Brushite-Metakaolin Composite Geopolymer Material as an Effective Adsorbent for Lead Removal from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Studies
3. Results and Discussion
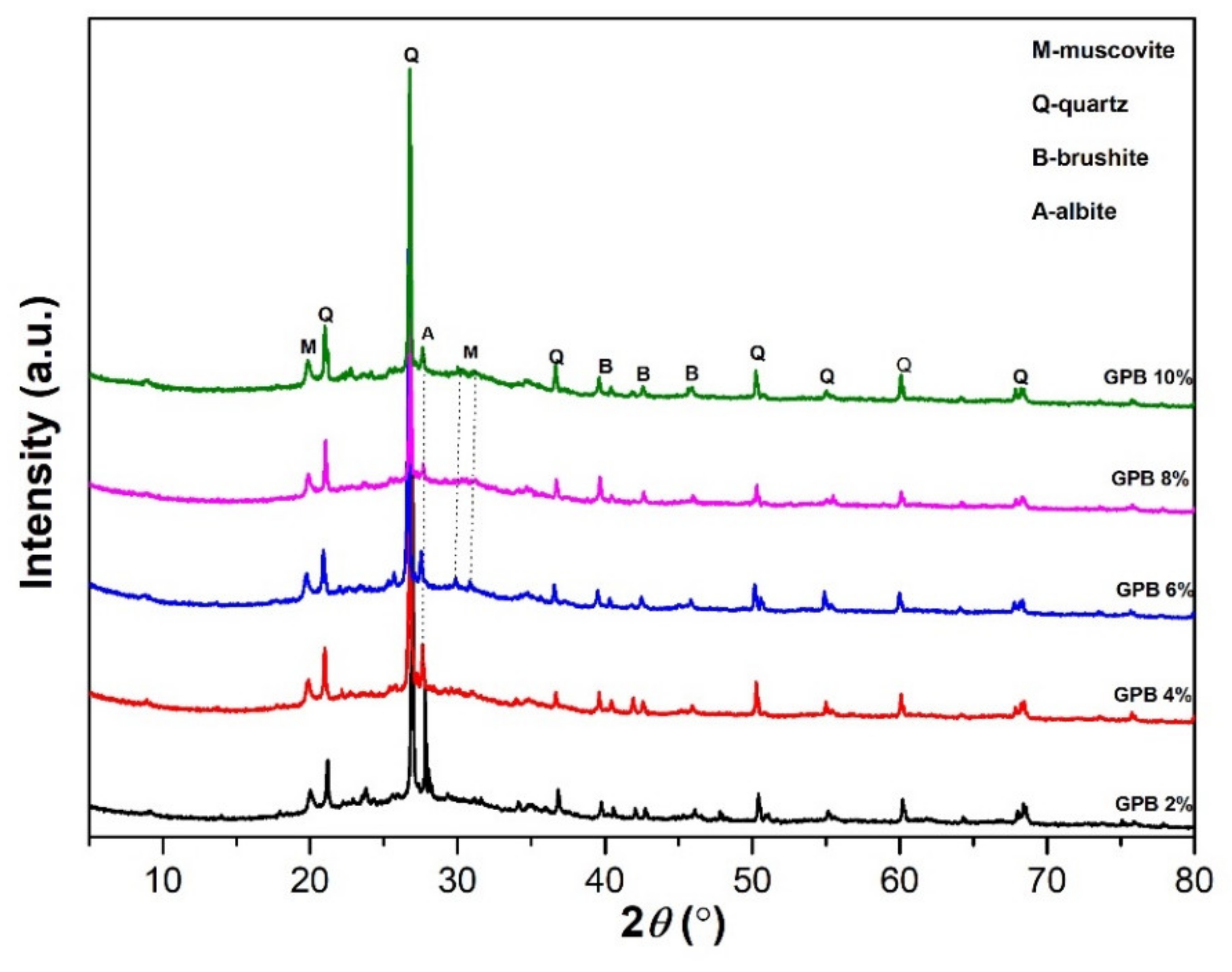
3.1. XRD Results
