Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Degradation of the Porous Magnesium Scaffold
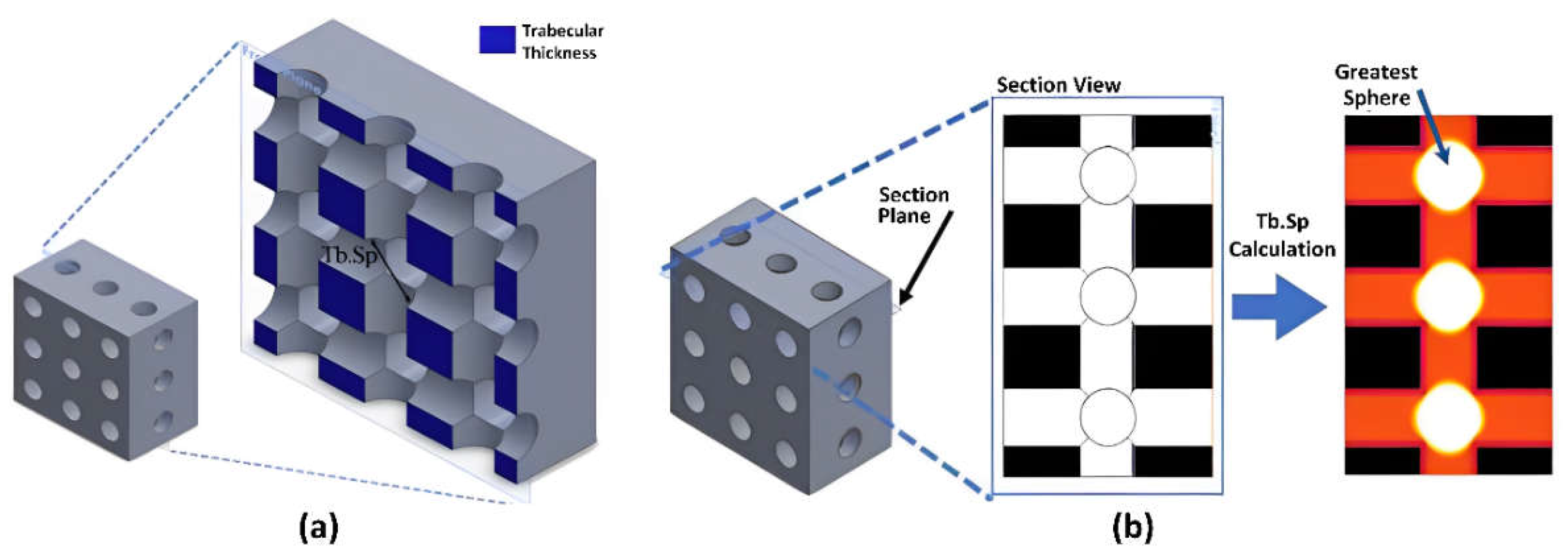
2.2. Morphological Characterization
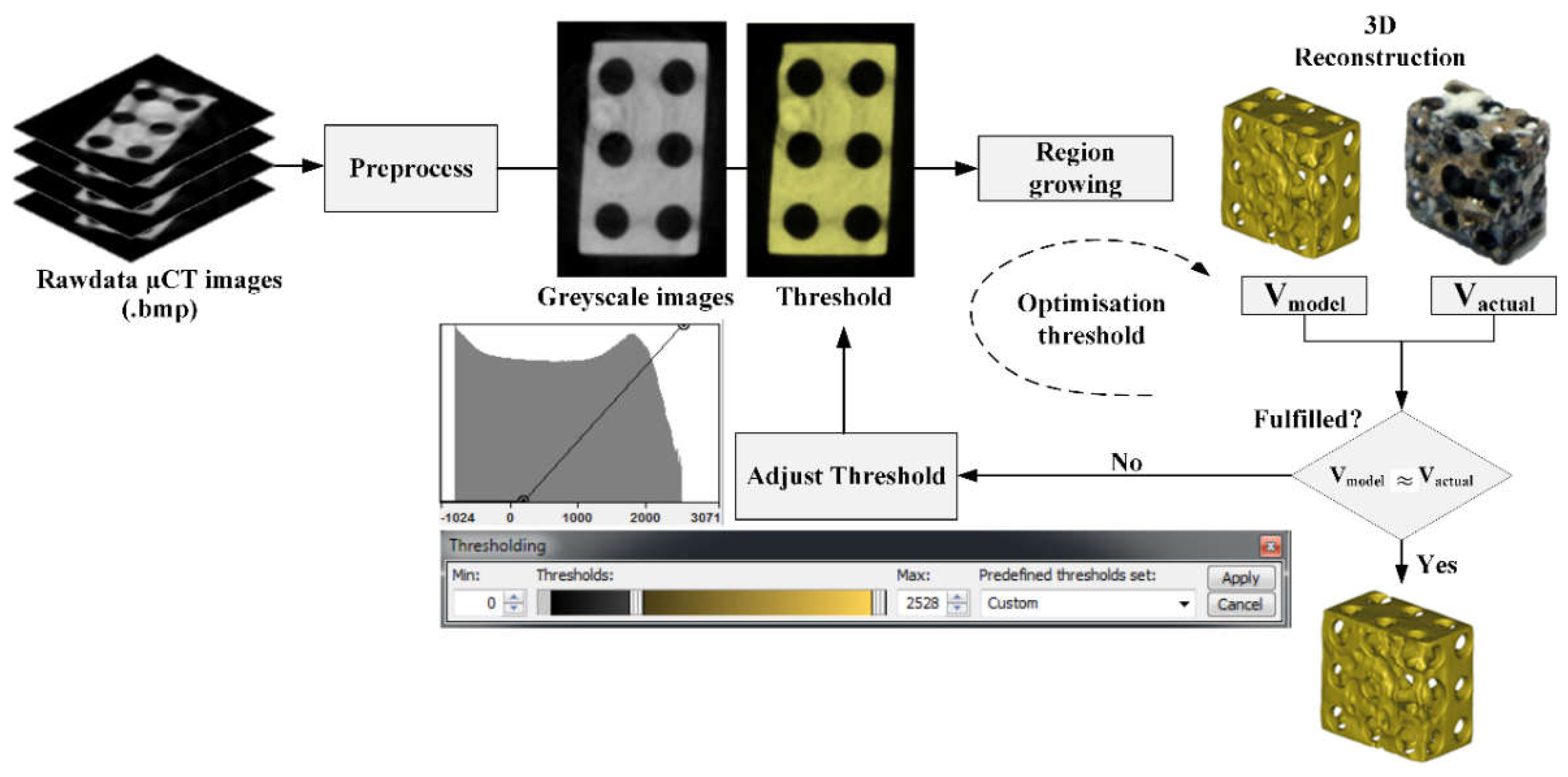
2.3. Segmentation Procedure
2.4. Monotonic Compression Test
2.5. FEA-Based Modeling of the Fatigue
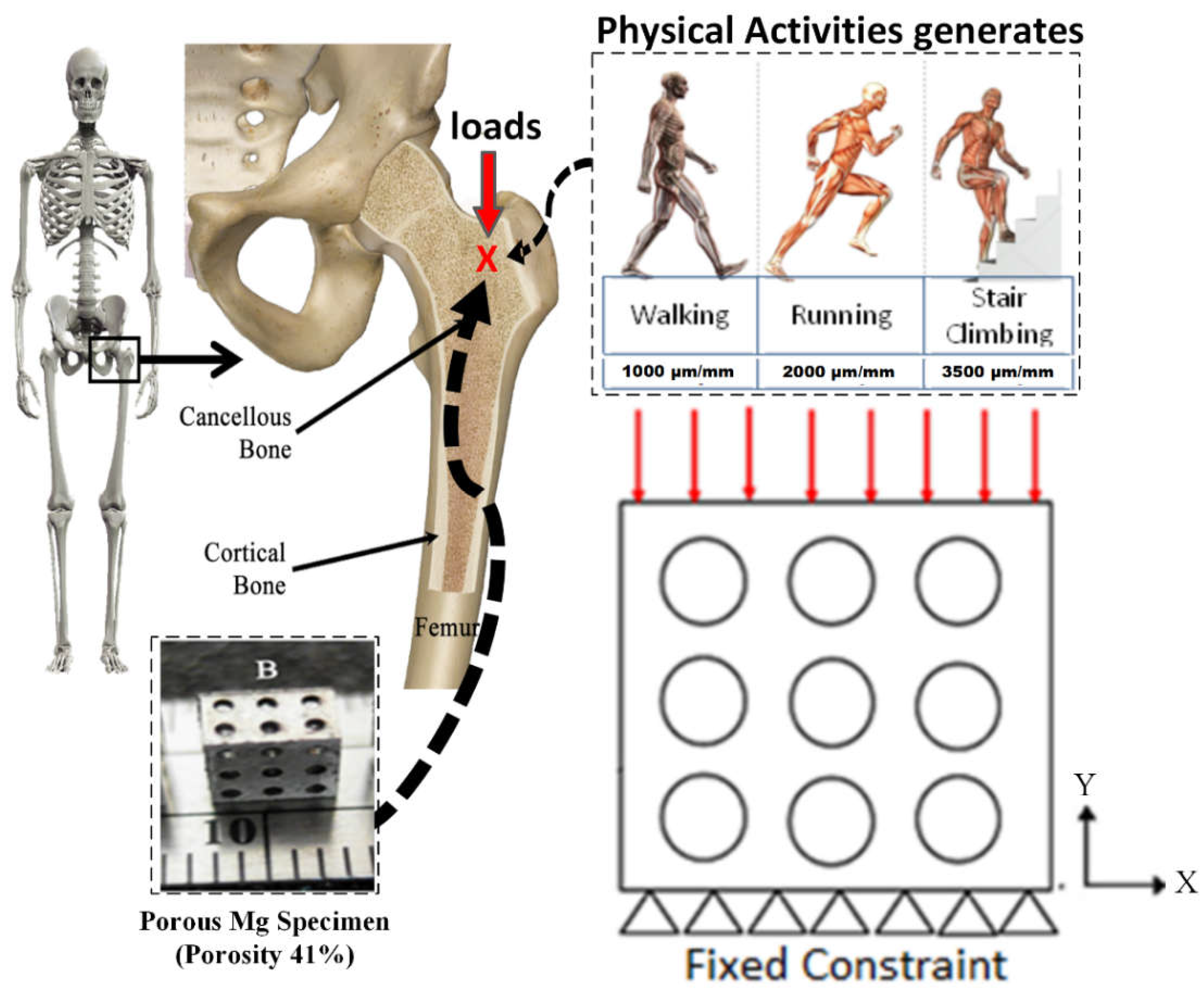
2.6. Boundary Condition and Material Input
3. Results
3.1. Simulation Result Using The Fatigue Method
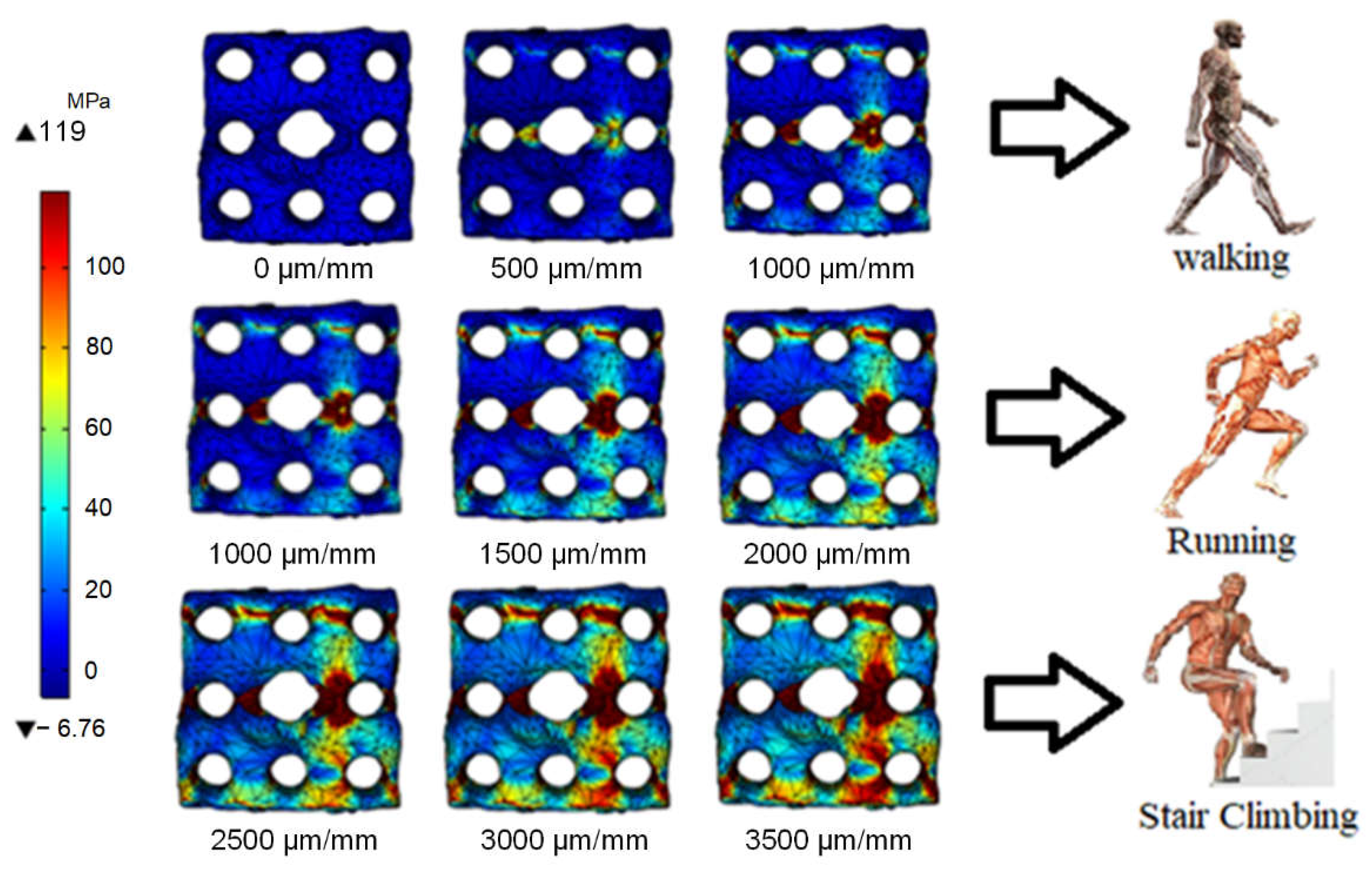
3.2. Stress Distribution of Porous Magnesium Implants on the Physiological Activity
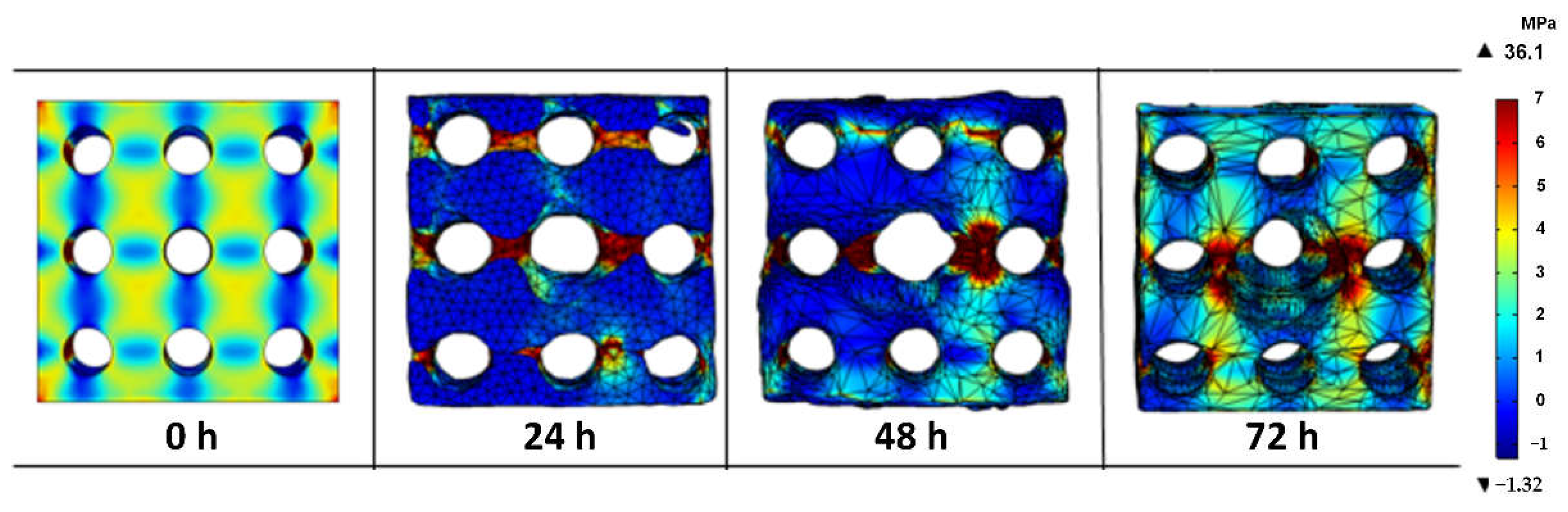
3.3. Stress Distribution on the Porous Magnesium Implant over Time
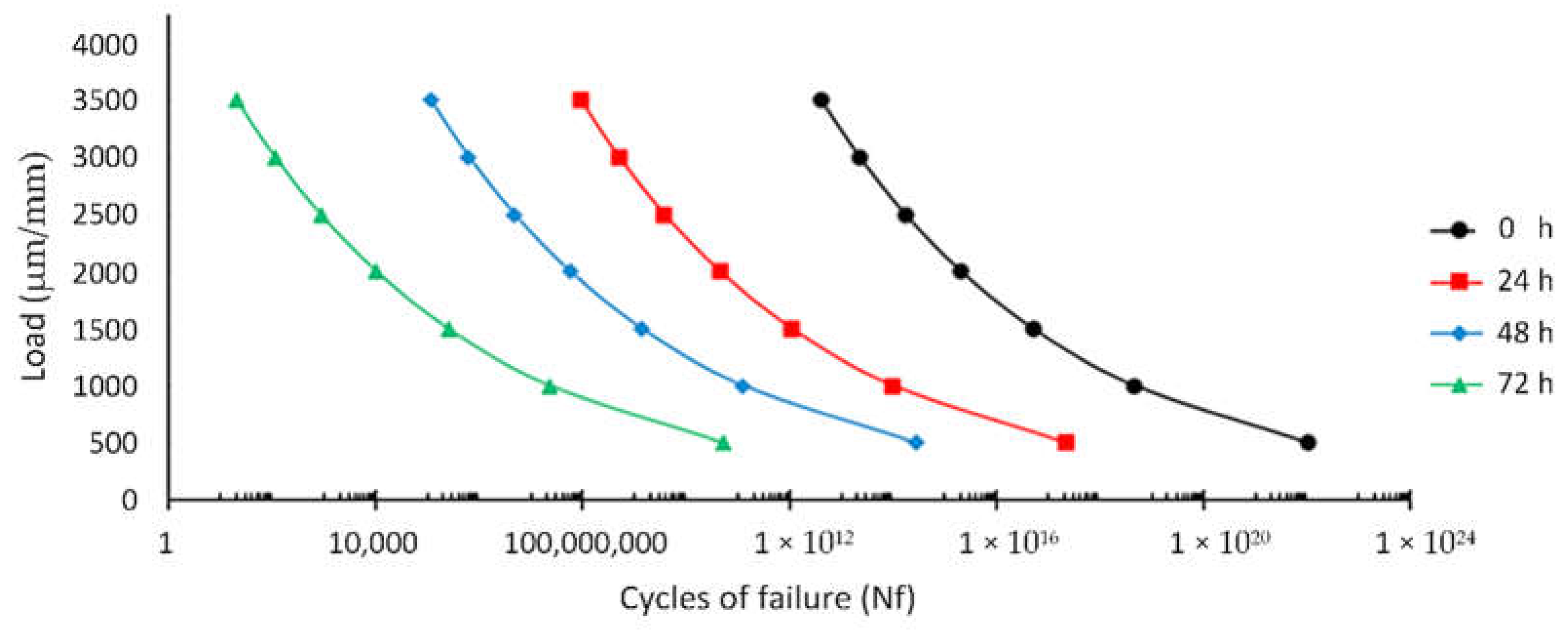
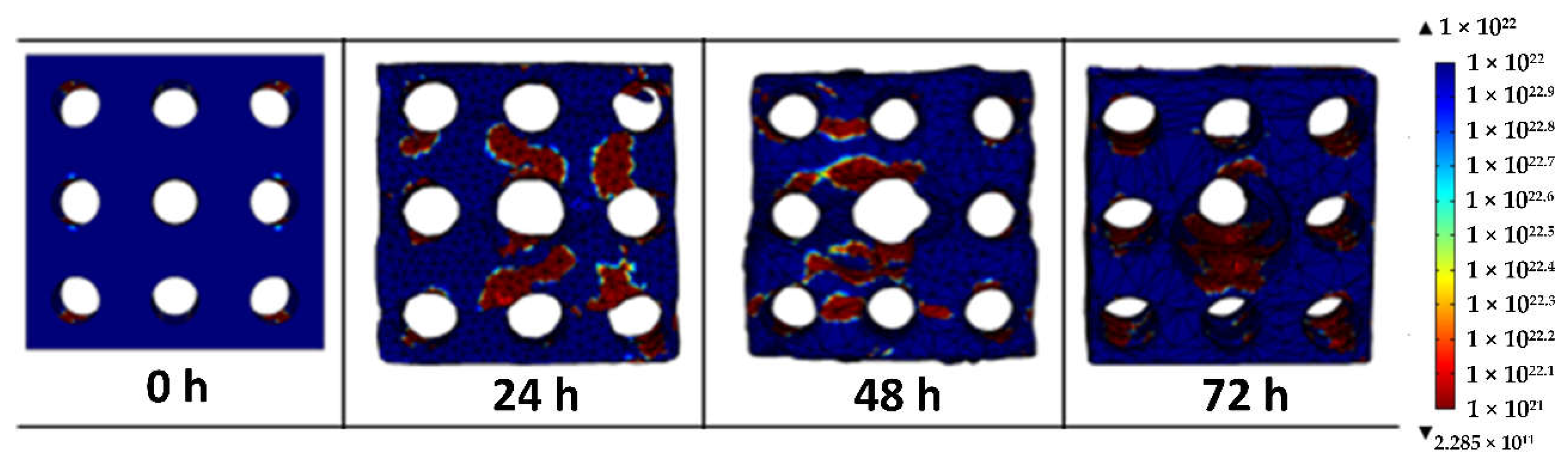
3.4. Fatigue Failure on the Porous Magnesium on Physiological Activity
3.5. Fatigue Failure on the Porous Magnesium Implant over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babuska, V.; Kasi, P.B.; Chocholata, P.; Wiesnerova, L.; Dvorakova, J.; Vrzakova, R.; Nekleionova, A.; Landsmann, L.; Kulda, V. Nanomaterials in Bone Regeneration. Appl. Sci. 2022, 12, 6793. [Google Scholar] [CrossRef]
- Rothweiler, R.M.; Zankovic, S.; Brandenburg, L.S.; Fuessinger, M.-A.; Gross, C.; Voss, P.J.; Metzger, M.-C. Feasibility of Implant Strain Measurement for Assessing Mandible Bone Regeneration. Micromachines 2022, 13, 1602. [Google Scholar] [CrossRef] [PubMed]
