, S.D.; Rudolph, V.; Underschultz, J. Geological storage of CO2 and acid gases dissolved at surface in production water. J. Petrol. Sci. Eng. 2022, 210, 110052. [Google Scholar] [CrossRef]
- Silva, T.L.S.; Morales-Torres, S.; Castro-Silva, S.; Figueiredo, J.L.; Silva, A.M. An overview on exploration and environmental impact of unconventional gas sources and treatment options for produced water. J. Environ. Manag. 2017, 200, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-L.; Wang, C.-W.; Chen, X.-F.; Jia, C.-S.; Chen, Z.; Zhou, F.-F.; Zhu, S.-Y. New insights into the beneficial use of coalbed-associated water: A feasibility study of co-produced water reinjection to coalbeds. J. Nat. Gas Sci. Eng. 2022, 103, 104622. [Google Scholar] [CrossRef]
- Frota, R.; Tanscheit, R.; Vellasco, M. Fuzzy logic for control of injector wells flow rates under produced water reinjection. J. Petrol. Sci. Eng. 2022, 215, 110574. [Google Scholar] [CrossRef]
- Kose, B.; Ozgun, H.; Ersahin, M.E.; Dizge, N.; Koseoglu-Imer, D.Y.; Atay, B.; Kaya, R.; Altınbas, M.; Sayılı, S.; Hoshan, P.; et al. Performance evaluation of a submerged membrane bioreactor for the treatment of brackish oil and natural gas field produced water. Desalination 2012, 285, 295–300. [Google Scholar] [CrossRef]
- Tan, B.; He, Z.; Fang, Y.; Zhu, L. Removal of organic pollutants in shale gas fracturing flowback and produced water: A review. Sci. Total Environ. 2023, 883, 163478. [Google Scholar] [CrossRef] [PubMed]
- Fard, A.K.; Mckay, G.; Chamoun, R.; Rhadfi, T.; Preud’Homme, H.; Atieh, M.A. Barium removal from synthetic natural and produced water using MXene as two dimensional (2-D) nanosheet adsorbent. Chem. Eng. J. 2017, 317, 331–342. [Google Scholar] [CrossRef]
- Underwood, E.C.; Ferguson, G.A.; Betcher, R.; Phipps, G. Elevated Ba concentrations in a sandstone aquifer. J. Hydrol. 2009, 376, 126–131. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Vilcáez, J. Effect of brine salinity and guar gum on the transport of barium through dolomite rocks: Implications for unconventional oil and gas wastewater disposal. J. Environ. Manag. 2018, 214, 370–378. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Vilcáez, J. Petroleum produced water disposal: Mobility and transport of barium in sandstone and dolomite rocks. Sci. Total Environ. 2018, 634, 1054–1063. [Google Scholar] [CrossRef]
