Leaching of Willemite Concentrate in Sulfuric Acid Solution at High Temperature
Abstract
1. Introduction
2. Experimental
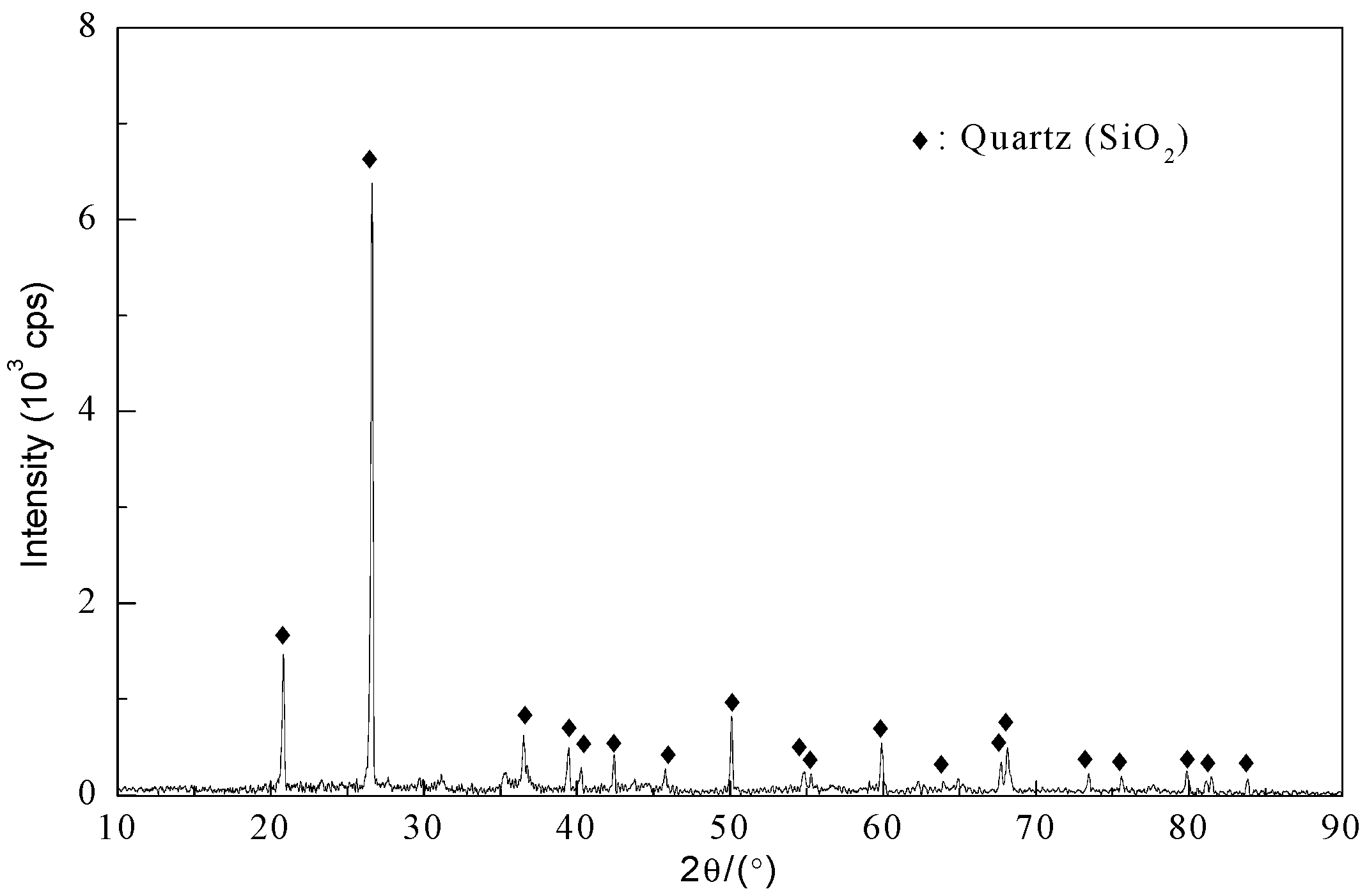
2.1. Materials
2.2. Equipment and Procedure
3. Results and Discussion
3.1. Effect of H2SO4 Concentration
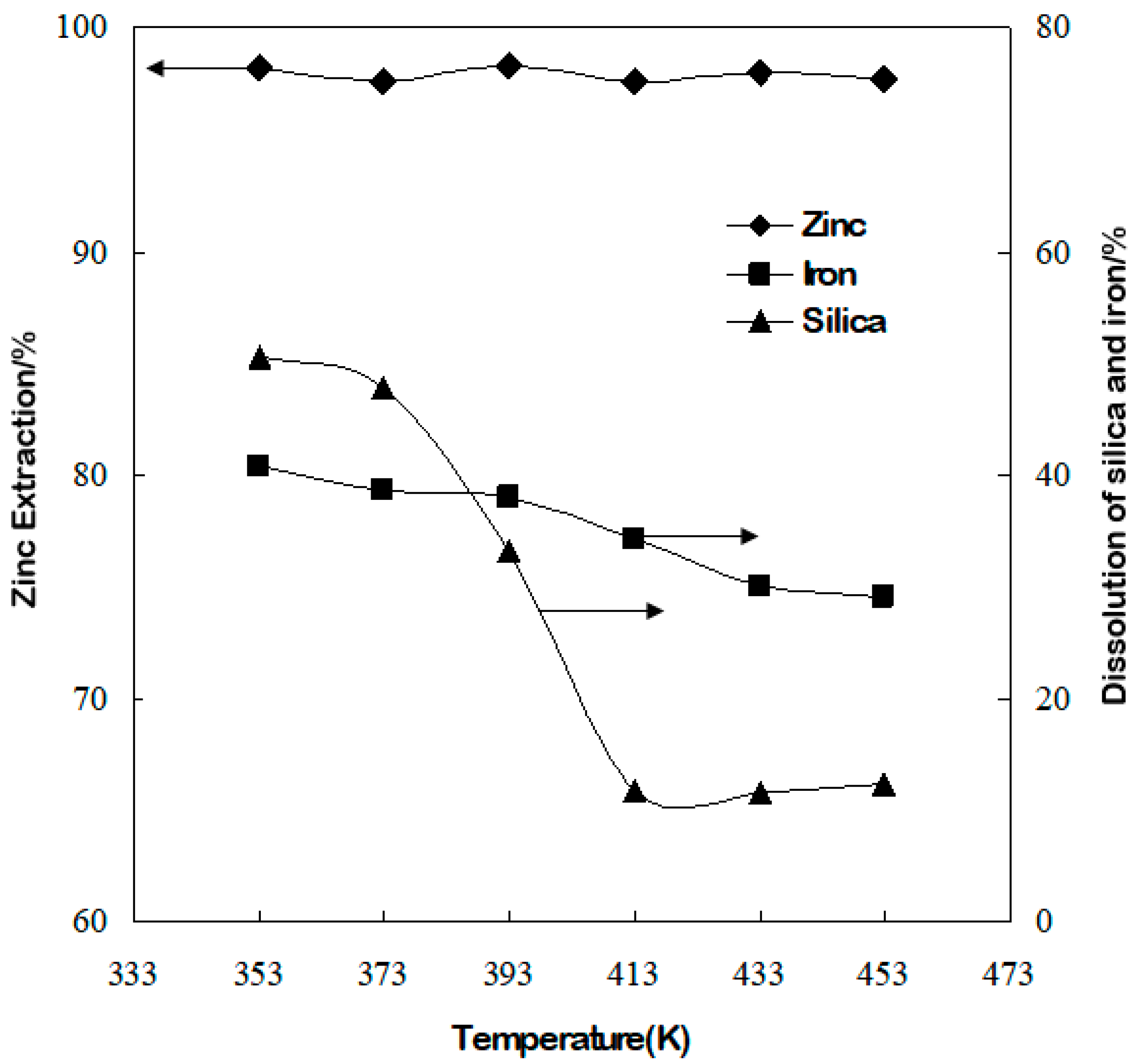
3.2. Effect of Temperature
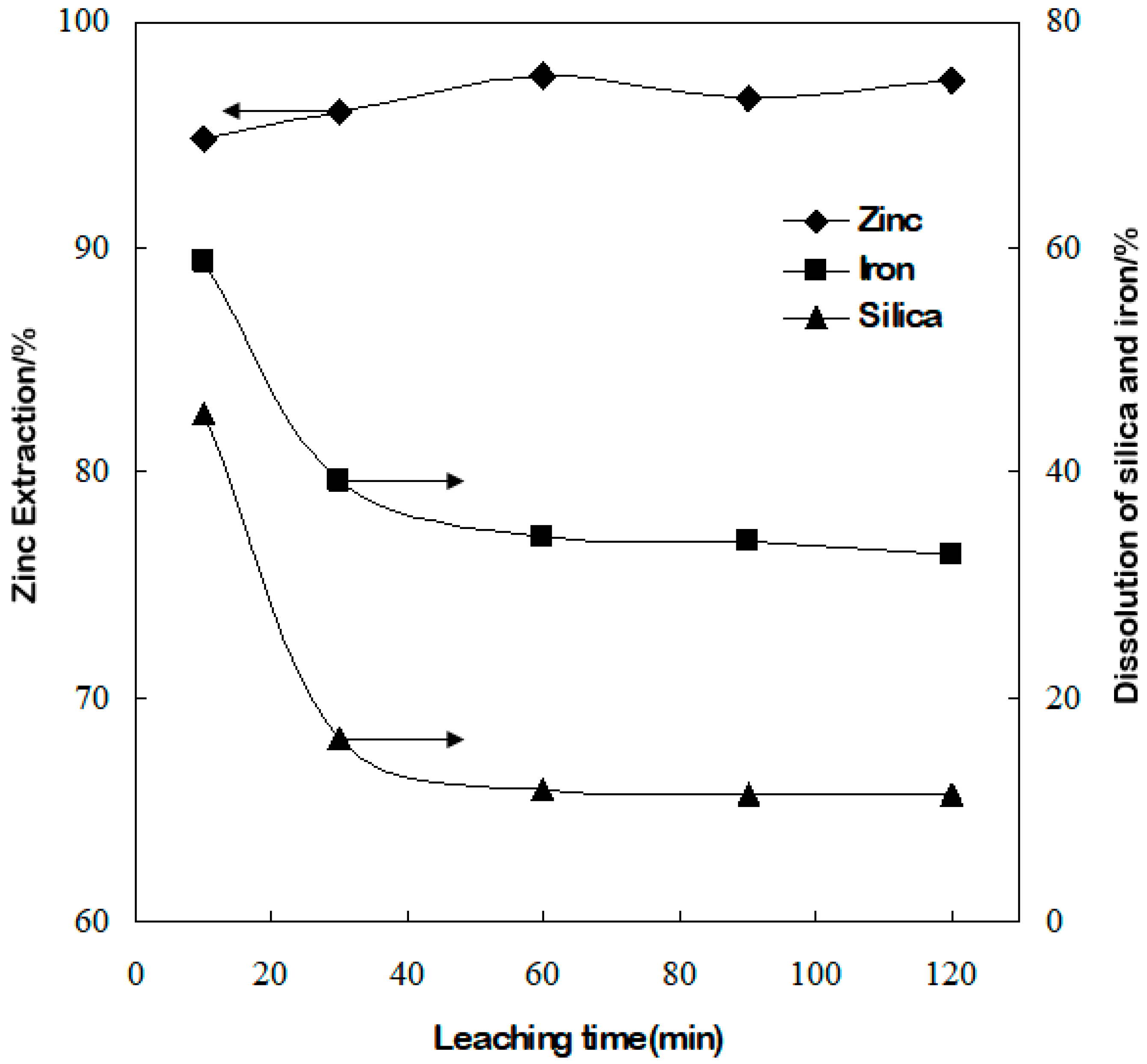
3.3. Effect of Leaching Time
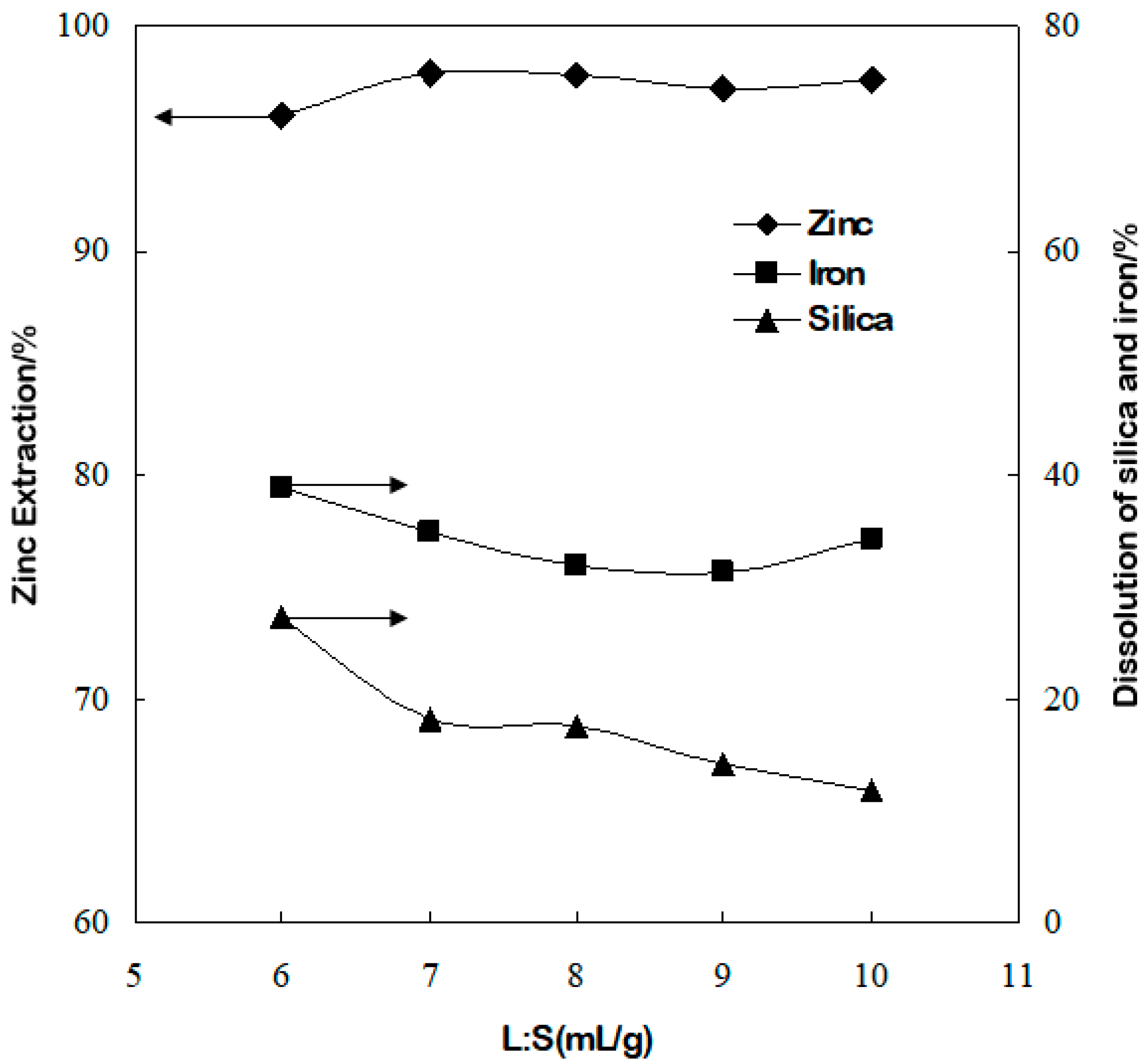
3.4. Effect of Liquid/Solid Ratio
3.5. Effect of Pressure
3.6. Effect of Particle Size
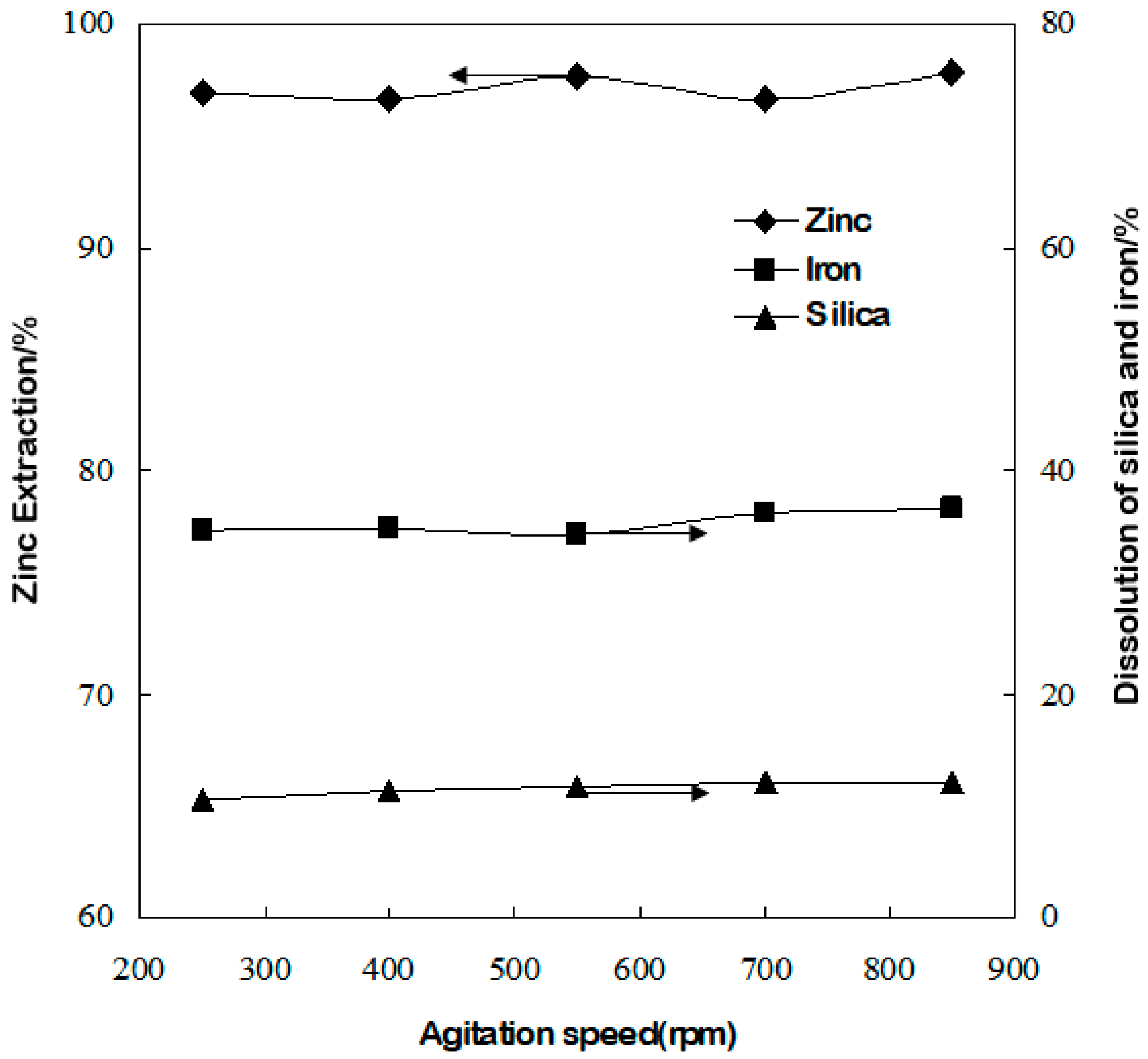
3.7. Effect of Agitation Speed
3.8. Integrated Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fedje, K.K.; Andersson, S. Zinc recovery from Waste-to-Energy fly ash—A pilot test study. Waste Manag. 2020, 118, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Roshanfar, M.; Khanlarian, M.; Rashchi, F.; Motesharezadeh, B. Phyto-extraction of zinc, lead, nickel, and cadmium from a zinc leach residue. J. Clean. Prod. 2020, 266, 121539. [Google Scholar] [CrossRef]
