A Technoeconomic Resilience and Exergy Analysis Approach for the Evaluation of a Vaccine Production Plant in North-East Colombia
Abstract
1. Introduction
2. Materials and Methods
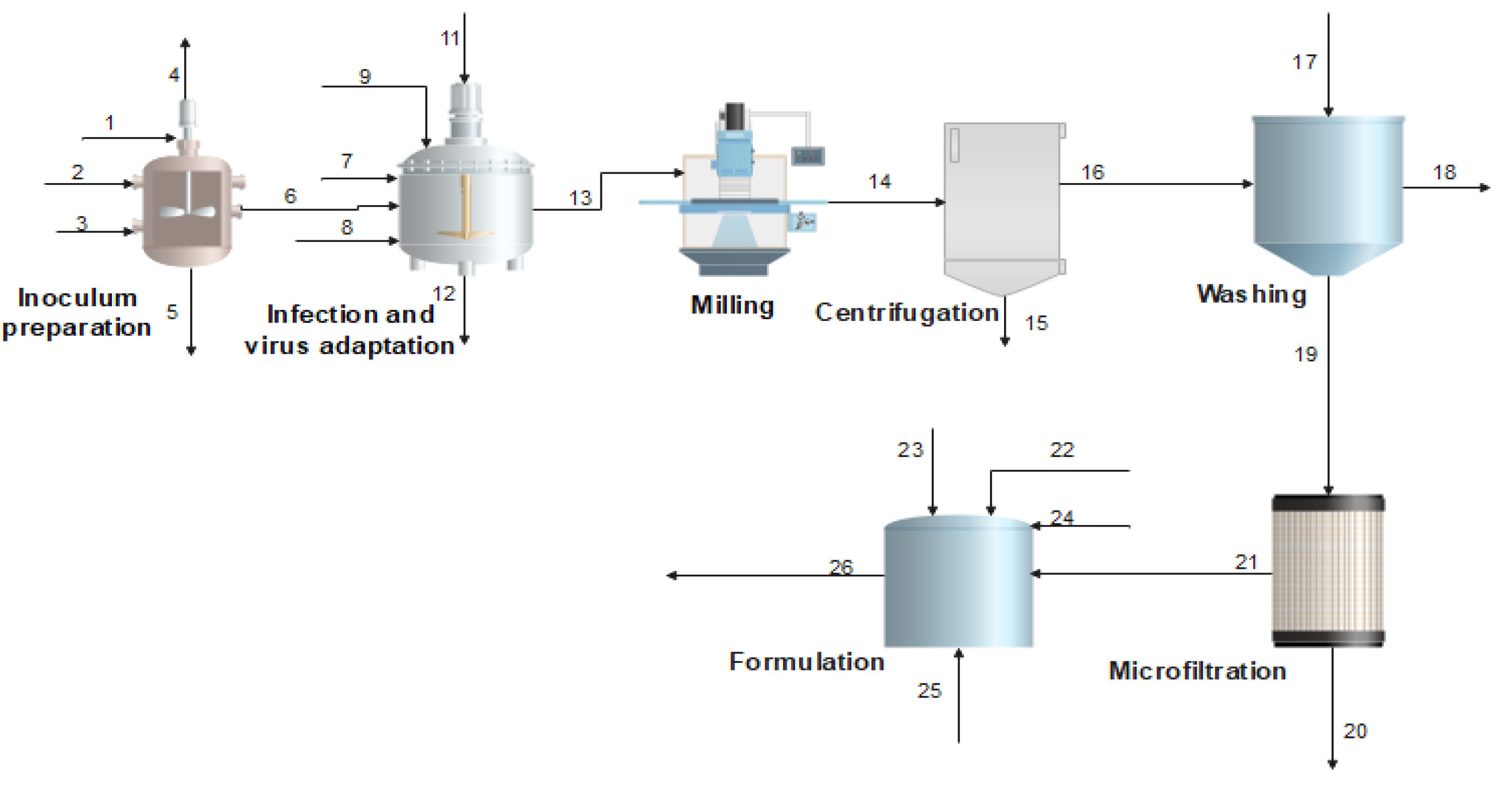
2.1. Process Description
2.2. Technoeconomic Analysis
2.3. Exergy Analysis
3. Results and Discussion
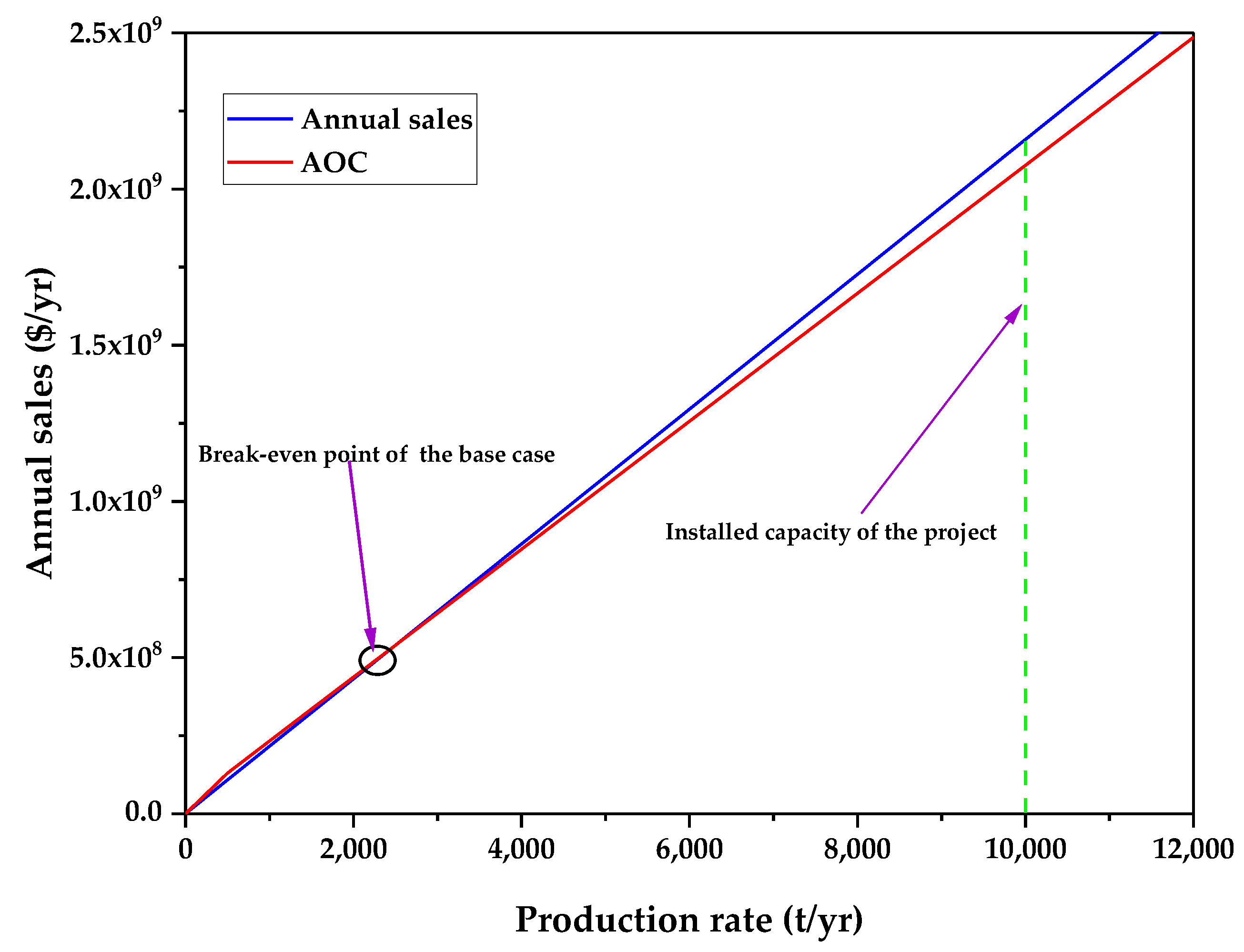
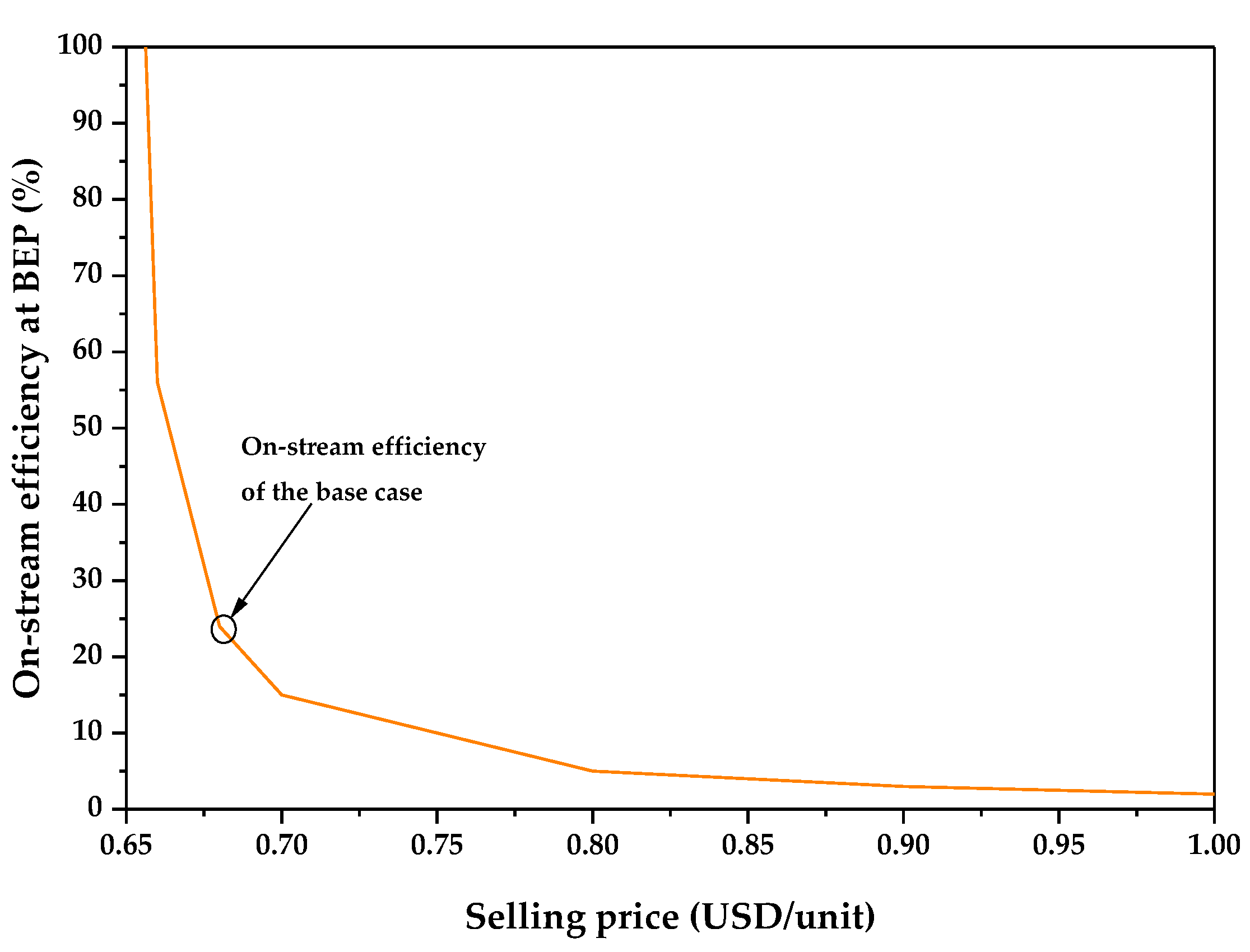
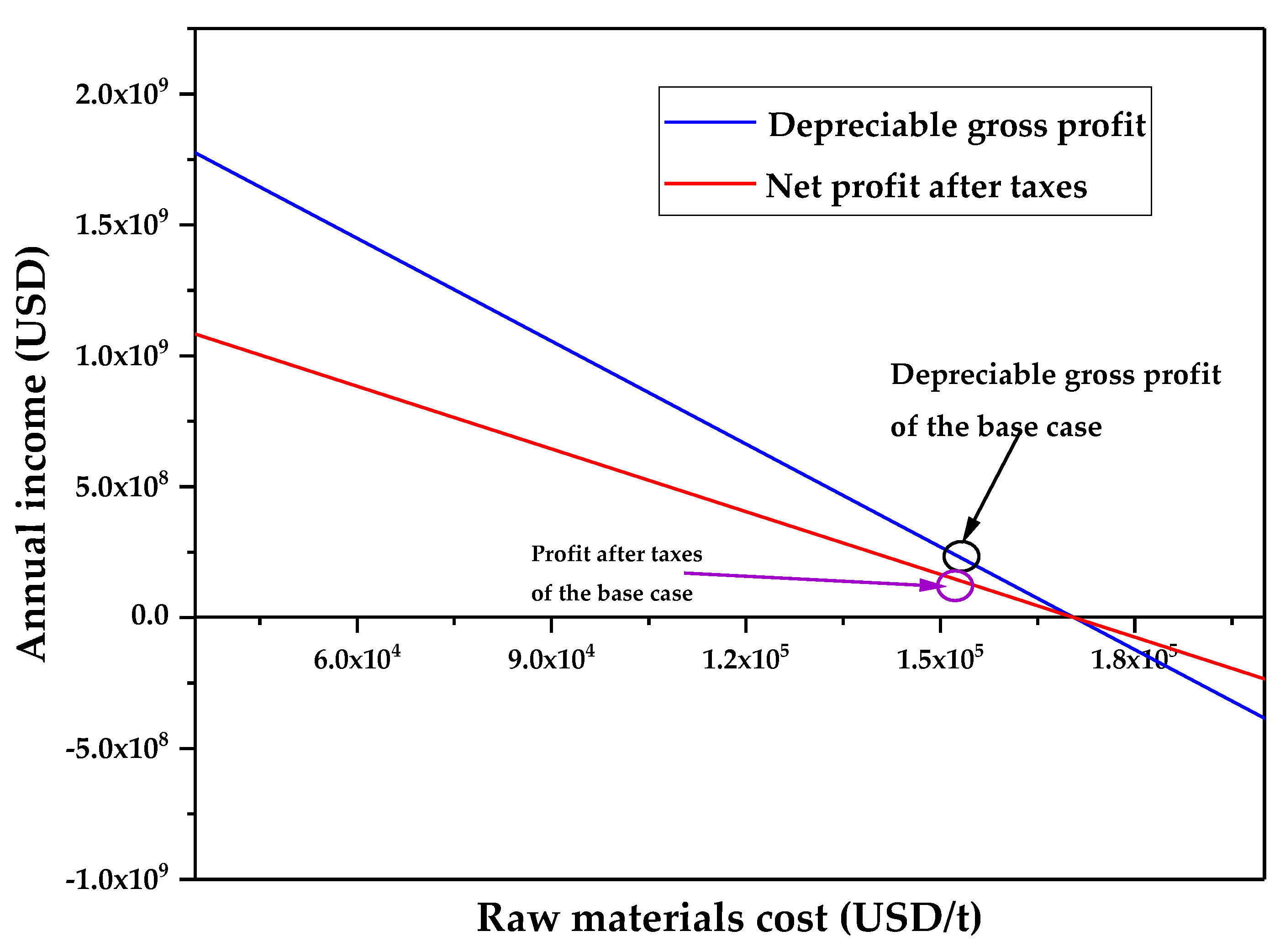
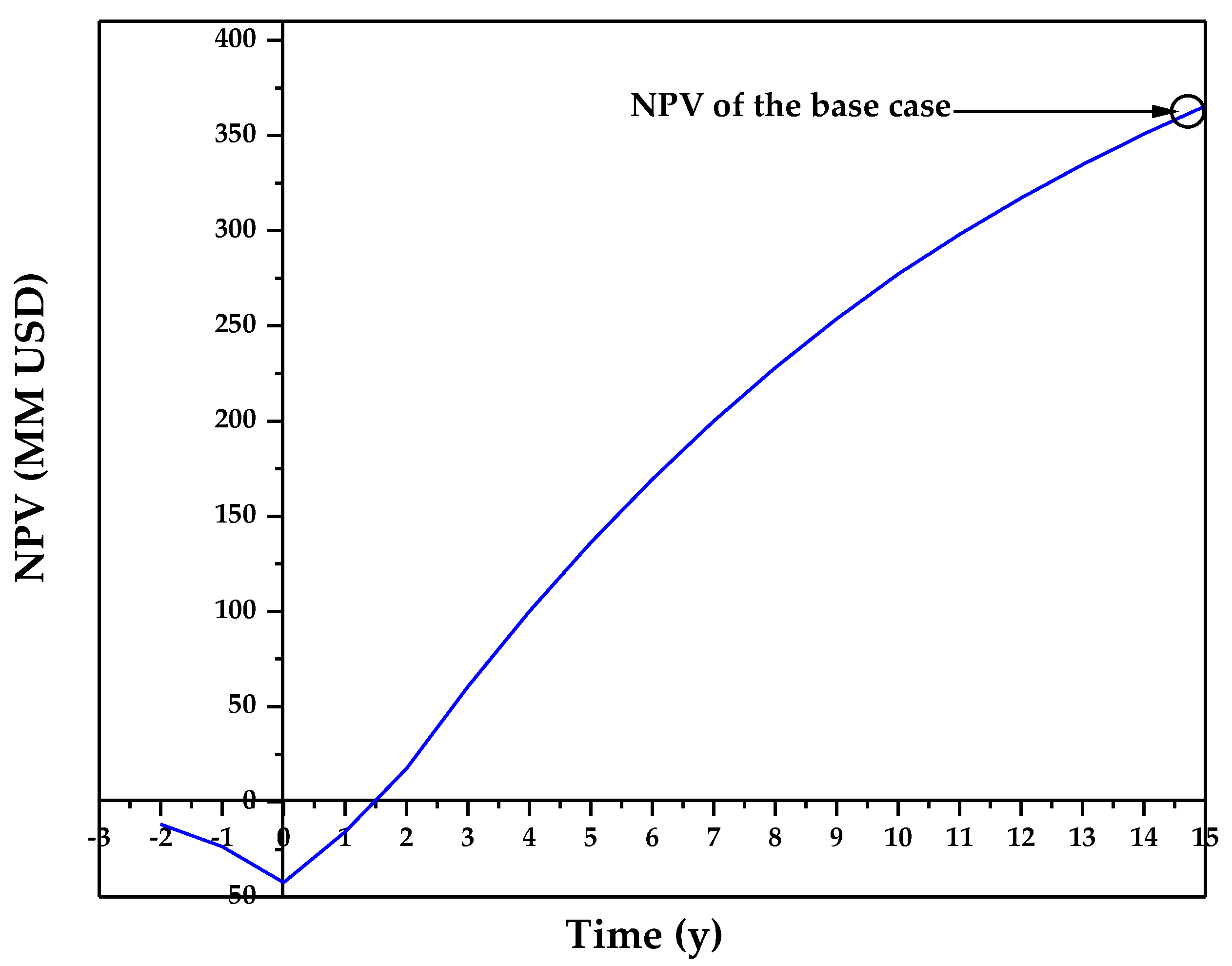
3.1. Technoeconomic Resilience Evaluation
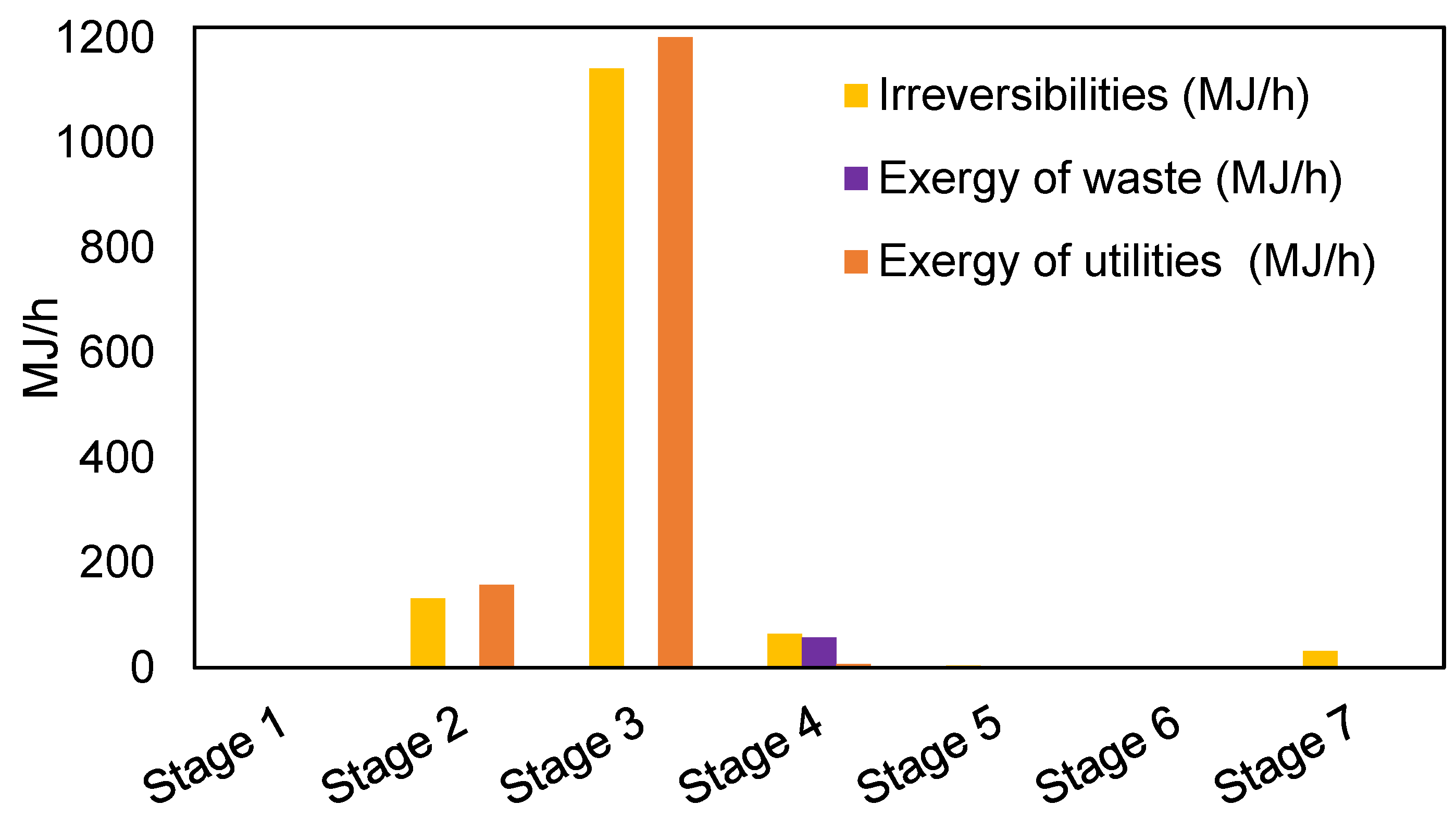
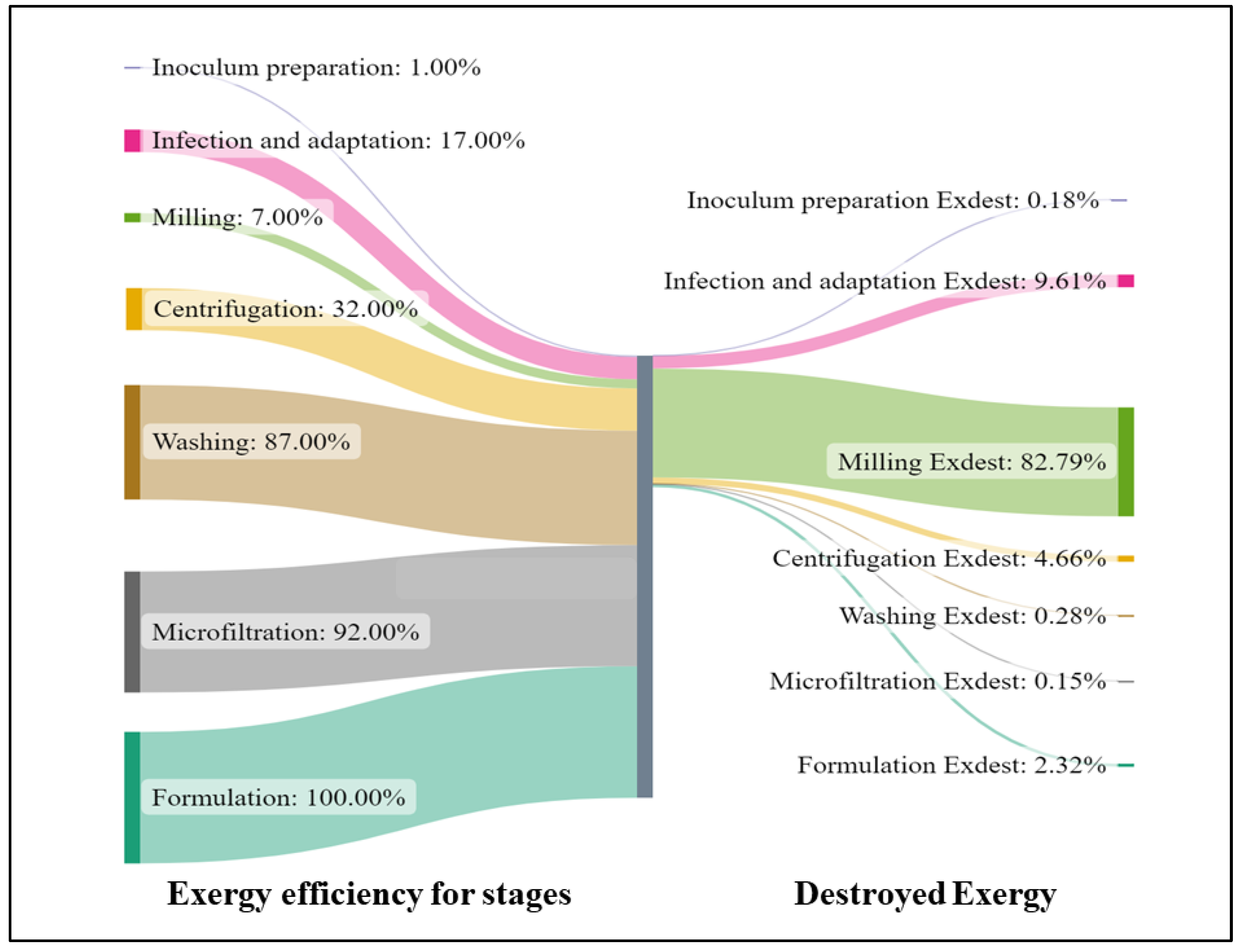
3.2. Exergy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| FCI | Fixed Capital Investment ($) |
| FCI0 | Initial Value of Depreciable Fixed Capital Investment ($) |
| FCIs | Salvage Value of Fixed Capital Investment ($) |
| OC | Operating Costs ($) |
| DPC | Direct Production Costs ($/y) |
| POH | Plant Overhead ($/y) |
| GE | General Expenses ($/y) |
| AFC | Annualized Fixed Costs ($/y) |
| ACF | Net Profit for Year n ($) |
| AOC | Annualized Operating Costs ($/y) |
| NVOC | Normalized Variable Operating Cost ($/t-rm) |
| PAT | Profit after Taxes ($/y) |
| CCF | Cumulative Cash Flow (1/y) |
| ACR | Annual Cost/Benefit Ratio |
| ROI | Return on Investment (%) |
| NPV | Net Present Value (MM$) |
| PBP | Payback Period (y) |
| DGP | Gross Profit (depreciation included) (MM$/y) |
| n | Years |
| i | Inflation Rate (%) |
| θi | Ratio between the quantity of product i obtained per unit of raw material |
| itr | Tax rate set by the government for income derived from the process (%) |
| Mass flow of raw material (t/y) | |
| Exergy of mass flow (MJ/h) | |
| Exergy of heat (MJ/h) | |
| Exergy of work (MJ/h) | |
| Physical Exergy (MJ/h) | |
| Chemical Exergy of the mixture (MJ/h) | |
| Chemical Exergy ((MJ/kg) | |
| Exergy of utilities (MJ/h) | |
| Potential Exergy (MJ/h) | |
| Kinetic Exergy (MJ/h) | |
| Exergy of products (MJ/h) | |
| Exergy of waste (MJ/h) | |
| Exergy efficiency (%) | |
| Gibbs free energy of formation (MJ/kmol) | |