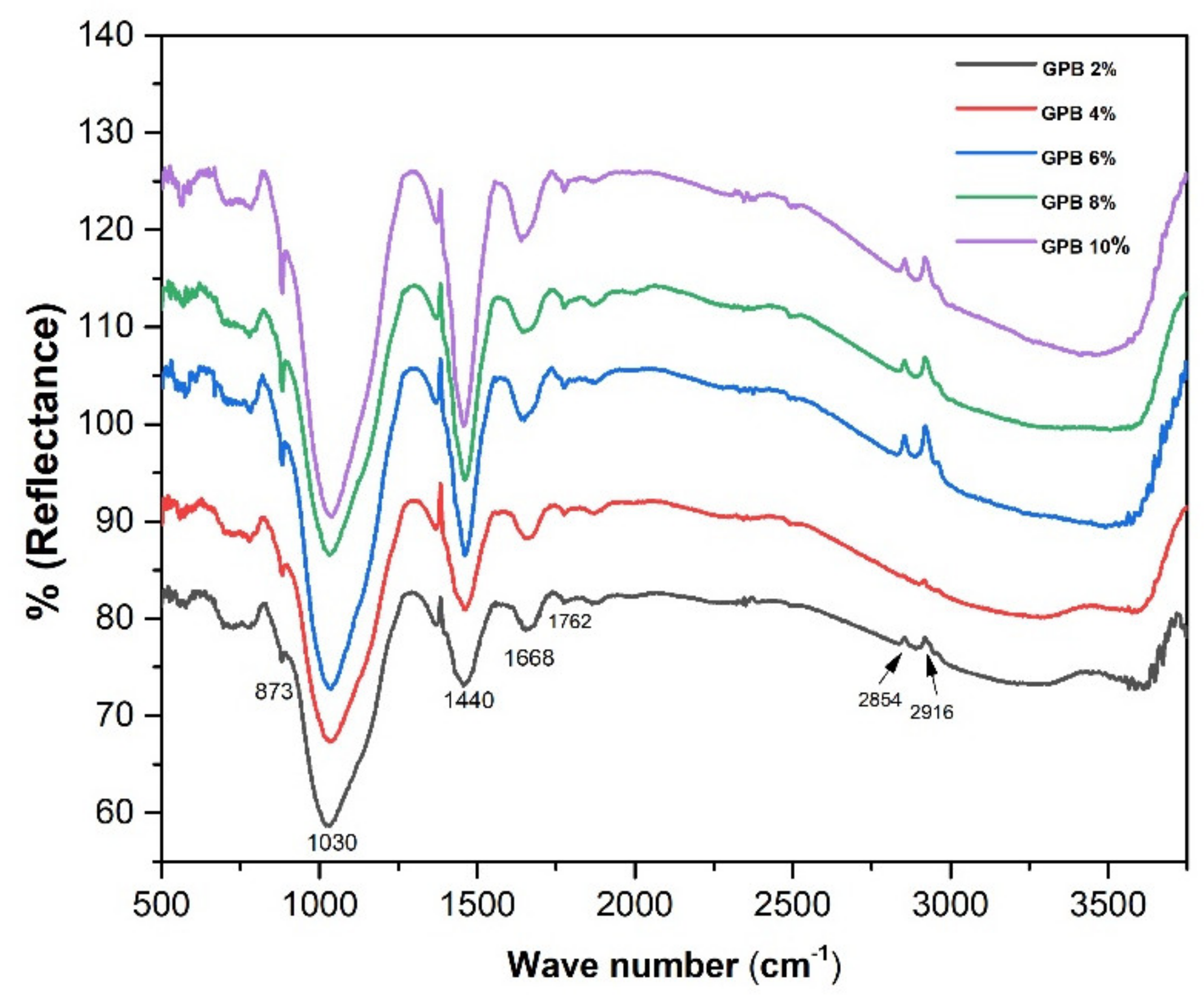
3.2. DRIFT Analysis Results
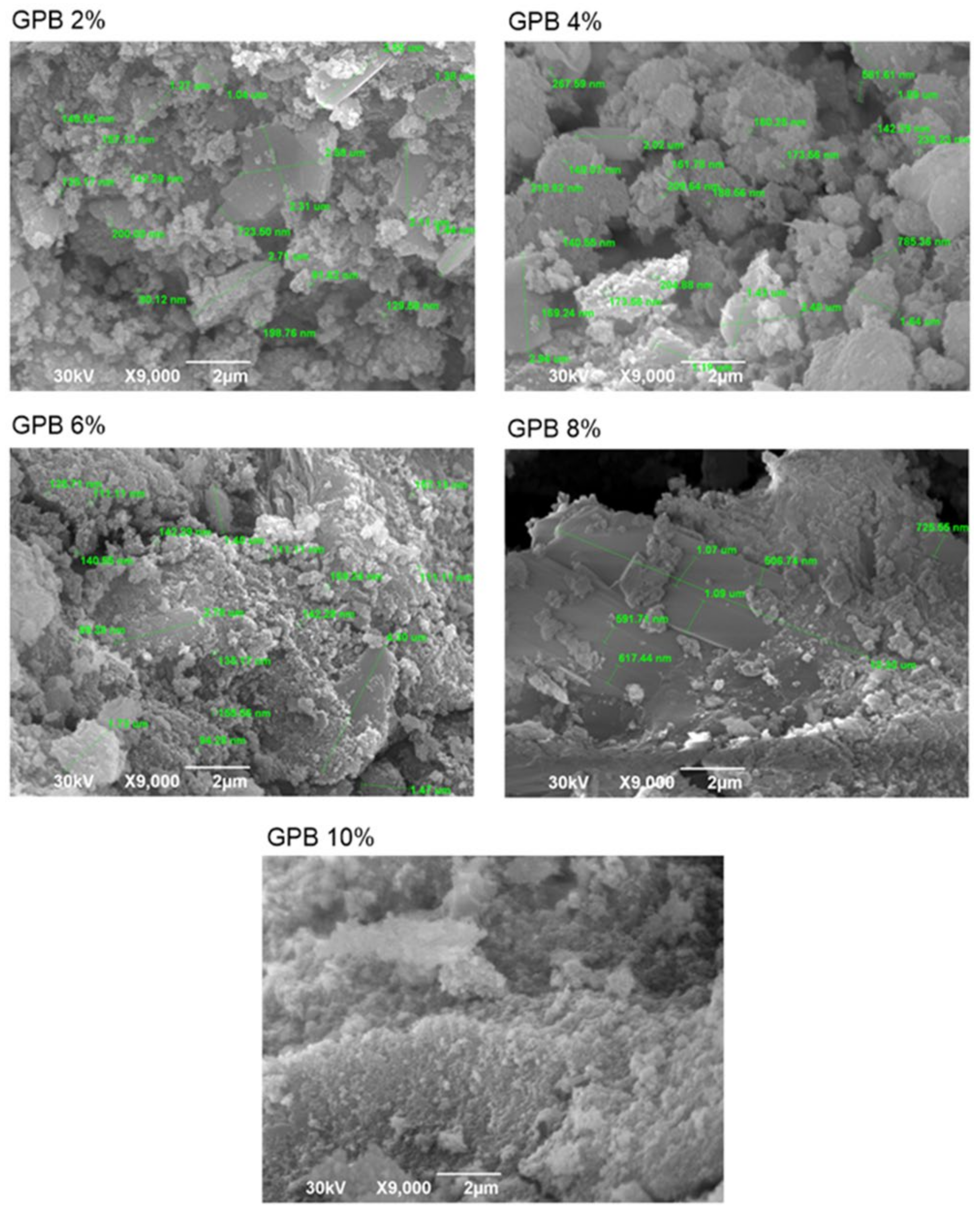
3.3. SEM-EDS Analysis Results
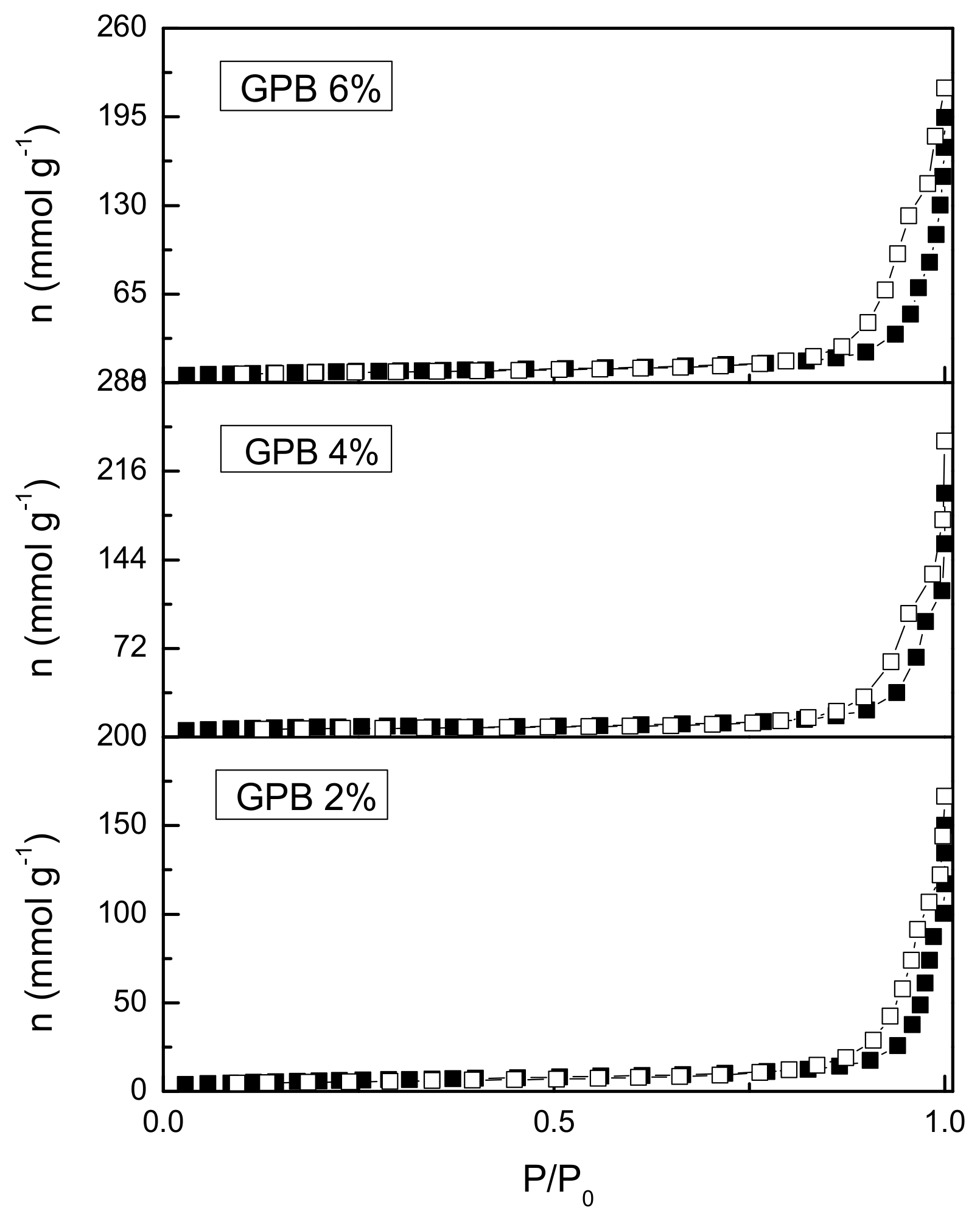
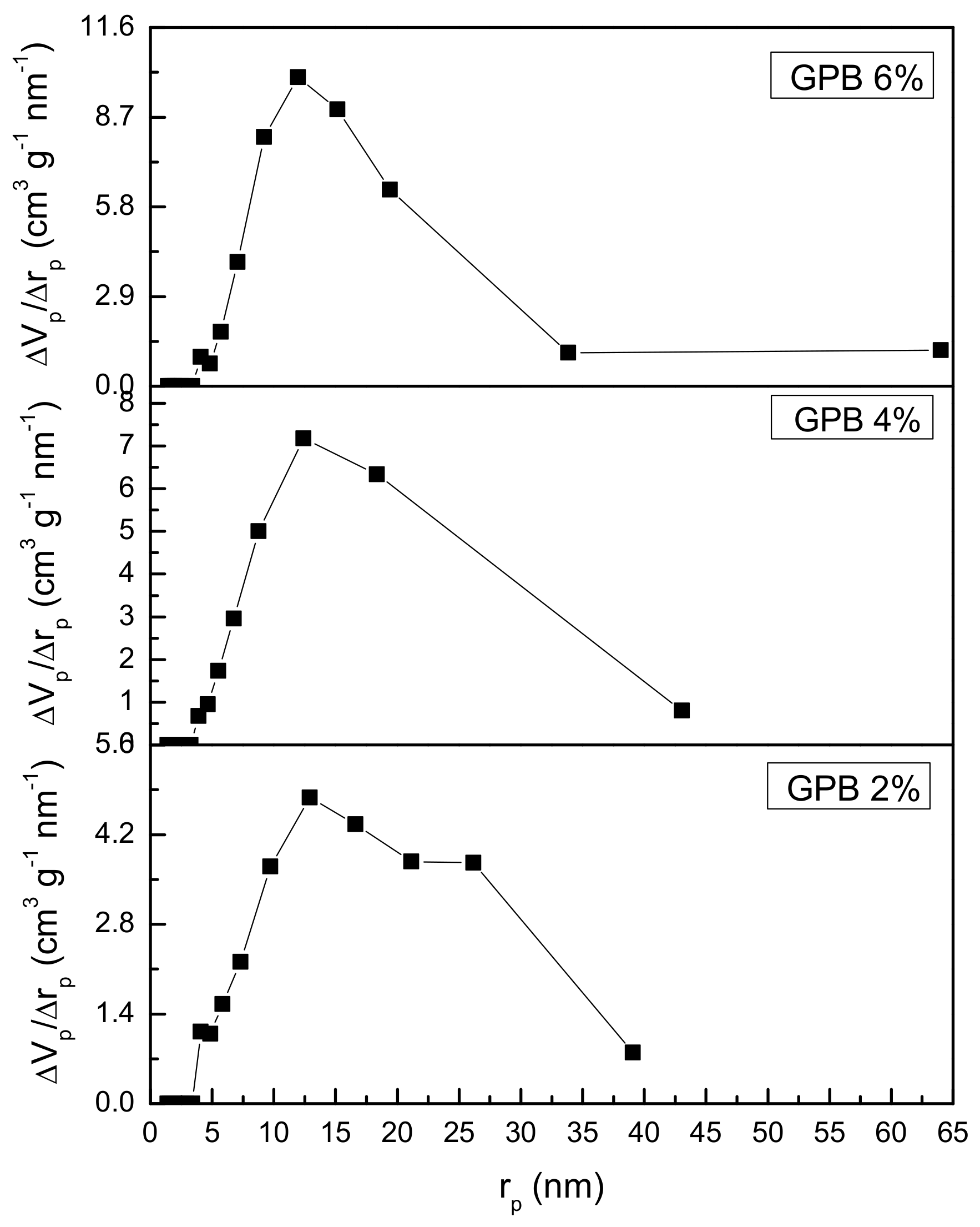
3.4. BET Analysis Results
