Inoculation of Prickly Pear Litter with Microbial Agents Promotes the Efficiency in Aerobic Composting
Abstract
:1. Introduction
2. Materials and Methods
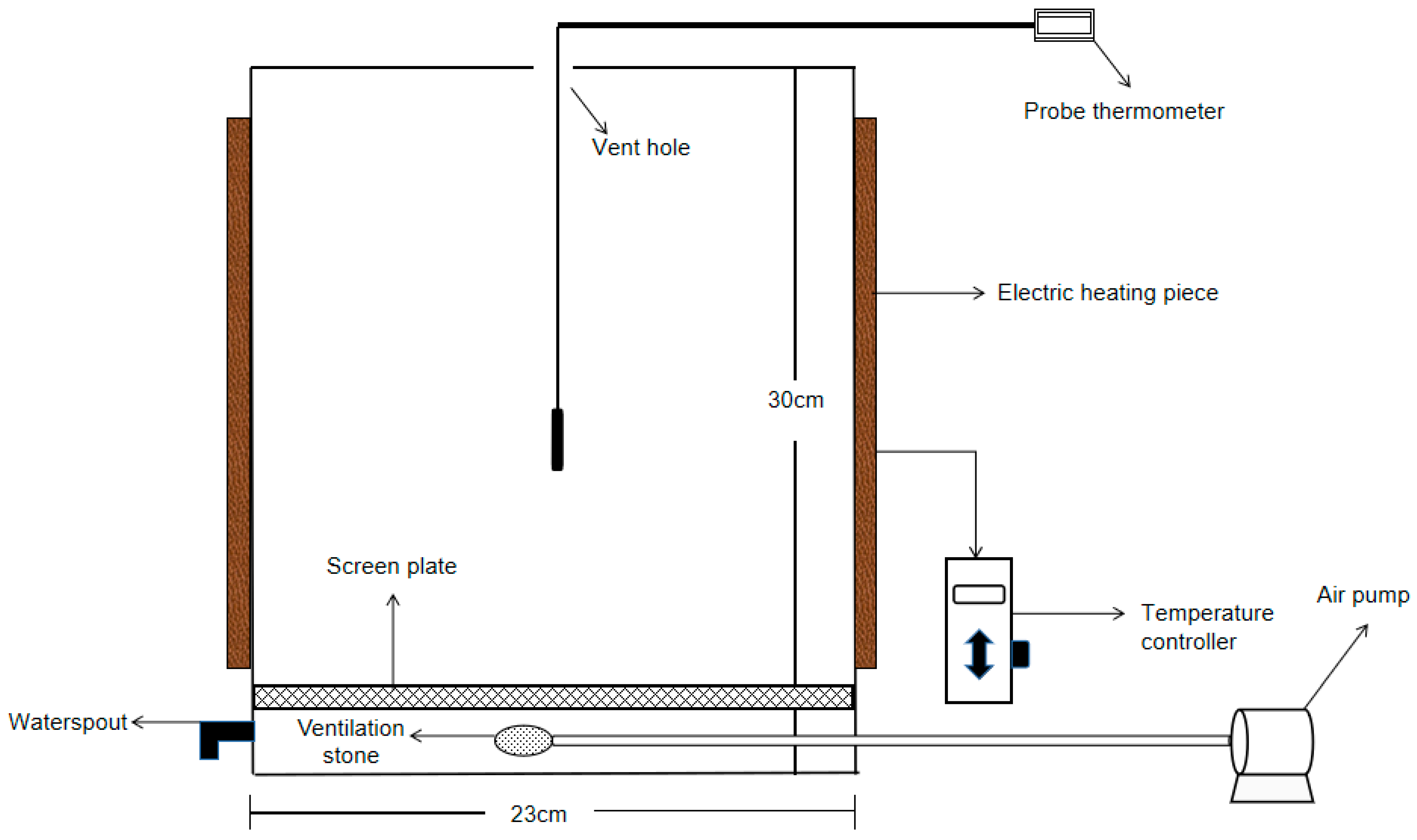
2.1. Compost Materials and Device
2.2. Treatment and Sample Collection
2.3. Determination of Physical and Chemical Properties
2.4. High-Throughput Sequencing and Analysis
2.5. Biological Information Processing
2.6. Statistical Analysis
3. Results
3.1. Microbial Composition of Decomposition Agent
3.2. Effects of Microbial Agents on Temperature, pH, Conductivity, and VS
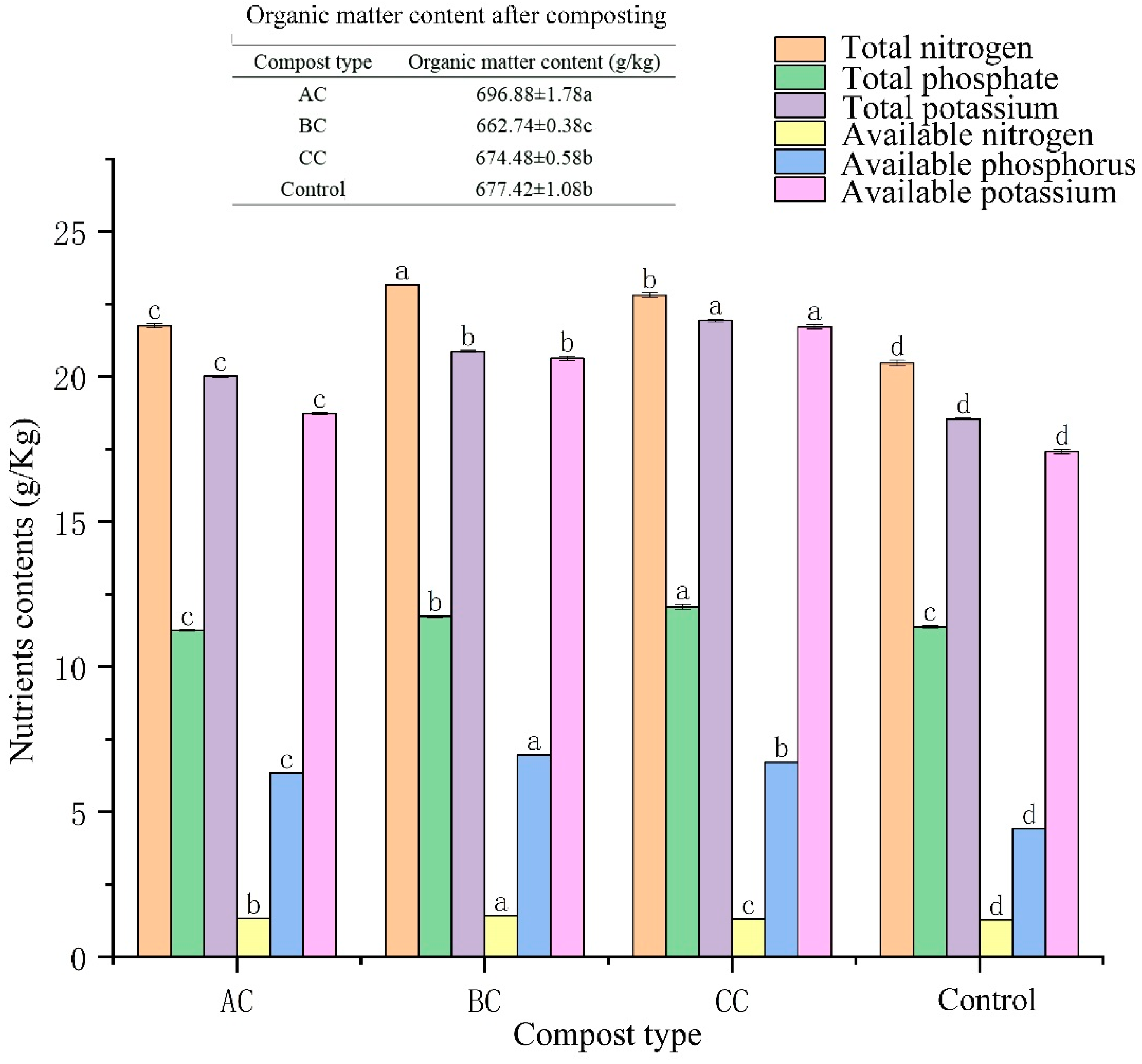
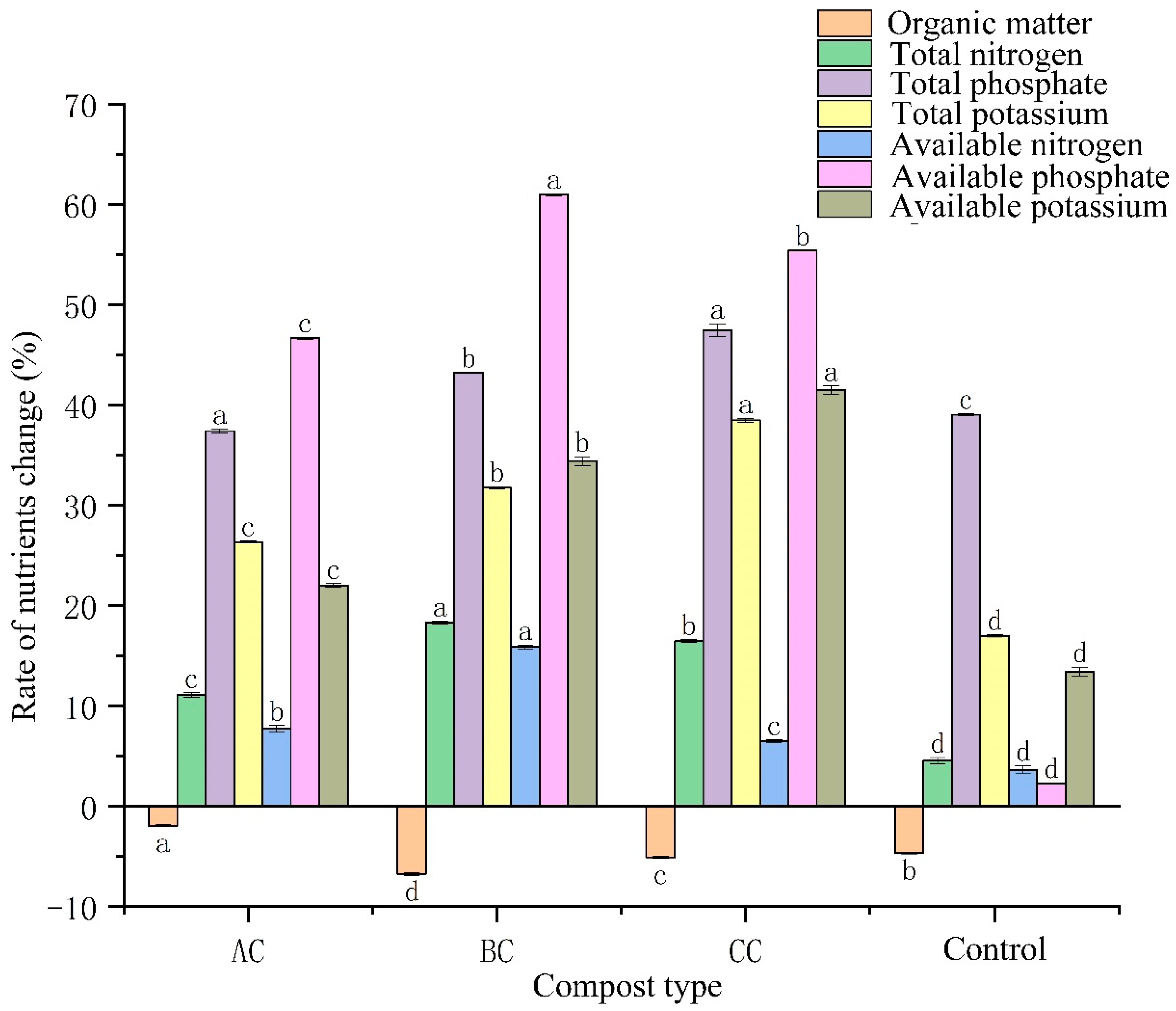
3.3. Effects of Microbial Agents on Organic Matter and Nutrients in Compost
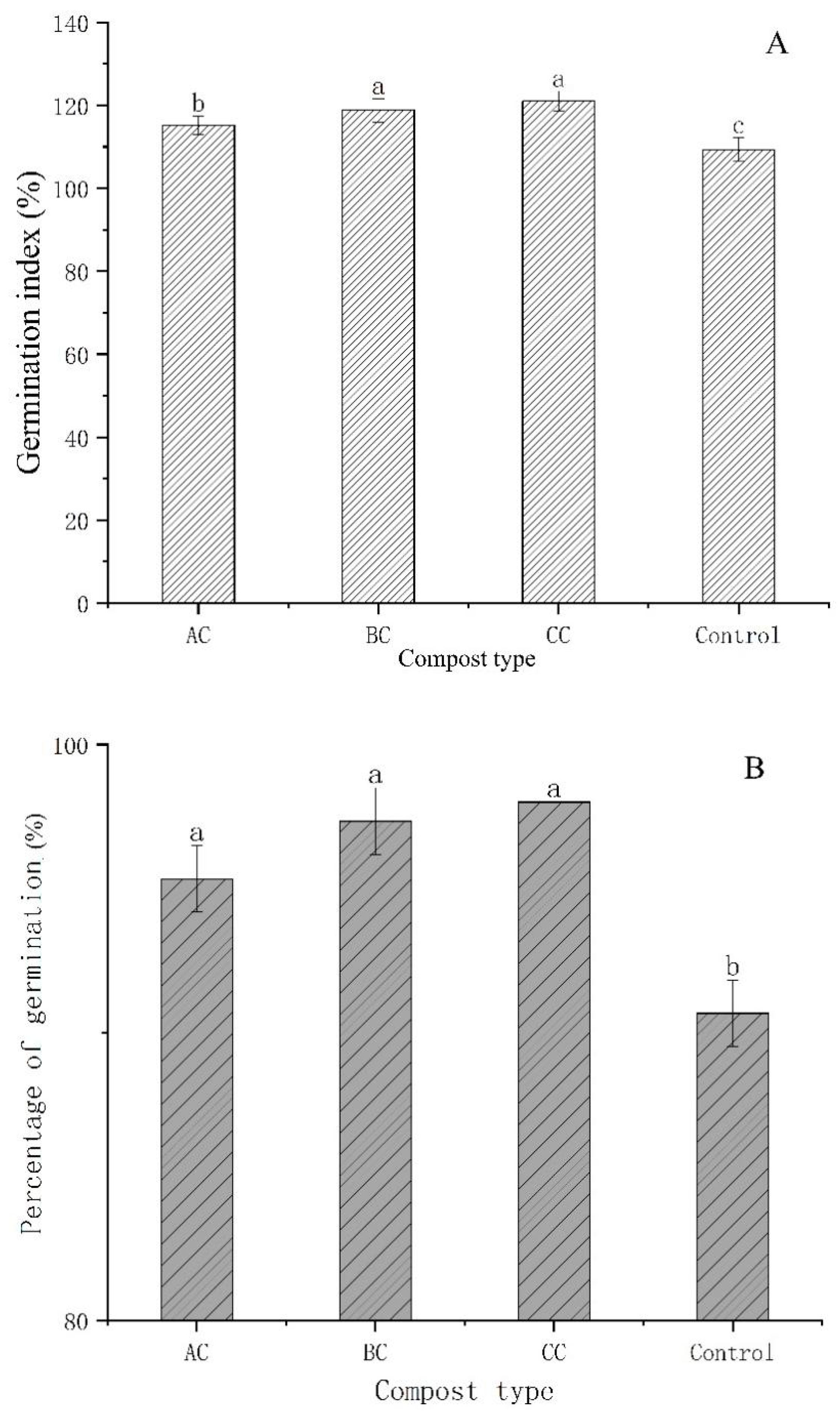
3.4. Effects of Microbial Agents on Seed Germination Index
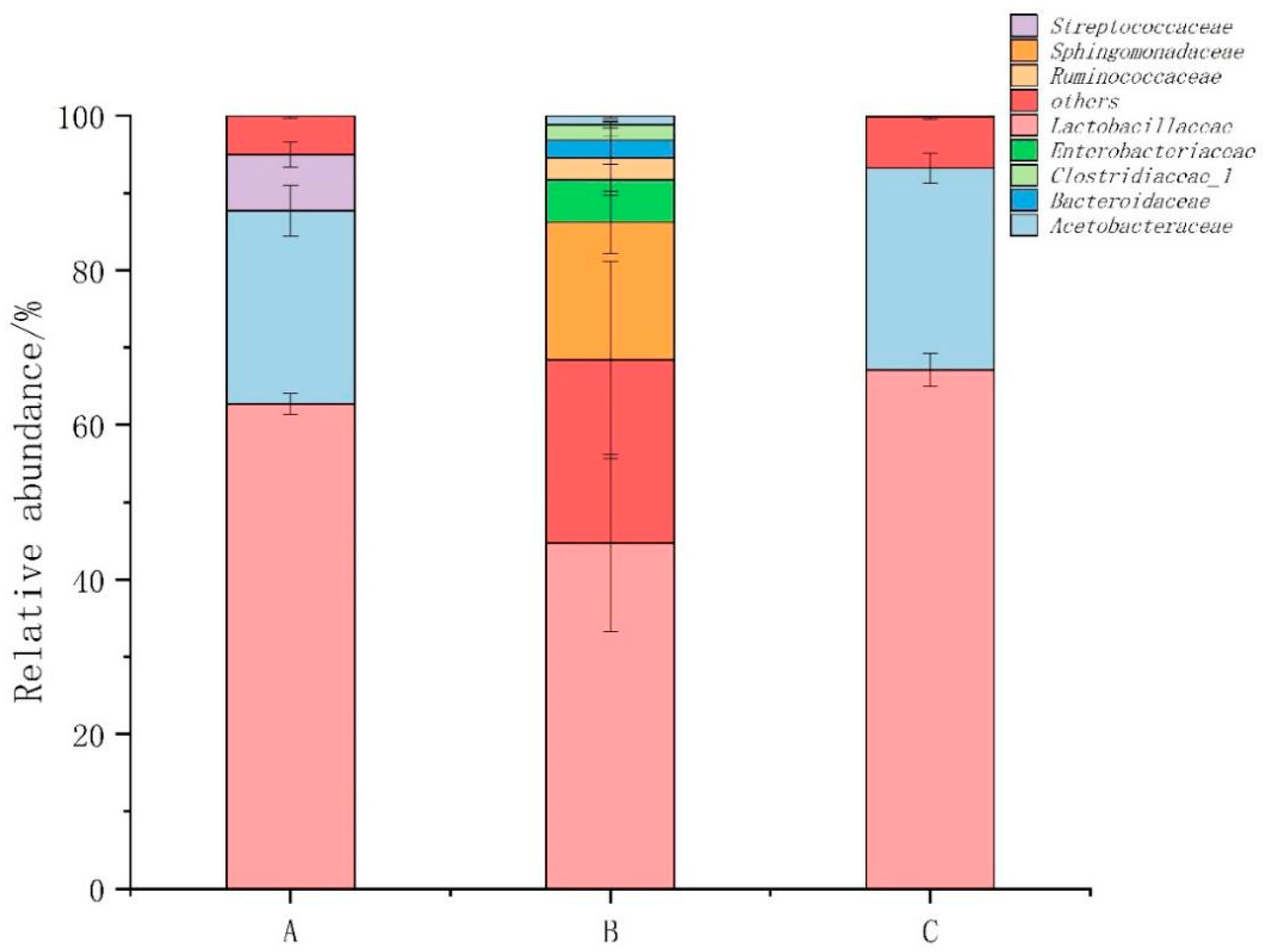
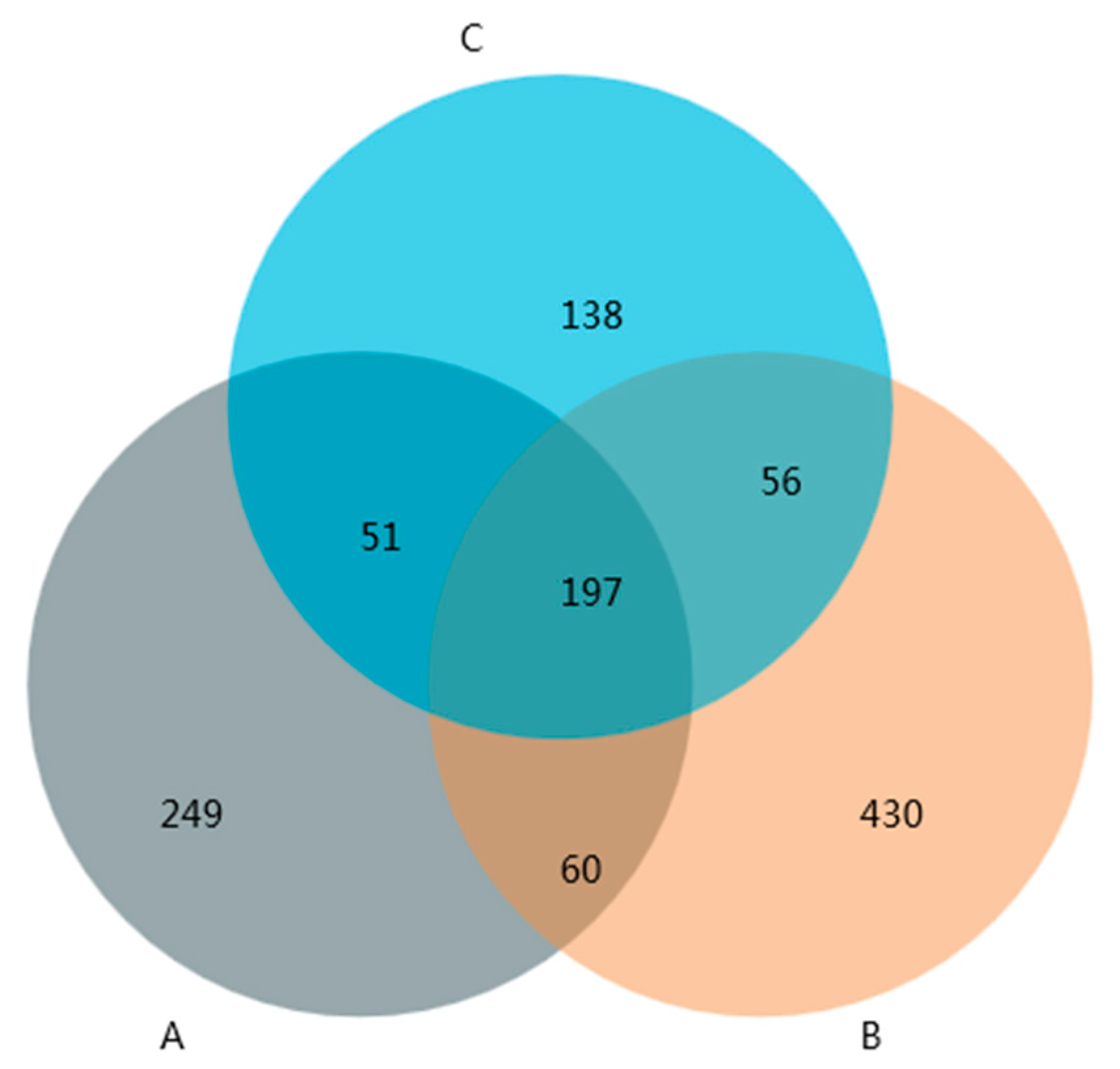
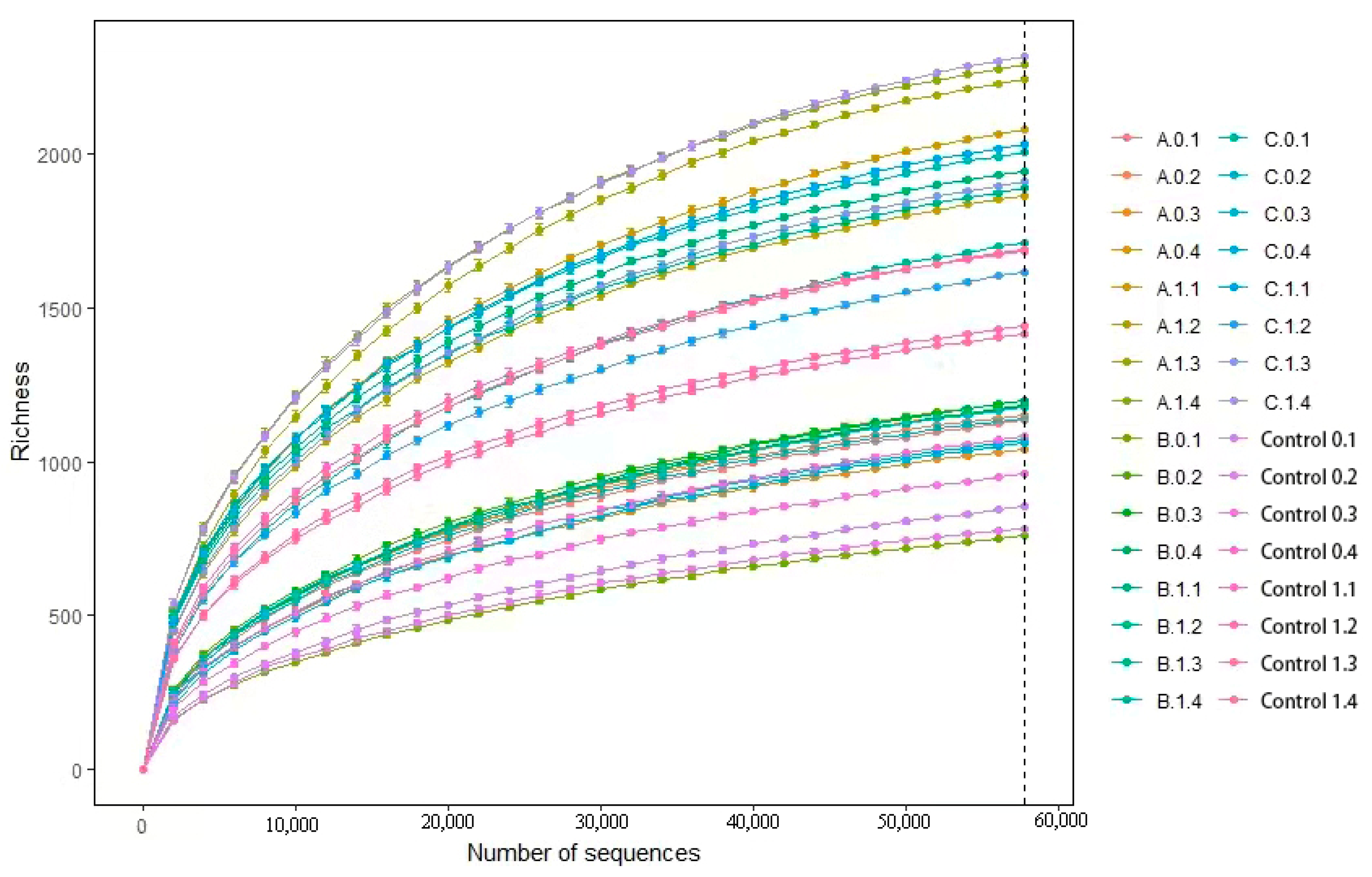
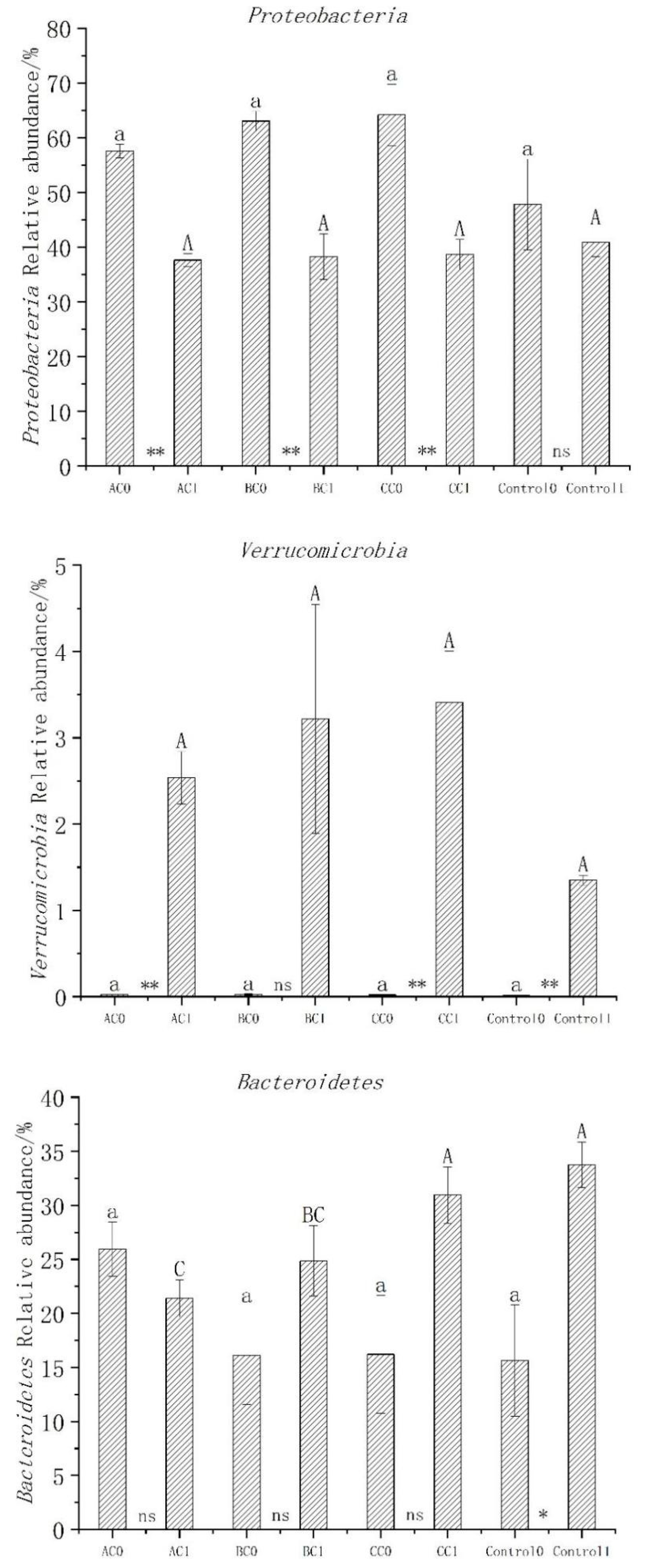
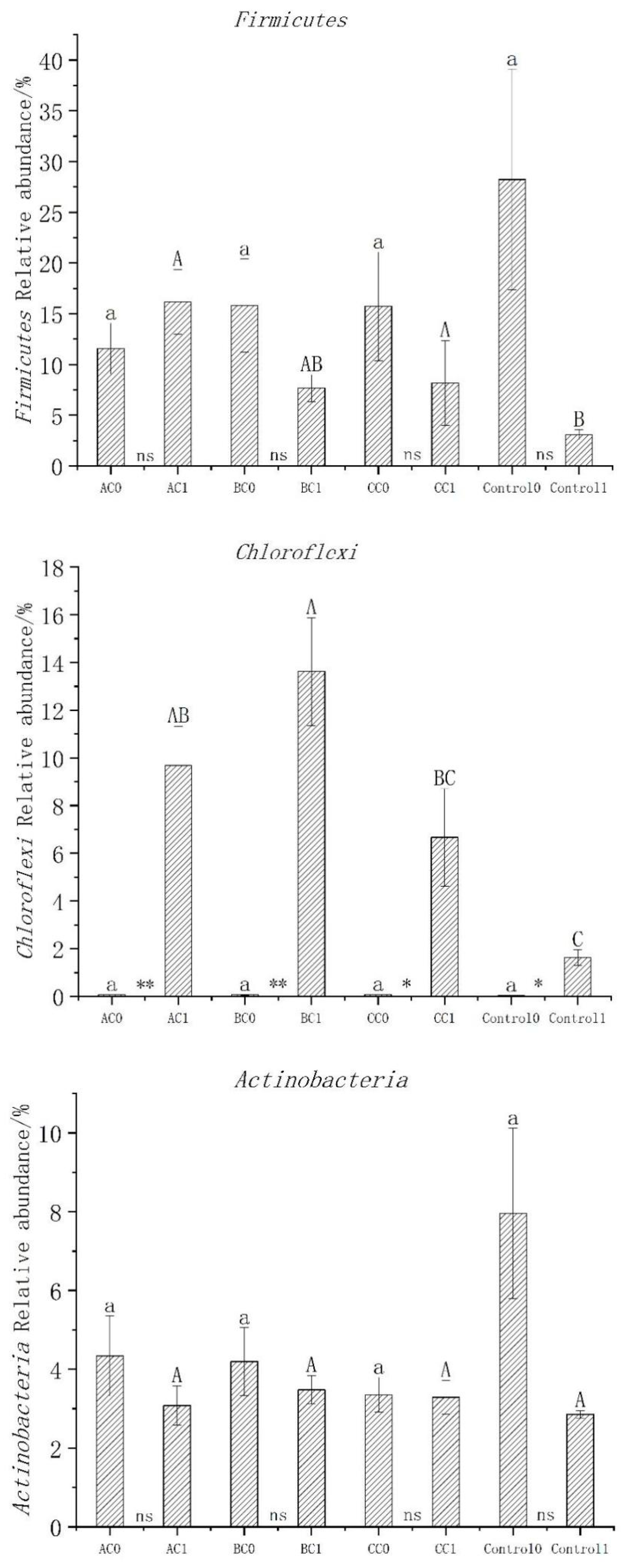
3.5. Abundance, Diversity, and Structural Differences of Bacterial Communities in Compost
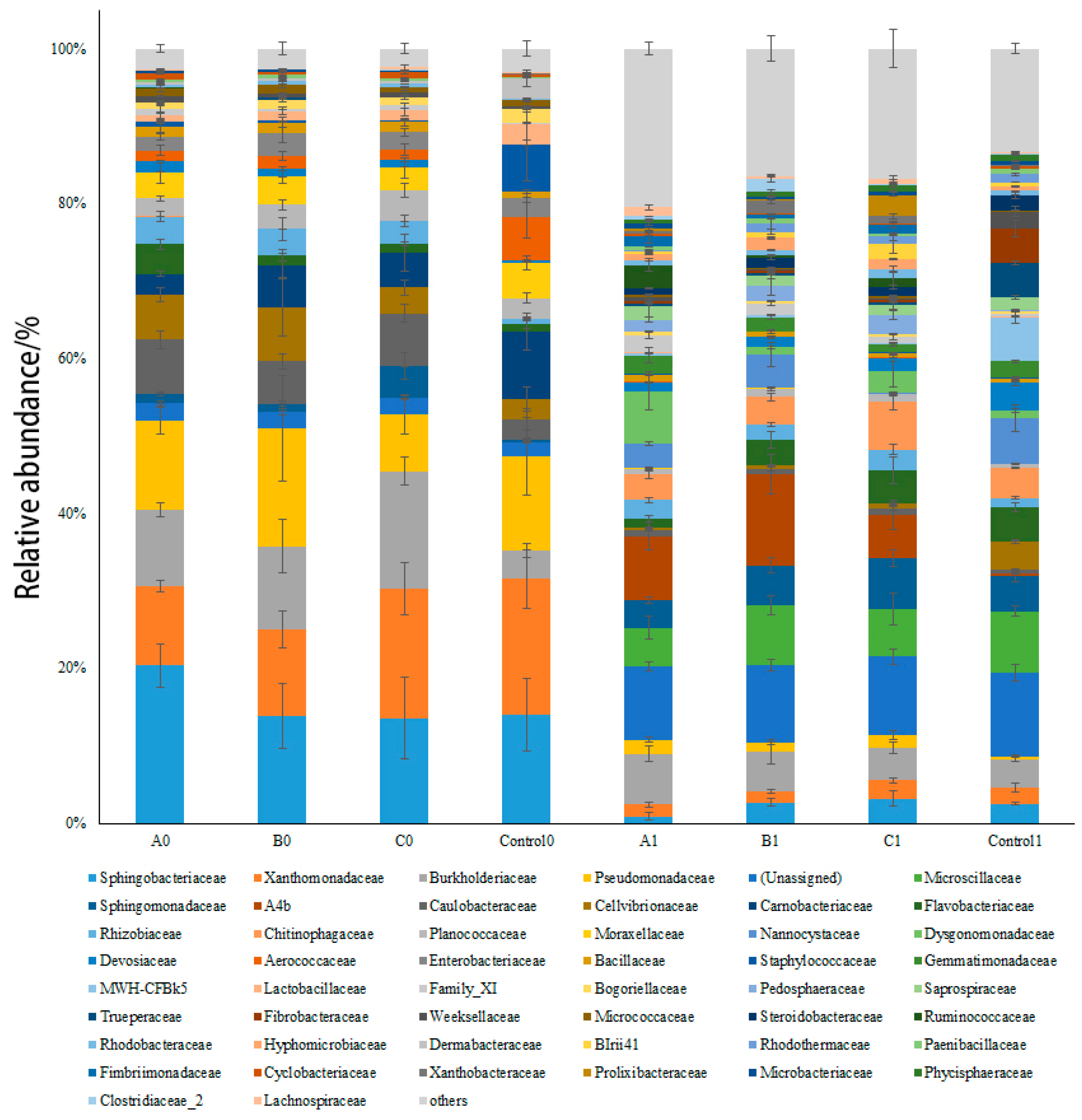
3.6. Bacterial Community Structure before and after Composting
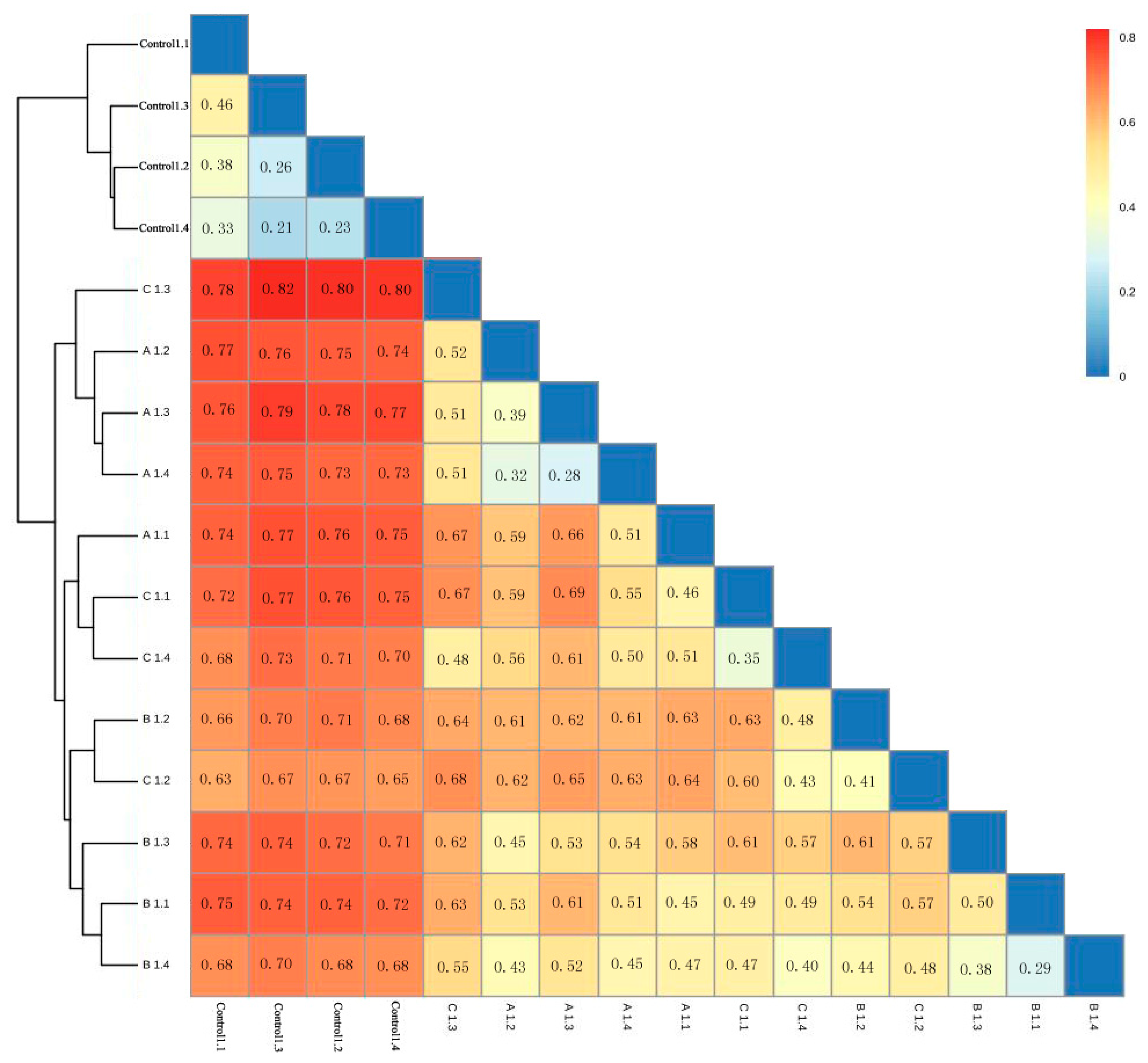
