Abstract
The black-odor phenomenon has been widely reported worldwide and recognized as a global ecological risk for aquatic environments. However, driving factors for black-odor-related microorganisms and potential self-remediation strategies are still poorly understood. This study collected eight water samples (sites A–H) disturbed by different factors from the Jishan River located in Jinmen, Hubei Province, China. Black-odor-related environmental factors and functional bacterial structure were further measured based on the basic physicochemical parameters. The results indicated that different types of disturbed conditions shape the distribution of water quality and microbial community structures. Site B, which was disturbed by dams, had the worst water quality, the lowest abundance of functional microbes for Mn, Fe, and S biotransformation, and the highest abundance of functional microbes for fermentation. The natural wetlands surrounding the terminus of the river (site H) were keys to eliminating the black-odor phenomenon. Potential black-odor-forming microorganisms include Lactococcus, Veillonella, Clostridium sensu stricto, Trichococcus, Rhodoferax, Sulfurospirillum, Desulfobulbus, and Anaeromusa-Anaeroarcus. Potential black-odor-repairing microbes include Acinetobacter, Mycobacterium, and Acidovorax. pH and COD were paramount physiochemical factors contributing to blackening-odor-related microorganisms. This study deepens our understanding of driving factors for black-odor-related microorganisms and provides a theoretical basis for eradicating the black-odor phenomenon.
1. Introduction
With rapid urbanization and industrialization, excessive pollutants have been released into river ecosystems. These increased anthropogenic pollutants dramatically exceeded the capacity of the river system for self-purification and further led to black-odorous rivers [1,2,3]. Waterbodies with unpleasant colors and malodor smell not only reduce the aesthetic value of landscapes but also destroy the ecological balance and raise severe hygienic problems. The black-odor phenomenon occurs worldwide in developing and developed countries, such as the meromictic Lower Mystic Lake in the United States, the Emscher River in Germany, and the Ganges River in India [1,4]. In China, more than 2000 urban rivers in 295 cities were recognized as black and odorous [5,6]. The black-odor phenomenon poses risks to the aquatic environment and residents’ health [4].
An interaction between physicochemical and biological factors causes the formation and remediation of black-odor phenomena in waterbodies [1,7]. According to the issuance of the Chinese Ministry of Housing and Urban–Rural Development, the definition of black-odorous water contains the value of oxidation reduction potential (ORP) < 50 mV [1,8]. Anaerobic microbial reactions are dominant in overlying water where the ORP is less than 50 mV. The proliferation, growth, and respiration of anaerobic microorganisms are associated with the decomposition and fermentation of pollutants, which enhance the production of gas substances with a foul odor, such as hydrogen sulfide (H2S), volatile organic sulfur compounds (thioethers and thiols), β-cyclocitral, β-ionone, and amines [9,10]. In addition, sulfate is eventually reduced to sulfide (S2−) by sulfate-reducing bacteria and further combined with reductive metals, such as Fe(II) and Mn(II), to form a blackish precipitate, which leads the water to turn black [11,12]. Suspended particles and floating materials absorb large amounts of negative colloids (FeS and MnS) in the overlying water, threatening the local water quality and the health of the inhabitants. In addition to inorganic compounds, chromophoric dissolved organic matter (CDOM) is a significant optical fraction of dissolved organic matter and is an important contributor to the blackening of waterbodies [3,13]. CDOM, which leaches from decaying macrophytes, comprises optically active substances, such as polysaccharides, amino acids, aromatic proteins, humic acid, and antibiotics [13,14]. There are different approaches to deal with polluted water and avoid the black-odor-forming microbes, such as the current developed solar-driven interfacial evaporation technique, photocatalysis, and the combination of these two techniques with antibacterial function [15,16,17]. These new technologies have achieved excellent wastewater treatment results [18,19,20]. In addition to this, microorganism (probiotics) application has been considered an economical, highly efficient, and eco-friendly treatment for remediating black-odor waterbodies [21]. Microorganisms, such as Mn(II)-oxidizing bacteria, Fe(II)-oxidizing bacteria, sulfur compound-oxidizing bacteria, and aerobic chemo-heterotrophic bacteria, have been applied directly to alleviate and eradicate the problem of black-odor waterbodies [22,23]. However, what kinds of microorganisms involved in the formation and remediation of black-odor waterbodies are unclear and require further research.
The primary sources of organic and inorganic contaminants are anthropogenic activities involving industrial discharges, domestic sewage, agricultural runoff, and aquacultural wastewater. Previous studies have revealed that the influx of different anthropogenic pollution types into rivers significantly affect chemical properties, microbial community structure, and nutrient element metabolism [24,25,26]. However, the contributions of different environmental disturbances to forming black-odor rivers have not been adequately addressed. Moreover, blackening occurs initially at the sediment-water interface and transfers to the water body [1]. The various contaminants absorbed into sediment further dynamically diffuse into the overlying water [27]. Therefore, it is necessary to investigate the harmful components and their metabolic processes in overlying water in direct contact with humans.
In this study, eight water samples were collected from the Jishan River [28], a typical black-odor river surrounded by concentrated anthropogenic activities. Black-odor-related environmental factors and functional bacterial structure were further detected based on the basic physicochemical parameters. The main objectives of this study were (1) to explore the contributions of different environmental conditions to the formation and remediation of the black-odor phenomenon; (2) to elucidate the important bacterial taxonomic genera related to the black-odor phenomenon in river overlying water; and (3) to identify the predominant water quality parameters affecting microbes contributing to blackening-odor formation and remediation of this river. The results can facilitate a complete biomechanism of the black-odor phenomenon and provide theoretical support for water quality restoration.