3.5. Adsorption Studies
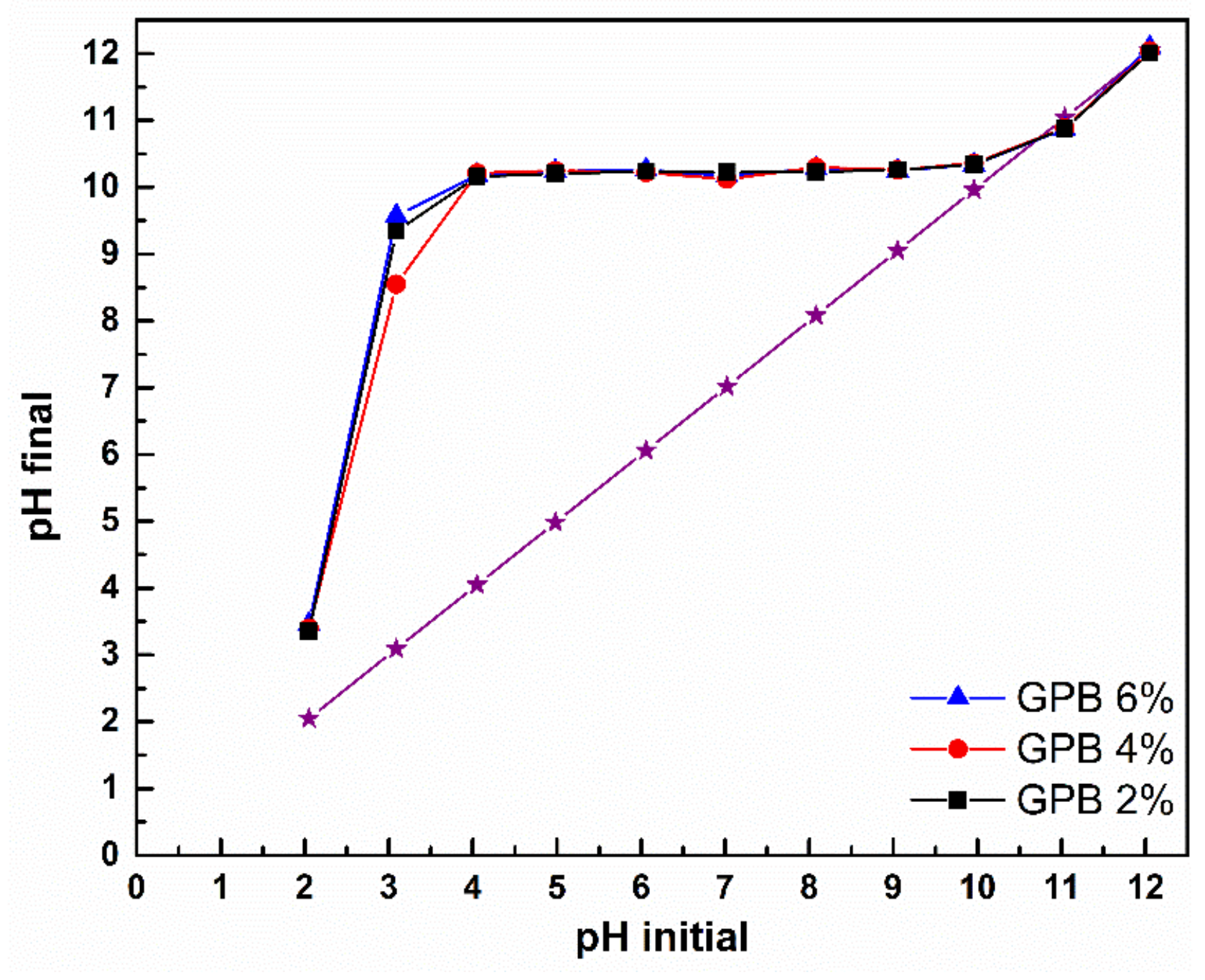
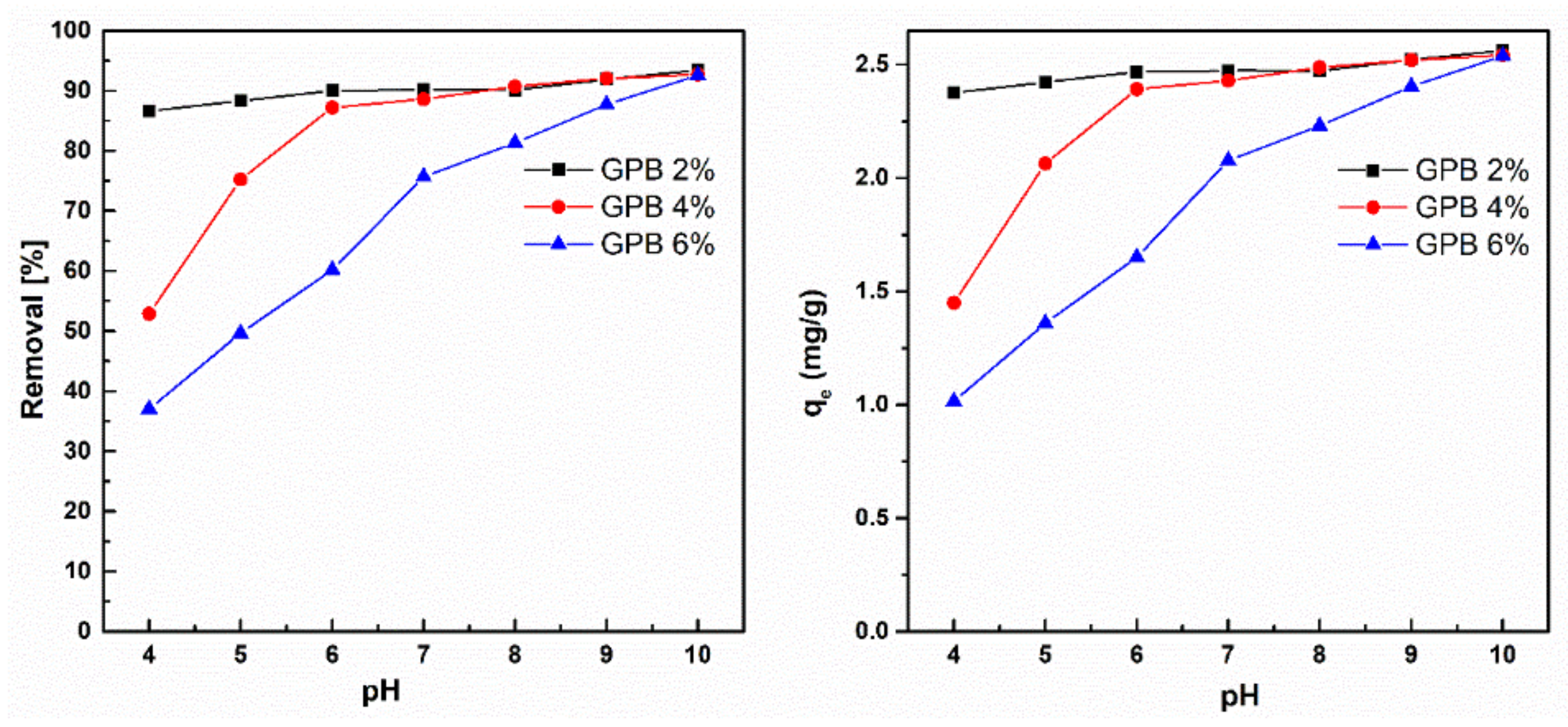
3.5.1. Effect of pH
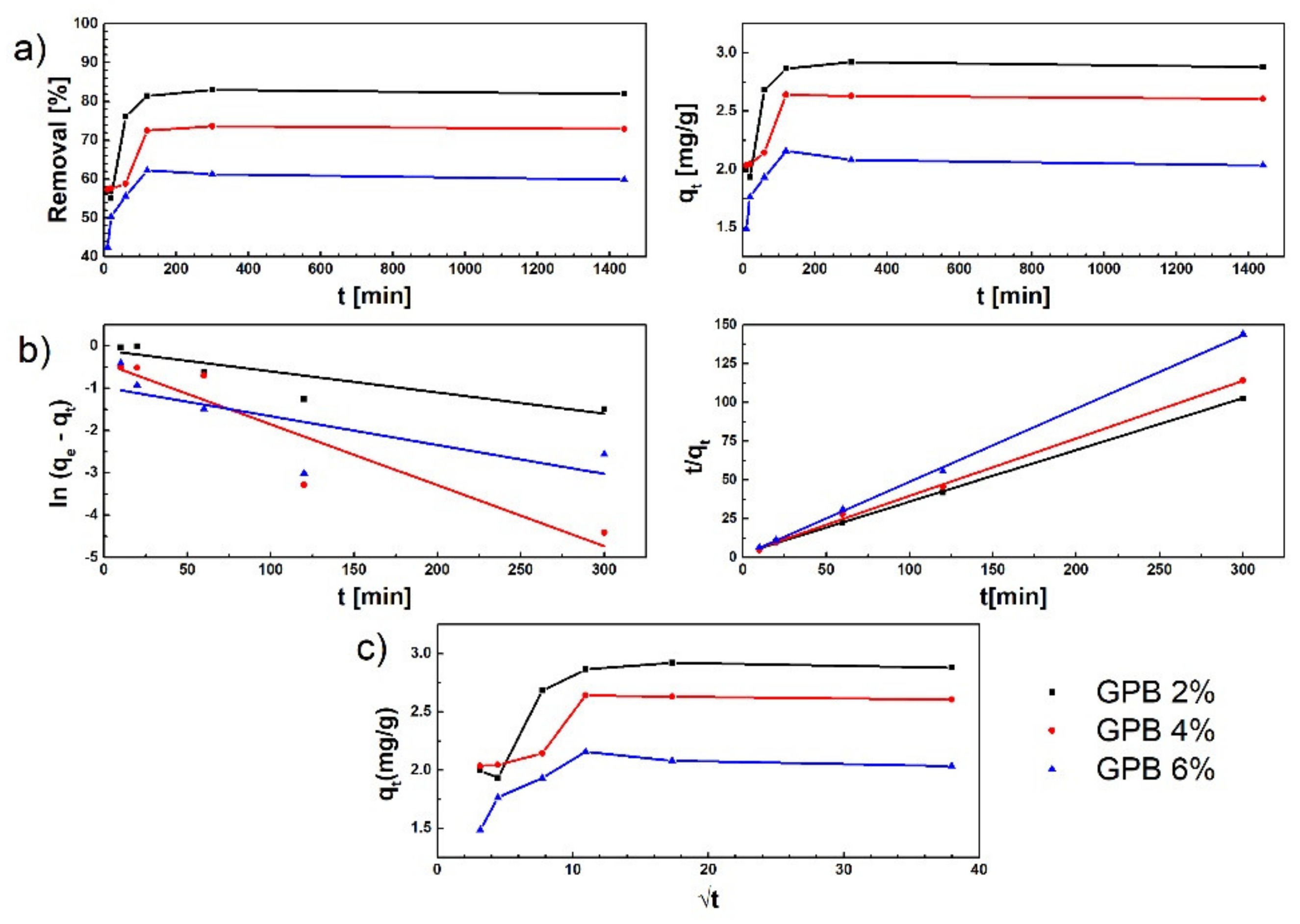
3.5.2. Adsorption Studies—Effect of Contact Time and Kinetic Study
- t is adsorption process time (min),
- qt is adsorption capacity of the GPB adsorbents, (mg g−1),
- qe is adsorption capacity of the adsorbent at equilibrium (mg g−1),
- k1 is the pseudo-first order model’s adsorption rate constant (min−1), and
- k2 is the pseudo-second order model’s adsorption rate constant [g (mg min)−1].
- kp is the rate constant of the intra-particle diffusion model (mg g−1 × min1/2) and
- C is a constant associated with thickness of the boundary layer (mg g−1).
