Effect of Peat-Perlite Substrate Compaction in Hiko V265 Trays on the Growth of Fagus sylvatica L. Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Levels of Compaction
2.2. Plant Growth
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
3.1. Growth
3.2. Dry Matter
3.3. Quality Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaworski, A. Hodowla Lasu. T. 3. Charakterystyka Hodowlana Drzew i Krzewów Leśnych; Powszechne Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 2019; ISBN 978-83-09-01117-0. [Google Scholar]
- Tomaś, Ł.; Jagodziński, A.M. Przebudowa Drzewostanów. ACADEMIA-Mag. Pol. Akad. Nauk. 2019, 3–4, 94–97. [Google Scholar]
- Zajączkowski, G.; Jabłoński, M.; Jabłoński, T.; Szmidla, H.; Kowalska, A.; Małachowska, J.; Piwnicki, J. Raport o Stanie Lasów w Polsce 2020 (Report on the State of Forests in Poland 2020); General Directorate of State Forests: Warszawa, Poland, 2021; pp. 1–162.
- Szabla, K.; Pabian, R. Szkółkarstwo Kontenerowe: Nowe Technologie i Techniki w Szkółkarstwie Leśnym; Centrum Informacji Lasów Państwowych: Warszawa, Poland, 2009; ISBN 978-83-89744-80-7.
- Zahreddine, H.G.; Struve, D.K.; Quigley, M. Growing Pinus Nigra Seedlings in SpinoutTM-Treated Containers Reduces Root Malformation and Increases Growth After Transplanting. J. Environ. Hortic. 2004, 22, 176–182. [Google Scholar] [CrossRef]
- Alameda, D.; Villar, R. Moderate Soil Compaction: Implications on Growth and Architecture in Seedlings of 17 Woody Plant Species. Soil Tillage Res. 2009, 103, 325–331. [Google Scholar] [CrossRef]
- Kormanek, M. Determination of the Impact of Soil Compaction on Germination and Seedling Growth Parameters of Common Beech in the Laboratory Conditions. Acta Sci. Pol. Silvarum. Colendarum. Ratio Et Ind. Lignaria 2013, 12, 14–27. [Google Scholar]
- Jourgholami, M.; Khoramizadeh, A.; Zenner, E. Effects of Soil Compaction on Seedling Morphology, Growth, and Architecture of Chestnut-Leaved Oak (Quercus castaneifolia). iForest 2017, 10, 145–153. [Google Scholar] [CrossRef]
- Banach, J.; Kormanek, M.; Jaźwiński, J. Quality of Scots Pine, European Beech and Pedunculate Oak Grown from Sowing on Soil with Different Compaction Levels. Leśne Pr. Badaw. 2020, 81, 167–174. [Google Scholar] [CrossRef]
- Onweremadu, E.U.; Eshett, E.T.; Ofoh, M.C.; Nwufo, M.I.; Obiefuna, J.C. Seedling Performance as Affected by Bulk Density and Soil Moisture on a Typic Tropaquept. J. Plant Sci. 2007, 3, 43–51. [Google Scholar] [CrossRef]
- Rykhlik, A.E.; Bezuglova, O.S. Method of Intra-Soil Pulse Continuous-Discrete Moistening (Model Experiment). Biogeosystem Tech. 2017, 4, 39–65. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and Use of Selenium Nanoparticles as Fertilizers. ACS Omega 2020, 5, 17767–17774. [Google Scholar] [CrossRef]
- Marshall, V.G. Impacts of forest harvesting on biological processes in northern forest soils. For. Ecol. Manag. 2000, 133, 43–60. [Google Scholar] [CrossRef]
- Gajda, A.; Przewłoka, B. Soil Biological Activity as Affected by Tillage Intensity. Int. Agrophysics 2012, 26, 15–23. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Soil Compaction and Growth of Woody Plants. Scand. J. For. Res. 1999, 14, 596–619. [Google Scholar] [CrossRef]
- Lipiec, J.; Medvedev, V.V.; Birkas, M.; Dumitru, E.; Lyndina, T.E.; Rousseva, S.; Fulajtár, E. Effect of Soil Compaction on Root Growth and Crop Yield in Central and Eastern Europe. Int. Agrophys. 2003, 17, 61–69. [Google Scholar]
- Passioura, J.B. Soil Conditions and Plant Growth’: Soil Conditions and Plant Growth. Plant Cell Environ. 2002, 25, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.A.; Anderson, W.K. Soil Compaction in Cropping Systems. Soil Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Towards a Thematic Strategy for Soil Protection; Commission of the European Communities: Brussels, Belgium, 2002; pp. 1–35.