2. Materials and Methods
2.1. Study Site and Sampling
In this study, eight overlying water samples were collected from the Jishan River (Figure 1), located in Jimen, Huibei Province, China (30°47′ N, 112°18′ E), in March 2021. The sampling sites were divided into six different types of natural and anthropogenic disturbances: industrial effluent (A), dam (B), the confluence (C, D, and F), aquacultural sewage (E), domestic wastewater (G), and wetland (H). At each sampling point, sampling and in situ water quality measurements were performed from the center and left and right sides of the river (1.5 m from the river bank).
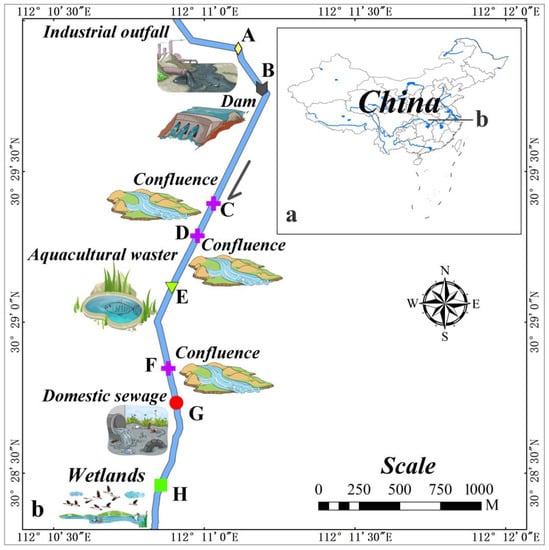
Figure 1.
Location of the study area and sampling points. (a) Geographical location of the study area in China; (b) Sampling sites in the Jishan River, the sampling sites were divided into six different types of disturbance conditions: industrial effluent (A), dam (B), the confluence (C, D, and F), aquacultural sewage (E), domestic wastewater (G), and wetland (H).
Water profiles were detected in situ by measuring water temperature (T), pH, total dissolved solids (TDS), redox potential (ORP), electrical conductivity (EC), and dissolved oxygen (DO) using pre-calibrated hand-held Hach LDOTM HQ10 multiprobe meters. All operations were repeated three times, and the mean values were finally represented as the overall water quality condition of the sampling site.
Overlying water was collected under the surface of river (0.5 m). Specifically, the water samples were collected from the three control points at each sampling cross-section, and subsequently were mixed in equal volumes; part of the mixed water samples were stored (−20 °C) in 1000 mL sterile polyethylene bottles for molecular analysis; the other mixed water samples were filtered in the presence and stored (4 °C) in 2000 mL polyethylene bottles for the determination of the other physicochemical parameters.
2.2. Physicochemical Analysis
The contents of ammonium nitrogen (NH4+-N), nitrite nitrogen (NO2−-N), nitrate nitrogen (NO3−-N), total nitrogen (TN), total phosphorus (TP), chemical oxygen demand (COD), and chromaticity were detected as previously described under the guidance of standard methods (Chinese National Environmental Protection Agency 2002). Sulfate (SO42−) was measured using sulfate–barium chromate spectrophotometry, while sulfide (S2−) was determined in situ using a HACH colorimeter [29]. UV–visible spectra of chromophoric DOM (CDOM) in water were recorded from 250 to 750 nm with a dual beam UV–VIS spectrophotometer (SHIMADZU 2550 Series, Kyoto, Japan) with quartz cells (path length, 10 cm) and Milli-Q water as a blank [30]. The spectral slope ratio SR (S275–295/S350–400) and a254 (m−1) were used to describe the quality and quantity of CDOM, respectively [31]. The spectral slope ratio SR has been used to represent the molecular weight, source, and exposure to photochemical degradation of CDOM in natural waters [32], and a254 (m−1) was proposed as an indicator of the relative CDOM concentration [33]. The Mn(II) and total Mn concentrations were determined with flame atomic absorption spectrophotometry and manganese–formaldehyde oxime spectrophotometry, respectively. The Fe(II) and total Fe concentrations were detected using the 1,10-phenanthroline method [34].
2.3. DNA Extraction and High-Throughput Sequencing
Water samples (500 mL) were filtered through 0.22 μm polycarbonate membranes (Shanghai Bandao Industrial Co., Ltd.). DNA was extracted from each filter using the DNeasy® PowerSoil® Kit (100T, Qiagen, Düsseldorf, Germany) according to the manufacturer’s instructions. The primers 338F (-ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT) were used to amplify the V3/V4 regions of the 16S rRNA gene. PCR amplicons were extracted from 2% agarose gels, purified using the QIAquick PCR purification kit (Qiagen, Düsseldorf, Germany), and quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, USA). Purified products were pooled in equimolar amounts and paired-end sequenced on an Illumina MiSeq PE250 platform following the manufacturer’s guidelines at Majorbio Technology Co., Ltd. (Shanghai, China).
2.4. Statistical Analysis
Spearman correlation analyses (vegan package in R) demonstrated the correlations between physiochemical parameters, dominant black-odor-related microorganisms, and microbial metabolic functions. The Mantel test (linkET package in R) [24] delineates the relationship between physiochemical factors and black-odor-related microorganisms. A heatmap was drawn by the “pheatmap” package of R. Through the R packages “tidyverse”, “ggplot2”, and “corrplot”, correlation combination figures were generated to show the relationships between black-odor-related microorganisms and physiochemical factors. Redundancy analysis (RDA) was performed using the R language vegan package for analyzing the effect of physicochemical parameters on the microbial community. The experimental data were statistically analyzed, calculated, and plotted using Origin 2021b, IBM SPSS 22 (SPSS, Chicago, USA), and ArcGIS 10.2.