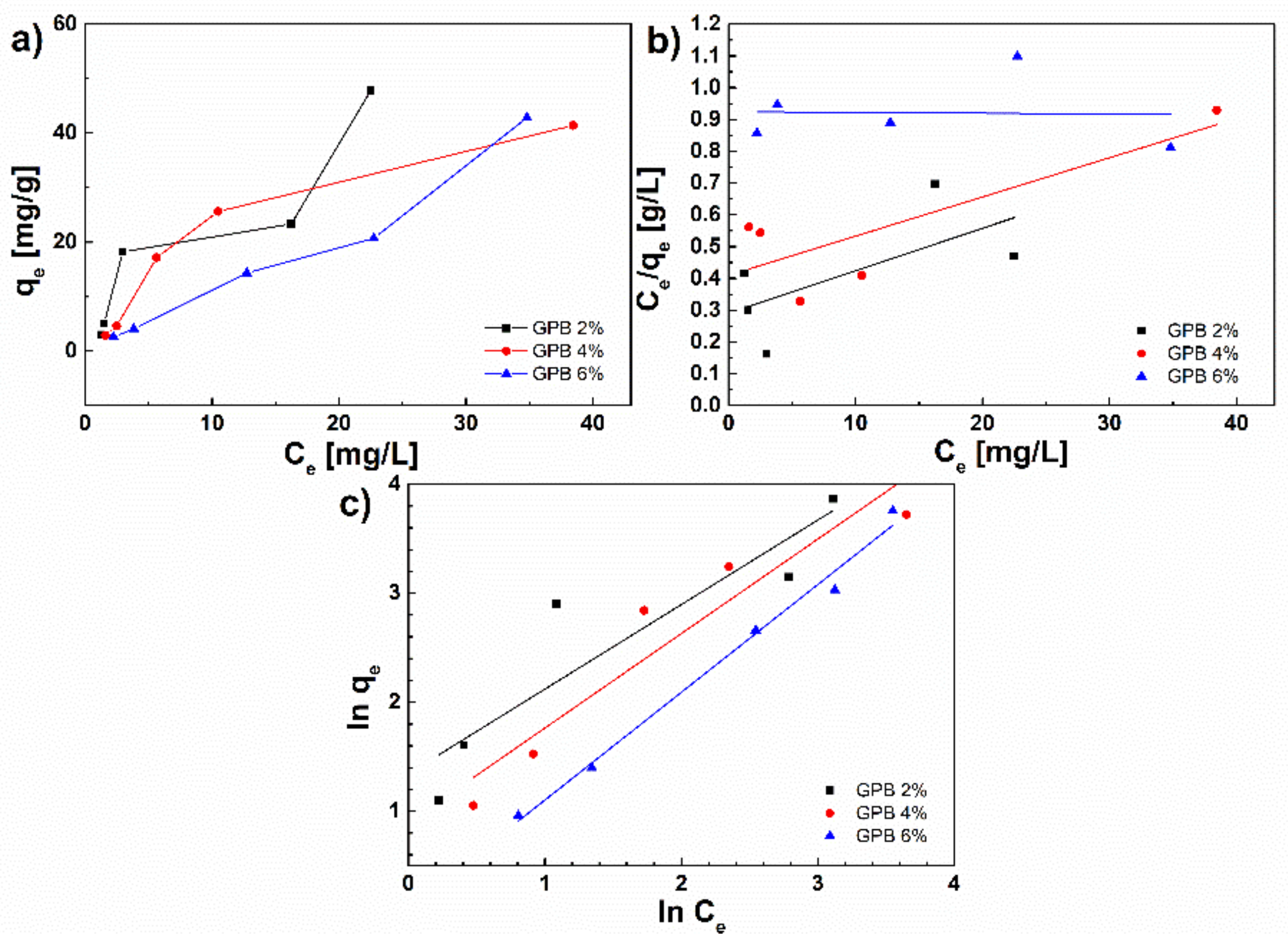
3.5.3. Adsorption Isotherms
- qe is adsorption capacity at equilibrium (mg g−1);
- qm is the maximum adsorption capacity (mg g−1);
- ce is concentration of lead solution after equilibrium concentration (mg dm−3);
- Kl is Langmuir isotherm constant (dm3 mg−1);
- is Freundlich isotherm constant (L mg−1);
- 1/n is heterogeneity factor and related to surface heterogeneity;
- αl is a parameter related to adsorption energy and is obtained from the linearly “fitted” graph.
- Kl/αl represents theoretical monolayer saturation capacity. Adsorption is favorable if is smaller than 1 and bigger than 0 [40].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melila, M.; Rajaram, R.; Ganeshkumar, A.; Kpemissi, M.; Pakoussi, T.; Agbere, S.; Lazar, I.M.; Lazar, G.; Amouzou, K.S.; Paray, B.A.; et al. Assessment of renal and hepatic dysfunction by co-exposure to toxic metals (Cd, Pb) and fluoride in people living nearby an industrial zone. J. Trace Elem. Med. Biol. 2021, 69, 126890. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C.; Forsthuber, M.; Szigeti, T.; Kakucs, R.; Mustieles, V.; Fernandez, M.F.; Bengtsen, E.; Vogel, U.; Hougaard, K.S.; Saber, A.T. Lead (Pb) and neurodevelopment: A review on exposure and biomarkers of effect (BDNF, HDL) and susceptibility. Int. J. Hyg. Environ. Health 2021, 238, 113855. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Tao, S.; Huang, Y.; Jin, Y.; Hu, Y.; Lu, J. Combined toxic effects of CBNPs and Pb on rat alveolar macrophage apoptosis and autophagy flux. Ecotoxicol. Environ. Saf. 2020, 205, 111062. [Google Scholar] [CrossRef] [PubMed]
- Rakhym, A.B.; Seilkhanova, G.A.; Kurmanbayeva, T.S. Adsorption of lead (II) ions from water solutions with natural zeolite and chamotte clay. Mater. Today Proc. 2020, 31, 482–485. [Google Scholar] [CrossRef]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z.; Lai, X. Adsorption mechanism of lead ions at ilmenite/water interface and its influence on ilmenite flotability. J. Ind. Eng. Chem. 2017, 53, 285–293. [Google Scholar] [CrossRef]
- Huda, B.N.; Wahyuni, E.T.; Mudasir, M. Eco-friendly immobilization of dithizone on coal bottom ash for the adsorption of lead(II) ion from water. Results Eng. 2021, 10, 100221. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Sun, M.; Li, X.; Qiu, H. Highly selective adsorption of lead ions by water-dispersible magnetic chitosan/graphene oxide composites. Colloids Surf. B Biointerfaces 2013, 103, 523–529. [Google Scholar] [CrossRef]
- Perná, I.; Šupová, M.; Hanzlíček, T.; Špaldoňová, A. The synthesis and characterization of geopolymers based on metakaolin and high LOI straw ash. Constr. Build. Mater. 2019, 228, 116765. [Google Scholar] [CrossRef]
- Nenadović, S.S.; Kljajević, L.M.; Nešić, M.A.; Petković, M.Ž.; Trivunac, K.V.; Pavlović, V.B. Structure analysis of geopolymers synthesized from clay originated from Serbia. Environ. Earth Sci. 2017, 76, 1–10. [Google Scholar] [CrossRef]
- Ivanović, M.; Kljajević, L.M.; Gulicovski, J.J.; Petković, M.; Janković-Častvan, I.; Bučevac, D.; Nenadović, S.S. The Effect of the Concentration of Alkaline Activator and Aging Time on the Structure of Metakaolin Based Geopolymer. Sci. Sinter. 2020, 52, 219–229. [Google Scholar] [CrossRef]
- Mladenović, N.; Kljajević, L.; Nenadović, S.; Ivanović, M.; Čalija, B.; Gulicovski, J.; Trivunac, K. The Applications of New Inorganic Polymer for Adsorption Cadmium from Waste Water. J. Inorg. Organomet. Polym. Mater. 2020, 30, 554–563. [Google Scholar] [CrossRef]
- Trivunac, K.; Kljajević, L.; Nenadović, S.; Gulicovski, J.; Mirković, M.; Babić, B.; Stevanović, S. Microstructural characterization and adsorption properties of alkali-activated materials based on metakaolin. Sci. Sinter. 2016, 48, 209–220. [Google Scholar] [CrossRef]
- Si, R.; Dai, Q.; Guo, S.; Wang, J. Mechanical Property, Nanopore Structure and Drying Shrinkage of Metakaolin-based Geopolymer with waste glass powder. J. Clean. Prod. 2019, 242, 118502. [Google Scholar] [CrossRef]
- Tan, J.; Lu, W.; Huang, Y.; Wei, S.; Xuan, X.; Liu, L.; Zheng, G. Preliminary study on compatibility of metakaolin-based geopolymer paste with plant fibers. Constr. Build. Mater. 2019, 225, 772–775. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Moghaddam, T.B.; Zhang, F.; Otto, F. Synergistic effects of iron (Fe) and biochar on light-weight geopolymers when used in wastewater treatment applications. J. Clean. Prod. 2021, 322, 129033. [Google Scholar] [CrossRef]