- Huang, Y.K.; Geng, Y.B.; Han, G.H.; Cao, Y.J.; Peng, W.J.; Zhu, X.F.; Zhang, T.A.; Dou, Z.H. A perspective of stepwise utilization of hazardous zinc plant purification residue based on selective alkaline leaching of zinc. J. Hazard. Mater. 2020, 389, 122090. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.A. Kinetics of sulfuric acid leaching of low-grade zinc silicate ore. Hydrometallurgy 2000, 55, 247–254. [Google Scholar] [CrossRef]
- Castro, I.M.; Fietto, J.L.R.; Vieira, R.X.; Trópia, M.J.M.; Campos, L.M.M.; Paniago, E.B.; Brandão, R.L. Bioleaching of zinc and nickel from silicates using Aspergillus niger cultures. Hydrometallurgy 2000, 57, 39–49. [Google Scholar] [CrossRef]
- Espiari, S.; Rashchi, F.; Sadrnezhaad, S.K. Hydrometallurgical treatment of tailings with high zinc content. Hydrometallurgy 2006, 82, 54–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Hua, Y.; Gao, X.; Xu, C.; Li, J.; Li, Y.; Zhang, Q.; Xiong, L.; Su, Z.; Wang, M.; et al. Recovery of zinc from a low-grade zinc oxide ore with high silicon by sulfuric acid curing and water leaching. Hydrometallurgy 2016, 166, 16–21. [Google Scholar] [CrossRef]
- Weidenkaff, A.; Reller, A.; Steinfeld, A. Solar Production of Zinc from the Zinc Silicate Ore Willemite. J. Sol. Energ.—T. ASME 2001, 123, 98–101. [Google Scholar] [CrossRef]