- Peña Fernández, M.; Black, C.; Dawson, J.; Gibbs, D.; Kanczler, J.; Oreffo, R.O.C.; Tozzi, G. Exploratory Full-Field Strain Analysis of Regenerated Bone Tissue from Osteoinductive Biomaterials. Materials 2020, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Noirrit-Esclassan, E.; Valera, M.-C.; Tremollieres, F.; Arnal, J.-F.; Lenfant, F.; Fontaine, C.; Vinel, A. Critical Role of Estrogens on Bone Homeostasis in Both Male and Female: From Physiology to Medical Implications. Int. J. Mol. Sci. 2021, 22, 1568. [Google Scholar] [CrossRef]
- Tavana, S.; Clark, J.N.; Newell, N.; Calder, J.D.; Hansen, U. In Vivo Deformation and Strain Measurements in Human Bone Using Digital Volume Correlation (DVC) and 3T Clinical MRI. Materials 2020, 13, 5354. [Google Scholar] [CrossRef]
- Semenova, I.P.; Modina, Y.M.; Stotskiy, A.G.; Polyakov, A.V.; Pesin, M.V. Fatigue Properties of Ti Alloys with an Ultrafine Grained Structure: Challenges and Achievements. Metals 2022, 12, 312. [Google Scholar] [CrossRef]
- Rajabinezhad, M.; Bahrami, A.; Mousavinia, M.; Seyedi, S.J.; Taheri, P. Corrosion-Fatigue Failure of Gas-Turbine Blades in an Oil and Gas Production Plant. Materials 2020, 13, 900. [Google Scholar] [CrossRef]
- Keramatian, A.; Bahrami, A.; Darougheh, A.H.; Zare, S. Root Cause Analysis of an Unexpected Brittle Failure in a Carbon Steel Slab. Eng. Fail. Anal. 2021, 122, 105205. [Google Scholar] [CrossRef]