- Humberto Tommasini Vieira Ramos, F.J.; Vieira Marques, M.D.F.; de Oliveira Aguiar, V.; Jorge, F.E. Performance of geopolymer foams of blast furnace slag covered with poly(lactic acid) for wastewater treatment. Ceram. Int. 2022, 48, 732–743. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Z.; Song, W.; Zhong, Y.; Sun, M.; Gan, T.; Bao, B. Quantifying gel properties of industrial waste-based geopolymers and their application in Pb2+ and Cu2+ removal. J. Clean. Prod. 2021, 315, 128203. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Fotio, D.; Rüscher, C.H.; Kamseu, E.; Djobo, J.N.Y.; Bignozzi, M.C.; Leonelli, C. The effects of synthesized calcium phosphate compounds on the mechanical and microstructural properties of metakaolin-based geopolymer cements. Constr. Build. Mater. 2018, 163, 776–792. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, C.; Rashid, S.; Li, S.; Ali, B.A.; Liu, J. Biopolymer-induced morphology control of brushite for enhanced defluorination of drinking water. J. Colloid Interface Sci. 2017, 491, 207–215. [Google Scholar] [CrossRef]
- Mirković, M.M.; Pašti, T.D.L.; Došen, A.M.; Čebela, M.Ž.; Rosić, A.A.; Matović, B.Z.; Babić, B.M. Adsorption of malathion on mesoporous monetite obtained by mechanochemical treatment of brushite. RSC Adv. 2016, 6, 12219–12225. [Google Scholar] [CrossRef]
- Possenti, E.; Colombo, C.; Conti, C.; Gigli, L.; Merlini, M.; Plaisier, J.R.; Realini, M.; Sali, D.; Gatta, G.D. Diammonium hydrogenphosphate for the consolidation of building materials. Investigation of newly-formed calcium phosphates. Constr. Build. Mater. 2019, 195, 557–563. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Removal of Cu(II) by natural and acid-activated clays: An insight of adsorption isotherm, kinetic and thermodynamics. Desalination 2011, 272, 66–75. [Google Scholar] [CrossRef]
- Salem, A.; Akbari Sene, R. Removal of lead from solution by combination of natural zeolite–kaolin–bentonite as a new low-cost adsorbent. Chem. Eng. J. 2011, 174, 619–628. [Google Scholar] [CrossRef]
- Liu, H.; Peng, S.; Shu, L.; Chen, T.; Bao, T.; Frost, R.L. Magnetic zeolite NaA: Synthesis, characterization based on metakaolin and its application for the removal of Cu2+, Pb2+. Chemosphere 2013, 91, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Lakherwal, D. Adsorption of heavy metals: A Review. Int. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Rigaku. PDXL Integrated X-ray Powder Diffraction Software, 2.8.3.0.; Rigaku: Tokyo, Japan, 2011. [Google Scholar]
- International Centre for Diffraction Data. International Crystallographical Database (ICDD); PDF-2 Release; International Centre for Diffraction Data: Newtown Square, PA, USA, 2012. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lippens, B.C.; Linsen, B.G.; de Boer, J.H. Studies on pore systems in catalysts I. The adsorption of nitrogen; apparatus and calculation. J. Catal. 1964, 3, 32–37. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, T.; Zhang, R.; Wang, M.; Krishnan, S.; Liu, S.; Zhou, Y.; Li, D.; Qi, Z. Effects of clay colloids on ciprofloxacin transport in saturated quartz sand porous media under different solution chemistry conditions. Ecotoxicol. Environ. Saf. 2020, 199, 110754. [Google Scholar] [CrossRef]
- Rüscher, C.H.M.; Elzbieta, M.; Wongpa, J.; Jaturapitakkul, C.; Jirasit, F.; Lohaus, L. Silicate-, aluminosilicate and calciumsilicate gels for building materials: Chemical and mechanical properties during ageing. Eur. J. Mineral. 2011, 23, 111–124. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dye Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Hamoudi, S.A.; Hamdi, B.; Brendlé, J. Chapter 4.7—Removal of Ions Pb2+ and Cd2+ from Aqueous Solution by Containment Geomaterials. In Exergetic, Energetic and Environmental Dimensions; Dincer, I., Colpan, C.O., Kizilkan, O., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1029–1043. [Google Scholar]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, N.K.; Asouhidou, D.D. Kinetics of sorptive removal of chromium(VI) from aqueous solutions by calcined Mg–Al–CO3 hydrotalcite. Water Res. 2003, 37, 2875–2882. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int. J. Environ. Sci. Technol. 2013, 10, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Yavari, S.; Mohammad, M.N.; Teymouri, P.; Shahmoradi, B.; Maleki, A. Cobalt ferrite nanoparticles: Preparation, characterization and anionic dye removal capability. J. Taiwan Inst. Chem. Eng. 2016, 59, 320–329. [Google Scholar] [CrossRef]