| P | Pressure (atm) |
| P0 | Pressure of the reference state (atm) |
| T | Temperature (K) |
| T0 | Temperature of the reference state (K) |
| Molar volume (m3/mol) | |
| Universal constant of gases (MJ/kmol·K) | |
| Molar fraction | |
| Number of atoms of elements j | |
| Heat capacity at constant pressure (J/kg·K) |
References
- Warmath, C.R.; Ortega-Sanchez, I.R.; Duca, L.M.; Porter, R.M.; Usher, M.G.; Bresee, J.S.; Lafond, K.E.; Davis, W.W. Comparisons in the health and economic assessments of using quadrivalent versus trivalent influenza vaccines: A systematic literature review. Value Health 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sellers, S.A.; Hagan, R.S.; Hayden, F.G.; Fischer, W.A. The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influ. Other Respir. Viruses 2017, 11, 372–393. [Google Scholar] [CrossRef] [PubMed]
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Viboud, C. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Walensky, R.P.; Jernigan, D.B.; Bunnell, R.; Layden, J.; Kent, C.K.; Gottardy, A.J.; Sanchez, J.N. Morbidity and Mortality Weekly Report Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–2022 Influenza Season Centers for Disease Control and Prevention. MMWR Recomm. Rep. 2021, 70, 1. [Google Scholar]
- Loeb, M.; Roy, A.; Dokainish, H.; Dans, A.; Palileo-Villanueva, L.; Karaye, K.; Zhu, J.; Liang, Y.; Goma, F.; Damasceno, A.; et al. Influenza vaccine to reduce adverse vascular events in patients with heart failure: A multinational randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 2022, 10, e1835–e1844. [Google Scholar] [CrossRef]
- Karlsson, E.A.; Ciuoderis, K.; Freiden, P.J.; Seufzer, B.; Jones, J.C.; Johnson, J.; Schultz-Cherry, S. Prevalence and characterization of influenza viruses in diverse species in Los Llanos, Colombia. Emerg. Microbes Infect. 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Gasparini, R.; Amicizia, D.; Lai, P.L.; Panatto, D. Influenza vaccination: From epidemiological aspects and advances in research to dissent and vaccination policies. J. Prev. Med. Hyg. 2016, 57, E1–E4. [Google Scholar]