- Mousavi Anijdan, S.H.; Sabzi, M. The Effect of Heat Treatment Process Parameters on Mechanical Properties, Precipitation, Fatigue Life, and Fracture Mode of an Austenitic Mn Hadfield Steel. J. Mater. Eng. Perform. 2018, 27, 5246–5253. [Google Scholar] [CrossRef]
- Salama, M.; Vaz, M.F.; Colaço, R.; Santos, C.; Carmezim, M. Biodegradable Iron and Porous Iron: Mechanical Properties, Degradation Behaviour, Manufacturing Routes and Biomedical Applications. J. Funct. Biomater. 2022, 13, 72. [Google Scholar] [CrossRef]
- Müller, M.; Šleger, V.; Kolář, V.; Hromasová, M.; Piš, D.; Mishra, R.K. Low-Cycle Fatigue Behavior of 3D-Printed PLA Reinforced with Natural Filler. Polymers 2022, 14, 1301. [Google Scholar] [CrossRef] [PubMed]
- Marcelino dos Santos, J.R.; Fernandes, M.F.; Velloso, V.M.d.O.; Voorwald, H.J.C. Fatigue Analysis of Threaded Components with Cd and Zn-Ni Anticorrosive Coatings. Metals 2021, 11, 1455. [Google Scholar] [CrossRef]
- Scacco, F.; Campagnolo, A.; Franceschi, M.; Meneghetti, G. Strain-Controlled Fatigue Behavior of a Nodular Cast Iron in Real Off-Highway Axles: Effects of Casting Skin and Strain Ratio. Metals 2022, 12, 426. [Google Scholar] [CrossRef]
