Contributions of Wetland Plants on Metal Accumulation in Sediment
Abstract
:1. Introduction
2. Materials and Methods
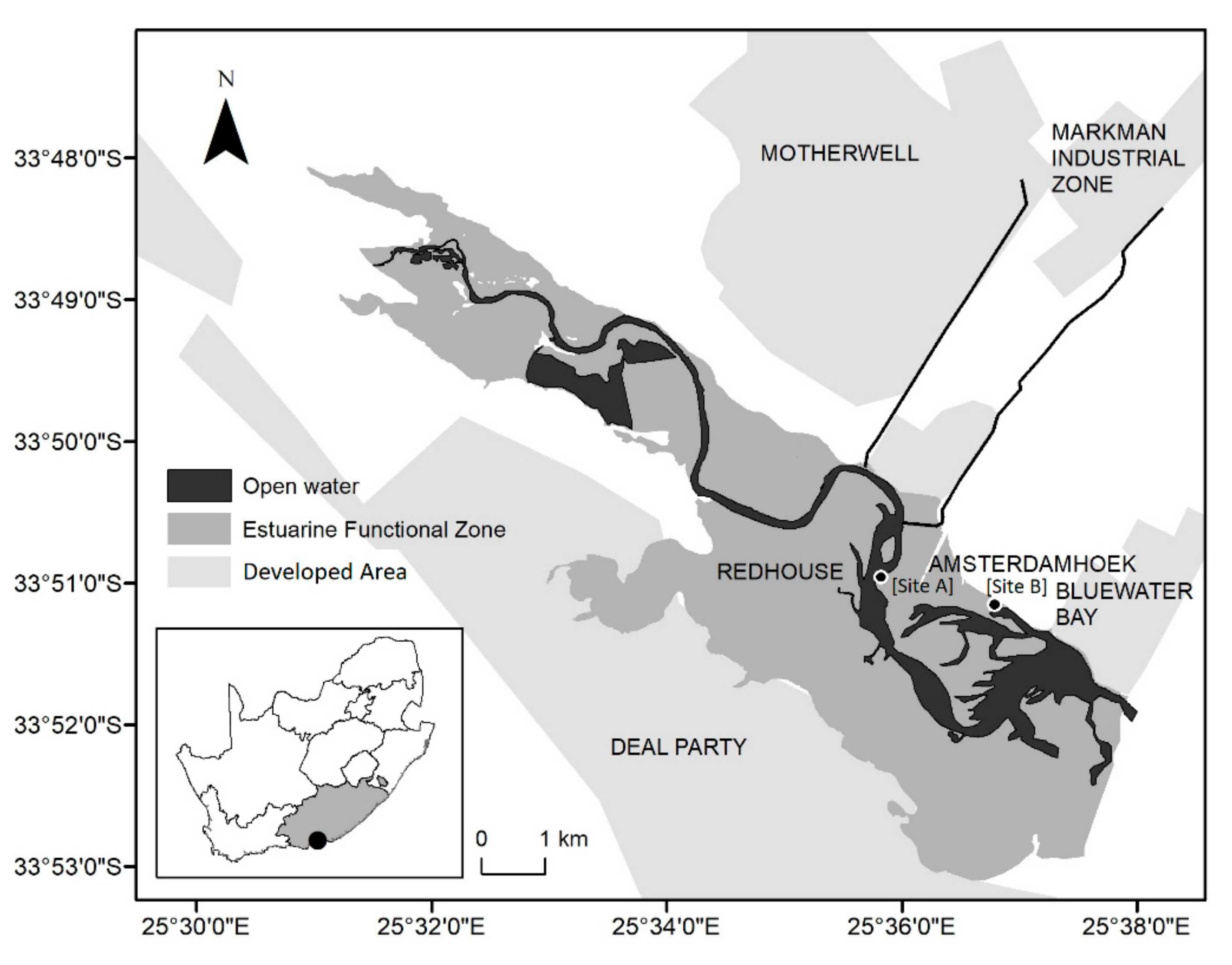
2.1. Study Site
2.2. Sampling
2.3. Analysis of Sediment Characteristics
2.4. Analysis of Metal Concentrations through TXRF Spectrometry
2.5. Statistical Analysis
3. Results
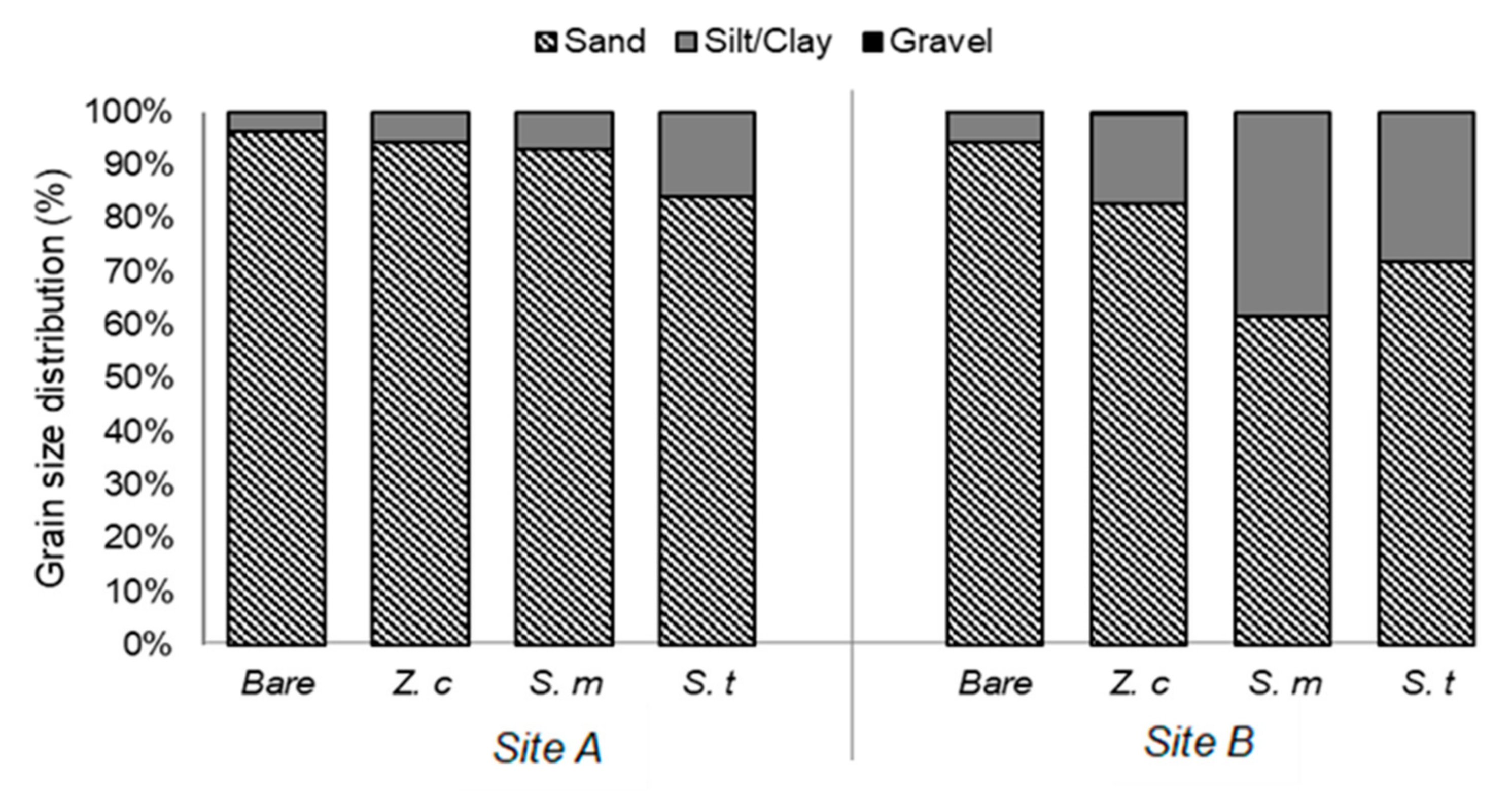
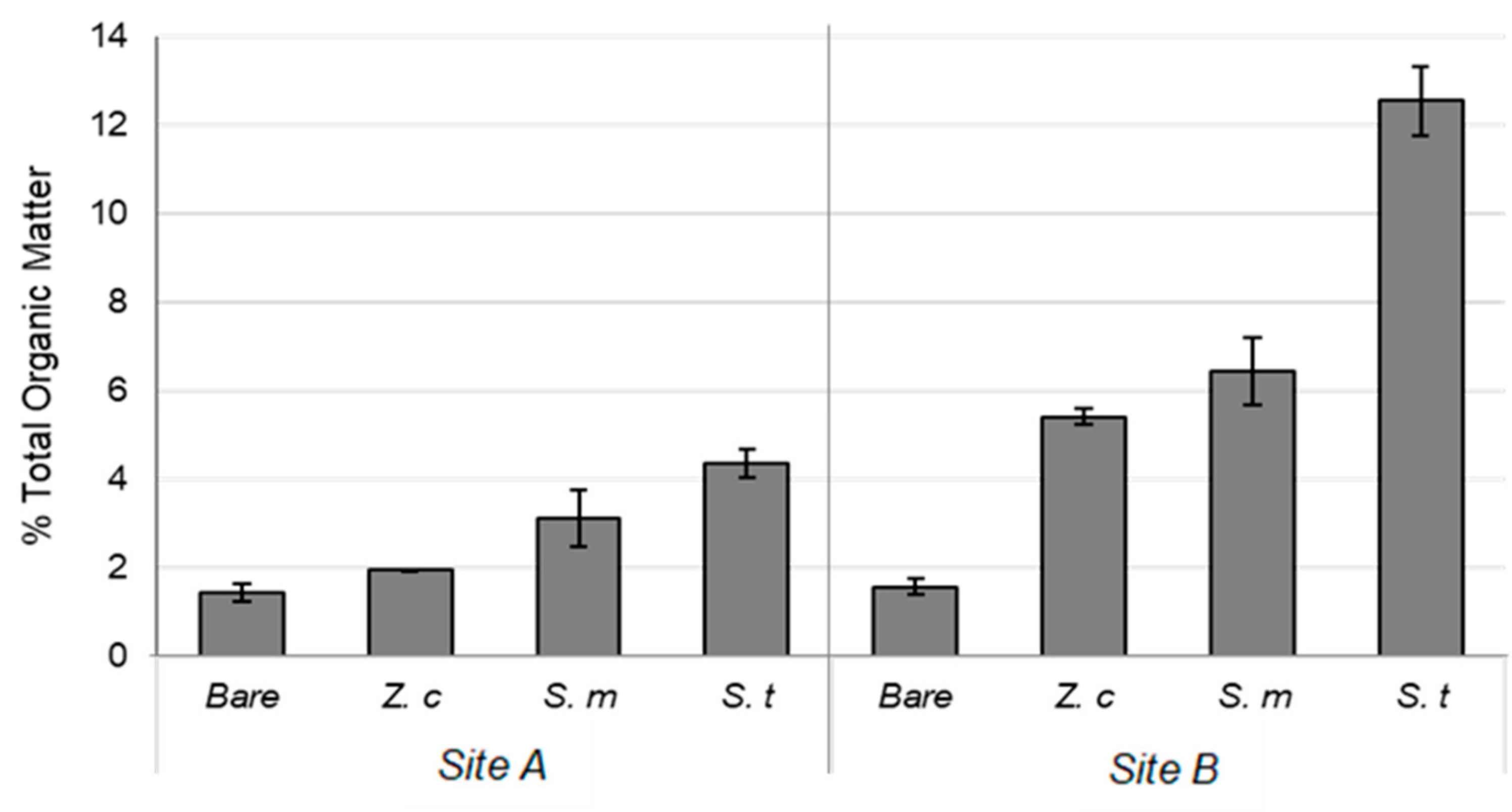
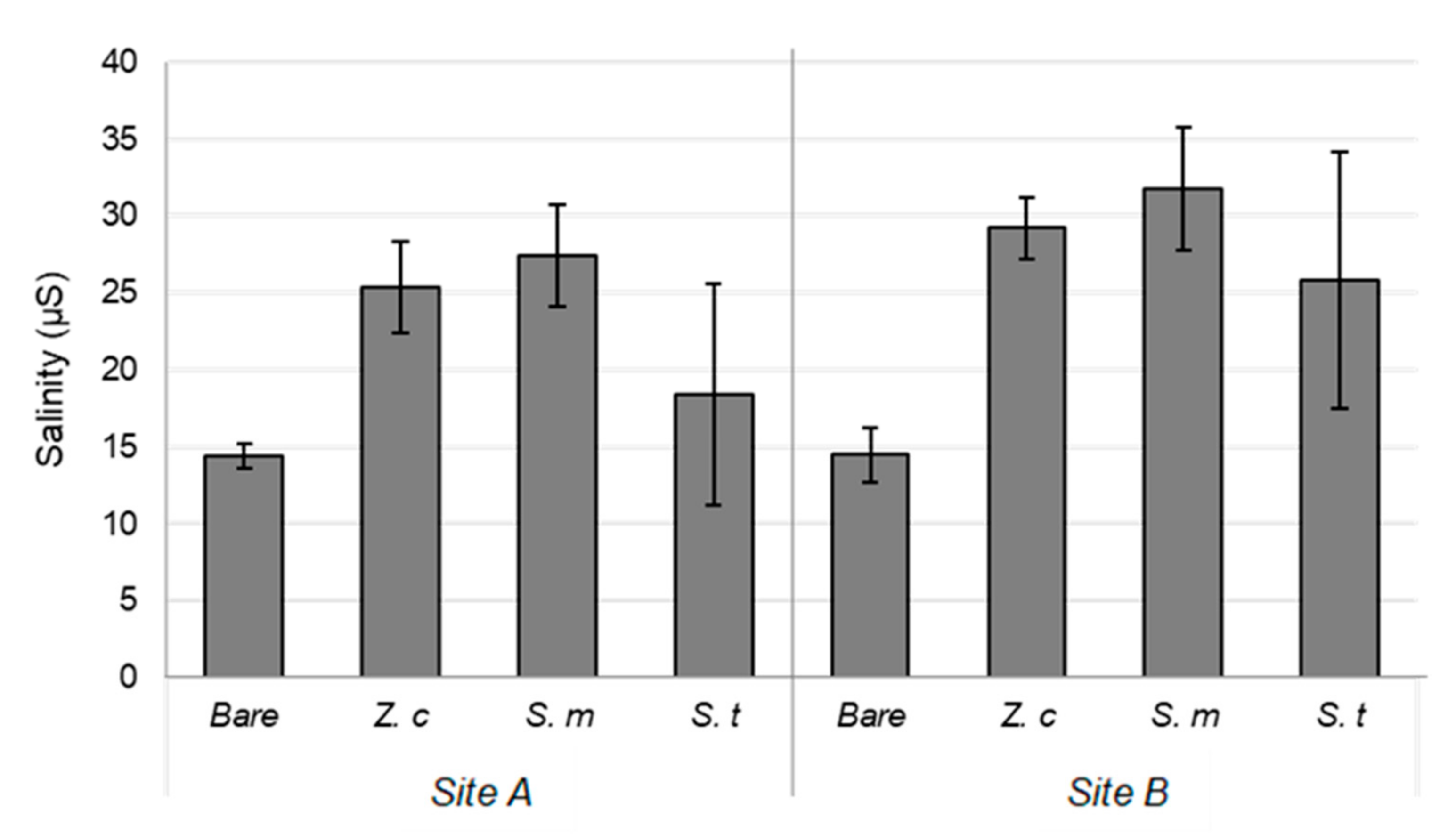
3.1. Sediment Characteristics
3.2. Total Metal Concentrations
3.3. nMDS Evaluation of Bare versus Vegetated Rhizosediment
4. Discussion
4.1. Sediment Characteristics
4.2. Metal Concentration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Niekerk, L.; Adams, J.B.; Bate, G.C.; Forbes, A.T.; Forbes, N.T.; Huizinga, P.; Lamberth, S.J.; MacKay, C.F.; Petersen, C.; Taljaard, S.; et al. Country-Wide Assessment of Estuary Health: An Approach for Integrating Pressures and Ecosystem Response in a Data Limited Environment. Estuar. Coast. Shelf Sci. 2013, 130, 239–251. [Google Scholar] [CrossRef]
- Doyle, M.; Otte, M. Organism-Induced Accumulation of Iron, Zinc and Arsenic in Wetland Soils. Environ. Pollut. 1997, 96, 1–11. [Google Scholar] [CrossRef]
- Reboreda, R.; Caçador, I. Halophyte Vegetation Influences in Salt Marsh Retention Capacity for Heavy Metals. Environ. Pollut. 2007, 146, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Duarte, B.; Caçador, I.; Sleimi, N.; Duarte, A.C.; Pereira, E. Biophysical and Biochemical Markers of Metal/Metalloid-Impacts in Salt Marsh Halophytes and Their Implications. Front. Environ. Sci. 2016, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Pedro, S.; Duarte, B.; Raposo, P.; Almeida, D.; Caçador, I. Metal Speciation in Salt Marsh Sediments: Influence of Halophyte Vegetation in Salt Marshes with Different Morphology. Estuar. Coast. Shelf Sci. 2015, 167, 248–255. [Google Scholar] [CrossRef]