- Arvidsson, J. Influence of Soil Texture and Organic Matter Content on Bulk Density, Air Content, Compression Index and Crop Yield in Field and Laboratory Compression Experiments. Soil Tillage Res. 1998, 49, 159–170. [Google Scholar] [CrossRef]
- Brais, S. Persistence of Soil Compaction and Effects on Seedling Growth in Northwestern Quebec. Soil Sci. Soc. Am. J. 2001, 65, 1263–1271. [Google Scholar] [CrossRef]
- Fleming, R.; Powers, R.; Foster, N.; Kranabetter, J.; Scott, A.; Jr, F.; Berch, S.; Chapman, W.; Kabzems, R.; Ludovici, K.; et al. Effects of Organic Matter Removal, Soil Compaction, and Vegetation Control on 5-Year Seedling Performance: A Regional Comparison of Long-Term Soil Productivity Sites. Can. J. For. Res. 2006, 36, 529–550. [Google Scholar] [CrossRef]
- Picchio, R.; Tavankar, F.; Nikooy, M.; Pignatti, G.; Venanzi, R.; Lo Monaco, A. Morphology, Growth and Architecture Response of Beech (Fagus orientalis Lipsky) and Maple Tree (Acer velutinum Boiss.) Seedlings to Soil Compaction Stress Caused by Mechanized Logging Operations. Forests 2019, 10, 771. [Google Scholar] [CrossRef]
- Zhao, Y.; Krzic, M.; Bulmer, C.E.; Schmidt, M.G.; Simard, S.W. Relative Bulk Density as a Measure of Compaction and Its Influence on Tree Height. Can. J. For. Res. 2010, 40, 1724–1735. [Google Scholar] [CrossRef]
- Kormanek, M.; Banach, J. Influence of unit pressure exerted on soil on quality of renewal of chosen species of trees. Acta Agroph. 2012, 19, 51–63. [Google Scholar]
- Lipiec, J.; Hajnos, M.; Świeboda, R. Estimating Effects of Compaction on Pore Size Distribution of Soil Aggregates by Mercury Porosimeter. Geoderma 2012, 179–180, 20–27. [Google Scholar] [CrossRef]
- Kalinitchenko, V.P.; Glinushkin, A.P.; Swidsinski, A.V.; Minkina, T.M.; Andreev, A.G.; Mandzhieva, S.S.; Sushkova, S.N.; Makarenkov, D.A.; Ilyina, L.P.; Chernenko, V.V.; et al. Thermodynamic Mathematical Model of the Kastanozem Complex and New Principles of Sustainable Semiarid Protective Silviculture Management. Environ. Res. 2021, 194, 110605. [Google Scholar] [CrossRef] [PubMed]
- Alameda, D.; Anten, N.P.R.; Villar, R. Soil Compaction Effects on Growth and Root Traits of Tobacco Depend on Light, Water Regime and Mechanical Stress. Soil Tillage Res. 2012, 120, 121–129. [Google Scholar] [CrossRef]
- Bartholomew, P.W.; Williams, R.D. Effects of Soil Bulk Density and Strength on Seedling Growth of Annual Ryegrass and Tall Fescue in Controlled Environment: Soil Bulk Density Effects on Grass Seedling Growth. Grass Forage Sci. 2010, 65, 348–357. [Google Scholar] [CrossRef]
- Ferree, D.C.; Streeter, J.G.; Yuncong, Y. Response of Container-Grown Apple Trees to Soil Compaction. HortScience 2004, 39, 40–48. [Google Scholar] [CrossRef]
- Pan, E.; Bassuk, N. Effects of Soil Type and Compaction on the Growth of Ailanthus Altissima Seedlings. J. Environ. Hortic. 1985, 3, 158–162. [Google Scholar] [CrossRef]
- Blouin, V.M.; Schmidt, M.G.; Bulmer, C.E.; Krzic, M. Effects of Compaction and Water Content on Lodgepole Pine Seedling Growth. For. Ecol. Manag. 2008, 255, 2444–2452. [Google Scholar] [CrossRef]
- Conlin, T.S.S.; van den Driessche, R. Soil Compaction Studies; FRDA Report; Canadian Forest Service and B.C. Ministry of Forests: Ottawa, ON, Canada, 1996; pp. 1–14.
- Siegel-Issem, C.M.; Burger, J.A.; Powers, R.F.; Ponder, F.; Patterson, S.C. Seedling Root Growth as a Function of Soil Density and Water Content. Soil Sci. Soc. Am. J. 2005, 69, 215–226. [Google Scholar] [CrossRef]
- Maupin, C.; Struve, D.K. Red Oak Transplanted to Different Bulk Density Soils Have Similar Water Use Characteristics. J. Arboric. 1997, 23, 233–238. [Google Scholar] [CrossRef]
- Jordan, D.; Ponder, F.; Hubbard, V.C. Effects of Soil Compaction, Forest Leaf Litter and Nitrogen Fertilizer on Two Oak Species and Microbial Activity. Appl. Soil Ecol. 2003, 23, 33–41. [Google Scholar] [CrossRef]
- Bejarano, M.D.; Villar, R.; Murillo, A.M.; Quero, J.L. Effects of Soil Compaction and Light on Growth of Quercus Pyrenaica Willd. (Fagaceae) Seedlings. Soil Tillage Res. 2010, 110, 108–114. [Google Scholar] [CrossRef]
- Kormanek, M.; Głąb, T.; Banach, J.; Szewczyk, G. Effects of Soil Bulk Density on Sessile Oak Quercus Petraea Liebl. Seedlings. Eur. J. For. Res 2015, 134, 969–979. [Google Scholar] [CrossRef]
- Kormanek, M.; Banach, J.; Ryba, M. Influence of Substrate Compaction in Nursery Containers on the Growth of Scots Pine (Pinus sylvestris L.) Seedlings. For. Res. Pap. 2013, 74, 307–314. [Google Scholar] [CrossRef]
- Kormanek, M.; Banach, J.; Leńczuk, D. Determination of the Impact of Soil Compaction on Growth Performance and Quality of Seedlings of European Beech Fagus sylvatica L. Grown in the Laboratory Conditions; SAGE Publishing: Thousand Oaks, CA, USA, 2013; pp. 67–78. [Google Scholar]
- Puértolas, J. Effects of Nutritional Status and Seedling Size on Field Performance of Pinus Halepensis Planted on Former Arable Land in the Mediterranean Basin. Forestry 2003, 76, 159–168. [Google Scholar] [CrossRef]
- Haase, D. Morphological and Physiological Evaluations of Seedling Quality. In National Proceedings: Forest and Conservation Nursery Associations—2006; Proc. RMRS-P-50; USDA Forest Service: Fort Collins, CO, USA, 2007; pp. 3–8. [Google Scholar]
- Grossnickle, S.C. Why Seedlings Survive: Influence of Plant Attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Ivetić, V.; Skorić, M. The Impact of Seeds Provenance and Nursery Production Method on Austrian Pine (Pinus Nigra Arn.) Seedlings Quality. Ann. For. Res. 2013, 3, 297–305. [Google Scholar]
- Johnson, J.D.; Cline, M.L. Seedling Quality of Southern Pines. In Forest Regeneration Manual; Duryea, M.L., Dougherty, P.M., Eds.; Forestry Sciences; Springer: Dordrecht, The Netherlands, 1991; Volume 36, pp. 143–159. ISBN 978-0-7923-0960-4. [Google Scholar]
- Roller, K.J. Suggested Minimum Standards for Containerized Seedlings in Nova Scotia; Information Report M-X-69; Maritimes Forest Research Centre: Fredericton, NB, Canada, 1977; pp. 1–18. [Google Scholar]
- PN-R-67025 Sadzonki Drzew i Krzewów do Upraw Leśnych i na Plantacje; Polski Komitet Normalizacyjny: Warszawa, Poland, 1999; ISBN 978-83-236-2771-5.