- Basri, H.; Prakoso, A.T.; Sulong, M.A.; Md Saad, A.P.; Ramlee, M.H.; Agustin Wahjuningrum, D.; Sipaun, S.; Öchsner, A.; Syahrom, A. Mechanical Degradation Model of Porous Magnesium Scaffolds under Dynamic Immersion. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2020, 234, 175–185. [Google Scholar] [CrossRef]
- Fritton, S.P.; McLeod, K.J.; Rubin, C.T. Quantifying the Strain History of Bone: Spatial Uniformity and Self-Similarity of Low-Magnitude Strains. J. Biomech. 2000, 33, 317–325. [Google Scholar] [CrossRef]
- Lynch, M.E.; Fischbach, C. Biomechanical Forces in the Skeleton and Their Relevance to Bone Metastasis: Biology and Engineering Considerations. Adv. Drug Deliv. Rev. 2014, 79–80, 119–134. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Kurdi, O.; Tauviqirrahman, M.; Winarni, T.I.; Jamari, J. Tresca Stress Study of CoCrMo-on-CoCrMo Bearings Based on Body Mass Index Using 2D Computational Model. J. Tribol. 2022, 33, 31–38. [Google Scholar]
- Roda-Casanova, V.; Pérez-González, A.; Zubizarreta-Macho, Á.; Faus-Matoses, V. Fatigue Analysis of NiTi Rotary Endodontic Files through Finite Element Simulation: Effect of Root Canal Geometry on Fatigue Life. J. Clin. Med. 2021, 10, 5692. [Google Scholar] [CrossRef]
- Bashiri, A.H.; Alshoaibi, A.M. Adaptive Finite Element Prediction of Fatigue Life and Crack Path in 2D Structural Components. Metals 2020, 10, 1316. [Google Scholar] [CrossRef]