- De Sá, A.; Abreu, A.S.; Moura, I.; Machado, A.V. 8—Polymeric materials for metal sorption from hydric resources. In Water Purification; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 289–322. [Google Scholar]
- Ghahremani, D.; Mobasherpour, I.; Mirhosseini, S.A. Sorption thermodynamic and kinetic studies of Lead removal from aqueous solutions by nano Tricalcium phosphate. Bull. Soc. R. Sci. Liège 2017, 86, 96–112. [Google Scholar] [CrossRef]
- Safatian, F.; Doago, Z.; Torabbeigi, M.; Rahmani Shams, H.; Ahadi, N. Lead ion removal from water by hydroxyapatite nanostructures synthesized from egg sells with microwave irradiation. Appl. Water Sci. 2019, 9, 108. [Google Scholar] [CrossRef] [Green Version]
- Lan, T.; Guo, S.; Li, X.; Guo, J.; Bai, T.; Zhao, Q.; Yang, W.; Li, P. Mixed precursor geopolymer synthesis for removal of Pb(II) and Cd(II). Mater. Lett. 2020, 274, 127977. [Google Scholar] [CrossRef]
| Element wt (%) | GPB 2% | GPB 4% | GPB 6% | GPB 8% | GPB 10% |
|---|---|---|---|---|---|
| Na | 27.62 | 27.02 | 27.44 | 26.94 | 27.62 |
| Al | 26.67 | 26.52 | 26.48 | 26.08 | 26.02 |
| Si | 42.71 | 42.68 | 42.86 | 41.94 | 41.83 |
| P | 0.64 | 0.72 | 0.79 | 0.96 | 0.99 |
| K | 0.67 | 0.45 | 0.48 | 0.86 | 0.57 |
| Ca | 0.86 | 0.93 | 1.02 | 1.72 | 1.90 |
| Fe | 0.83 | 1.68 | 0.93 | 1.50 | 1.07 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Sample | Model | Parameter | Value |
|---|---|---|---|
| GBP2% | Pseudo-first order | k1 | −0.0050 |
| qe | 0.9014 | ||
| R2 | 0.8660 | ||
| Pseudo-second order | k2 | 0.0428 | |
| qe | 2.9949 | ||
| R2 | 1.0000 | ||
| GBP4% | Pseudo-first order | k1 | −0.0144 |
| qe | 1.5083 | ||
| R2 | 0.8250 | ||
| Pseudo-second order | k2 | 0.0542 | |
| qe | 2.6923 | ||
| R2 | 0.9997 | ||
| GBP6% | Pseudo-first order | k1 | −0.0068 |
| qe | 0.3755 | ||
| R2 | 0.3976 | ||
| Pseudo-second order | k2 | 0.1422 | |
| qe | 2.1169 | ||
| R2 | 0.9999 |
| Sample | Model | Parameter | Value |
|---|---|---|---|
| GBP2% | Langmuir | KL | 3.4447 |
| αL | 0.0462 | ||
| qm | 74.627 | ||
| R2 | 0.4340 | ||
| Freundlich | KF | 3.8359 | |
| 1/n | 0.7757 | ||
| R2 | 0.8405 | ||
| GBP4% | Langmuir | KL | 2.4402 |
| αL | 0.0300 | ||
| qm | 81.301 | ||
| R2 | 0.6716 | ||
| Freundlich | KF | 2.4493 | |
| 1/n | 0.8670 | ||
| R2 | 0.9033 | ||
| GBP6% | Langmuir | KL | 1.0814 |
| αL | −0.0002 | ||
| R2 | 0.0009 | ||
| Freundlich | KF | 1.1196 | |
| 1/n | 0.9889 | ||
| R2 | 0.9901 |
| CoPb(II) (mg/L) | Adsorbent Dosage (mg) | Adsorbent | Removal (%) | Saturation Time (min) | Adsorption Efficiency * (mg g−1 min−1) | Ref. |
|---|---|---|---|---|---|---|
| 400 | 30 | nano-TCP | 37.5 | 60 | 0.08 | [42] |
| 20 | 10 | nano-HAp | 99.8 | 15 | 0.13 | [43] |
| 50 | 500 | MK | 98 | 60 | 0.002 | [12] |
| 50 | 120 | MK-GP | 99 | 60 | 0.007 | [12] |
| 1000 | 100 | FA 35%/MK 65% | 65.44 | 240 | 0.027 | [44] |
| 142.1 | 50 | GPB 2% | 84.18 | 120 | 0.020 | This work |
| 142.1 | 50 | GPB 4% | 72.95 | 120 | 0.017 | This work |
| 142.1 | 50 | GPB 6% | 75.52 | 120 | 0.018 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djukić, D.; Krstić, A.; Jakovljević, K.; Butulija, S.; Andjelković, L.; Pavlović, V.; Mirković, M. Brushite-Metakaolin Composite Geopolymer Material as an Effective Adsorbent for Lead Removal from Aqueous Solutions. Sustainability 2022, 14, 4003. https://doi.org/10.3390/su14074003
Djukić D, Krstić A, Jakovljević K, Butulija S, Andjelković L, Pavlović V, Mirković M. Brushite-Metakaolin Composite Geopolymer Material as an Effective Adsorbent for Lead Removal from Aqueous Solutions. Sustainability. 2022; 14(7):4003. https://doi.org/10.3390/su14074003
Chicago/Turabian StyleDjukić, Dunja, Aleksandar Krstić, Ksenija Jakovljević, Svetlana Butulija, Ljubica Andjelković, Vladimir Pavlović, and Miljana Mirković. 2022. "Brushite-Metakaolin Composite Geopolymer Material as an Effective Adsorbent for Lead Removal from Aqueous Solutions" Sustainability 14, no. 7: 4003. https://doi.org/10.3390/su14074003
APA StyleDjukić, D., Krstić, A., Jakovljević, K., Butulija, S., Andjelković, L., Pavlović, V., & Mirković, M. (2022). Brushite-Metakaolin Composite Geopolymer Material as an Effective Adsorbent for Lead Removal from Aqueous Solutions. Sustainability, 14(7), 4003. https://doi.org/10.3390/su14074003