- Dufresne, R.E. Quick leaching of siliceous zinc ore. J. Met. 1976, 28, 8–12. [Google Scholar]
- Matthew, I.G.; Elsner, D. The hydrometallurgical treatment of zinc silicate ores. Metall. Mater. Trans. B 1977, 8, 73–83. [Google Scholar] [CrossRef]
- Terry, B.; Monhemius, A.J. Acid dissolution of willemite ((Zn, Mn)2SiO4) and hemimorphite (Zn4Si2O7(OH)2H2O). Metall. Mater. Trans. B 1983, 14, 335–345. [Google Scholar] [CrossRef]
- Bodas, M.G. Hydrometallurgical treatment of zinc silicate ore from Thailand. Hydrometallurgy 1996, 40, 37–49. [Google Scholar] [CrossRef]
- Gnoinski, J. Skorpion zinc optimization and innovation. J. S. Afr. Inst. Min. Metall. 2007, 107, 657–662. [Google Scholar]
- Souza, A.D.; Pina, P.S.; Santos, F.M.F.; da Silva, C.A.; Leão, V.A. Effect of iron in zinc silicate concentrate on leaching with sulphuric acid. Hydrometallurgy 2009, 95, 207–214. [Google Scholar] [CrossRef]
- Li, C.X.; Xu, H.S.; Deng, Z.G.; Li, X.B.; Li, M.T.; Wei, C. Pressure leaching of zinc silicate ore in sulfuric acid medium. Trans. Nonferrous Met. Soc. China 2010, 20, 918–923. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Long, S.; Chen, A.L.; Huo, G.H.; Li, H.G.; Jia, X.J.; Chen, X.Y. Mechanochemical leaching of refractory zinc silicate (hemimorphite) in alkaline solution. Hydrometallurgy 2009, 99, 255–258. [Google Scholar] [CrossRef]
- Santos, F.M.F.; Pina, P.S.; Porcaro, R.; Oliveira, V.A.; Silva, C.A.; Leão, V.A. The kinetics of zinc silicate leaching in sodium hydroxide. Hydrometallurgy 2010, 102, 43–49. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Liu, Z.H.; Li, Q.H.; Cao, Z.Y.; Yang, T.Z. Dissolution behavior of willemite in the (NH4)2SO4-NH3-H2O system. Hydrometallurgy 2012, 125–126, 50–54. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Yin, Z.L.; Wu, X.F.; Hu, H.P.; Chen, Q.Y. Leaching Kinetics of Willemite in Ammonia-Ammonium Chloride Solution. Metall. Mater. Trans. B 2011, 42, 633–641. [Google Scholar] [CrossRef]
- Abdel Aal, E.A.; Shukry, Z.E. Application of quick leaching method to an Egyptian zinc silicate ore. Trans. Inst. Min. Metall. 1997, 106, 89–90. [Google Scholar]
- Al-Harahsheh, M.; Kingman, S.W. Microwave-assisted leaching—A review. Hydrometallurgy 2004, 73, 189–203. [Google Scholar] [CrossRef]