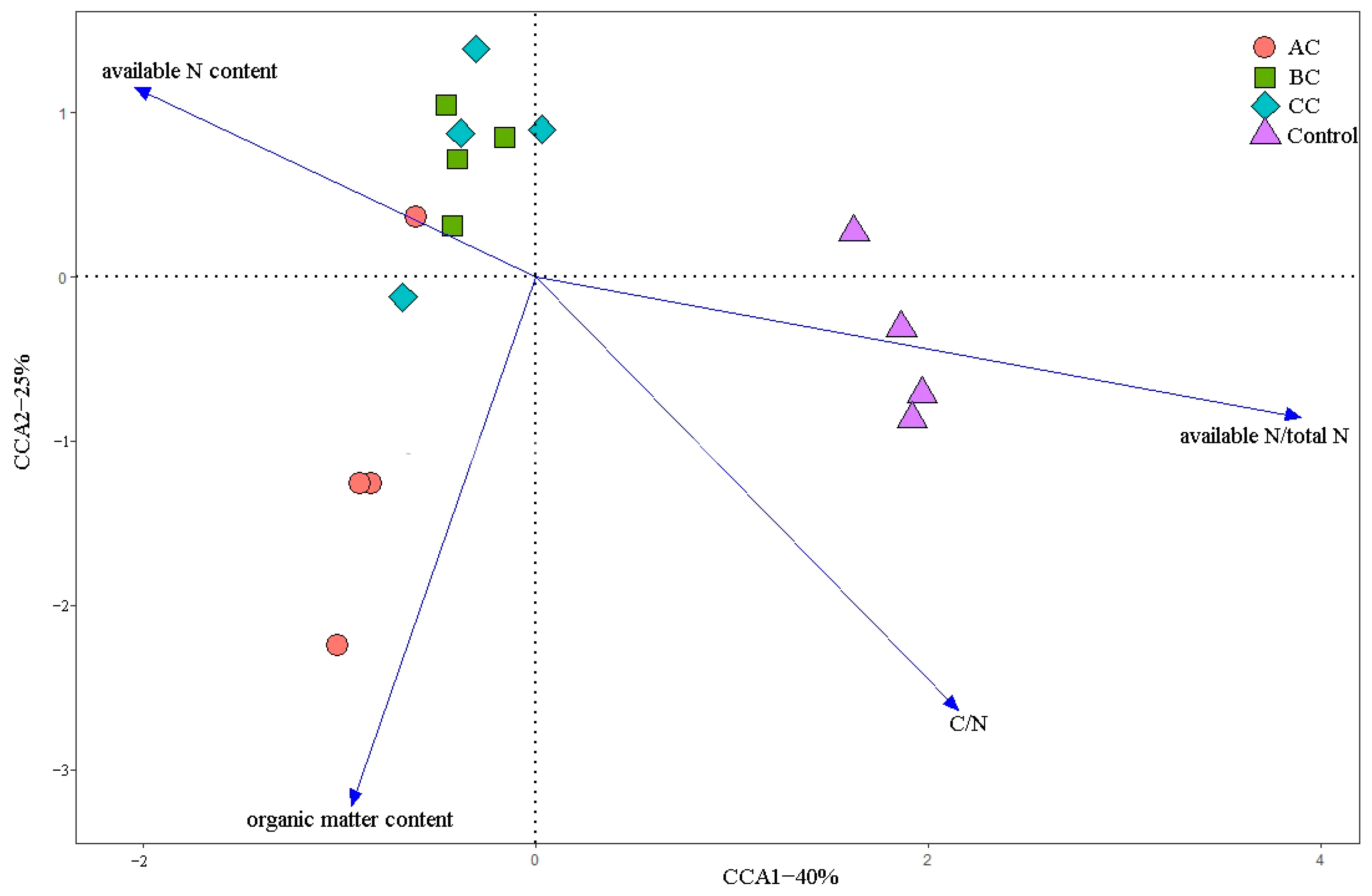
3.7. Correlation Analysis between the Community Structure and Physicochemical Factors
4. Discussion
4.1. Effect of Microbial Agents on Physical Properties of Composting
4.2. Effect of Microbial Agents on Nutrient Content
4.3. Changes in Microbial Diversity during Composting
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, N.; Nie, M.; Li, B.; Wu, J.; Zhao, J. Contrasting effects of the aboveground litter of native Phragmites australis and invasive Spartina alterniflora on nitrification and denitrification. Sci. Total Environ. 2020, 764, 144283. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Scandellari, F.; Bonora, E.; Tagliavini, M. Nutrient release during decomposition of leaf litter in a peach (Prunus persica L.) orchard. Nutr. Cycl. Agroecosyst. 2010, 87, 115–125. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, Z.; Tan, W. Plant litter quality regulates soil eco-enzymatic stoichiometry and microbial nutrient limitation in a citrus orchard. Plant Soil 2021, 466, 179–191. [Google Scholar] [CrossRef]
- Xu, J.; Vidyarthi, S.K.; Bai, W.; Pan, Z. Nutritional constituents, health benefits and processing of Rosa Roxburghii: A review. J. Funct. Foods 2019, 60, 103456. [Google Scholar] [CrossRef]