- Skrzyszewska, K.; Banach, J.; Bownik, G. Wpływ sposobu przedsiewnego przygotowania żołędzi i terminu siewu na kiełkowanie nasion i wzrost sadzonek dębu szypułkowego. Sylwan 2019, 163, 716–725. [Google Scholar] [CrossRef]
- Makita, N.; Hirano, Y.; Mizoguchi, T.; Kominami, Y.; Dannoura, M.; Ishii, H.; Finér, L.; Kanazawa, Y. Very Fine Roots Respond to Soil Depth: Biomass Allocation, Morphology, and Physiology in a Broad-Leaved Temperate Forest. Ecol. Res. 2011, 26, 95–104. [Google Scholar] [CrossRef]
- Farahnak, M.; Mitsuyasu, K.; Hishi, T.; Katayama, A.; Chiwa, M.; Jeong, S.; Otsuki, K.; Sadeghi, S.M.M.; Kume, A. Relationship between Very Fine Root Distribution and Soil Water Content in Pre- and Post-Harvest Areas of Two Coniferous Tree Species. Forests 2020, 11, 1227. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System); TIBCO Software Inc.: Sydney, NSW, Australia, 2017. [Google Scholar]
- Tworkoski, T.; Burger, J.; Wm, D. Soil Texture and Bulk Density Affect Early Growth of White Oak Seedlings. Tree Plant. Note 1983, 34, 22–25. [Google Scholar]
- Grossnickle, S.C. Ecophysiology of Northern Spruce Species: The Performance of Planted Seedlings; NRC Research Press: Ottawa, ON, Canada, 2000; ISBN 978-0-660-17959-9. [Google Scholar]
- Wrzesiński, P. The Influence of Seedling Density in Containers on Morphological Characteristics of European Beech. For. Res. Pap. 2015, 76, 304–310. [Google Scholar] [CrossRef][Green Version]