- Bonanno, G.; Vymazal, J.; Cirelli, G.L. Translocation, Accumulation and Bioindication of Trace Elements in Wetland Plants. Sci. Total Environ. 2018, 631–632, 252–261. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Spencer, K.; Kloas, W.; Toffolon, M.; Zarfl, C. Metal Fate and Effects in Estuaries: A Review and Conceptual Model for Better Understanding of Toxicity. Sci. Total Environ. 2016, 541, 268–281. [Google Scholar] [CrossRef]
- Williams, T.P.; Bubb, J.M.; Lester, J.N. Metal Accumulation within Salt Marsh Environments: A Review. Mar. Pollut. Bull. 1994, 28, 277–290. [Google Scholar] [CrossRef]
- Caçador, I.; Vale, C.; Catarino, F. Seasonal Variation of Zn, Pb, Cu and Cd Concentrations in the Root- Sediment System of Spartina Maritima and Halimione Portulacoides from Tagus Estuary Salt Marshes. Mar. Environ. Res. 2000, 49, 279–290. [Google Scholar] [CrossRef]
- Bonanno, G.; Raccuia, S.A. Comparative Assessment of Trace Element Accumulation and Bioindication in Seagrasses Posidonia Oceanica, Cymodocea Nodosa and Halophila Stipulacea. Mar. Pollut. Bull. 2018, 131, 260–266. [Google Scholar] [CrossRef]
- Duarte, B.; Caetano, M.; Almeida, P.R.; Vale, C.; Caçador, I. Accumulation and Biological Cycling of Heavy Metal in Four Salt Marsh Species, from Tagus Estuary (Portugal). Environ. Pollut. 2010, 158, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, G.; Vymazal, J. Compartmentalization of Potentially Hazardous Elements in Macrophytes: Insights into Capacity and Efficiency of Accumulation. J. Geochem. Explor. 2017, 181, 22–30. [Google Scholar] [CrossRef]
- du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace Metal Behaviour in Estuarine and Riverine Floodplain Soils and Sediments: A Review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.G.; Kemp, M.W.; Boynton, W.R. The Influence of Waves and Seagrass Communities on Suspended Particulates in an Estuarine Embayment. Mar. Geol. 1984, 59, 85–103. [Google Scholar] [CrossRef]