- Bu, X.N.; Danstan, J.K.; Hassanzadeh, A.; Vakylabad, A.B.; Chelgani, S.C. Metal extraction from ores and waste materials by ultrasound-assisted leaching—An overview. Min. Proc. Ext. Met. Rev. 2022, 43, 1–18. [Google Scholar] [CrossRef]
- Hua, Y.; Lin, Z.; Yan, Z. Application of microwave irradiation to quick leach of zinc silicate ore. Miner. Eng. 2002, 15, 451–456. [Google Scholar] [CrossRef]
- Wang, X.; Yang, D.J.; Srinivasakannan, C.; Peng, J.H.; Duan, X.H.; Ju, S.H. A comparison of the conventional and ultrasound-augmented leaching of zinc residue using sulphuric acid. Arab. J. Sci. Eng. 2014, 39, 163–173. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Ram, R.; Pownceby, M.; Grocott, S.; Ring, B.; Tardio, J.; Jones, L. A review of acid leaching of uraninite. Hydrometallurgy 2015, 151, 10–24. [Google Scholar] [CrossRef]
- Parker, E.G. Oxidative pressure leaching of zinc concentrates. CIM. Bull. 1987, 74, 145–150. [Google Scholar]
- Xu, Z.F.; Qiu, D.F.; Lu, H.M.; Wang, H.B. Review on research of oxidic–acidic pressure leaching of zinc concentrates. Nonferr. Metal. 2005, 57, 101–105. (In Chinese) [Google Scholar]