- Kormanek, M.; Banach, J.; Leńczuk, D. Influence of Soil Compaction on the Growth of Silver Fir (Abies alba Mill.) under a Forest Canopy. EQ 2015, 22, 47–54. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Importance of Root Growth in Overcoming Planting Stress. New For. 2005, 30, 273–294. [Google Scholar] [CrossRef]
- Ivetić, V.; Grossnickle, S.; Škorić, M. Forecasting the Field Performance of Austrian Pine Seedlings Using Morphological Attributes. Iforest-Biogeosci. For. 2016, 10, 99–107. [Google Scholar] [CrossRef]
- Rodríguez-Ortiz, J.C.; Carballo-Méndez, F.d.J.; Preciado-Rangel, P.; Rodríguez-Fuentes, H.; Lozano-Cavazos, C.J. Broccoli Seedling Production in Response to Recognised Organic Inputs. Int. J. Agric. Biol. 2021, 26, 8. [Google Scholar]
- Zisa, R.R.; Halverson, H.G.; Stout, B.B. Establishment and Early Growth of Conifers on Compact Soils in Urban Areas; Forest Service Research Paper; Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Amherst, MA, USA, 1980; Volume NE-451, pp. 1–8. [Google Scholar]
- Misra, R.K.; Gibbons, A.K. Growth and Morphology of Eucalypt Seedling-Roots, in Relation to Soil Strength Arising from Compaction. Plant Soil 1996, 182, 1–11. [Google Scholar] [CrossRef]
- Mósena, M.; Dillenburg, L.R. Early Growth of Brazilian Pine (Araucaria Angustifolia [Bertol.] Kuntze) in Response to Soil Compaction and Drought. Plant Soil 2004, 258, 293–306. [Google Scholar] [CrossRef]