3. Results and Discussion
3.1. Characteristics of Black-Odor Factors at Different Sampling Sites
The physicochemical characteristics of the overlying water samples are revealed in Table S1. The results illustrated that the water quality of the Jishan River is classified as Grade V according to Environmental Quality Standards for Surface Water for China (GB 3838-2002) [35]. High concentrations of COD (39.52–1138 mg/L), TN (3.25–24.68 mg/L), TP (0.49–2.39 mg/L), and NH4+-N (0.64–13.69 mg/L) were detected in all overlying water samples. According to the classifications and standards for the degree of pollution in urban black-odor waterbodies, the Jishan River was defined as a typical black-odor water body [36]. To date, those standards have focused only on basic water quality parameters. However, previous studies have proven that biotransformations associated with the Mn, Fe, S, and C cycles significantly contribute to blackening, odor formation, and remediation [1,5]. Therefore, detecting these key black-odor factors at different sampling sites is necessary.
Figure 2 shows the distribution and concentration of different black-odor factors at different sampling sites. The SO42− values ranged from 2.8 to 104.0 mg/L, averaging 48.8 mg/L. The S2− concentrations were 0–11.17 mg/L (mean 4.07 mg/L), much higher than the maximum allowable limit of S2− (0.02 mg/L), according to the International Standards For Drinking Water [37]. The dissolved concentrations of SO42− and S2− were similar to other black-odor rivers in China, such as the Jinchuan River, Taihu Lake, and Dihe River [4,38,39]. The SR and a254 varied from 0.01–1.38 (mean 0.53) and 16.3–95.55 m−1 (mean 39.15 m−1), respectively. High total Fe (0.007–2.434 mg/L) and total Mn (0.012–1.234 mg/L) concentrations were measured, indicating heavy metal contamination (the maximum allowable levels of Fe and Mn in the national life potable water are 0.5 mg/L and 0.3 mg/L, respectively, according to the hygienic standard of drinking water in China). Thus far, in waterbodies that are not influenced by the black-odor phenomenon, Fe(II) concentrations are normally less than 0.05 mg/L [38], while Mn(II) concentrations are normally less than 0.3 mg/L [40]. In contrast, excessive Fe(II) and Mn(II) concentrations were detected, with averages of 0.40 mg/L and 0.17 mg/L, respectively. When these heavy metal contaminations accumulate and biomagnify to a certain degree in human bodies, they destroy human health through various diseases [41]. Excessive Mn can threaten the nervous system and cause IQ deficits, and high Fe can lead to tissue damage and neurodegenerative diseases [42].
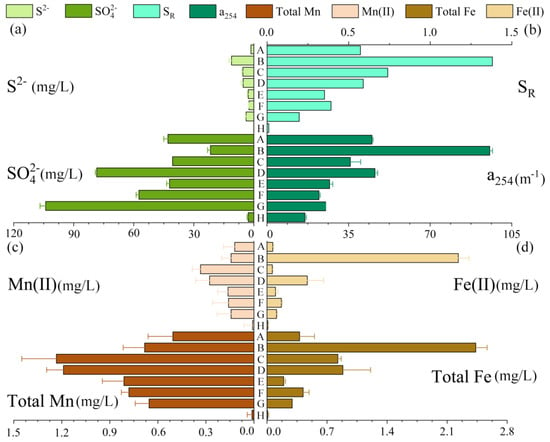
Figure 2.
The distribution and concentration of different black-odor factors at different sampling sites. Variation in (a) sulfide (S2−) and sulfate (SO42−); (b) SR and a254; (c) Mn(II) and total Mn; (d) Fe(II) and total Fe. A, B, ..., H represent the corresponding eight points in Figure 1.
3.2. Distribution and Diversity of Black-Odor-Related Microorganisms
The main blackening and odor formation and restoration mechanisms are shown in Table 1. Microorganisms associated with Mn, Fe, S, and C may be involved in the phenomena of blackening and odorization in waterbodies, such as Mn(IV)-reducing bacteria (MRBs), Fe(III)-respirating bacteria (FRBs), different sulfur compound-respirating bacteria (SRBs), and fermentative bacteria (FBs) (Figure 3). In contrast, microorganisms involved in restoring the self-purification ability and ecosystem health of waterbodies may contain Mn(II)-oxidizing bacteria (MOBs), Fe(II)-oxidizing bacteria (FOBs), sulfur compound-oxidizing bacteria (SOBs), and aerobic chemo-heterotrophic bacteria (ACBs) (Figure 3). Tables S2–S4 summaries the reported black-odor-related microorganisms from the literature. By alignment with Tables S2–S4 and literature confirmation, there are 14,19, 32, and 100 bacterial genera associated with the Mn, Fe, S and C biotransformation, respectively, based on 16S rRNA gene sequence data [4,43]. Within the bacterial community structures observed (Figure 4), bacterial genera associated with Mn, Fe, S, and C biotransformation accounted for 4.53–44.64, 0.61–15.03, 0.63–39.03, and 25.80–94.71%, respectively. The relative abundance of MRBs, FRBs, SRBs, and FBs ranged from 0.21–8.49, 0.60–14.46, 0.38–12.54, and 3.10–94.32%, respectively. However, the relative abundance of MOBs, FOBs, SOBs, and ACBs varied at 0.06–44.29, 0.01–9.42, 0.07–38.65, and 0.40–56.34%, respectively. Site B had the lowest abundances of functional microbes for Mn, Fe, and S biotransformation and the highest abundances for fermentation. As shown in the abundances and observed richness (Sobs) of different black-odor-related bacterial genera in Table S1, site B possessed the highest abundances and lowest diversities. Biodiversity, which increases the resistance of communities to environmental perturbations and allows the restoration of microbial functions after pollution, is the key to ecological insurance [44]. Moreover, combined with the physiochemical parameters, site B, which was disturbed by dams, had the worst water quality. This result revealed that dams could alter the hydrological and environmental conditions of natural rivers, reshape the structures of microbial communities, and destroy the self-purification ability, thereby deteriorating river water quality. It is interesting to find that site H, surrounded by natural wetlands, has self-remediation potentials (Figure 2). Site H showed relatively high levels of microbes with metal oxidation, oxidation of sulfur compounds, and aerobic heterotrophic growing abilities (Figure 4). The results suggested that natural wetlands possess potentials to reduce the black-odor phenomenon. Therefore, we should spare no effort to protect against wetland degradation and improve wetland ecosystem health.