- Yang, Z.P.; Jin, X.Z.; Huang, J.H. Extraction of zinc concentrates under oxygen stressing and acid leaching. Miner. Resour. Geol. 2005, 8, 449–451. (In Chinese) [Google Scholar]
- The Test Institute of Beijing General Research Institute of Mining & Metallurgy. Nonferrous Metallurgy Analysis Manual; Metallurgical Industry Press: Beijing, China, 2004; ISBN 7502435263. (In Chinese) [Google Scholar]
- Zhang, H.B. Chemical Phase Analysis of Ore and Industrial Product; Metallurgical Industry Press: Beijing, China, 1992; ISBN 9787502410353. (In Chinese) [Google Scholar]
- Iler, R.K. The Colloid Chemistry of Silica and Silicates; Cornell University Press: Ithaca, NY, USA, 1955; pp. 92–93. [Google Scholar]
- Xu, H.S.; Wei, C.; Li, C.X.; Fan, G.; Deng, Z.G.; Li, M.T.; Li, X.B. Sulfuric acid leaching of zinc silicate ore under pressure. Hydrometallurgy 2010, 105, 186–190. [Google Scholar] [CrossRef]
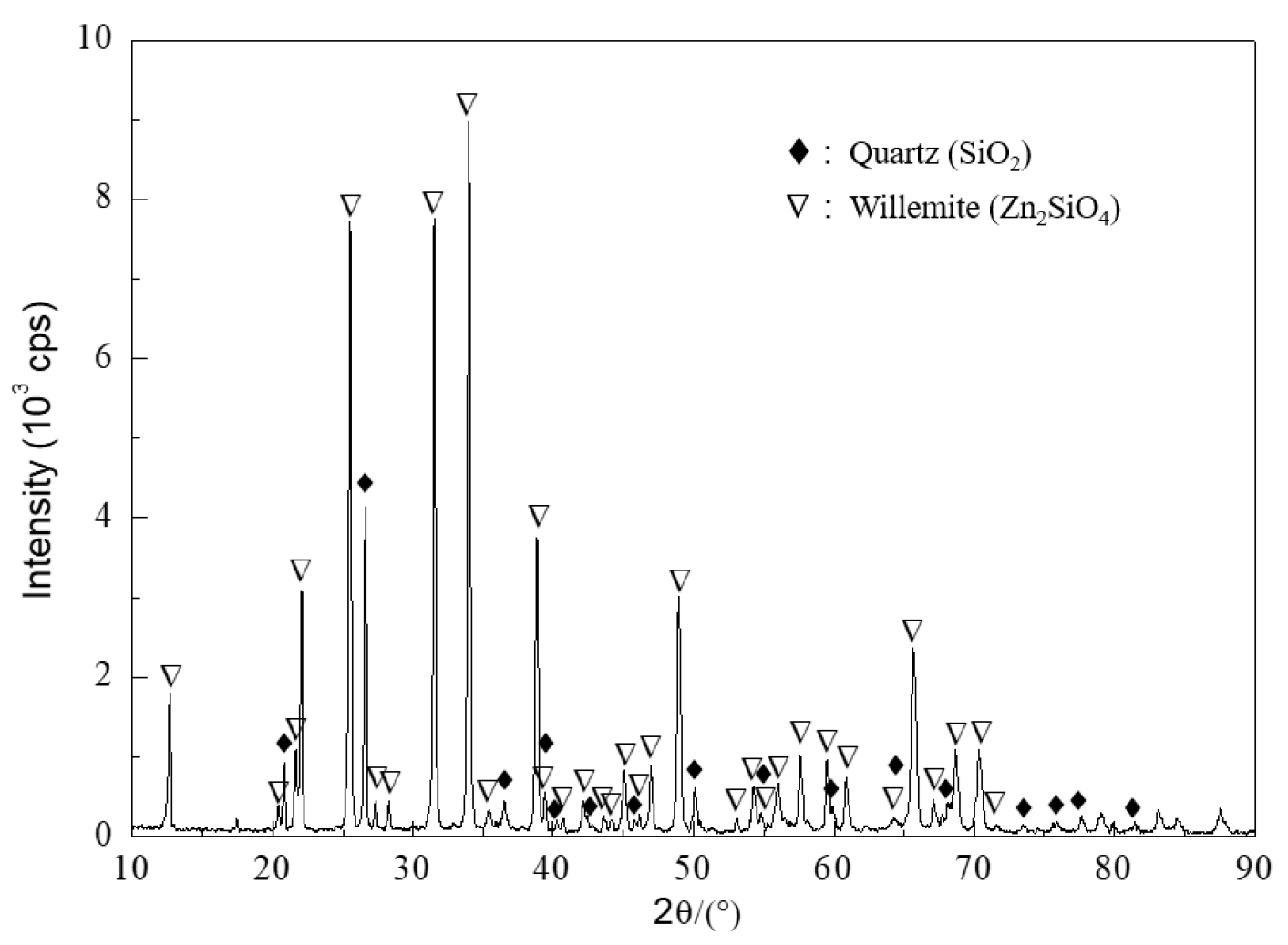
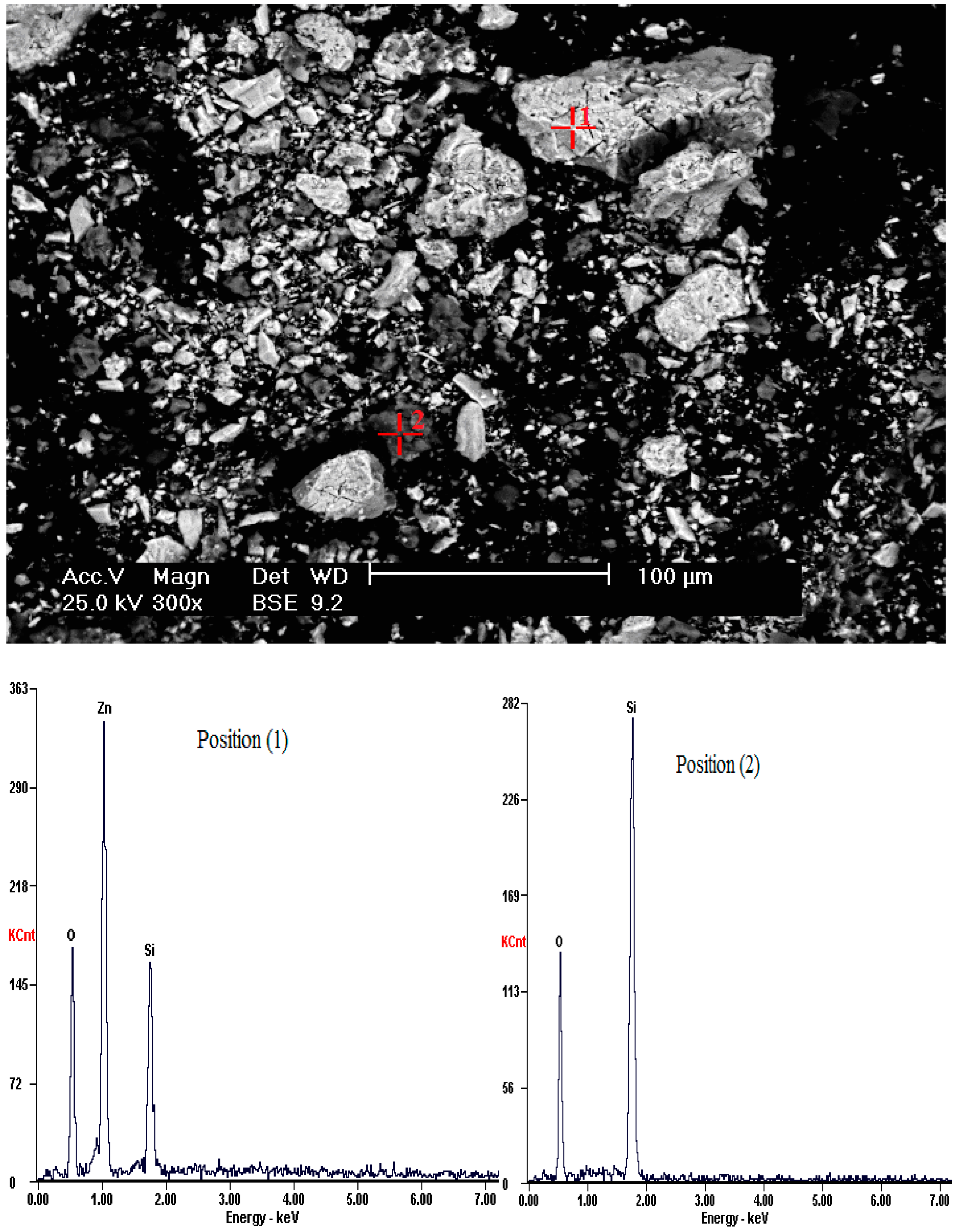




| Component | Zn | Fe | Pb | Cu | S | As | SiO2 | Al2O3 | K2O | MgO | CaO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 43.96 | 1.23 | 0.78 | 0.03 | 0.06 | <0.1 | 38.9 | 0.91 | 1.06 | 0.27 | 1.22 |