- Contreras, J.E.; Ruiz, S.L.; García, J.B.; Urbina, N.A.; López, G.L.; Barajas, A.F.; Zuorro, A. A simulation analysis of an influenza vaccine production plant in areas of high humanitarian flow. A preliminary study for the region of Norte de Santander (Colombia). Appl.Sci. 2022, 12, 183. [Google Scholar]
- Barragan, D.; Morales, S.A.; Kafarov, V. Exergetic analysis of combustion processes of variable mixtures of refinery residual gas: Effect of propane. Chem. Eng. Trans. 2018, 70, 1183–1188. [Google Scholar]
- Klemeš, J.J.; Jiang, P.; Fan, Y.; Van Bokhari, A.; Wang, X.C. COVID-19 pandemics Stage II—Energy and environmental impacts of vaccination. Renew. Sustain. Energy Rev. 2021, 150, 111400. [Google Scholar] [CrossRef] [PubMed]
- Crager, S.E. Improving global access to new vaccines: Intellectual property, technology transfer, and regulatory pathways. Am. J. Public Health 2018, 108, S414–S420. [Google Scholar] [CrossRef]
- Kawakami, T.; Kawamura, K.; Fujimori, K.; Koike, A.; Amano, F. Influence of the culture medium on the production of nitric oxide and expression of inducible nitric oxide synthase by activated macrophages in vitro. Biochem. Biophy. Rep. 2016, 5, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.S.; Peters, J.I. Plant Design and Economics for Chemical Engineers; Engineering Economics; McGraw-Hill: New York, NY, USA, 2002; Volume 5. [Google Scholar]
- Li, G.; Chang, Y.; Liu, T.; Yu, Z.; Liu, F.; Ma, S.; Weng, Y.; Zhang, Y. Hydrogen element flow and economic analyses of a coal direct chemical looping hydrogen generation process. Energy 2020, 206, 118243. [Google Scholar] [CrossRef]
- Romero, J.; Vergara, L.; Peralta, Y.; González, A. A Techno-Economic Sensitivity Approach for Development of a Palm-based Biorefineries in Colombia. Chem. Eng. Trans. 2017, 57, 13–18. [Google Scholar] [CrossRef]
- Herrera-Rodriguez, T.; Parejo-Palacio, V.; González-Delgado, A. Techno-economic Evaluation of Creole Avocado Biomass Valorization via Oil Production in North-colombia. Chem. Eng. Trans. 2022, 92, 409–414. [Google Scholar] [CrossRef]