Table 1.
The formation and remediation reactions contributing to black-odor waterbodies.
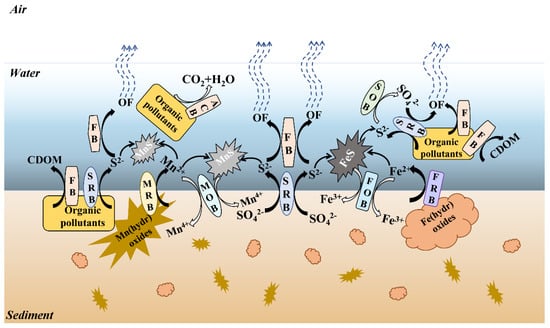
Figure 3.
A schematic showing Mn, Fe, S, and C biotransformation and biological mechanisms of black-odor formation and remediation in overlying water. The black arrows represent the black-odor formation processes, while the white solid arrows represent the black-odor remediation processes. The full name of functional bacteria that were not mentioned in text was provided in the follow, MOB, Mn(II)-oxidizing bacteria that can oxidize Mn(II) to Mn(IV); MRB, Mn(IV)-reducing bacteria that can reduce Mn(IV) to Mn(II); FOB, Fe(II)-oxidizing bacteria that can oxidize Fe(II) to Fe(III); FRB, Fe(III)-respirating bacteria that can reduce Fe(III) to Fe(II); SOB, sulfur compound-oxidizing bacteria that can oxidize different types of sulfur compounds; SRB, sulfur compound-respirating bacteria that can reduce different types of sulfur compounds; ACB, aerobic chemo-heterotrophic bacteria that can utilize organic matter as an electron donor and oxygen as an electron acceptor under aerobic conditions to produce carbon dioxide and water.
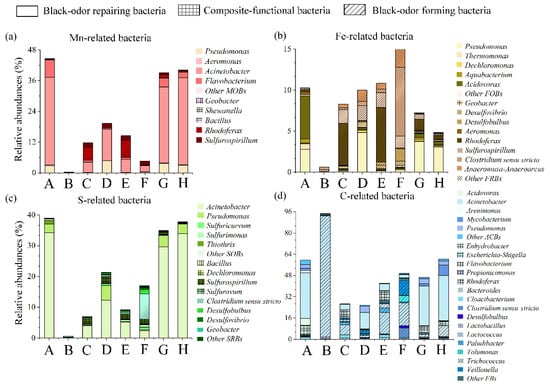
Figure 4.
The distribution and diversity of black-odor-related microorganisms. The relative abundance of (a) Mn-related functional bacterial genera, containing Mn(II)-oxidizing bacteria and Mn(IV)-reducing bacteria; (b) Mn-related functional bacterial genera, containing Fe(II)-oxidizing bacteria and Fe(III)-respirating bacteria; (c) S-related functional bacterial genera, containing sulfur compound-oxidizing bacteria and different sulfur compound-respirating bacteria; (d) C-related functional bacterial genera, containing fermentative bacteria and aerobic chemo-heterotrophic bacteria. A, B, ..., H represent the corresponding eight points in Figure 1.
As shown in Figure 4, apparently dominant metal-reducing bacteria and sulfate-reducing bacteria, such as Rhodoferax (0–6.63%), Sulfurospirillum (0.002–1.84%), Desulfobulbus (0–1.52%), and Anaeromusa-Anaeroarcus (0–2.23%), are the primary genera contributing to the precipitation of black metal sulfide. Moreover, fermenting bacteria, such as Lactococcus (0.24–91.34%), Veillonella (0–10.96%), Tolumonas (0–4.82%), Clostridium sensu stricto (0.08–8.36%), and Trichococcus (0.096–8.19%), play a vital role in CDOM and odor formation. Previous studies have reported that Clostridium sensu stricto, Rhodoferax, and Desulfobulbus are commonly found in alga-induced black-odor waterbodies and sediments [2,5]. In addition, the genus Clostridium sensu stricto can participate in the anaerobic degradation of Micocystis blooms, thus producing CDOM [51]. The genus Lactococcus has a significant correlation with bovine mastitis and fish lactococcal disease and has been regarded as a biomarker for the microecological balance and health of river systems [25]. In contrast, metal sulfide-oxidizing bacteria and aerobic chemo-heterotrophic bacteria (Tables S2 and S3), such as Acinetobacter (0.01–34.27%), Mycobacterium (0.004–7.91%), and Acidovorax (0.005–5.22%), are significantly related to eliminating the black-odor phenomenon of this river. Among these genera, Acinetobacter and Acidovorax have been reported to degrade refractory organics in the restoration process [8,52,53]. The genus Mycobacterium, which can utilize morpholine, a chemical industry pollutant, as its sole source of carbon, nitrogen, and energy, has been recognized as an ecological indicator of its pollution [25].
The relationships between black-odor-related microorganisms and physiochemical parameters were investigated by redundancy analysis (RDA). The results (Figure 5) revealed that black-odor-related microorganisms were significantly correlated with physiochemical parameters. Fe(II), NO3−-N, pH, and TP were primary drivers in black-odor-forming microbes. The association between physiochemical parameters and dominant black-odor-forming microbes demonstrated that the distributions of Lactococcus, Clostridium sensu stricto, Tolumonas, Veillonella, and Desulfobulbus were negatively correlated with pH but positively associated with TN, TP, Fe(II), and Mn(II) (Figure S1). RDA also indicated that total manganese (TMn), TP, S2−, and pH were the environmental factors that shaped the black-odor-repairing microbes. Correlation analysis demonstrated that dominant black-odor-repairing microbes, including Arenimonas, Acidovorax, Acinetobacter, Mycobacterium, and Pseudomonas, were negatively related to all detected physiochemical factors except pH and SO42− (Figure S1). This suggested that relatively higher pH and lower nutrient levels might facilitate the recruitment of microorganisms for black-odor remediation. However, further investigation of other key factors that could contribute to the formation and remediation of black-odor waterbodies in different ecosystems is still necessary.