| Constituent | Carbonate | Silicate | Sulfide | Others | Total |
|---|---|---|---|---|---|
| Zinc content (wt.%) | 0.63 | 41.16 | 0.04 | 1.86 | 43.69 |
| Zinc distribution ratio (%) | 1.44 | 94.21 | 0.09 | 4.26 | 100 |
| Particle Size (Mesh) | Zn (%) | Fe (%) | SiO2 (%) |
|---|---|---|---|
| −100 + 150 | 42.79 | 2.17 | 37.6 |
| −150 + 200 | 43.35 | 1.43 | 38.4 |
| −200 + 250 | 43.23 | 1.21 | 38.5 |
| −250 + 300 | 43.96 | 1.23 | 38.9 |
| −300 + 340 | 43.43 | 1.38 | 39.0 |
| Experiment No. | 1 | 2 | 3 | 4 | Average | |
|---|---|---|---|---|---|---|
| Chemical composition of leaching residue (wt.%) | Zn | 2.15 | 1.63 | 1.97 | 1.74 | 1.87 |
| Fe | 2.13 | 1.89 | 2.06 | 1.95 | 2.01 | |
| Pb | 0.94 | 1.13 | 1.03 | 1.2 | 1.07 | |
| As | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | |
| SiO2 | 77.04 | 75.6 | 76.3 | 74.69 | 75.9 | |
| Al2O3 | 1.09 | 1.22 | 1.18 | 1.27 | 1.19 | |
| K2O | 0.53 | 0.52 | 0.42 | 0.48 | 0.49 | |
| MgO | 0.2 | 0.22 | 0.23 | 0.22 | 0.22 | |
| CaO | 2.26 | 2.3 | 2.04 | 2.1 | 2.2 | |
| Leaching rate (%) | Zn | 97.6 | 98.2 | 97.8 | 98.09 | 97.9 |
| Fe | 15.58 | 27.01 | 18.35 | 23.51 | 21.11 | |