- El-Halwagi, M. Sustainable Design through Process Integration: Fundamentals and Applications to Industrial Pollution Prevention, Resource Conservation, and Profitability Enhancement; Elsevier: Amsterdam, The Netherland, 2011. [Google Scholar]
- El-Halwagi, M. A return on investment metric for incorporating sustainability in process integration and improvement projects. Clean Technol. Environ. Policy 2016, 19, 611–617. [Google Scholar] [CrossRef]
- Terzi, R. Application of Exergy Analysis to Energy Systems; Application of Exergy: London, UK, 2018. [Google Scholar] [CrossRef]
- González-Delgado, Á.D.; Moreno-Sader, K.A.; Martínez-Consuegra, J.D. Biorrefinación Sostenible del Camarón: Desarrollos desde la Ingeniería de Procesos Asistida por Computador; Corporación Universitaria Minuto de Dios-UNIMINUTO: Bogotá, Colombia, 2022. [Google Scholar]
- Herrera-Rodriguez, T.; Parejo-Palacio, V.; González-Delgado, A. Quality of Energy Conservation in an Avocado Oil Extraction Via Exergy Analysis. Chem. Eng. Trans. 2022, 91, 241–246. [Google Scholar] [CrossRef]
- Moreno, K.; Meramo, S.I.; González, A. Computer-aided environmental and exergy analysis as decisionmaking tools for selecting bio-oil feedstocks. Renew. Sustain. Energy Rev. 2019, 112, 42–57. [Google Scholar] [CrossRef]
- Arteaga-Díaz, S.J.; Meramo, S.; González-Delgado, Á.D. Computer-Aided Modeling, Simulation, and Exergy Analysis of Large-Scale Production of Magnetite (Fe3O4) Nanoparticles via Coprecipitation. ACS Omega 2021, 6, 30666–30673. [Google Scholar] [CrossRef]
- Szargut, J. Chemical exergies of the elements. Appl. Energy 1989, 32, 269–286. [Google Scholar] [CrossRef]
- Reed, J.J. Digitizing “The NBS tables of chemical thermodynamic properties: Selected values for inorganic and C1 and C2 organic substances in SI Units”. J. Res. Nat. Inst. Stand. Technol. 2020, 125, 125007. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.; Minceva, M. A thermodynamic insight into viral infections: Do viruses in a lytic cycle hijack cell metabolism due to their low Gibbs energy. Heliyon 2020, 6, e03933. [Google Scholar] [CrossRef] [PubMed]