- Ye, Z.; Prigiobbe, V. Effect of ionic strength on barium transport in porous media. J. Contam. Hydrol. 2018, 209, 24–32. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Vilcaez, J. Transport of barium in fractured dolomite and sandstone saline aquifers. Sci. Total Environ. 2019, 647, 323–333. [Google Scholar] [CrossRef]
- Vilcaez, J. Reactive transport modeling of produced water disposal into dolomite saline aquifers: Controls of barium transport. J. Contam. Hydrol. 2020, 233, 103600. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Q. CO2 breakthrough pressure and permeability for unsaturated low-permeability sandstone of the Ordos Basin. J. Hydrol. 2017, 550, 331–342. [Google Scholar] [CrossRef]
- Liu, H.; Hou, Z.; Were, P.; Gou, Y.; Sun, X. Simulation of CO2 plume movement in multilayered saline formations through multilayer injection technology in the Ordos Basin, China. Environ. Earth Sci. 2014, 71, 4447–4462. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.; Agofack, N.; Gawel, K.M.; Cerasi, P.R. Micro-and macroscale consequences of interactions between CO2 and shale rocks. Energies 2020, 13, 1167. [Google Scholar] [CrossRef]
- Sharma, H.; Mohanty, K.K. An experimental and modeling study to investigate brine-rock interactions during low salinity water flooding in carbonates. J. Petrol. Sci. Eng. 2018, 165, 1021–1039. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Q. The role of fluid-rock interactions in permeability behavior of shale with different pore fluids. Int. J. Rock Mech. Min. Sci. 2022, 150, 105023. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, J.; Kamali, F.; Othman, F.; Wang, Y.; Le-Hussain, F. Wettability of sandstone rocks and their mineral components during CO2 injection in aquifers: Implications for fines migration. J. Nat. Gas Sci. Eng. 2019, 73, 103050. [Google Scholar] [CrossRef]
- Tanikawa, W.; Shimamoto, T. Comparison of Klinkenberg-corrected gas permeability and water permeability in sedimentary rocks. Int. J. Rock Mech. Min. Sci. 2009, 46, 229–238. [Google Scholar] [CrossRef]
- Vinogradov, J.; Jackson, M.D. Zeta potential in intact natural sandstones at elevated temperatures. Geophys. Res. Lett. 2015, 42, 6287–6294. [Google Scholar] [CrossRef]