- Kashyzadeh, K.R.; Rahimian Koloor, S.S.; Omidi Bidgoli, M.; Petrů, M.; Amiri Asfarjani, A. An Optimum Fatigue Design of Polymer Composite Compressed Natural Gas Tank Using Hybrid Finite Element-Response Surface Methods. Polymers 2021, 13, 483. [Google Scholar] [CrossRef]
- Shah, G.J.; Nazir, A.; Lin, S.-C.; Jeng, J.-Y. Design for Additive Manufacturing and Investigation of Surface-Based Lattice Structures for Buckling Properties Using Experimental and Finite Element Methods. Materials 2022, 15, 4037. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, M.M.; Chen, D.A. Bio-Inspired Sutures: Using Finite Element Analysis to Parameterize the Mechanical Response of Dovetail Sutures in Simulated Bending of a Curved Structure. Biomimetics 2022, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.R.; Rubin, C.T.; Rubin, J. Mechanical Regulation of Signaling Pathways in Bone. Gene 2012, 503, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Shokry, A.; Mulki, H.; Kharmanda, G. A Logarithmic Formulation for Anisotropic Behavior Characterization of Bovine Cortical Bone Tissue in Long Bones Undergoing Uniaxial Compression at Different Speeds. Materials 2021, 14, 5045. [Google Scholar] [CrossRef]
- Ahlhelm, M.; Latorre, S.H.; Mayr, H.O.; Storch, C.; Freytag, C.; Werner, D.; Schwarzer-Fischer, E.; Seidenstücker, M. Mechanically Stable β-TCP Structural Hybrid Scaffolds for Potential Bone Replacement. J. Compos. Sci. 2021, 5, 281. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, H.; Schlangen, E.; Šavija, B. Lattice Fracture Model for Concrete Fracture Revisited: Calibration and Validation. Appl. Sci. 2020, 10, 4822. [Google Scholar] [CrossRef]
- Zargarian, A.; Esfahanian, M.; Kadkhodapour, J.; Ziaei-Rad, S. Numerical Simulation of the Fatigue Behavior of Additive Manufactured Titanium Porous Lattice Structures. Mater. Sci. Eng. C 2016, 60, 339–347. [Google Scholar] [CrossRef]
- Weinkamer, R.; Eberl, C.; Fratzl, P. Mechanoregulation of Bone Remodeling and Healing as Inspiration for Self-Repair in Materials. Biomimetics 2019, 4, 46. [Google Scholar] [CrossRef]
- Silva, C.L.P.; Camara, M.A.; Hohenwarter, A.; Figueiredo, R.B. Mechanical Behavior and In Vitro Corrosion of Cubic Scaffolds of Pure Magnesium Processed by Severe Plastic Deformation. Metals 2021, 11, 1791. [Google Scholar] [CrossRef]
- Sakthiabirami, K.; Soundharrajan, V.; Kang, J.-H.; Yang, Y.P.; Park, S.-W. Three-Dimensional Zirconia-Based Scaffolds for Load-Bearing Bone-Regeneration Applications: Prospects and Challenges. Materials 2021, 14, 3207. [Google Scholar] [CrossRef]
- Olăreț, E.; Stancu, I.-C.; Iovu, H.; Serafim, A. Computed Tomography as a Characterization Tool for Engineered Scaffolds with Biomedical Applications. Materials 2021, 14, 6763. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, S.A.; Tamaddon, M.; Marghoub, A.; Wang, K.; Babamiri, B.B.; Hazeli, K.; Xu, W.; Lu, X.; Sun, C.; Wang, L.; et al. Mechanical Characterisation and Numerical Modelling of TPMS-Based Gyroid and Diamond Ti6Al4V Scaffolds for Bone Implants: An Integrated Approach for Translational Consideration. Bioengineering 2022, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Madhukarϯ, S.; Harshith Reddy, B.R.; Kumar, G.A.; Naikϯ, R.P. A Study on Improvement of Fatigue Life of Materials by Surface Coatings. Int. J. Curr. Eng. Technol. 2018, 8, 5–9. [Google Scholar] [CrossRef][Green Version]
- Li, N.; Tian, Y.; Ma, B.; Hu, D. Experimental Investigation of Water-Retaining and Mechanical Behaviors of Unbound Granular Materials under Infiltration. Sustainability 2022, 14, 1174. [Google Scholar] [CrossRef]
- Li, Y.; Retraint, D.; Gao, P.; Xue, H.; Gao, T.; Sun, Z. Effect of Surface Mechanical Attrition Treatment on Torsional Fatigue Properties of a 7075 Aluminum Alloy. Metals 2022, 12, 785. [Google Scholar] [CrossRef]

| Porosity | Surface Area | Volume | Mass per Surface Area |
|---|---|---|---|
| 41% | 209.81 mm2 | 44.57 mm3 | 0.34 kg/m2 |
| Iterative | Threshold Values (HU) | Volume of Mimics Model (mm3) | Actual Volume (mm3) | Percentage Error (%) |
|---|---|---|---|---|
| #1 | −592 | 50.58 | 39.89 | 26.82 |
| #2 | −407 | 46.32 | 39.89 | 16.13 |
| #3 | 0 | 41.61 | 39.89 | 4.33 |