| Parameter | Variant | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 | V9 | |
| Wet substrate weight (60% H2O) (g) | 52 | 56 | 60 | 64 | 68 | 72 | 76 | 80 | 84 |
| Actual bulk density (g∙cm−1) | 0.196 | 0.211 | 0.226 | 0.242 | 0.257 | 0.272 | 0.287 | 0.302 | 0.317 |
| Dry bulk density (g∙cm−1) * | 0.078 | 0.085 | 0.091 | 0.097 | 0.103 | 0.109 | 0.115 | 0.121 | 0.127 |
| Porosity | 95.13 | 94.69 | 94.31 | 93.94 | 93.56 | 93.19 | 92.81 | 92.44 | 92.06 |
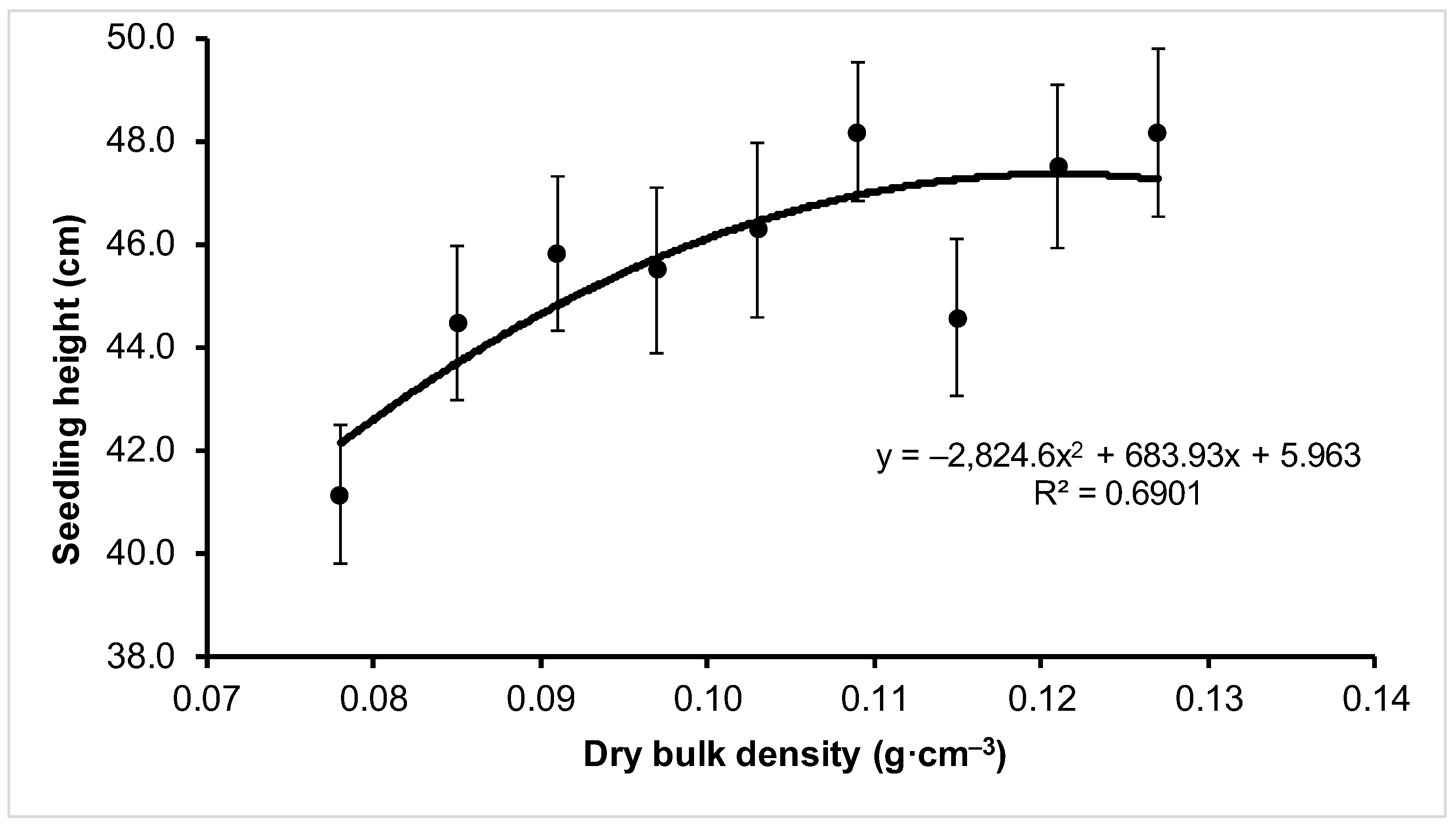
| Variant | Growth Parameter | ||
|---|---|---|---|
| SH (cm) | RCD (mm) | LA (cm2) | |
| V1 | 41.13 ± 9.12 b | 5.38 ± 1.37 a | 257.35 ± 134.99 a |
| V2 | 44.47 ± 10.31 ab | 4.74 ± 1.32 a | 210.29 ± 139.91 a |
| V3 | 45.82 ± 10.08 ab | 5.00 ± 1.25 a | 231.15 ± 131.02 a |
| V4 | 45.49 ± 9.68 ab | 5.00 ± 1.20 a | 223.13 ± 159.41 a |
| V5 | 46.27 ± 10.89 ab | 4.77 ± 1.53 a | 224.26 ± 150.45 a |
| V6 | 48.19 ± 9.85 a | 4.81 ± 1.39 a | 205.11 ± 131.10 a |
| V7 | 44.57 ± 8.98 ab | 4.89 ± 1.33 a | 176.50 ± 135.86 a |
| V8 | 47.51 ± 10.57 ab | 5.08 ± 1.46 a | 215.73 ± 162.83 a |
| V9 | 48.18 ± 11.27 a | 5.40 ± 1.59 a | 248.85 ± 164.97 a |
| Mean | 45.81 ± 10.37 | 5.01 ± 1.41 | 221.88 ± 147.70 |
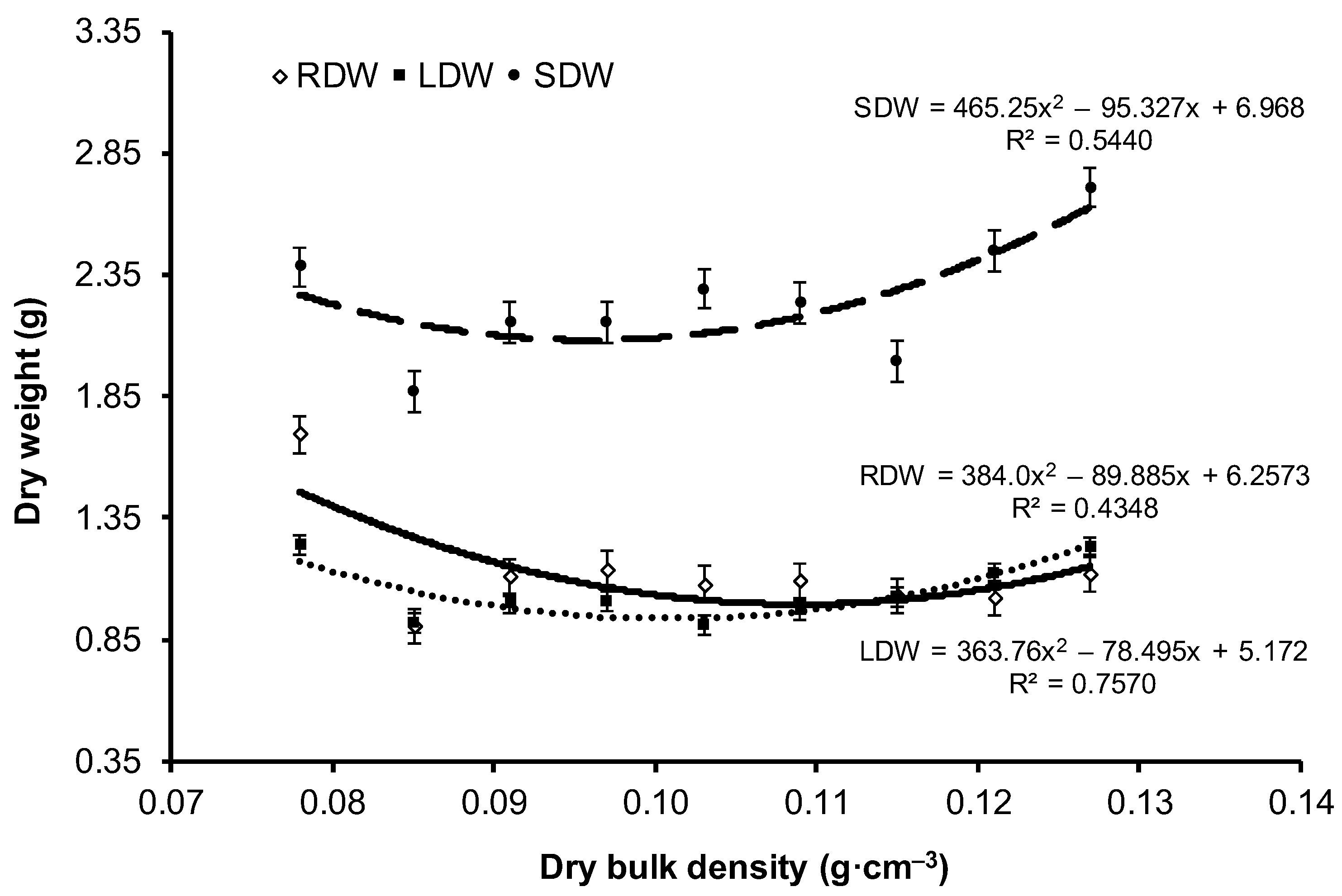
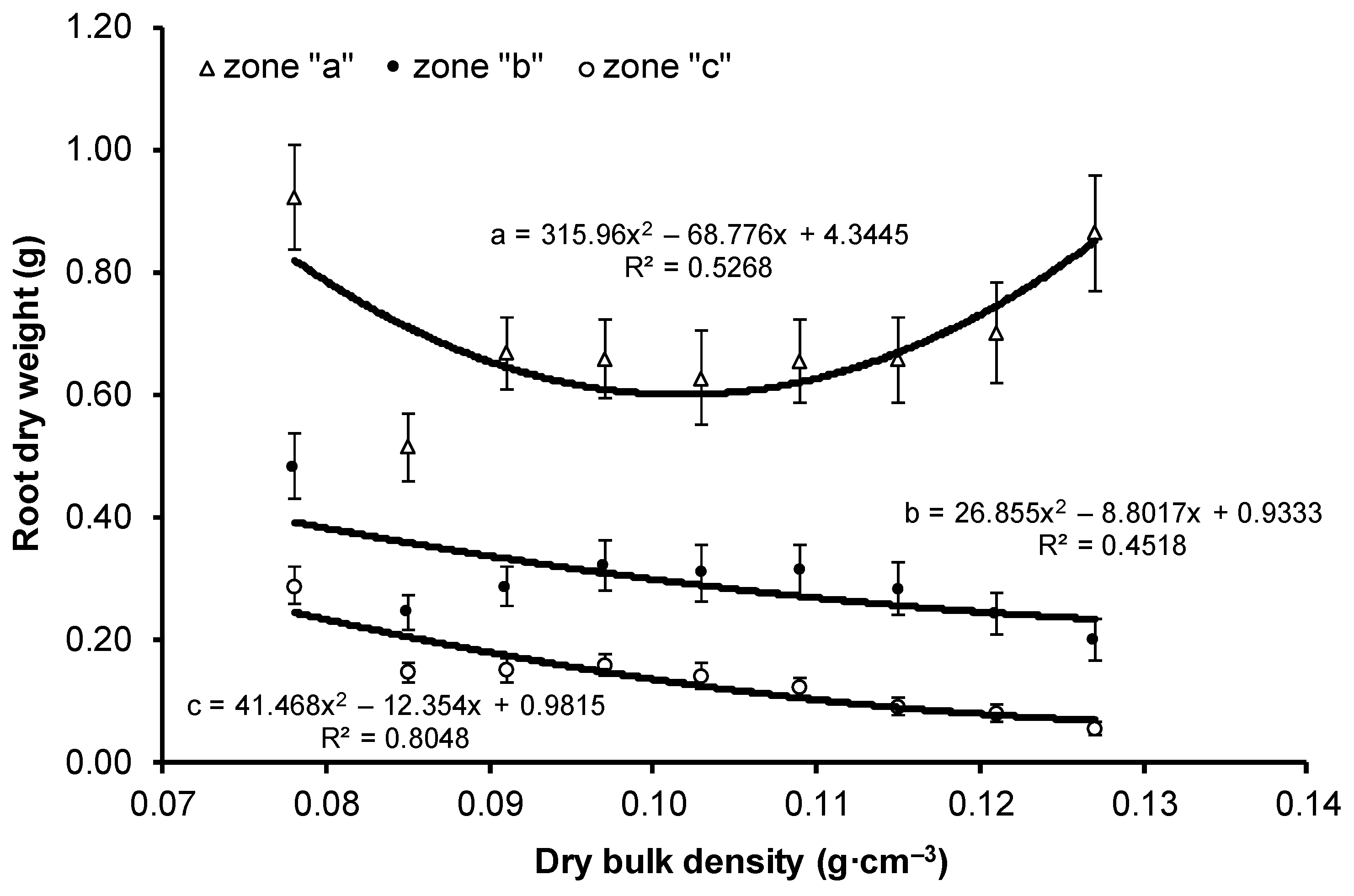
| Variant | Dry Weight (g) | Dry Weight of Root Parts (g) | ||||
|---|---|---|---|---|---|---|
| Stems | Leaves | Roots | (a) | (b) | (c) | |