- Awan, F.U.R.; Al-Yaseri, A.; Akhondzadeh, H.; Iglauer, S.; Keshavarz, A. Influence of mineralogy and surfactant concentration on zeta potential in intact sandstone at high pressure. J. Colloid Interface Sci. 2022, 607, 401–411. [Google Scholar] [CrossRef]
- Arif, M.; Abu-Khamsin, S.; Iglauer, S. Wettability of rock/CO2/brine and rock/oil/CO2-enriched-brine systems: Critical parametric analysis and future outlook. Adv. Colloid Interface Sci. 2019, 268, 91–113. [Google Scholar] [CrossRef]
- Fauziah, C.A.; Al-Yaseri, A.Z.; Beloborodov, R.; Siddiqui, M.A.Q.; Lebedev, M.; Parsons, D.F.; Roshan, H.; Barifcani, A.; Iglauer, S. Carbon dioxide/brine, nitrogen/brine and oil/brine wettability of montmorillonite, illite and kaolinite at elevated pressure and temperature. Energy Fuels 2019, 33, 441–448. [Google Scholar] [CrossRef]
- Mirchi, V.; Saraji, S.; Goual, L.; Piri, M. Dynamic interfacial tension and wettability of shale in the presence of surfactants at reservoir conditions. Fuel 2015, 148, 127–138. [Google Scholar] [CrossRef]
- Shehata, A.M.; Nasr-El-Din, H.A. Zeta Potential Measurements: Impact of Salinity on Sandstone Minerals. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 13–15 April 2015; SPE-173763-MS. pp. 1–17. [Google Scholar] [CrossRef]
- Kazempour, M.; Sundstrom, E.; Alvarado, V. Geochemical modeling and experimental evaluation of high-pH floods: Impact of water–rock interactions in sandstone. Fuel 2012, 92, 216–230. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Miao, X.; Gan, M.; Li, X. Geochemistry in geologic CO2 utilization and storage: A brief review. Adv. Geo-Energy Res. 2019, 3, 304–313. [Google Scholar] [CrossRef]
- Kango, S.; Kumar, R. Low-cost magnetic adsorbent for As (III) removal from water: Adsorption kinetics and isotherms. Environ. Monit. Assess. 2016, 188, 60. [Google Scholar] [CrossRef] [PubMed]
- Sauve, S.; Hendershot, W.; Allen, H.E. Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environ. Sci. Technol. 2000, 34, 1125–1131. [Google Scholar] [CrossRef]
- Wissocq, A.; Beaucaire, C.; Latrille, C. Application of the multi-site ion exchanger model to the sorption of Sr and Cs on natural clayey sandstone. Appl. Geochem. 2017, 98, 167–177. [Google Scholar] [CrossRef]