- Lamers, P.; Roni, M.S.; Tumuluru, J.S.; Jacobson, J.J.; Cafferty, K.G.; Hansen, J.K.; Bals, B. Techno-economic analysis of decentralized biomass processing depots. Biores. Technol. 2015, 194, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Nallaperuma, W.; Ekanayake, U.; Punchi-Manage, R. Identifying Factors that Affect the Downtime of a Production Process. In Proceedings of the 15th Open University Research Sessions (OURS 2017), Colombo, Sri Lanka, 16–17 November 2017. [Google Scholar]
- Herrera-Rodriguez, T.; Parejo-Palacio, V.; González-Delgado, Á.D. Technoeconomic sensibility analysis of industrial agar production from red algae. Chem. Eng. Trans. 2018, 70, 2029–2034. [Google Scholar]
- Moreno Sader, K.; León Pulido, J.; González-Delgado, Á. Evaluación de la producción de aceite crudo de palma y palmiste en el norte de Colombia mediante el análisis de exergía asistido por computador. Revista ION 2021, 34, 31–41. [Google Scholar] [CrossRef]
- Rajemi, M.F.; Mativenga, P.T.; Aramcharoen, A.; Velchev, S.; Kolev, I.; Ivanov, K.; Rajemi, M.F. Energy Analysis in Turning and Milling. J. Clean. Prod. 2010, 18, 149–152. [Google Scholar]
- González-Delgado, Á.D.; García-Martínez, J.B.; Barajas-Solano, A.F. Inherent Safety Analysis and Sustainability Evaluation of a Vaccine Production Topology in North-East Colombia. Sustainability 2022, 14, 9985. [Google Scholar] [CrossRef]




| Parameters | Value/Description |
|---|---|
| Main product flow (t/y) | 3326.4 |
| Raw materials cost ($/t) | 163,891.7201 |
| Useful life of the plant (years) | 15 |
| Salvage value | 10% of depreciable FCI |
| Construction time of the plant (years) | 3 |
| Income tax rate (itr) | 39% |
| Interest | 9% |
| Type of process | New and unproven |
| Process control | Digital |
| Project type | Plant on non-built land |
| Soil type | Soft clay |
| Selling price per unit (USD) | 0.68 |
| Cost of Capital Investment | Total (USD) |
|---|---|
| Equipment Purchase Cost | 7,755,000.00 |