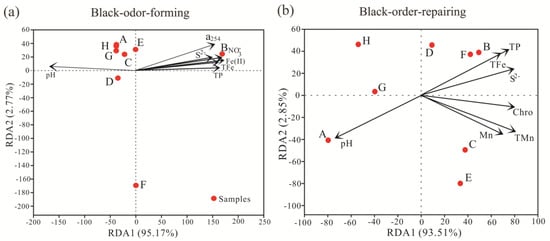
Figure 5.
Redundancy analysis (RDA) of the effect of physiochemical parameters on (a) black-odor-forming microbes and (b) black-odor-repairing microbes.
3.3. Paramount of Physiochemical Factors Contributing to Black-Odor of Jishan River
Correlation analysis (Figure 5 and Figure 6) showed that TP was positively correlated with chromaticity, NO3−-N, total Fe, S2−, and a254. Previous study has demonstrated that the reduction of Fe(III) oxyhydroxides and the competitive adsorption of dissolved organic matter to minerals are the primary factors in the release of phosphorus from sediments to pore water [54]. Chromaticity positively correlated with total Fe, S2−, a254, and SR. The formation of black color in water is accompanied by an increase in S2− concentrations, which combines with Fe(II), forming black metal sulfides (FeS). Moreover, the qualitative and quantitative characteristics of CDOM could be used to identify and monitor black-odor phenomena [55]. Total Fe was significantly positively associated with S2−, a254, and SR. The reductive dissolution of Fe oxyhydroxides promotes the release of CDOM and Fe(II) into overlying water. Fe(II) then rapidly combines with S2− to form black precipitates of FeS [7]. In addition, pH disturbance significantly affected black and odor issues by changing the concentration of other water quality parameters. Previous studies have reported that acidic water environments promote the release of organic matter and the dissolution of minerals, leading to the enrichment of heavy metals and high concentrations of nutrient elements [6,41,56], such as sulfur, nitrogen, and phosphorus [56]. These substances have been reported to be the main component in blackening and the odor formation of waterbodies.

Figure 6.
The Mantel test was performed to discriminate the predominant water quality parameters affecting microbes contributing to blackening-odor formation and remediation of this river. Significant difference was denoted with ** p < 0.01, * p < 0.05.
The Mantel test (Figure 6) was performed to discriminate the predominant water quality parameters affecting microbes contributing to blackening-odor formation and remediation of this river. The results illustrated that pH had a significant correlation with black-odor-related microorganisms. pH has been considered a primary factor in driving microorganisms’ distribution and function in different ecosystems. It can change the ionization state of metabolites via the change in the charge of their functional groups, which may lead to metabolic alteration [56]. Meanwhile, excessively low pH might also lead to further activation of heavy metals and organic compounds to the ionic state, which could inhibit or transform biochemical processes by affecting enzyme activity [56]. COD was observably associated with black-odor-related microorganisms. Since COD is an index reflecting organic matter when the inorganic reductant is limited, organic matter is the primary contributor to the black-odor water [5,57]. The excess organic matter would cause microbes to utilize the dissolved oxygen in water rapidly, thereby leading the waterbodies to remain anaerobic. Moreover, organic matter served as an electron donor during fermentation, while high-valence metals and sulfur served as electron acceptors, and sulfide was then combined with metal ions to form black suspended substances (metal sulfide complexes). Similarly, this electron transfer mechanism (via sulfate reduction) in anaerobic digestion also reduced effluent treatment efficiency and exacerbated the difficulty of biogas purification [58,59]. Fortunately, according to the recent report from Tsui et al., the production of hydrogen sulfide could be effectively inhibited by direct injection of conductive materials (e.g., biochar) into the anaerobic digestion system [44], with approximately 26.6% of the total electrons being migrated from hydrogen sulfide production back to methane generation [58]. It provided a highly important contribution to the prevention and treatment of black smelly waterbodies. This study also probed that organic matter was a key factor related to the self-remediation of black-odor water. The biological removal of sulfides is mainly based on chemo-lithotrophic or photoautotrophic sulfur-oxidizing bacteria [60]. The equation for the reaction of this process is 2 CO2 + 2 H2S + 2 H2O → 2 (CH2O) + 2 H++ SO42−. Sulfur-oxidizing bacteria can oxidize sulfide into sulfate using CO2 produced by aerobic chemo-heterotrophic processes as an electron acceptor. Therefore, organic matter as a substrate for aerobic chemo-heterotrophic processes could also influence the black-odor remediation process. Physiochemical factors, such as TP, Fe2+, and S2− were significantly correlated with black-odor-forming microbes. Previous studies have reported that phosphorus could alter the occurrence of black-odor water by reducing the activity of organic matter decomposers [57]. The activity of Fe2+ is higher than that of Mn2+, Cu2+, and other metal ions; hence, Fe2+ promptly combines with S2− to precipitate iron sulfide (FeS), which has been proven to be the predominant contributor to black water. Therefore, pH and COD were paramount physiochemical factors contributing to blackening-odor-related microorganisms, while TP, Fe2+, and S2− were flagship parameters linked to black-odor-forming microbes.