| V1 | 2.384 ± 1.43 a | 1.236 ± 0.72 a | 1.695 ± 1.09 b | 0.924 ± 0.58 a | 0.483 ± 0.35 b | 0.288 ± 0.20 c |
| V2 | 1.871 ± 1.19 a | 0.918 ± 0.62 a | 0.905 ± 0.64 a | 0.514 ± 0.37 b | 0.245 ± 0.19 a | 0.146 ± 0.12 a |
| V3 | 2.157 ± 1.25 a | 1.000 ± 0.61 a | 1.105 ± 0.69 a | 0.668 ± 0.40 ab | 0.287 ± 0.22 a | 0.150 ± 0.13 a |
| V4 | 2.155 ± 1.27 a | 1.007 ± 0.60 a | 1.139 ± 0.71 ab | 0.659 ± 0.39 ab | 0.320 ± 0.25 ab | 0.160 ± 0.11 a |
| V5 | 2.293 ± 1.75 a | 0.912 ± 0.70 a | 1.078 ± 0.92 a | 0.628 ± 0.50 ab | 0.309 ± 0.31 ab | 0.141 ± 0.14 a |
| V6 | 2.238 ± 1.53 a | 0.975 ± 0.70 a | 1.089 ± 0.89 a | 0.654 ± 0.49 ab | 0.313 ± 0.31 a | 0.122 ± 0.11 ab |
| V7 | 1.997 ± 1.28 a | 1.027 ± 0.72 a | 1.029 ± 0.71 a | 0.657 ± 0.41 ab | 0.283 ± 0.25 a | 0.090 ± 0.08 ab |
| V8 | 2.451 ± 1.64 a | 1.123 ± 0.83 a | 1.021 ± 0.81 a | 0.701 ± 0.54 ab | 0.243 ± 0.23 a | 0.079 ± 0.10 ab |
| V9 | 2.712 ± 1.86 a | 1.232 ± 0.93 a | 1.120 ± 0.89 a | 0.865 ± 0.66 a | 0.200 ± 0.23 a | 0.055 ± 0.07 b |
| Mean | 2.261 ± 1.51 | 1.050 ± 0.74 | 1.134 ± 0.86 | 0.699 ± 0.51 | 0.298 ± 0.28 | 0.137 ± 0.14 |
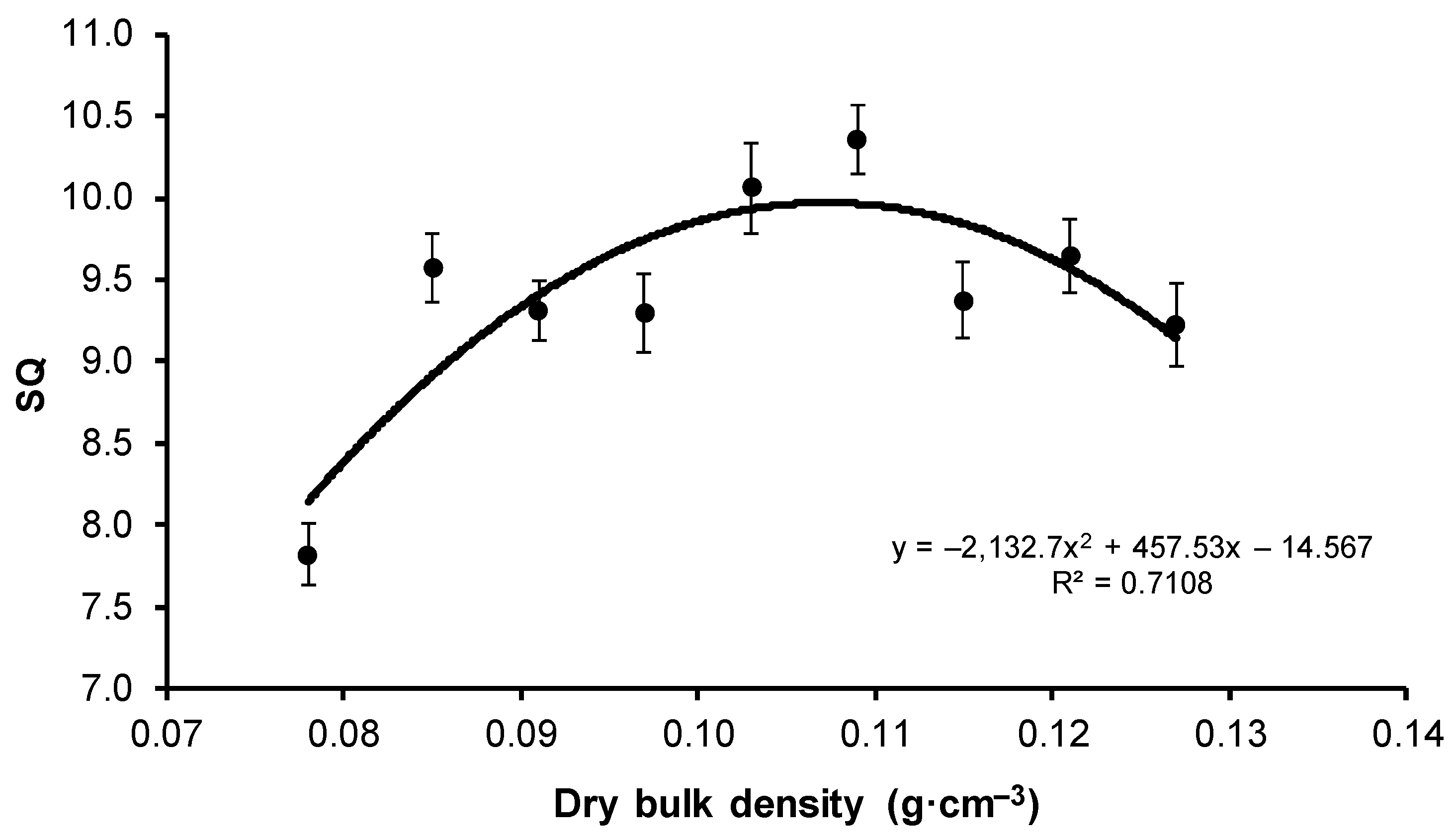
| Variant | Quality Indicator | |
|---|---|---|
| SQ | S/R | |
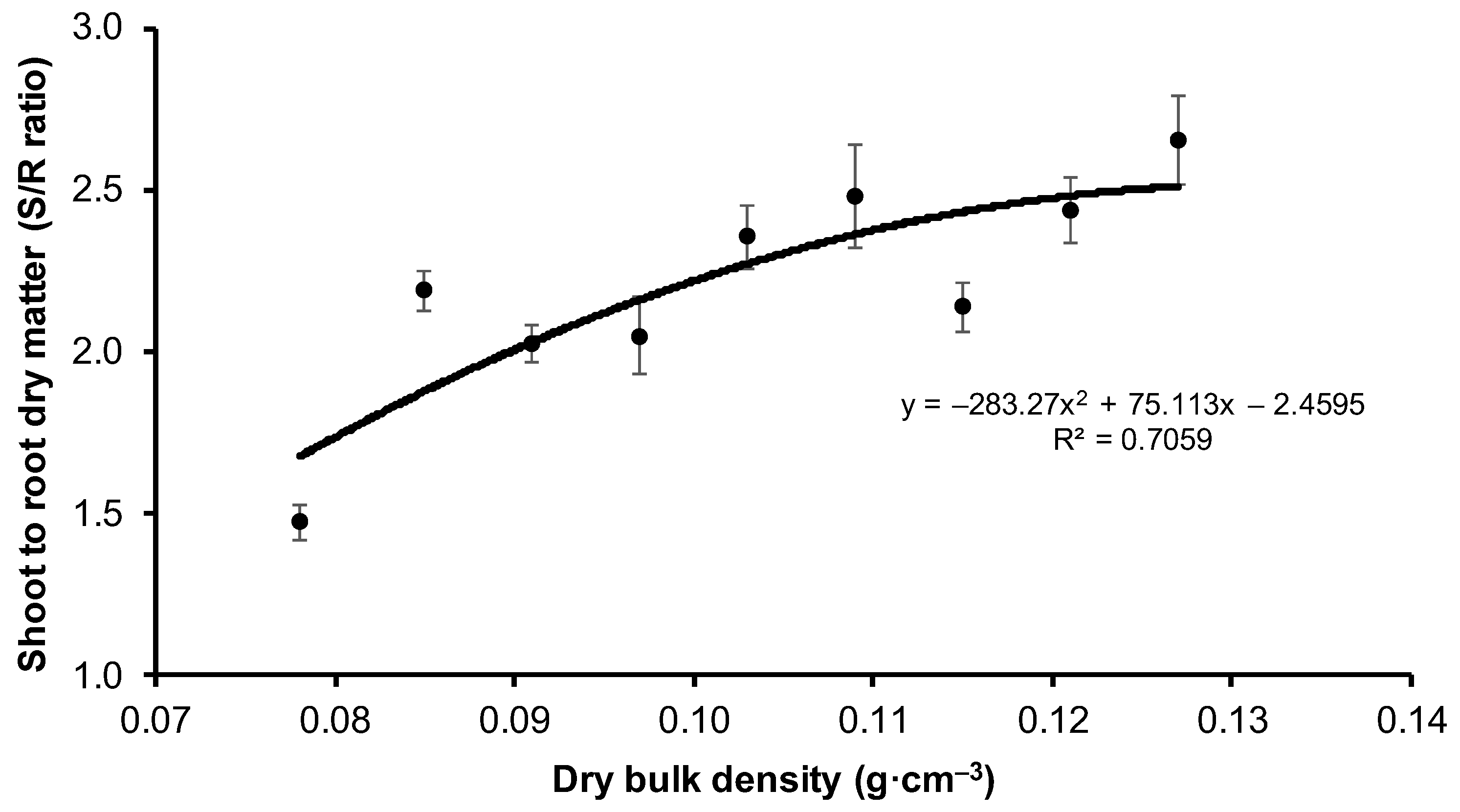
| V1 | 7.82 ± 1.27 c | 1.47 ± 0.36 e |
| V2 | 9.58 ± 1.42 ab | 2.19 ± 0.46 abc |
| V3 | 9.31 ± 1.20 a | 2.03 ± 0.41 a |
| V4 | 9.30 ± 1.45 a | 2.05 ± 0.80 ab |
| V5 | 10.1 ± 1.76 ab | 2.35 ± 0.66 abcd |
| V6 | 10.4 ± 1.53 b | 2.48 ± 1.17 bcd |
