Impact of the Manufacturing Processes of Aromatic-Polymer-Based Carbon Fiber on Life Cycle Greenhouse Gas Emissions
Abstract
:1. Introduction
2. Materials and Methods
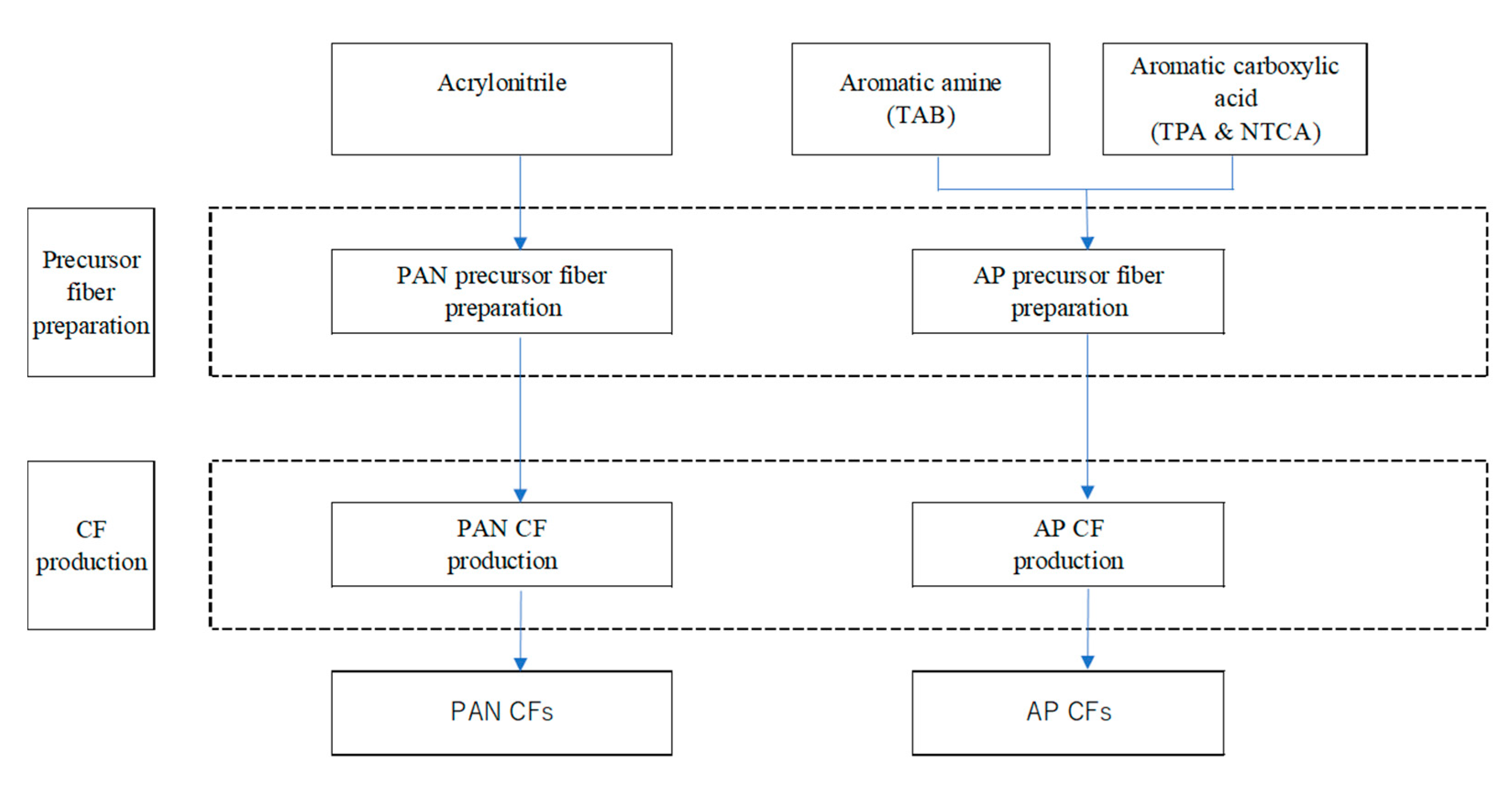
2.1. CF Production Processes
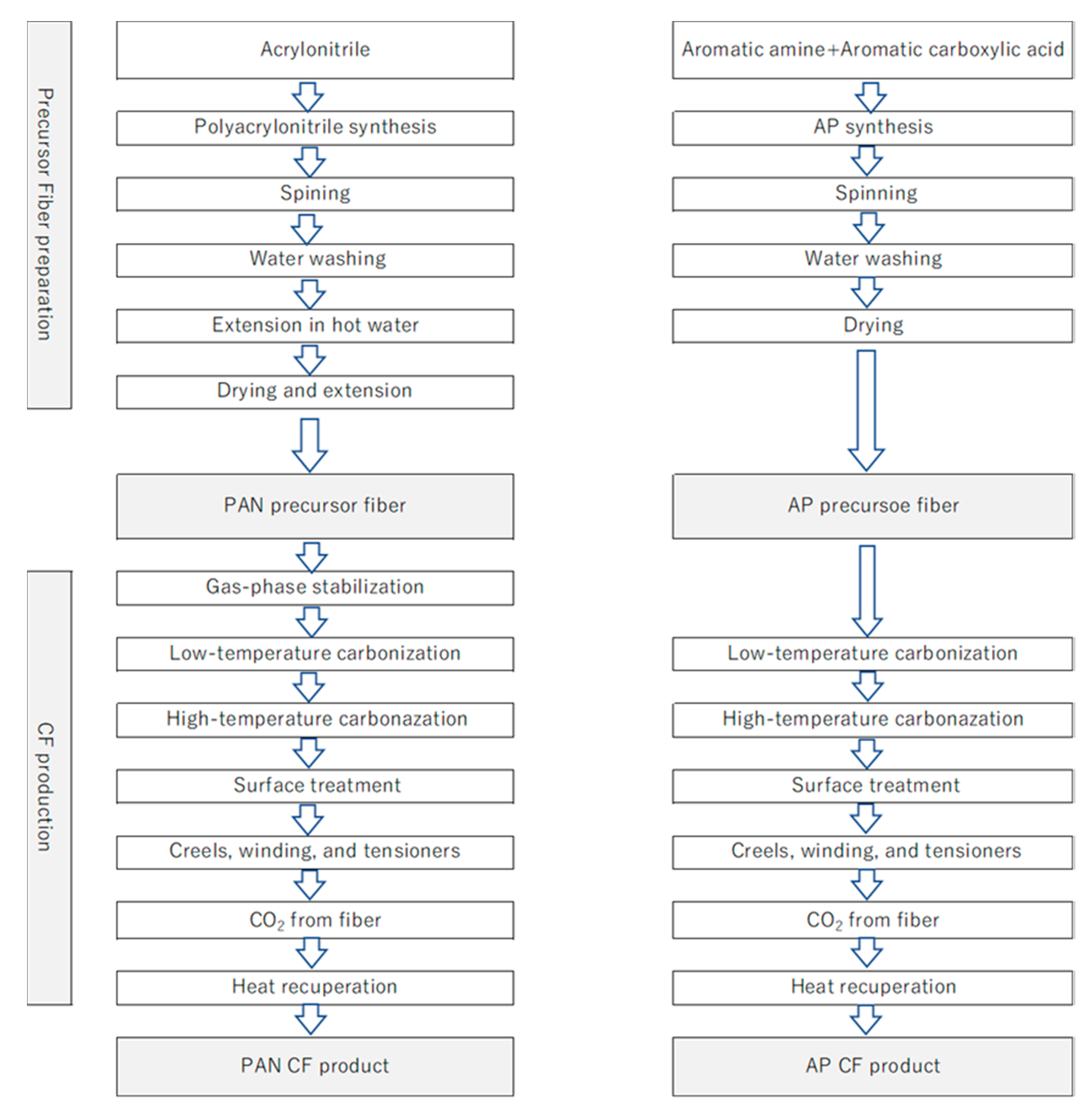
2.1.1. PAN CF Production Process
2.1.2. Aromatic Polymer CF Production Process
2.2. Functional Unit and System Boundary
2.3. Inventory Analysis
2.4. Assumptions
- Ratio of reused solvent: 95% [32];
- Ratio of reused catalyst: 95%;
- Carbonization efficiency: PAN CF = 50% and AP CF = 75%;
- Possibility of heat reduction was fixed;
- Power-reduction ratio by LT (large tow): 75% of RT (regular tow);
- Sizes of RT and LT models were 12 k and 48 k, respectively;
- Weight ratio of PBI to PBN was 1:1.