- Wen, X.; Deng, X. Characterization of genotypes and genetic relationship of Cili (Rosa roxburghii) and its relatives using RAPD markers. J. Agric. Biotechnol. 2003, 11, 605–611. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.J.; Liu, J.; Liu, J.; Zhang, E.; Li, W. Inhibition of metastasis and invasion of ovarian cancer cells by crude polysaccharides from Rosa Roxburghii Tratt in vitro. Asian Pac. J. Cancer Prev. Prev. 2014, 15, 10351–10355. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zhang, B.; Wang, B.; Li, C.; Xiong, F.; Huang, Q. In-vitro inhibitory effects of flavonoids in Rosa roxburghii and R. sterilis fruits on α-glucosidase: Effect of stomach digestion on flavonoids alone and in combination with acarbose. J. Funct. Foods 2019, 54, 13–21. [Google Scholar] [CrossRef]
- Li, H.; Fang, W.; Wang, Z.; Chen, Y. Physicochemical, biological properties, and flavour profile of Rosa roxburghii Tratt, Pyracantha fortuneana, and Rosa laevigata Michx fruits: A comprehensive review. Food Chem. 2022, 366, 130509. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, A.; Ajmal, M.; Ye, L.; Awais, M. Comprehensive review on agricultural waste utilization and high-temperature fermentation and composting. Biomass Convers. Biorefin. 2021, 7046, 1–24. [Google Scholar]