| #4 | 110 | 40.57 | 39.89 | 1.72 |
| #5 | 150 | 40.19 | 39.89 | 0.77 |
| #6 | 186 | 39.87 | 39.89 | 0.03 |
| #7 | 200 | 39.73 | 39.89 | 0.39 |
| #8 | 240 | 39.41 | 39.89 | 1.19 |
| #9 | 267 | 39.16 | 39.89 | 1.82 |
| #10 | 300 | 38.89 | 39.89 | 2.49 |
| #11 | 1076 | 32.53 | 39.89 | 18.53 |
| #12 | 1416 | 28.83 | 39.89 | 27.72 |
| Mechanical Properties | Magnesium |
|---|---|
| Monotonic Properties | |
| Young’s modulus, E (GPa) | 3.5 |
| Poisson’s ratio | 0.35 |
| (MPa) | 147 |
| 0.05E | |
| Fatigue Properties | |
| 0.425 | |
| −1.3 | |
| (MPa) | 180 |
| −0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. https://doi.org/10.3390/su15010823
Putra RU, Basri H, Prakoso AT, Chandra H, Ammarullah MI, Akbar I, Syahrom A, Kamarul T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability. 2023; 15(1):823. https://doi.org/10.3390/su15010823
Chicago/Turabian StylePutra, Risky Utama, Hasan Basri, Akbar Teguh Prakoso, Hendri Chandra, Muhammad Imam Ammarullah, Imam Akbar, Ardiyansyah Syahrom, and Tunku Kamarul. 2023. "Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time" Sustainability 15, no. 1: 823. https://doi.org/10.3390/su15010823
APA StylePutra, R. U., Basri, H., Prakoso, A. T., Chandra, H., Ammarullah, M. I., Akbar, I., Syahrom, A., & Kamarul, T. (2023). Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability, 15(1), 823. https://doi.org/10.3390/su15010823