- Maricle, B.R.; Lee, R.W. Aerenchyma Development and Oxygen Transport in the Estuarine Cordgrasses Spartina Alterniflora and S. Anglica. Aquat. Bot. 2002, 74, 109–120. [Google Scholar] [CrossRef]
- Yang, J.; Ye, Z. Metal Accumulation and Tolerance in Wetland Plants. Front. Biol. China 2009, 4, 282–288. [Google Scholar] [CrossRef]
- Pezeshki, S.R.; DeLaune, R.D. Soil Oxidation-Reduction in Wetlands and Its Impact on Plant Functioning. Biology 2012, 1, 196–221. [Google Scholar] [CrossRef] [Green Version]
- Fakoya, O.T.; Oluyemi, E.A.; Olabanji, I.O.; Eludoyin, A.O.; Makinde, O.W.; Oyinloye, J.A. Seasonal Variation of Heavy Metal Speciation in Soil and Stream Sediments from Hospital Waste Dumpsite in Ilesa, Southwestern Nigeria. Afr. J. Environ. Sci. Technol. 2018, 12, 312–322. [Google Scholar] [CrossRef]
- Reboreda, R.; Caçador, I. Copper, Zinc and Lead Speciation in Salt Marsh Sediments Colonised by Halimione Portulacoides and Spartina Maritima. Chemosphere 2007, 69, 1655–1661. [Google Scholar] [CrossRef]
- Peppicelli, C.; Cleall, P.; Sapsford, D.; Harbottle, M. Changes in Metal Speciation and Mobility during Electrokinetic Treatment of Industrial Wastes: Implications for Remediation and Resource Recovery. Sci. Total Environ. 2018, 624, 1488–1503. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781420093681. [Google Scholar]
- Colloty, B.M.; Adams, J.B.; Bate, G.C. The Use of a Botanical Importance Rating to Assess Changes in the Flora of the Swartkops Estuary over Time. Water SA 2000, 26, 171–180. [Google Scholar]
- van Niekerk, L.; Adams, J.B.; Lamberth, S.J.; MacKay, C.F.; Taljaard, S.; Turpie, J.K.; Raimondo, D.C. South African National Biodiversity Assessment 2018: Technical Report. Volume 3: Estuarine Realm; CSIR: Stellenbosch, South Africa, 2019; Volume 3. [Google Scholar]
- Enviro-Fish Africa. Swartkops Integrated Environmental Management Plan; CAPE: Port Elizabeth, South Africa, 2009. [Google Scholar]
- Enviro-Fish Africa. Integrated Management Plan: Swartkops Estuary and the Swartkops River Valley and Aloes Nature Reserves; CAPE: Grahamstown, South Africa, 2011. [Google Scholar]