4. Conclusions
This study used several approaches to reveal the effects of different disturbance conditions on black-odor-related microorganisms and establish driving factors for environmental management. Different types of disturbed conditions shape the distribution of water quality and microbial community structures. Site B, disturbed by dams, had the worst water quality, the lowest abundance of functional microbes for Mn, Fe, and S biotransformation, and the highest abundance of functional microbes for fermentation. Natural wetlands surrounded by the terminus of the river (site H) were keys to restoring the self-purification ability and eliminating the black-odor phenomenon. Potential black-odor-repairing microbes, including metal sulfide-oxidizing bacteria and aerobic chemo-heterotrophic bacteria, such as Acinetobacter, Mycobacterium, and Acidovorax, are significantly related to eliminating the black-odor phenomenon of this river. The Mantel test showed that pH and COD were paramount physiochemical factors contributing to blackening-odor-related microorganisms, while TP, Fe2+, and S2− were flagship parameters to black-odor-forming microbes. A relative higher pH and lower levels of other chemical factors could be beneficial for recruiting black-odor-repairing microbes. Notably, only a typical black smelly river was selected as the subject of this study; although, these results aid our knowledge of the biological mechanisms contributing to the formation and remediation of black-odor waterbodies and provide a theoretical basis for bioremediation. However, to obtain more scientific, rigorous, and accepted conclusions, the research scenarios and sample amounts should be further added in next step.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15010521/s1, Table S1. Observed richness (Sobs) and water quality parameters of water samples. Table S2. Reported the bacterial genera related to Mn and Fe cycle in the environment. Table S3. Reported the bacterial genera related to S cycle in the environment. Table S4. Reported the bacterial genera related to C cycle in the environment. Figure S1. Correlation analysis of different physicochemical parameters; red symbolizes a positive correlation, while blue represents a negative correlation; color intensity indicates correlation strength. Significant difference was denoted with ** p < 0.01, * p < 0.05.
Author Contributions
X.Z. (Xun Zhang): methodology, data analysis, data curation, writing—review and editing. Y.R.: methodology, writing—review, funding acquisition and supervision. X.Z. (Xianbin Zhu): conceptualization, methodology, investigation, writing—original draft, writing—review and editing. H.P.: project administration, funding acquisition, and supervision. H.Y.: funding acquisition and supervision. J.W.: methodology, data analysis. M.L.: data analysis, data curation. M.H.: data analysis, data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by National Natural Science Foundation of China (Grant Number 41902257), the Hubei Key Laboratory of Intelligent Yangtze and Hydroelectric Science (No. ZH2002000113, ZH2102000113), the Educational Commission of Hubei Province of China (No. Q20211310), the Department of Ecology and Environmental of Hubei Province of China (No. 2015HB17), and the Natural Science Foundation of Hubei Province of China (No. 2020CFB750).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cao, J.; Sun, Q.; Zhao, D.; Xu, M.; Shen, Q.; Wang, D.; Wang, Y.; Ding, S. A critical review of the appearance of black-odorous waterbodies in China and treatment methods. J. Hazard. Mater. 2020, 385, 121511. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, J.; Lou, Y.; Fang, A.; Zhou, H.; Liu, B.; Xie, G.; Xing, D. MnO2/tourmaline composites as efficient cathodic catalysts enhance bioelectroremediation of contaminated river sediment and shape biofilm microbiomes in sediment microbial fuel cells. Appl. Catal. B Environ. 2020, 278, 119331. [Google Scholar] [CrossRef]
- Miao, S.; Lyu, H.; Xu, J.; Bi, S.; Guo, H.; Mu, M.; Lei, S.; Zeng, S.; Liu, H. Characteristics of the chromophoric dissolved organic matter of urban black-odor rivers using fluorescence and UV–visible spectroscopy. Environ. Pollut. 2021, 268, 115763. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Li, Y.; Shen, Y.; Wang, C.; Wang, P.; Wang, L.; Niu, L.; Zhang, W. Vertical distribution and assemblages of microbial communities and their potential effects on sulfur metabolism in a black-odor urban river. J. Environ. Manag. 2019, 235, 368–376. [Google Scholar] [CrossRef]
- Liang, Z.; Fang, W.; Luo, Y.; Lu, Q.; Juneau, P.; He, Z.; Wang, S. Mechanistic insights into organic carbon-driven water blackening and odorization of urban rivers. J. Hazard. Mater. 2021, 405, 124663. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.-H.; Lü, H.; Li, H.; Li, Y.-W.; Mo, C.-H.; Cai, Q.-Y. Persistent contamination of polycyclic aromatic hydrocarbons (PAHs) and phthalates linked to the shift of microbial function in urban river sediments. J. Hazard. Mater. 2021, 414, 125416. [Google Scholar] [CrossRef]
- Song, C.; Liu, X.; Song, Y.; Liu, R.; Gao, H.; Han, L.; Peng, J. Key blackening and stinking pollutants in Dongsha River of Beijing: Spatial distribution and source identification. J. Environ. Manag. 2017, 200, 335–346. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Z.; Qi, K.; Zhao, C. Different pollutant removal efficiencies of artificial aquatic plants in black-odor rivers. Environ. Sci. Pollut. Res. 2019, 26, 33946–33952. [Google Scholar] [CrossRef]
- Huang, H.; Xu, X.; Liu, X.; Han, R.; Liu, J.; Wang, G. Distributions of four taste and odor compounds in the sediment and overlying water at different ecology environment in Taihu Lake. Sci. Rep. 2018, 8, 6179. [Google Scholar] [CrossRef]
- Yu, D.; Xie, P.; Zeng, C.; Xie, L.; Chen, J. In situ enclosure experiments on the occurrence, development and decline of black bloom and the dynamics of its associated taste and odor compounds. Ecol. Eng. 2016, 87, 246–253. [Google Scholar] [CrossRef]