- Liang, B.; Chang, Q.; Fan, L.; Wang, Y.; Yuan, Y. Soil amendment alters soil physicochemical properties and bacterial community structure of a replanted apple orchard. Microbiol. Res. 2018, 216, 1–11. [Google Scholar] [CrossRef]
- Hu, T.; Wang, X.; Zhen, L.; Gu, J.; Zhang, K.; Wang, Q.; Ma, J.; Peng, H.; Lei, L.; Zhao, W. Effects of inoculating with lignocellulose-degrading consortium on cellulose-degrading genes and fungal community during co-composting of spent mushroom substrate with swine manure. Bioresour. Technol. 2019, 291, 121876. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; López, M.J.; Suárez-Estrella, F.; Vargas-García, M.C.; Moreno, J. Exploiting composting biodiversity: Study of the persistent and biotechnologically relevant microorganisms from lignocellulose-based composting. Bioresour. Technol. 2014, 162, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, D.; Wei, D.; Zhao, Y.; Wu, J.; Xie, X.; Zhang, R.; Wei, Z. Improved lignocellulose-degrading performance during straw composting from diverse sources with actinomycetes inoculation by regulating the key enzyme activities. Bioresour. Technol. 2018, 71, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Kumar, A.; Singh, B.; Nain, L.; Joshi, M.; Satya, S. Bioaugmented composting of Jatropha de-oiled cake and vegetable waste under aerobic and partial anaerobic conditions. J. Basic Microbiol. 2013, 53, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Qin, X.; Tian, X.; Zhao, Y.; Huang, J. Inoculating with the microbial inoculums to start up the aerobic composting of mushroom residue and wood chips at low temperature. J. Environ. Chem. Eng. 2021, 9, 105294. [Google Scholar] [CrossRef]
- Yeoh, C.Y.; Chin, N.L.; Tan, C.S.; Ooi, H.S. Acceleration effects of microbial inoculum on palm oil mill organic waste composting. Compost. Sci. Util. 2011, 19, 135–142. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, B.; Tan, C.; Ding, L.; Shao, M.; Chen, C.; Fu, X.; Huang, Q. Effect of Rosa Roxburghii juice on starch digestibility: A focus on the binding of polyphenols to amylose and porcine pancreatic α-amylase by molecular modeling. Food Hydrocoll. 2021, 123, 106966. [Google Scholar] [CrossRef]
- Pan, H.; Nie, S.; Kou, P.; Wang, L.; Wang, Z.; Liu, Z.; Zhao, C.; Wang, X.; Fu, Y. An enhanced extraction and enrichment phytochemicals from Rosa roxburghii Tratt leaves with ultrasound-assist CO2-based switchable-solvent and extraction mechanism study. J. Mol. Liq. 2021, 337, 116591. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, C.; Zeng, Q.; Yao, M.; Zhang, A. Assessing the potential value of Rosa Roxburghii Tratt in arsenic-induced liver damage based on elemental imbalance and oxidative damage. Environ. Geochem. Health 2021, 43, 1165–1175. [Google Scholar] [CrossRef]
- Wang, L.; Lv, A.J.; Fan, X.; Dong, M.; Zhang, S.; Wang, J.; Wang, Y.; Cai, Z.; Fu, Y. Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef]
- Wu, P.; Han, S.; Wu, M. Beneficial effects of hydroalcoholic extract from Rosa Roxburghii Tratt fruit on hyperlipidemia in high-fat-fed rats. Acta Cardiol. Sin. 2020, 36, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Fang, C.; Sun, X.; Han, L.; He, X.; Huang, G. Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane under slight positive pressure. Bioresour. Technol. 2018, 59, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Xi, B.; Huang, C.; Tan, W.; Yuan, W. Linking phytoavailability of heavy metals with microbial community dynamics during municipal sludge composting. Process Saf. Environ. Prot. 2019, 130, 288–296. [Google Scholar] [CrossRef]
- Lu, R.K. Agrochemical Analysis Methods of Soil; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Bao, S.D. Soil and Agriculture Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Zhao, H.; Li, J.; Liu, J.; Lv, Y.; Wang, X.; Cui, Z. Microbial community dynamics during biogas slurry and cow manure compost. J. Integr. Agric. 2013, 12, S2095–S3119. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Woldesenbe, S.; Bayabil, H.K.; Garcia, M.; Gao, M.; Ampim, P.; Awal, R.; Fares, A. Evaluation of phytotoxicity of three organic amendments to collard greens using the seed germination bioassay. Environ. Sci. Pollut. Res. 2019, 26, 5454–5462. [Google Scholar] [CrossRef] [Green Version]
- Dennis, K.L.; Wang, Y.; Blatner, N.R.; Wang, S.; Saadalla, A.; Trudeau, E.; Roers, A.; Weaver, C.T.; Lee, J.J.; Gillbert, J.A.; et al. Adenomatous polyps are driven by microbe- instigated focal inflammation and are controlled by IL-10 producing T cells. Cancer Res. 2013, 73, 5905–5913. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Chu, C.; Li, X.; Wang, W.; Ren, N. Succession of bacterial community function in cow manure composing. Bioresour. Technol. 2018, 267, 63–70. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1979, 54, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, R.B. Vegan: Community Ecology Package Version 2007, 1.8-6. Available online: https://cran.r-project.org/src/contrib/Archive/vegan/ (accessed on 5 June 2021).