| V7 | 9.37 ± 1.34 ab | 2.14 ± 0.49 abc |
| V8 | 9.64 ± 1.48 ab | 2.60 ± 0.66 cd |
| V9 | 9.22 ± 1.74 a | 2.80 ± 0.95 d |
| Mean | 9.42 ± 1.63 | 2.25 ± 0.81 |
| Variant | Mean Total Root Length | Coarse Roots (Diameter > 2 mm) | Fine Roots (Diameter 2–0.5 mm) | Very Fine Roots (Diameter ≤ 0.5 mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (a) | (b) | (c) | (a) | (b) | (c) | (a) | (b) | (c) | ||
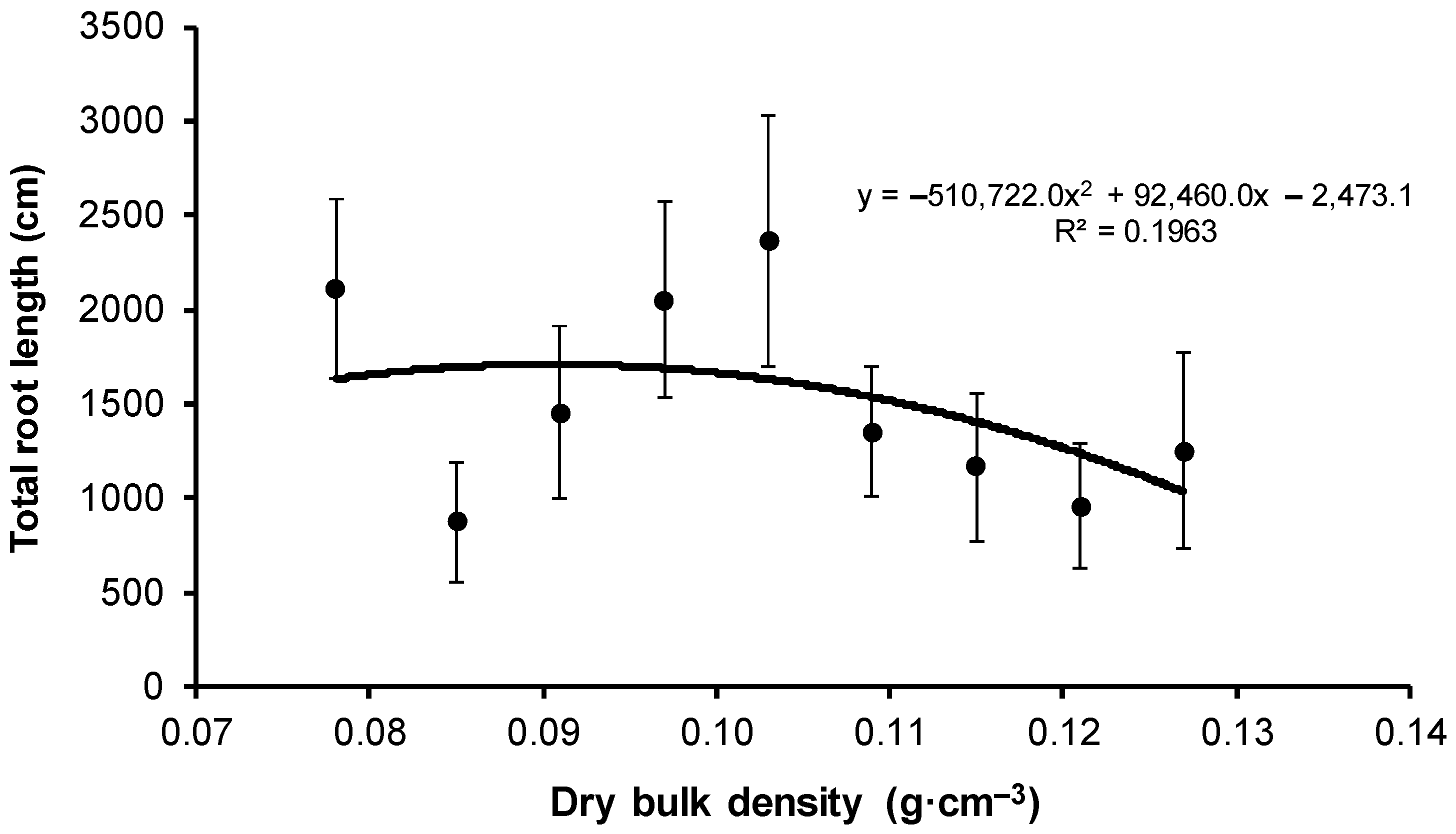
| V1 | 2108.3 ± 1774.9 b | 22.9 ± 8.7 | 11.0 ± 5.2 b | 2.6 ± 2.9 b | 41.2 ± 30.3 | 52.8 ± 47.0 b | 35.2 ± 26.8 b | 415.5 ± 458.7 | 728.3 ± 701.5 | 872.5 ± 787.9 |
| V2 | 871.9 ± 1204.6 a | 15.8 ± 11.1 | 4.6 ± 6.7 ab | 0.8 ± 1.8 ab | 24.6 ± 15.0 | 17.3 ± 11.7 a | 10.2 ± 10.8 a | 173.2 ± 190.2 | 245.4 ± 308.3 | 380.0 ± 720.6 |
| V3 | 1455.5 ± 1463.5 a | 22.6 ± 8.5 | 8.6 ± 8.5 ab | 0.9 ± 2.3 ab | 22.7 ± 12.2 | 15.8 ± 9.8 a | 9.3 ± 5.8 a | 434.9 ± 460.9 | 519.8 ± 720.4 | 421.1 ± 500.3 |
| V4 | 2056.7 ± 1480.1 a | 18.7 ± 6.4 | 4.1 ± 3.5 ab | 0.2 ± 0.3 a | 28.6 ± 11.2 | 24.6 ± 17.2 ab | 31.2 ± 39.9 ab | 416.2 ± 299.9 | 416.2 ± 299.9 | 923.2 ± 814.2 |
| V5 | 2361.3 ± 1895.3 a | 23.4 ± 7.4 | 8.9 ± 7.3 ab | 0.3 ± 0.6 ab | 27.8 ± 14.6 | 27.2 ± 22.6 ab | 20.6 ± 21.6 ab | 585.1 ± 531 | 787.2 ± 732.9 | 880.8 ± 728.9 |
| V6 | 1353.0 ± 966.0 a | 18.4 ± 7.4 | 6.4 ± 4.3 ab | 0.0 ± 0.1 a | 25.8 ± 13.0 | 29.6 ± 21.7 ab | 13.8 ± 10.5 ab | 352.9 ± 259.2 | 484.3 ± 392.9 | 421.8 ± 365.4 |