- Binning, K.; Baird, D. Survey of Heavy Metals in the Sediments of the Swartkops River Estuary, Port Elizabeth South Africa. Water SA 2001, 27, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Fourqurean, J.W.; Johnson, B.; Kauffman, J.B.; Kennedy, H.; Lovelock, C.E.; Megonigal, J.P.; Rahman, A.; Saintilan, N.; Simard, M. Coastal Blue Carbon; Howard, J., Hoyt, S., Isensee, K., Emily, P., Telszewski, M., Eds.; International Union for Conservation of Nature: Arlington, VA, USA, 2017; ISBN 9782831717623. [Google Scholar]
- Wentworth, C.K. A Scale of Grade and Class Terms for Clastic Sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Buchanan, J.B. Sediment Analysis. In Methods for the Study of Marine Benthos; Holme, N.A., McIntyre, A.D., Eds.; Blackwell: Oxford, UK, 1984; pp. 41–65. [Google Scholar]
- Briggs, D. Soils: Sources and Methods in Geography; Butterworths: London, UK, 1977; ISBN 0408709111. [Google Scholar]
- Veres, D.S. A Comparitive Study between Loss on Ignition and Total Carbon Analysis on Minerogenic Sediments. Studia Univ. Babes-Bolyai 2002, 67, 171–182. [Google Scholar] [CrossRef]
- Bernard, R.O. Handbook of Standard Soil Testing Methods for Advisory Purposes; Sediment Science Society of South Africa: Pretoria, South Africa, 1990; ISBN 0620148004. [Google Scholar]
- du Laing, G.; Tack, F.M.G.; Verloo, M.G. Performance of Selected Destruction Methods for the Determination of Heavy Metals in Reed Plants (Phragmites australis). Anal. Chim. Acta 2003, 497, 191–198. [Google Scholar] [CrossRef]
- Phillips, D.P.; Human, L.R.D.; Adams, J.B. Wetland Plants as Indicators of Heavy Metal Contamination. Mar. Pollut. Bull. 2015, 92, 227–232. [Google Scholar] [CrossRef]
- Vilhena, J.C.E.; Amorim, A.; Ribeiro, L.; Duarte, B.; Pombo, M. Baseline Study of Trace Element Concentrations in Sediments of the Intertidal Zone of Amazonian Oceanic Beaches. Front. Mar. Sci. 2021, 8, 570. [Google Scholar] [CrossRef]