- Core, R.; Rdct, R.; Team, R.A. Language and Environment for Statistical Computing. Computing 2015, 1, 12–21. [Google Scholar] [CrossRef]
- Caceres, R.; Flotats, X.; Marfa, O. Changes in the chemical and physicochemical properties of the solid fraction of cattle slurry during composting using different aeration strategies. Waste Manag. 2006, 26, 1081–1091. [Google Scholar] [CrossRef]
- Varma, V.S.; Prasad, R.; Deb, S.; Kalamdhad, A.S. Effects of aeration during pile composting of water hyacinth operated at agitated, passive and forced aerated condition. Waste Biomass Valorization 2018, 9, 1339–1347. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Zhang, S.; Wang, Y.; Shao, X.; Wu, D. Analysis of raw materials and products characteristics from composting and anaerobic digestion in rural areas. J. Clean. Prod. 2022, 338, 130455. [Google Scholar] [CrossRef]
- Laubr, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Zhang, R.; Wu, H.; Xu, D.; Zhu, T.; Yu, G.; Xu, Z.; Shen, Q. Changes in biochemical and microbiological parameters during the period of rapid composting of dairy manure with rice chaff. Bioresour. Technol. 2011, 102, 9040–9049. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, H.; Song, J.; Lv, X.; Li, W.; Li, B.; Shi, J. Impact of saline soil improvement measures on salt content in the abandonment-reclamation process. Soil Tillage Res. 2021, 208, 104867. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Puig-Ventosa, I.M.; López, M.; Martínez, F.X.; Bertrán, T.G. The impact of improper materials in biowaste on the quality of compost. J. Clean. Prod. 2019, 251, 119601. [Google Scholar] [CrossRef]
- Zhu, N.; Zhu, Y.; Kan, Z.; Li, B.; Cao, Y.; Jin, H. Effects of two-stage microbial inoculation on organic carbon turnover and fungal community succession during co-composting of cattle manure and rice straw. Bioresour. Technol. 2021, 341, 125842. [Google Scholar] [CrossRef]
- Nigussie, A.; Dume, B.; Ahmed, M.; Mamuye, M. Effect of microbial inoculation on nutrient turnover and lignocellulose degradation during composting: A meta-analysis. Waste Manag. 2021, 125, 220–234. [Google Scholar] [CrossRef]
- Fang, M.; Wong, J.; Ma, K.; Wong, M. Co-composting of sewage sludge and coal fly ash: Nutrient transformations. Bioresour. Technol. 1999, 67, 19–24. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Yao, T.; Su, M.; Gun, S. Effects of microbial inoculation on enzyme activity, available nitrogen content, and bacterial succession during pig manure composting. Bioresour. Technol. 2020, 306, 123167. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Yao, T.; Su, M.; Gun, S. Microbial inoculation influences bacterial community succession and physicochemical characteristics during pig manure composting with corn straw. Bioresour. Technol. 2019, 289, 121653. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhao, Y.; Fan, Y.; Lu, Q.; Li, M.; Wei, Q.; Zhao, Y.; Cao, Z.; Wei, Z. Impact of phosphate-solubilizing bacteria inoculation methods on phosphorus transformation and long-term utilization in composting. Bioresour. Technol. 2017, 241, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E. The Family Chitinophagaceae. In The Prokaryotes: Other Major Lineages of Bacteria and the Archaea; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.; Dou, Y.; Chen, M.; Ping, S.; Peng, J.; Lu, W.; Zhang, W.; Yao, Z.; Li, H.; et al. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc. Natl. Acad. Sci. USA 2008, 105, 7564–7569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, D.; Sharma, J.; Kaur, G.; Bhojiya, A.A.; Maharjan, E. Phenetic and molecular diversity of nitrogen fixating plant growth promoting Azotobacter isolated from semiarid regions of India. BioMed Res. Int. 2021, 2021, 6686283. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Yu, H.; Cheng, J.; Miao, L. Community composition and cellulase activity of cellulolytic bacteria from forest soils planted with broad-leaved deciduous and evergreen trees. Appl. Microbiol. Biotechnol. 2014, 98, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Beguin, P. Molecular biology of cellulose degradation. Annu. Rev. Microbiol. 1990, 44, 219–248. [Google Scholar] [CrossRef]
- Spiers, A.J.; Deeni, Y.Y.; Folorunso, A.O.; Koza, A.; Moshynets, O.; Zawadzki, K. Cellulose expression in Pseudomonas fluorescens SBW25 and other environmental Pseudomonads. In Cellulose: Medical, Pharmaceutical and Electronic Applications; InTech: Dundee, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Kirchman, D.L. The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiol. Ecol. 2002, 39, S0168–S6496. [Google Scholar] [CrossRef]
- Herrera, L.M.; Braña, V.; Fraguas, L.F.; Castro-Sowinski, S. Characterization of the cellulase-secretome produced by the Antarctic bacterium Flavobacterium sp. AUG42. Microbiol. Res. 2019, 223–225, 13–21. [Google Scholar] [CrossRef]
- Williams, T.J.; Wilkins, D.; Long, E.; Evans, F.; DeMaere, M.Z.; Raftery, M.J.; Cavicchioil, R. The role of planktonic Flavobacteria in processing algal organic matter in coastal East Antarctica revealed using metagenomics and metaproteomics. Environ. Microbiol. 2013, 15, 1302–1317. [Google Scholar] [CrossRef]
- Wang, C.; Dong, D.; Wang, H.S.; Müller, K. Metagenomic analysis of microbial consortia enriched from compost: New insights into the role of Actinobacteria in lignocellulose decomposition. Biotechnol. Biofuels 2016, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Castignetti, D.; Hollocher, T.C. Heterotrophic nitrification among denitrifiers. Appl. Environ. Microbiol. 1984, 47, 620–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Compost Material | Total C (g·kg−1) | Total N (g·kg−1) | Total P (g·kg−1) | Total K (g·kg−1) | Cellulose (%) | Lignin (%) |
|---|---|---|---|---|---|---|
| Rosa roxburghii litter | 519.75 ± 5.30 | 15.43 ± 1.10 | 7.52 ± 0.19 | 11.38 ± 0.51 | 24.35 ± 0.08 | 24.32 ± 0.51 |
| Chicken feces | 859.52 ± 25.20 | 28.11 ± 0.01 | 11.28 ± 0.22 | 24.26 ± 1.35 | 0.08 ± 0.04 | 0.44 ± 0.11 |
| Agent | Source | Main Components |
|---|---|---|
| AC | Junde Bio. Co., Ltd. (Weifang, China) | Bacillus natto, Bacillus sp., Actinomycetes sp., Saccharomyces sp., Trichoderma sp., Azotobacter sp., and Lactobacillus sp. |
| BC | Nongfukang Bio. Co., Ltd. (Nanyang, China) | Bacillus subtilis, Lactobacillaceae sp., Bacillus licheniformis, Saccharomyces sp., and Enterococcus faecalis |
| CC | Junde Bio. Co., Ltd. (Weifang, China) | Bacillus sp., Actinomycetes sp., Lactobacillaceae sp., Saccharomyces sp., and Trichoderma sp. |
| Control | Prepared in laboratory | Sterilized pure water |
| Period | Treatment | Number of Optimized Sequences | 97% Similarity Level | ||||
|---|---|---|---|---|---|---|---|
| Cover Degree | Richness | Chao1 | Shannon | Simpson | |||
| Before composting | AC | 77,641.5 | 0.994 | 1128 ± 30.39 a | 1129.45 ± 30.33 a | 6.22 ± 0.11 a | 0.04 ± 0.004 a ** |
| BC | 73,762.5 | 0.994 | 1084.75 ± 108.97 a | 1085.98 ± 108.81 a | 5.88 ± 0.33 a | 0.05 ± 0.01 a | |
| CC | 72,727.25 | 0.994 | 1113.25 ± 28.31 a | 1114.78 ± 28.16 a | 5.87 ± 0.14 a | 0.05 ± 0.01 a ** | |
| Control | 81,982.75 | 0.994 | 920 ± 65.25 a | 921.98 ± 65.12 a | 5.40 ± 0.22 a | 0.06 ± 0.01 a ** | |
| Total | 1,224,456 | ||||||
| After composting | AC | 62,317.75 | 0.992 | 2120 ± 96.18 a ** | 2120.4 ± 96.11 a ** | 7.86 ± 0.15 a ** | 0.02 ± 0.002 a |
| BC | 63,969.75 | 0.992 | 1888.25 ± 63.5 a ** | 1888.88 ± 63.43 a ** | 7.61 ± 0.27 b ** | 0.02 ± 0.01 a | |
| CC | 64,712 | 0.992 | 1970 ± 145.28 a ** | 1970.58 ± 145.14 a ** | 7.74 ± 0.23 b ** | 0.02 ± 0.003 a | |
| Control | 67,192.5 | 0.993 | 1558.25 ± 75.39 a ** | 1558.98 ± 75.38 a ** | 7.33 ± 0.04 c ** | 0.02 ± 0.001 a | |
| Total | 1,032,768 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, C.; Zhao, B.; Zhang, J.; Qiu, R. Inoculation of Prickly Pear Litter with Microbial Agents Promotes the Efficiency in Aerobic Composting. Sustainability 2022, 14, 4824. https://doi.org/10.3390/su14084824
Liu Y, Li C, Zhao B, Zhang J, Qiu R. Inoculation of Prickly Pear Litter with Microbial Agents Promotes the Efficiency in Aerobic Composting. Sustainability. 2022; 14(8):4824. https://doi.org/10.3390/su14084824
Chicago/Turabian StyleLiu, Yiliang, Chao Li, Benliang Zhao, Jiaen Zhang, and Rongliang Qiu. 2022. "Inoculation of Prickly Pear Litter with Microbial Agents Promotes the Efficiency in Aerobic Composting" Sustainability 14, no. 8: 4824. https://doi.org/10.3390/su14084824
APA StyleLiu, Y., Li, C., Zhao, B., Zhang, J., & Qiu, R. (2022). Inoculation of Prickly Pear Litter with Microbial Agents Promotes the Efficiency in Aerobic Composting. Sustainability, 14(8), 4824. https://doi.org/10.3390/su14084824