| V7 | 1167.1 ± 1109.4 a | 15.6 ± 5.6 | 3.4 ± 3.3 ab | 0.2 ± 0.5 a | 25.9 ± 8.8 | 12.8 ± 6.3 a | 4.8 ± 5.6 a | 343.2 ± 339.6 | 272.5 ± 135.2 | 488.6 ± 708.5 |
| V8 | 956.0 ± 881.2 a | 16.4 ± 7.0 | 5.1 ± 5.7 ab | 0.0 ± 0.0 a | 25.6 ± 18.9 | 16.8 ± 11.0 a | 4.6 ± 2.7 a | 456.6 ± 556.7 | 273.5 ± 233.7 | 157.5 ± 113.2 |
| V9 | 1248.6 ± 1383.1 a | 19.8 ± 9.6 | 1.6 ± 2.4 a | 0.0 ± 0.0 a | 46.4 ± 40.7 | 10.9 ± 7.9 a | 4.1 ± 5.3 a | 808.9 ± 1028.1 | 220.9 ± 218.4 | 136.2 ± 173.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pająk, K.; Kormanek, M.; Małek, S.; Banach, J. Effect of Peat-Perlite Substrate Compaction in Hiko V265 Trays on the Growth of Fagus sylvatica L. Seedlings. Sustainability 2022, 14, 4585. https://doi.org/10.3390/su14084585
Pająk K, Kormanek M, Małek S, Banach J. Effect of Peat-Perlite Substrate Compaction in Hiko V265 Trays on the Growth of Fagus sylvatica L. Seedlings. Sustainability. 2022; 14(8):4585. https://doi.org/10.3390/su14084585
Chicago/Turabian StylePająk, Katarzyna, Mariusz Kormanek, Stanisław Małek, and Jacek Banach. 2022. "Effect of Peat-Perlite Substrate Compaction in Hiko V265 Trays on the Growth of Fagus sylvatica L. Seedlings" Sustainability 14, no. 8: 4585. https://doi.org/10.3390/su14084585
APA StylePająk, K., Kormanek, M., Małek, S., & Banach, J. (2022). Effect of Peat-Perlite Substrate Compaction in Hiko V265 Trays on the Growth of Fagus sylvatica L. Seedlings. Sustainability, 14(8), 4585. https://doi.org/10.3390/su14084585