- Li, K.; Yang, M.; Peng, J.; Liu, R.; Joshi, T.P.; Bai, Y.; Liu, H. Rapid control of black and odorous substances from heavily-polluted sediment by oxidation: Efficiency and effects. Front. Environ. Sci. Eng. 2019, 13, 87. [Google Scholar] [CrossRef]
- Wang, W.-H.; Wang, Y.; Fan, P.; Chen, L.-F.; Chai, B.-H.; Zhao, J.-C.; Sun, L.-Q. Effect of calcium peroxide on the water quality and bacterium community of sediment in black-odor water. Environ. Pollut. 2019, 248, 18–27. [Google Scholar] [CrossRef]
- He, Y.; Song, N.; Jiang, H.-L. Effects of dissolved organic matter leaching from macrophyte litter on black water events in shallow lakes. Environ. Sci. Pollut. Res. 2018, 25, 9928–9939. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.; Stauber, J.; Wood, C.M.; Trenfield, M.; Jolley, D.F. Dissolved organic matter signatures vary between naturally acidic, circumneutral and groundwater-fed freshwaters in Australia. Water Res. 2018, 137, 184–192. [Google Scholar] [CrossRef]
- Feng, K.; Gong, J.; Qu, J.; Niu, R. Dual-Mode-Driven Micromotor Based on Foam-like Carbon Nitride and Fe3O4 with Improved Manipulation and Photocatalytic Performance. ACS Appl. Mater. Interfaces 2022, 14, 44271–44281. [Google Scholar] [CrossRef] [PubMed]
- Niu, R.; Ding, Y.; Hao, L.; Ren, J.; Gong, J.; Qu, J. Plant-Mimetic Vertical-Channel Hydrogels for Synergistic Water Purification and Interfacial Water Evaporation. ACS Appl. Mater. Interfaces 2022, 14, 45533–45544. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xing, H.H.; Qing, M.; Shi, Y.; Ling, Y.; Li, N.B.; Luo, H.Q. From the perspective of high-throughput recognition: Sulfur quantum dots-based multi-channel sensing platform for metal ions detection. Chem. Eng. J. 2023, 452, 139594. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.-J.; Fang, B. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Tian, N.; Huang, H.; Du, X.; Dong, F.; Zhang, Y. Rational nanostructure design of graphitic carbon nitride for photocatalytic applications. J. Mater. Chem. A 2019, 7, 11584–11612. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, P.; Ma, R.; Luo, C.; Wen, T.; Zhao, G.; Cheng, W.; Wang, X. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: A critical review. Catal. Today 2019, 335, 65–77. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Z.; Li, J.; Gao, H.; Qin, S.; Guo, J. Alterations in microbial community during the remediation of a black-odorous stream by acclimated composite microorganisms. J. Environ. Sci. 2022, 118, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Liang, Y.; Cheng, M.; He, Z.; Yu, G. Coupling oxidation of acid volatile sulfide, ferrous iron, and ammonia nitrogen from black-odorous sediment via autotrophic denitrification-anammox by nitrate addition. Sci. Total Environ. 2021, 790, 147972. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.-F.; Zhang, T.; Fang, H.H.-P. Sulfur-driven autotrophic denitrification: Diversity, biochemistry, and engineering applications. Appl. Microbiol. Biotechnol. 2010, 88, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Fang, W.; Cheng, Y.; Cai, H.; Zhang, S. Impact of different types of anthropogenic pollution on bacterial community and metabolic genes in urban river sediments. Sci. Total Environ. 2021, 793, 148475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, F.; Li, X.; Lu, W. Contribution of influent rivers affected by different types of pollution to the changes of benthic microbial community structure in a large lake. Ecotoxicol. Environ. Saf. 2020, 198, 110657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, Z.; Sun, Q.; Ding, Y.; Ding, Z.; Sun, L. Response of chemical properties, microbial community structure and functional genes abundance to seasonal variations and human disturbance in Nanfei River sediments. Ecotoxicol. Environ. Saf. 2019, 183, 109601. [Google Scholar] [CrossRef]
- Wang, L.; Yu, L.; Xiong, Y.; Li, Z.; Geng, J. Study on the governance of black-odor water in Chinese cities. J. Clean. Prod. 2021, 308, 127290. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L.; Zhang, X.; He, M.; Wang, D.; Ren, Y.; Yao, H.; net Victoria Ngegla, J.; Pan, H. Effects of different types of anthropogenic disturbances and natural wetlands on water quality and microbial communities in a typical black-odor river. Ecol. Indic. 2022, 136, 108613. [Google Scholar] [CrossRef]
- Guo, Q.; Planer-Friedrich, B.; Liu, M.; Li, J.; Zhou, C.; Wang, Y. Arsenic and thioarsenic species in the hot springs of the Rehai magmatic geothermal system, Tengchong volcanic region, China. Chem. Geol. 2017, 453, 12–20. [Google Scholar] [CrossRef]
- Basili, M.; Campanelli, A.; Frapiccini, E.; Luna, G.M.; Quero, G.M. Occurrence and distribution of microbial pollutants in coastal areas of the Adriatic Sea influenced by river discharge. Environ. Pollut. 2021, 285, 117672. [Google Scholar] [CrossRef]
- Li, D.; Pan, B.; Han, X.; Li, J.; Zhu, Q.; Li, M. Assessing the potential to use CDOM as an indicator of water quality for the sediment-laden Yellow river, China. Environ. Pollut. 2021, 289, 117970. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xie, X.; Peng, K.; Wang, N.; Zhang, Y.; Deng, Y.; Gan, Y.; Li, Q.; Zhang, Y. Sources and compositional characterization of chromophoric dissolved organic matter in a Hainan tropical mangrove-estuary. J. Hydrol. 2021, 600, 126572. [Google Scholar] [CrossRef]
- Li, S.; Fan, R.; Luo, D.; Xue, Q.; Li, L.; Yu, X.; Huang, T.; Yang, H.; Huang, C. Variation in quantity and quality of rainwater dissolved organic matter (DOM) in a peri-urban region: Implications for the effect of seasonal patterns on DOM fates. Atmos. Environ. 2020, 239, 117769. [Google Scholar] [CrossRef]
- Amonette, J.E.; Charles Templeton, J. Improvements to the Quantitative Assay of Nonrefractory Minerals for Fe(II) and Total Fe Using 1,10-Phenanthroline. Clays Clay Miner. 1998, 46, 51–62. [Google Scholar] [CrossRef]
- GB 3838-2002; Environmental Quality Standard for Surface Water. China Environmental Science Press: Beijing, China, 2002.