- Montero Alvarez, A.; Estévez Alvarez, J.R.; Padilla Alvarez, R. Heavy Metal Analysis of Rainwaters: A Comparison of TXRF and ASV Analytical Capabilities. J. Radioanal. Nucl. Chem. 2007, 273, 427–433. [Google Scholar] [CrossRef]
- Matusiak, K.; Skoczen, A.; Setkowicz, Z.; Kubala-Kukus, A.; Stabrawa, I.; Ciarach, M.; Janeczko, K.; Jung, A.; Chwiej, J. The Elemental Changes Occurring in the Rat Liver after Exposure to PEG-Coated Iron Oxide Nanoparticles: Total Reflection x-Ray Fluorescence (TXRF) Spectroscopy Study. Nanotoxicology 2017, 11, 1225–1236. [Google Scholar] [CrossRef]
- Esterhuysen, K.; Rust, I.C. Channel Migration in the Lower Swartkops Estuary. S. Afr. J. Sci. 1987, 83, 521–525. [Google Scholar]
- Reddering, J.S.V.; Esterhuysen, K.; Rust, I.C. The Sedimentary Ecology of the Swartkops Estuary; University of Port Elizabeth: Gqeberha, South Africa, 1981; ISBN 0869882589. [Google Scholar]
- Bornman, T.G.; Schmidt, J.; Adams, J.B.; Mfikili, A.N.; Farre, R.E.; Smit, A.J. Relative Sea-Level Rise and the Potential for Subsidence of the Swartkops Estuary Intertidal Salt Marshes, South Africa. South Afr. J. Bot. 2016, 107, 91–100. [Google Scholar] [CrossRef]
- Reddering, J.S.; Esterhuysen, K. The Swartkops Estuary: Physical Description and History. Swart. Estuary 1988, 7–15. [Google Scholar]
- Adams, J.B.; Pretorius, L.; Snow, G.C. Deterioration in the Water Quality of an Urbanised Estuary with Recommendations for Improvement. Water SA 2019, 45, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Els, J. Carbon and Nutrient Storage of the Swartkops Estuary Salt Marsh and Seagrass Habitats; Nelson Mandela University: Port Elizabeth, South Africa, 2020. [Google Scholar]
- Nel, L.; Strydom, N.A.; Bouwman, H. Preliminary Assessment of Contaminants in the Sediment and Organisms of the Swartkops Estuary, South Africa. Mar. Pollut. Bull. 2015, 101, 878–885. [Google Scholar] [CrossRef]
- Watling, R.J.; Watling, H.R. Metal Surveys in South African Estuaries. I. Swartkops River. Water SA 1982, 8, 26. [Google Scholar]