| Total direct plant cost (TPDC) | 18,786,000.00 |
| Contractor’s fee | 1,267,000.00 |
| Land | 775,500.00 |
| Contingency | 2,533,000.00 |
| Total Plant Indirect Cost (TPIC) | 14,074,500.00 |
| FCI | 32,860,500.00 |
| Start up (SU) | 3,286,050.00 |
| WCI | 26,288,400.00 |
| Total Capital Investment (TCI) | 62,434,950.00 |
| Total Product Cost (TPC) | Total (USD/y) |
|---|---|
| Raw materials | 1,716,852,131.18 |
| Utilities (U) | 48,445.00 |
| Maintenance and repairs (MR) | 1,643,025.00 |
| Operating supplies | 246,453.75 |
| Operating labor (OL) | 8,537,291.00 |
| Direct supervision and clerical labor | 1,280,593.65 |
| Laboratory charges | 853,729.10 |
| Patents and royalties | 328,605.00 |
| Direct production cost (DPC) | 1,729,790,273.68 |
| Depreciation (D) | 2,190,700.00 |
| Local taxes | 985,815.00 |
| Insurance | 328,605.00 |
| Interest/rent | 624,349.50 |
| Fixed charges (FCH) | 4,129,469.50 |
| Plant overhead (POH) | 5,122,374.60 |
| Total Manufacturing Cost (TMC) | 1,739,042,117.78 |
| General expenses (GE) | 434,760,529.45 |
| Total product cost (TPC) | 2,173,802,647.23 |
| Economic Parameters of the Base Case | Value |
|---|---|
| Gross Profit (depreciation not included) (GP) (USD/y) | 90,340,052.77 |
| Gross Profit (depreciation included) (DGP) | 88,149,352.77 |
| Profit After Taxes (PAT) (USD/y) | 53,771,105.19 |
| Payback Period (PBP) (years) | 0.61 |
| %ROI | 86% |
| NPV (MM USD) | 388.87 |
| Annual Cost/Revenue | 48.24 |
| Stream | 1 | 4 | 6 | 12 | 13 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|
| T °C | 25.00 | 111.00 | 100.00 | 39.64 | 39.62 | 25.00 | 25.00 | 25.00 |
| P (atm) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mass flow (kg/h) | 2.99 | 0.36 | 3.32 | 0.32 | 3.24 | 0.89 | 0.99 | 1.12 |
| Exchemical (MJ/h) | 0.00 | 0.00 | 0.01 | 0.00 | 27.41 | 30.11 | 0.07 | 3.19 |
| Exphysical (MJ/h) | 0.00 | 0.01 | 0.09 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 |
| Components | ||||||||
| Cholesterol | 0.000 | 0.000 | 3.1 × 10−6 | 0.000 | 3.3 × 10−5 | 8.4 × 10−5 | 0.000 | 1.2 × 10−5 |