- Lu, J.; Mickler, P.J.; Nicot, J.P.; Choi, W.; Esch, W.L.; Darvari, R. Geochemical interactions of shale and brine in autoclave experiments-Understanding mineral reactions during hydraulic fracturing of Marcellus and Eagle Ford Shales. AAPG Bull. 2017, 101, 1567–1597. [Google Scholar] [CrossRef]
- Tessier, A.; Carignan, R.; Dubreuil, B.; Rapin, F. Partitioning of zinc between the water column and the oxic sediments in lakes. Geochim. Cosmochim. Acta 1989, 53, 1511–1522. [Google Scholar] [CrossRef]
- Walker, E.; Glover, P. Measurements of the relationship between microstructure, pH, and the streaming and zeta potentials of sandstones. Transp. Porous Med. 2018, 121, 183–206. [Google Scholar] [CrossRef]
- Johnson, B.B. Effect of pH, temperature, and concentration on the adsorption of cadmium on goethite. Environ. Sci. Technol. 1990, 24, 112–118. [Google Scholar] [CrossRef]
- Shahwan, T.; Suzer, S.; Erten, H.N. Sorption of Cs+ and Ba2+ cations on magnesite. Appl. Radiat. Isot. 1998, 49, 915–921. [Google Scholar] [CrossRef]
- Arias, F.; Sen, T.K. Removal of zinc metal ion (Zn2+) from its aqueous solution by kaolin clay mineral: A kinetic and equilibrium study. Colloid Surface A 2009, 348, 100–108. [Google Scholar] [CrossRef]
- Fan, Q.; Shao, D.; Lu, Y.; Wu, W.; Wang, X. Effect of pH, ionic strength, temperature and humic substances on the sorption of Ni (II) to Na-attapulgite. Chem. Eng. J. 2009, 150, 188–195. [Google Scholar] [CrossRef]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, P.; Borrok, D.M. Mobility of Ba, Sr, Se and as under simulated conditions of produced water injection in dolomite. Appl. Geochem. 2020, 118, 104640. [Google Scholar] [CrossRef]
- Hatje, V.; Payne, T.; Hill, D.; McOrist, G.; Birch, G.; Szymczak, R. Kinetics of trace element uptake and release by particles in estuarine waters: Effects of pH, salinity, and particle loading. Environ. Int. 2003, 29, 619–629. [Google Scholar] [CrossRef]
- Vinogradov, J.; Jaafar, M.Z.; Jackson, M.D. Measurement of streaming potential coupling coefficient in sandstones saturated with natural and artificial brines at high salinity. J. Geophys. Res. 2010, 115, B12204. [Google Scholar] [CrossRef]
- Van Cappellen, P.; Charlet, L.; Stumm, W.; Wersin, P. A surface complexation model of the carbonate mineral-aqueous solution interface. Geochim. Cosmochim. Acta 1993, 57, 3505–3518. [Google Scholar] [CrossRef]