- Ministry of Housing and Urban-Rural Development, China. Working Guidelines for the Treatment of Urban Black-Odorous Water. Available online: https://www.mohurd.gov.cn/gongkai/fdzdgknr/bzgf/index.html (accessed on 2 February 2022).
- World Health Organization. A Global Overview of National Regulations and Standards for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2021; ISSN 978-92-4-002364-2. [Google Scholar]
- Shen, Q.; Zhou, Q.; Shang, J.; Shao, S.; Zhang, L.; Fan, C. Beyond hypoxia: Occurrence and characteristics of black blooms due to the decomposition of the submerged plant Potamogeton crispus in a shallow lake. J. Environ. Sci. 2014, 26, 281–288. [Google Scholar] [CrossRef]
- Sheng, Y.; Qu, Y.; Ding, C.; Sun, Q.; Mortimer, R.J.G. A combined application of different engineering and biological techniques to remediate a heavily polluted river. Ecol. Eng. 2013, 57, 1–7. [Google Scholar] [CrossRef]
- Wang, M.; Lu, H.; Li, H.; Qian, X. Pollution level and ecological risk assessment of heavy metals in typical rivers of Taihu basin. Environ. Chem. 2016, 35, 2025–2035. [Google Scholar] [CrossRef]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci. Total Environ. 2020, 749, 141555. [Google Scholar] [CrossRef]
- Chai, N.; Yi, X.; Xiao, J.; Liu, T.; Liu, Y.; Deng, L.; Jin, Z. Spatiotemporal variations, sources, water quality and health risk assessment of trace elements in the Fen River. Sci. Total Environ. 2021, 757, 143882. [Google Scholar] [CrossRef]
- Yuan, J.; Shentu, J.; Feng, J.; Lu, Z.; Xu, J.; He, Y. Methane-associated micro-ecological processes crucially improve the self-purification of lindane-polluted paddy soil. J. Hazard. Mater. 2021, 407, 124839. [Google Scholar] [CrossRef]
- Li, E.; Deng, T.; Yan, L.; Zhou, J.; He, Z.; Deng, Y.; Xu, M. Elevated nitrate simplifies microbial community compositions and interactions in sulfide-rich river sediments. Sci. Total Environ. 2021, 750, 141513. [Google Scholar] [CrossRef] [PubMed]
- Dahl, C. A biochemical view on the biological sulfur cycle. Environ. Technol. Treat Sulfur Pollut. Princ. Eng. 2020, 2, 55–96. [Google Scholar] [CrossRef]
- Lovley, D. Dissimilatory Fe (III) and Mn (IV) reduction. Microbiol. Rev. 1991, 55, 259–287. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Zhang, C.; Ge, Y.; Yao, H.; Chen, X.; Hu, M. Iron oxidation-reduction and its impacts on cadmium bioavailability in paddy soils: A review. Front. Environ. Sci. Eng. 2012, 6, 509–517. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Seager, S.; Schrenk, M.; Bains, W. An astrophysical view of Earth-based metabolic biosignature gases. Astrobiology 2012, 12, 61–82. [Google Scholar] [CrossRef]
- Xing, P.; Guo, L.; Tian, W.; Wu, Q.L. Novel Clostridium populations involved in the anaerobic degradation of Microcystis blooms. ISME J. 2011, 5, 792–800. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, H.; Lan, S.; Wang, C.; Li, X.; Xing, Y.; Yue, H.; Li, Q.; Wang, L.; Xie, Y. Evolution of urban black and odorous water: The characteristics of microbial community and driving-f actors. J. Environ. Sci. 2022, 112, 94–105. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, D.; Liu, X.; Xie, H.; Lou, X.; Zeng, C. Combined microbial degradation of crude oil under alkaline conditions by Acinetobacter baumannii and Talaromyces sp. Chemosphere 2021, 273, 129666. [Google Scholar] [CrossRef]
- Kong, M.; Han, T.; Chen, M.; Zhao, D.; Chao, J.; Zhang, Y. High mobilization of phosphorus in black-odor river sediments with the increase of temperature. Sci. Total Environ. 2021, 775, 145595. [Google Scholar] [CrossRef]
- Ding, X.-L.; Li, Y.-M.; Lü, H.; Zhu, L.; Wen, S.; Lei, S.-H. Analysis of Absorption Characteristics of Urban Black-odor Water. Huan Jing Ke Xue 2018, 39, 4519–4529. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarinasab, S.; Motamedian, E. Improving ethanol production by studying the effect of pH using a modified metabolic model and a systemic approach. Biotechnol. Bioeng. 2021, 118, 2934–2946. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, L.; Yang, L.; Liu, C.; Li, J.; Li, N. Combined impact of organic matter, phosphorus, nitrate, and ammonia nitrogen on the process of blackwater. Environ. Sci. Pollut. Res. 2021, 28, 32831–32843. [Google Scholar] [CrossRef] [PubMed]
- Tsui, T.-H.; Zhang, L.; Zhang, J.; Dai, Y.; Tong, Y.W. Engineering interface between bioenergy recovery and biogas desulfurization: Sustainability interplays of biochar application. Renew. Sustain. Energy Rev. 2022, 157, 112053. [Google Scholar] [CrossRef]
- Tsui, T.-H.; Zhang, L.; Zhang, J.; Dai, Y.; Tong, Y.W. Methodological framework for wastewater treatment plants delivering expanded service: Economic tradeoffs and technological decisions. Sci. Total Environ. 2022, 823, 153616. [Google Scholar] [CrossRef]
- Hao, T.-W.; Xiang, P.-Y.; Mackey, H.R.; Chi, K.; Lu, H.; Chui, H.-K.; van Loosdrecht, M.C.M.; Chen, G.-H. A review of biological sulfate conversions in wastewater treatment. Water Res. 2014, 65, 1–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).