| Urea | 0.000 | 0.000 | 2.3 × 10−6 | 0.000 | 2.5 × 10−5 | 6.4 × 10−5 | 0.000 | 9.3 × 10−6 |
| Carbon Dioxide | 0.000 | 1.000 | 0.963 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Water | 0.990 | 0.000 | 0.036 | 0.000 | 0.864 | 0.436 | 0.989 | 0.920 |
| Sodium bicarbonate | 0.004 | 0.000 | 9.9 × 10−5 | 0.000 | 0.001 | 0.007 | 0.000 | 0.000 |
| Sodium chloride | 0.006 | 0.000 | 1.7 × 10−4 | 0.000 | 0.002 | 0.001 | 0.008 | 0.007 |
| D-glucose | 0.000 | 0.000 | 4.9 × 10−6 | 0.000 | 5.4 × 10−5 | 1.4 × 10−4 | 0.001 | 0.001 |
| Sodium phosphate | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 |
| Nitrogen | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Oxygen | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Penicillin | 0.000 | 0.000 | 0.000 | 0.000 | 0.009 | 0.022 | 0.000 | 0.000 |
| Potassium alum | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Organelles | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.089 | 0.000 | 0.071 |
| Virus | 0.000 | 0.000 | 0.000 | 0.000 | 0.123 | 0.449 | 0.000 | 0.000 |
| Stream | 19 | 20 | 21 | 22 | 23 | 25 | 26 |
|---|---|---|---|---|---|---|---|
| T °C | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| P (atm) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mass flow (kg/h) | 0.76 | 0.38 | 0.37 | 297.62 | 89.29 | 453.04 | 840.32 |
| Exchemical (MJ/h) | 26.33 | 1.18 | 24.30 | 11,322.53 | 3396.91 | 3401.18 | 18,112.89 |
| Exphysical (MJ/h) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Components | |||||||
| Cholesterol | 8.1 × 10−5 | 1.6 × 10−4 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Urea | 6.2 × 10−5 | 1.2 × 10−4 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Carbon Dioxide | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Water | 0.458 | 0.916 | 0.000 | 0.000 | 0.000 | 0.522 | 0.281 |
| Sodium bicarbonate | 0.001 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Sodium chloride | 0.001 | 0.003 | 0.000 | 0.000 | 0.000 | 0.005 | 0.003 |