- Strawn, D.G.; Sparks, D.L. Effects of soil organic matter on the kinetics and mechanisms of Pb (II) sorption and desorption in soil. Soil Sci. Soc. Am. J. 2000, 64, 144–156. [Google Scholar] [CrossRef]

| Types | Quartz | Plagioclase | Microcline | Chlorite | Mica | Calcite |
|---|---|---|---|---|---|---|
| Chemical formula (a) | SiO2 | (Na, Ca), Al(Al, Si)Si2O8 | KAlSi3O8 | (Fe, Mg)5Al2Si3O10(OH)8 | KAl2(AlSi3O10)(OH)2 | CaCO3 |
| Content | 38% | 39% | 11% | 4% | 4% | 4% |
| Types | SiO2 | Al2O3 | CO2 | Fe2O3 | Na2O | CaO | K2O | MgO | TiO2 | P2O5 | BaO | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 56.1 | 15.5 | 7.9 | 4.9 | 4.1 | 3.9 | 3.4 | 3.1 | 0.62 | 0.19 | 0.1 | 0.19 |
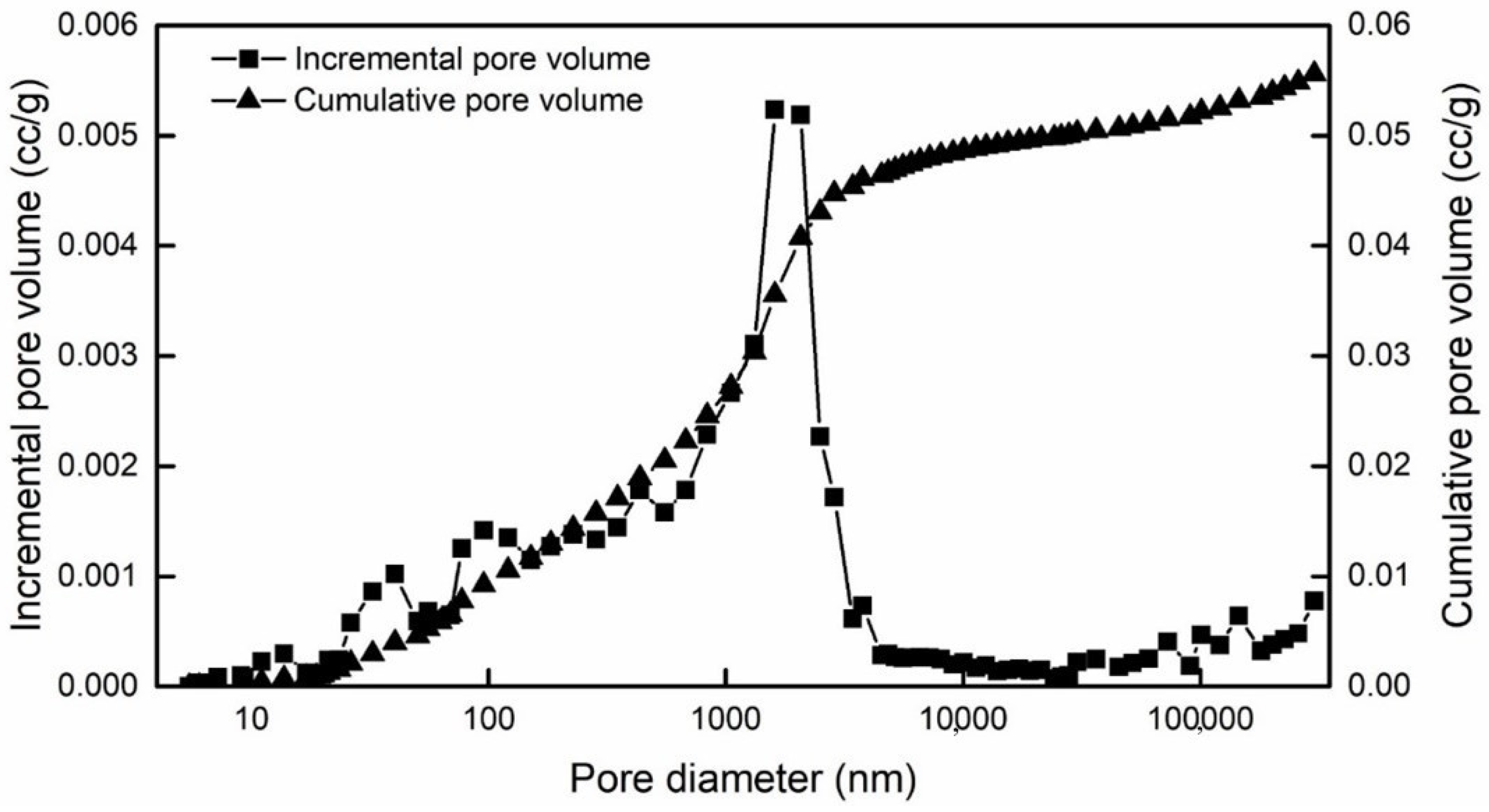
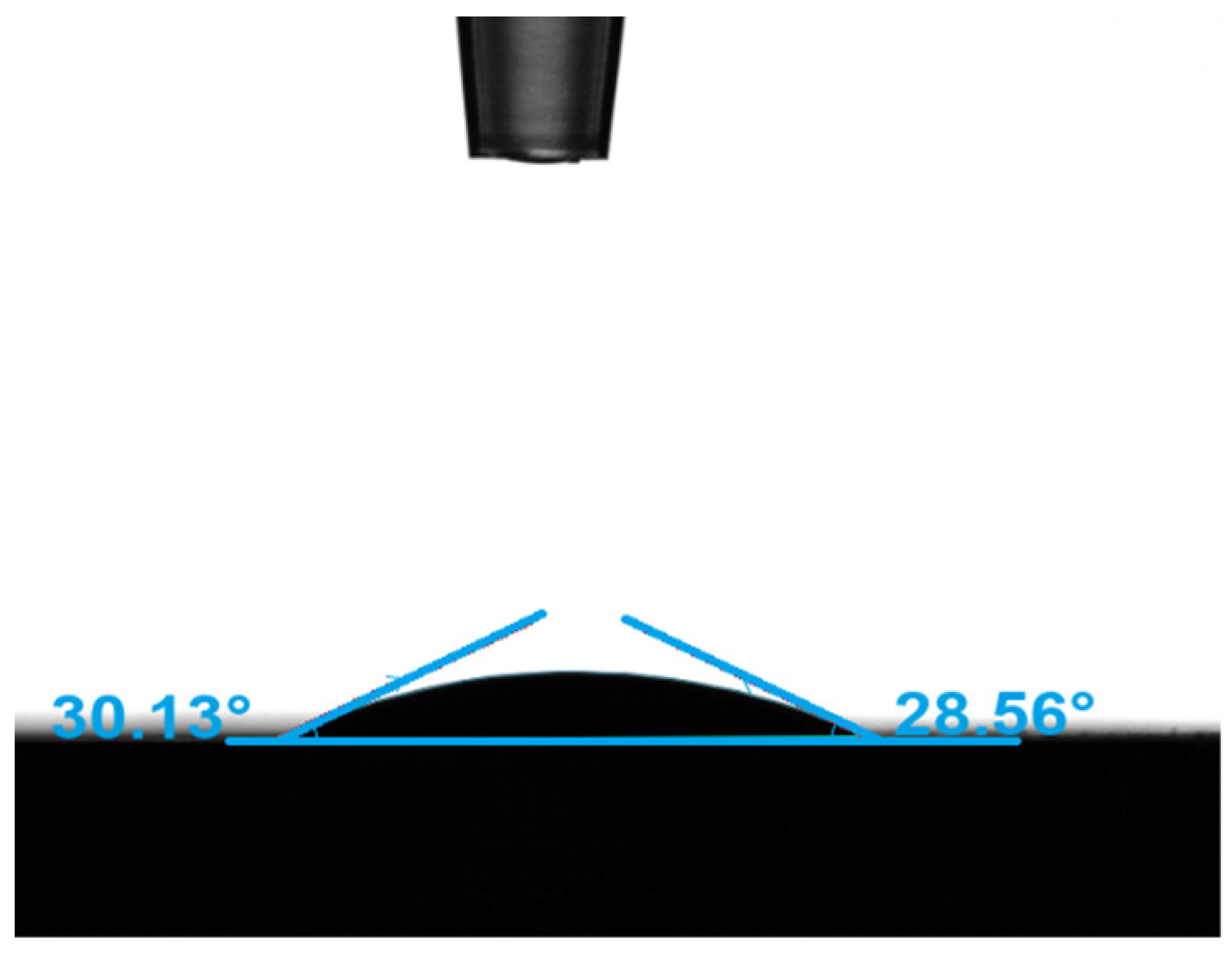
| Types | Most Probable Pore Diameter | Cumulative Specific Surface Area | Porosity | Average Contact Angle | Water Permeability | Zeta Potential |
|---|---|---|---|---|---|---|
| Value | 1.4 μm | 1.17 m2/g | 12.8% | 29.35° | 0.24 mD | −20.9 mV |
| NO. | Purpose | Rock (g) | Brine (mL) | Brine/Rock Ratio | Brine Composition (K = 1000 mg/L) | pH | Temperature (°C) |
|---|---|---|---|---|---|---|---|
| 1 | Effect of Brine/rock ratio | 20 | 100 | 5:1 | 0.2 K BaCl2 | 6.21 | 20 |
| 2 | 20 | 200 | 10:1 | 0.2 K BaCl2 | 6.21 | 20 | |
| 3 | 10 | 200 | 20:1 | 0.2 K BaCl2 | 6.21 | 20 | |
| 4 | 4 | 200 | 50:1 | 0.2 K BaCl2 | 6.21 | 20 | |
| 5 | 2 | 200 | 100:1 | 0.2 K BaCl2 | 6.21 | 20 | |
| 6 | 1 | 200 | 200:1 | 0.2 K BaCl2 | 6.21 | 20 | |
| 7 | 1 | 500 | 500:1 | 0.2 K BaCl2 | 6.21 | 20 | |
| 8 | Effect of pH | 20 | 100 | 5:1 | 0.2 K BaCl2 | 3.11 | 20 |
| 9 | 20 | 100 | 5:1 | 0.2 K BaCl2 | 5.06 | 20 | |
| 10 | 20 | 100 | 5:1 | 0.2 K BaCl2 | 7.06 | 20 | |
| 11 | 20 | 100 | 5:1 | 0.2 K BaCl2 | 9.22 | 20 | |
| 12 | 20 | 100 | 5:1 | 0.2 K BaCl2 | 10.1 | 20 | |
| 13 | Effect of Temperature | 20 | 100 | 5:1 | 0.2 K BaCl2 | 10.25 | 20 |
| 14 | 20 | 100 | 5:1 | 0.2 K BaCl2 | 10.25 | 50 | |
| 15 | 20 | 100 | 5:1 | 0.2 K BaCl2 | 10.25 | 80 | |
| 16 | Effect of Cations competition | 20 | 100 | 5:1 | 0.2 K BaCl2 + 0.5 K MgCl2 | 10.05 | 20 |
| 17 | 20 | 100 | 5:1 | 0.2 K BaCl2 + 0.5 K SrCl2 | 10.05 | 20 | |
| 18 | Effect of Salinity | 20 | 100 | 5:1 | 0.2 K BaCl2 + 1 K NaCl | 10.05 | 20 |
| 19 | 20 | 100 | 5:1 | 0.2 K BaCl2 + 5 K NaCl | 10.05 | 20 | |
| 20 | 20 | 100 | 5:1 | 0.2 K BaCl2 + 10 K NaCl | 10.05 | 20 | |