3. Results
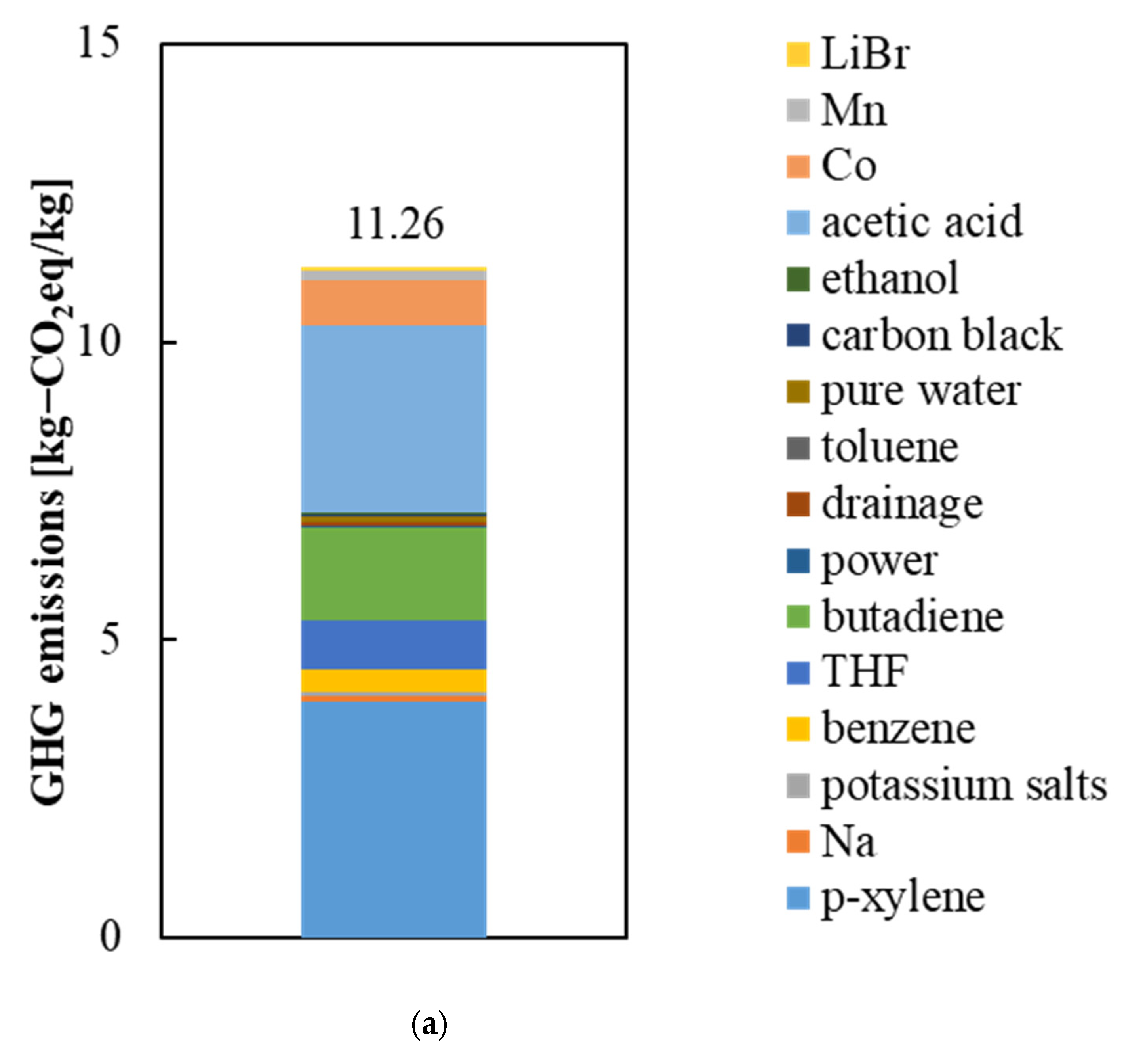