| SiO2 | 3.45 | 7.69 | 4.38 | 7.36 | 5.72 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Qian, Y.; Zhou, Q.; Wei, C.; Wang, Q.; Zhao, W.; Zhu, B.; Tong, F.; Ren, F.; Zhang, M.; et al. Leaching of Willemite Concentrate in Sulfuric Acid Solution at High Temperature. Sustainability 2023, 15, 161. https://doi.org/10.3390/su15010161
Xu H, Qian Y, Zhou Q, Wei C, Wang Q, Zhao W, Zhu B, Tong F, Ren F, Zhang M, et al. Leaching of Willemite Concentrate in Sulfuric Acid Solution at High Temperature. Sustainability. 2023; 15(1):161. https://doi.org/10.3390/su15010161
Chicago/Turabian StyleXu, Hongsheng, Yanan Qian, Quanfa Zhou, Chang Wei, Qi Wang, Wenjie Zhao, Binglong Zhu, Fei Tong, Fang Ren, Min Zhang, and et al. 2023. "Leaching of Willemite Concentrate in Sulfuric Acid Solution at High Temperature" Sustainability 15, no. 1: 161. https://doi.org/10.3390/su15010161
APA StyleXu, H., Qian, Y., Zhou, Q., Wei, C., Wang, Q., Zhao, W., Zhu, B., Tong, F., Ren, F., Zhang, M., & Xu, J. (2023). Leaching of Willemite Concentrate in Sulfuric Acid Solution at High Temperature. Sustainability, 15(1), 161. https://doi.org/10.3390/su15010161