- Veldkornet, D.A.; Potts, A.J.; Adams, J.B. The Distribution of Salt Marsh Macrophyte Species in Relation to Physicochemical Variables. S. Afr. J. Bot. 2016, 107, 84–90. [Google Scholar] [CrossRef]
- Smillie, C. Salicornia Spp. as a Biomonitor of Cu and Zn in Salt Marsh Sediments. Ecol. Indic. 2015, 56, 70–78. [Google Scholar] [CrossRef]
- Furtado, B.U.; Golebiewski, M.; Skorupa, M.; Hulisz, P.; Hrynkiewicz, K. Bacterial and Fungal Endophytic Microbiomes of Salicornia Europaea. Appl. Environ. Microbiol. 2019, 85, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Nel, M.A.; Rubidge, G.; Adams, J.B.; Human, L.R.D. Rhizosediments of Salicornia Tegetaria Indicate Metal Contamination in the Intertidal Estuary Zone. Front. Environ. Sci. 2020, 8, 175. [Google Scholar] [CrossRef]
- Watling, R.J.; Watling, H.R. Metal Surveys in Southern African Estuaries: 1. Swartkops Estuary. CSIR Spec. Rep. FIS 1979, 183. [Google Scholar]
- Bonanno, G.; Borg, J.A.; di Martino, V. Levels of Heavy Metals in Wetland and Marine Vascular Plants and Their Biomonitoring Potential: A Comparative Assessment. Sci. Total Environ. 2017, 576, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root Development and Nutrient Uptake. Crit. Rev. Plant Sci. 2006, 25, 279–301. [Google Scholar] [CrossRef]
- Koop-Jakobsen, K.; Fischer, J.; Wenzhöfer, F. Survey of Sediment Oxygenation in Rhizospheres of the Saltmarsh Grass - Spartina Anglica. Sci. Total Environ. 2017, 589, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Holmer, M.; Gribsholt, B.; Kristensen, E. Effects of Sea Level Rise on Growth of Spartina Anglica and Oxygen Dynamics in Rhizosphere and Salt Marsh Sediments. Mar. Ecol. Prog. Ser. 2002, 225, 197–204. [Google Scholar] [CrossRef]