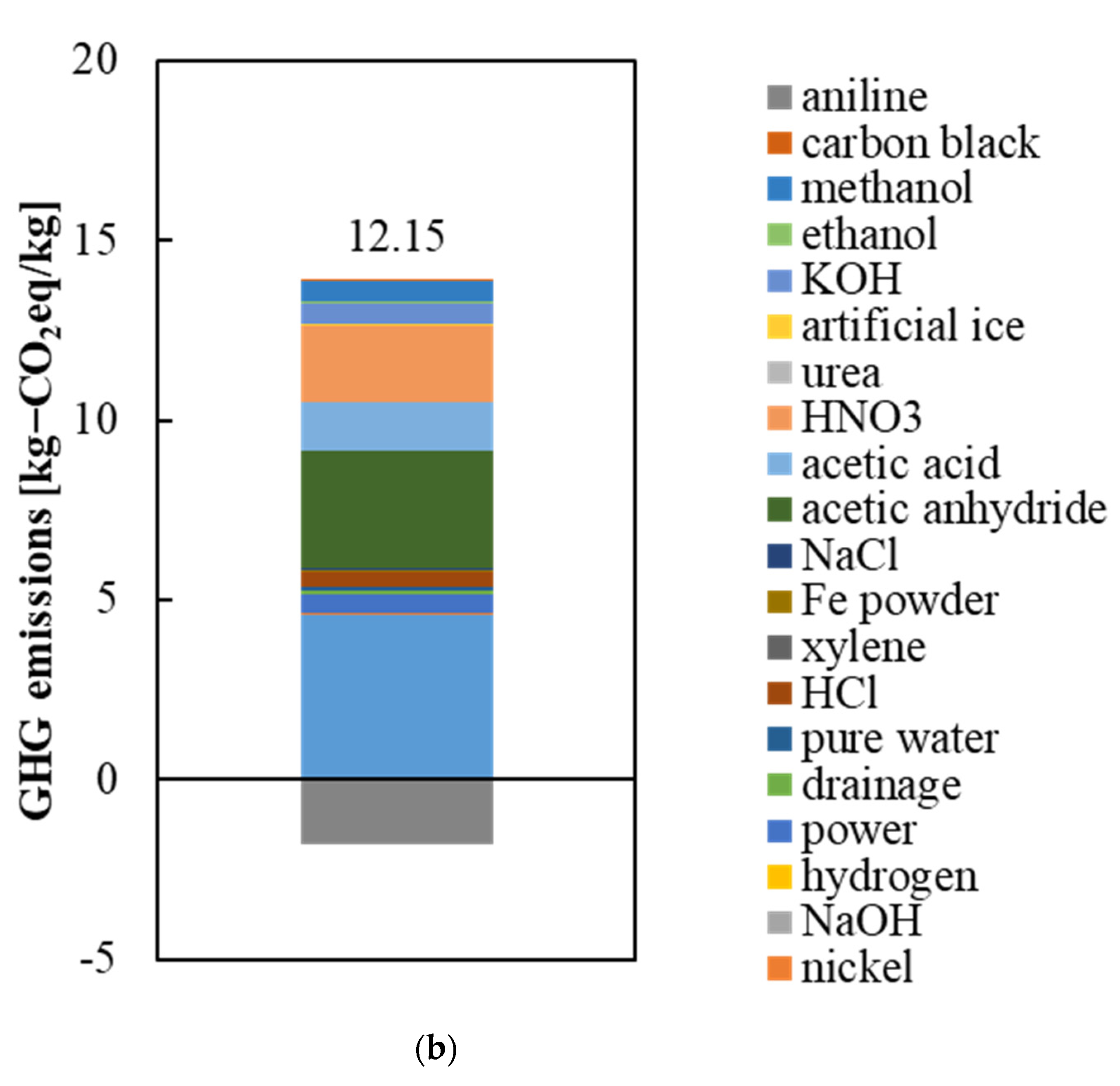
3.1. GHG Emissions from the Raw Materials