| D-glucose | 1.3 × 10−4 | 2.6 × 10−4 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Sodium phosphate | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Nitrogen | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Oxygen | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Penicillin | 0.026 | 0.026 | 0.000 | 1.000 | 1.000 | 0.158 | 0.546 |
| Potassium alum | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.316 | 0.170 |
| Organelles | 0.000 | 0.053 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Virus | 0.513 | 0.000 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Component | Chemical Exergy (MJ/kg) |
|---|---|
| Cholesterol | 57.36645078 |
| Urea | 0.011465201 |
| Carbon Dioxide | 0.000454545 |
| Water | 0.05 |
| Sodium bicarbonate | 0.257142857 |
| Sodium chloride | 0.244695414 |
| D-glucose | 15.50434068 |
| Sodium phosphate | 0.41695122 |
| Nitrogen | 0.025714286 |
| Oxygen | 0.1240625 |
| Penicillin | 38.04359354 |
| Potassium alum | 4.831223629 |
| Organelles | 39.20553822 |
| Virus | 65.68514885 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Delgado, Á.D.; García-Martínez, J.B.; Barajas-Solano, A.F. A Technoeconomic Resilience and Exergy Analysis Approach for the Evaluation of a Vaccine Production Plant in North-East Colombia. Sustainability 2023, 15, 287. https://doi.org/10.3390/su15010287
González-Delgado ÁD, García-Martínez JB, Barajas-Solano AF. A Technoeconomic Resilience and Exergy Analysis Approach for the Evaluation of a Vaccine Production Plant in North-East Colombia. Sustainability. 2023; 15(1):287. https://doi.org/10.3390/su15010287
Chicago/Turabian StyleGonzález-Delgado, Ángel Darío, Janet B. García-Martínez, and Andrés F. Barajas-Solano. 2023. "A Technoeconomic Resilience and Exergy Analysis Approach for the Evaluation of a Vaccine Production Plant in North-East Colombia" Sustainability 15, no. 1: 287. https://doi.org/10.3390/su15010287
APA StyleGonzález-Delgado, Á. D., García-Martínez, J. B., & Barajas-Solano, A. F. (2023). A Technoeconomic Resilience and Exergy Analysis Approach for the Evaluation of a Vaccine Production Plant in North-East Colombia. Sustainability, 15(1), 287. https://doi.org/10.3390/su15010287