- Frederiksen, M.S.; Glud, R.N. Oxygen Dynamics in the Rhizosphere of Zostera Marina: A Two-Dimensional Planar Optode Study. Limnol. Oceanogr. 2006, 51, 1072–1083. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.; Ferreira, A.M.; Vale, C. The Influence of Sarcocornia Fruticosa on Retention of PAHs in Salt Marsh Sediments (Sado Estuary, Portugal). Chemosphere 2008, 71, 1599–1606. [Google Scholar] [CrossRef]
- Tranvik, L.J. Dystrophy in Freshwater Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780124095489. [Google Scholar]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and Bioavailability of Heavy Metals and Metalloids in Soil Environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, K.H.; Stevenson, R.J. Effects of Acidification and Alkalinization on a Periphytic Algal Community in an Alaskan Wetland. Wetlands 2010, 30, 1193–1202. [Google Scholar] [CrossRef]
- Human, L.; Feijão, E.; de Carvalho, R.C.; Caçador, I.; Reis-Santos, P.; Fonseca, V.; Duarte, B. Mediterranean Salt Marsh Sediment Metal Speciation and Bioavailability Changes Induced by the Spreading of Non-Indigenous Spartina Patens. Estuar. Coast. Shelf Sci. 2020, 243, 1–9. [Google Scholar] [CrossRef]
- Lin, J.G.; Chen, S.Y.; Su, C.R. Assessment of Sediment Toxicity by Metal Speciation in Different Particle-Size Fractions of River Sediment. Water Sci. Technol. 2003, 47, 233–241. [Google Scholar] [CrossRef]
- Ujević, I.; Odžak, N.; Barić, A. Trace Metal Accumulation in Different Grain Size Fractions of the Sediments from a Semi-Enclosed Bay Heavily Contaminated by Urban and Industrial Wastewaters. Water Res. 2000, 34, 3055–3061. [Google Scholar] [CrossRef]
- Elderfield, H.; Hepworth, A.; Edwards, P.N.; Holliday, L.M. Zinc in the Conwy River and Estuary. Estuar. Coast. Mar. Sci. 1979, 9, 403–422. [Google Scholar] [CrossRef]
- Talbot, M.M.J.F. Trace Metals in the Marine Environment and Their Effect on Nitrogen Cycling; Nelson Mandela University: Port Elizabeth, South Africa, 1988. [Google Scholar]
- Fritioff, Å.; Kautsky, L.; Greger, M. Influence of Temperature and Salinity on Heavy Metal Uptake by Submersed Plants. Environ. Pollut. 2005, 133, 265–274. [Google Scholar] [CrossRef] [PubMed]