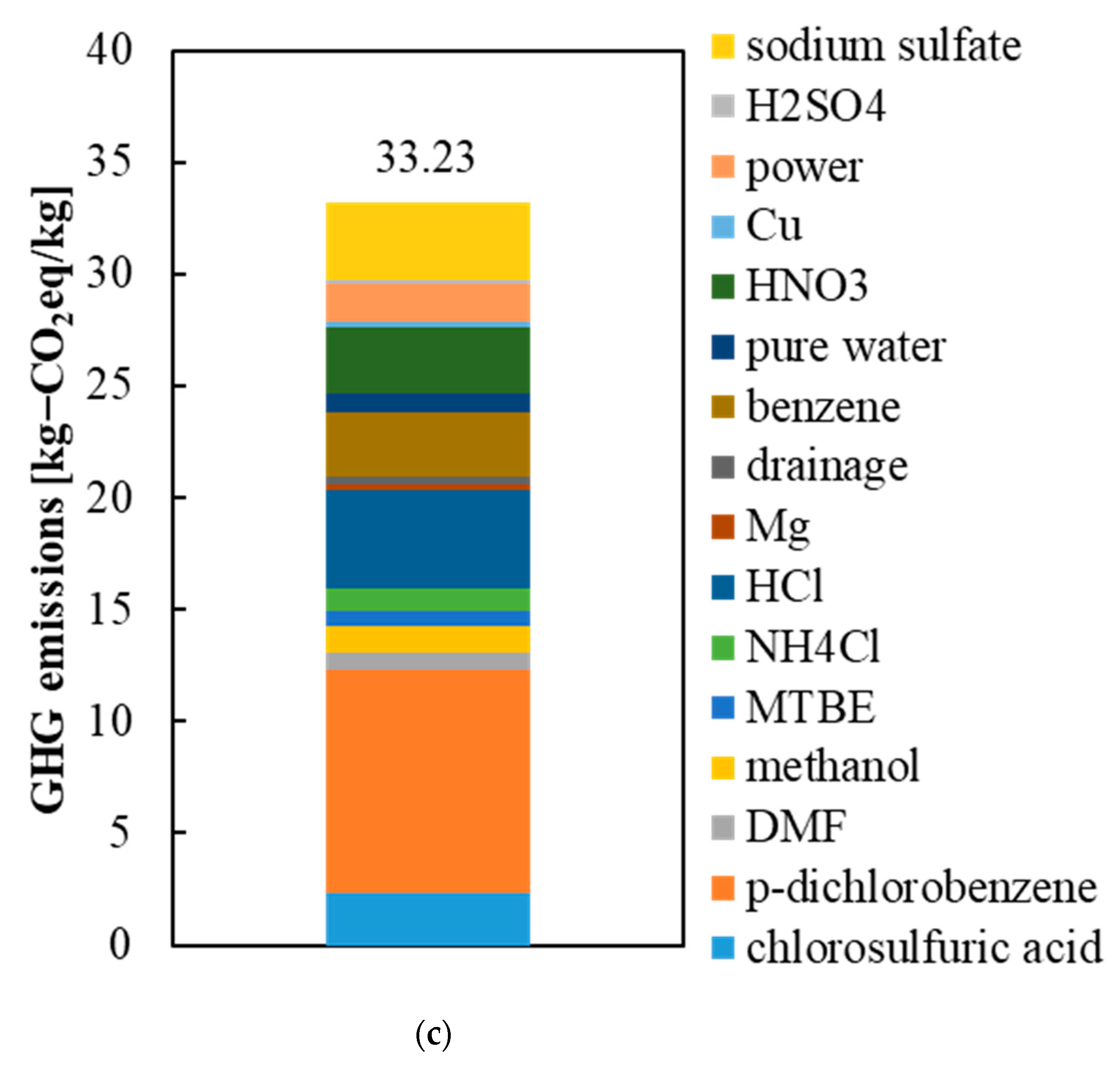