| 21 | 20 | 100 | 5:1 | 0.2 K BaCl2 + 50 K NaCl | 10.05 | 20 | |
| 22 | 20 | 100 | 5:1 | 0.2 K BaCl2 + 100 K NaCl | 10.05 | 20 | |
| 23 | Effect of Methanol | 20 | 200 | 10:1 | 0.2 K BaCl2 | 3.5 | 30 |
| 24 | 20 | 200 | 10:1 | 0.2 K BaCl2 + 1 K methanol | 3.5 | 30 | |
| 25 | 20 | 200 | 10:1 | 0.2 K BaCl2 + 2 K methanol | 3.5 | 30 | |
| 26 | 20 | 200 | 10:1 | 0.2 K BaCl2 + 5 K methanol | 3.5 | 30 | |
| 27 | 20 | 200 | 10:1 | 0.2 K BaCl2 | 8.5 | 30 | |
| 28 | 20 | 200 | 10:1 | 0.2 K BaCl2 + 1 K methanol | 8.5 | 30 | |
| 29 | 20 | 200 | 10:1 | 0.2 K BaCl2 + 2 K methanol | 8.5 | 30 | |
| 30 | 20 | 200 | 10:1 | 0.2 K BaCl2 + 5 K methanol | 8.5 | 30 |
| NO. | Experiments | Pseudo-First-Order Kinetics Model Parameters | Pseudo-Second-Order Kinetics Model Parameters | |||||
|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | K1 (1/h) | R2 | qe (mg/g) | K2 (mg/(g·h)) | R2 | |||
| 1 | Effect of brine/rock ratio | 5:1 | 0.9278 | 11.109 | 0.9999 | 0.9290 | 354.926 | 0.9999 |
| 2 | 10:1 | 1.0580 | 7.201 | 0.9998 | 1.0637 | 58.622 | 0.9999 | |
| 3 | 20:1 | 0.8377 | 7.800 | 0.9981 | 0.8445 | 62.813 | 0.9988 | |
| 4 | 50:1 | 1.3713 | 7.351 | 0.9982 | 1.3839 | 32.720 | 0.9991 | |
| 5 | 100:1 | 2.1326 | 5.832 | 0.9915 | 2.1728 | 9.768 | 0.9951 | |
| 6 | 200:1 | 2.2518 | 4.852 | 0.9953 | 2.2981 | 7.178 | 0.9984 | |
| 7 | 500:1 | 1.4512 | 0.751 | 0.9647 | 1.5992 | 0.671 | 0.9824 | |
| 8 | Effect of pH | 3.11 | 0.6900 | 5.590 | 0.9974 | 0.7006 | 32.298 | 0.9994 |
| 9 | 5.06 | 0.7153 | 9.386 | 0.9998 | 0.7172 | 207.711 | 0.9999 | |
| 10 | 7.06 | 0.7436 | 4.821 | 0.9905 | 0.7626 | 18.611 | 0.9967 | |
| 11 | 9.22 | 0.8310 | 6.687 | 0.9972 | 0.8410 | 40.144 | 0.9987 | |
| 12 | 10.1 | 0.8390 | 6.782 | 0.9979 | 0.8474 | 45.681 | 0.9990 | |
| 13 | Effect of temperature | 20 | 0.7552 | 4.892 | 0.9929 | 0.7727 | 19.980 | 0.9978 |
| 14 | 50 | 0.7442 | 5.568 | 0.9974 | 0.7558 | 30.455 | 0.9995 | |
| 15 | 80 | 0.7396 | 6.232 | 0.9989 | 0.7472 | 46.041 | 0.9997 | |
| 16 | Effect of cations competition | 0.2 K BaCl2 + 0.5 K MgCl2 | 0.4638 | 4.686 | 0.9929 | 0.4751 | 30.006 | 0.9981 |
| 17 | 0.2 K BaCl2 + 0.5 K SrCl2 | 0.4174 | 6.073 | 0.9980 | 0.4228 | 67.607 | 0.9996 | |
| 18 | Effect of salinity | 0.2 K BaCl2 + 1 K NaCl | 0.6372 | 4.657 | 0.9966 | 0.6495 | 25.150 | 0.9981 |