| Sites | Metals (µg.g−1) | Unvegetated | Rhizosediment | ||
|---|---|---|---|---|---|
| Bare Mudflat | Z. capensis | S. maritima | S. tegetaria | ||
| Site A | Fe | 3468 ± 385 | 3152 ± 171 | ↑5407 ± 985 * | ↑6923 ± 1222 * |
| Mn | 31.5 ± 4.1 | 30.4 ± 2.5 | ↑63.7 ± 14.2 * | ↑112.9 ± 28.6 * | |
| Zn | 48.8 ± 12.7 | 38.1 ± 0.8 | 57.4 ± 3.8 | 53.7 ± 1.8 | |
| Cr | 8.5 ± 1.1 | 10.6 ± 0.5 | ↑19.0 ± 4.0 * | 11.6 ± 1.7 | |
| Pb | 3.9 ± 0.6 | 2.8 ± 1.0 | 5.2 ± 1.3 | 7.1 ± 1.9 | |
| Ni | 0.9 ± 0.5 | ↑4.1 ± 1.7 * | 1.2 ± 0.4 | 1.2 ± 0.3 | |
| Cu | 2.9 ± 0.7 | ↑1.4 ± 0.2 * | 2.9 ± 0.7 | 3.0 ± 0.4 | |
| Site B | Fe | 5120± 223 | ↑14073 ± 341 * | ↑19445 ± 2 850 * | ↑28947 ± 858 * |
| Mn | 29.3 ± 1.8 | ↑76.8 ± 10.4 * | ↑108.6 ± 21.2 * | ↑367.2 ± 34.4 * | |
| Zn | 51.2 ± 2.6 | ↑96.7 ± 16.0 * | ↑81.0 ± 10.3 * | ↑128.2 ± 9.0 * | |
| Cr | 10.3 ± 0.7 | ↑36.8 ± 2.3 * | ↑37.5 ± 4.1 * | ↑72.1 ± 1.5 * | |
| Pb | 7.1 ± 0.9 | ↑29.8 ± 5.5 * | ↑30.3 ± 5.0 * | ↑59.0 ± 1.5 * | |
| Ni | 3.5 ± 0.1 | 9.6 ± 0.7 | ↑11.2 ± 1.8 * | ↑21.2 ± 0.7 * | |
| Cu | 3.5 ± 0.1 | ↑11.6 ± 0.6 * | ↑8.7 ± 1.2 * | ↑13.9 ± 0.8 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nel, M.A.; Rubidge, G.; Adams, J.B.; Human, L.R.D. Contributions of Wetland Plants on Metal Accumulation in Sediment. Sustainability 2022, 14, 3679. https://doi.org/10.3390/su14063679
Nel MA, Rubidge G, Adams JB, Human LRD. Contributions of Wetland Plants on Metal Accumulation in Sediment. Sustainability. 2022; 14(6):3679. https://doi.org/10.3390/su14063679
Chicago/Turabian StyleNel, Marelé A., Gletwyn Rubidge, Janine B. Adams, and Lucienne R. D. Human. 2022. "Contributions of Wetland Plants on Metal Accumulation in Sediment" Sustainability 14, no. 6: 3679. https://doi.org/10.3390/su14063679
APA StyleNel, M. A., Rubidge, G., Adams, J. B., & Human, L. R. D. (2022). Contributions of Wetland Plants on Metal Accumulation in Sediment. Sustainability, 14(6), 3679. https://doi.org/10.3390/su14063679