| 19 | 0.2 K BaCl2 + 5 K NaCl | 0.4704 | 5.177 | 0.9942 | 0.4799 | 37.188 | 0.9979 | |
| 20 | 0.2 K BaCl2 + 10 K NaCl | 0.4065 | 4.729 | 0.9929 | 0.4162 | 35.027 | 0.9979 | |
| 21 | 0.2 K BaCl2 + 50 K NaCl | 0.1606 | 1.913 | 0.9521 | 0.1732 | 17.035 | 0.9876 | |
| 22 | 0.2 K BaCl2 + 100 K NaCl | 0.0932 | 2.409 | 0.8888 | 0.1014 | 28.507 | 0.9327 | |
| 23 | Effect of methanol (pH = 3.5) | 0.2 K BaCl2 | 0.7259 | 4.588 | 0.9966 | 0.7385 | 21.863 | 0.9998 |
| 24 | 0.2 K BaCl2 + 1 K methanol | 0.7428 | 4.477 | 0.9946 | 0.7578 | 18.936 | 0.9950 | |
| 25 | 0.2 K BaCl2 + 2 K methanol | 0.7336 | 4.496 | 0.9926 | 0.7493 | 18.499 | 0.9992 | |
| 26 | 0.2 K BaCl2 + 5 K methanol | 0.7184 | 4.471 | 0.9963 | 0.7315 | 20.837 | 0.9998 | |
| 27 | Effect of methanol (pH = 8.5) | 0.2 K BaCl2 | 0.9833 | 7.432 | 0.9994 | 0.9888 | 59.708 | 0.9999 |
| 28 | 0.2 K BaCl2 + 1 K methanol | 0.9850 | 6.028 | 0.9994 | 0.9931 | 35.730 | 0.9998 | |
| 29 | 0.2 K BaCl2 + 2 K methanol | 0.9889 | 6.974 | 0.9992 | 0.9959 | 47.274 | 0.9999 | |
| 30 | 0.2 K BaCl2 + 5 K methanol | 0.9837 | 7.351 | 0.9994 | 0.9893 | 58.063 | 0.9999 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Liu, S.; Zhang, K.; Cai, M.; Chen, C.; Zhao, X. The Sorption Behaviors of Barium during Reinjection of Gas Field Produced Water into Sandstone Reservoir: An Experimental Water-Rock Interaction Study. Sustainability 2023, 15, 8132. https://doi.org/10.3390/su15108132
Yang S, Liu S, Zhang K, Cai M, Chen C, Zhao X. The Sorption Behaviors of Barium during Reinjection of Gas Field Produced Water into Sandstone Reservoir: An Experimental Water-Rock Interaction Study. Sustainability. 2023; 15(10):8132. https://doi.org/10.3390/su15108132
Chicago/Turabian StyleYang, Shugang, Shuangxing Liu, Kunfeng Zhang, Mingyu Cai, Changzhao Chen, and Xinglei Zhao. 2023. "The Sorption Behaviors of Barium during Reinjection of Gas Field Produced Water into Sandstone Reservoir: An Experimental Water-Rock Interaction Study" Sustainability 15, no. 10: 8132. https://doi.org/10.3390/su15108132
APA StyleYang, S., Liu, S., Zhang, K., Cai, M., Chen, C., & Zhao, X. (2023). The Sorption Behaviors of Barium during Reinjection of Gas Field Produced Water into Sandstone Reservoir: An Experimental Water-Rock Interaction Study. Sustainability, 15(10), 8132. https://doi.org/10.3390/su15108132