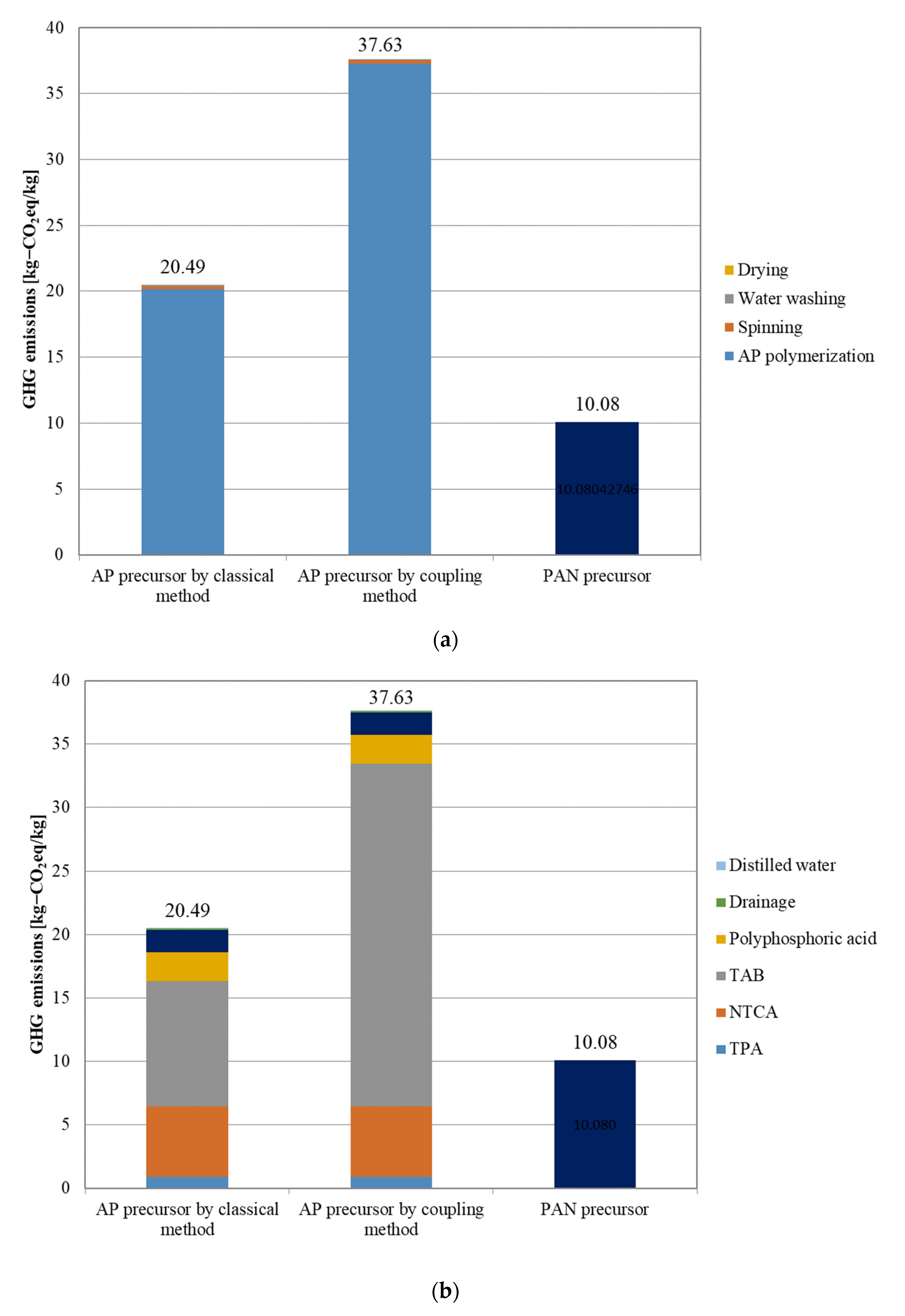
3.2. GHG Emissions from the Precursors
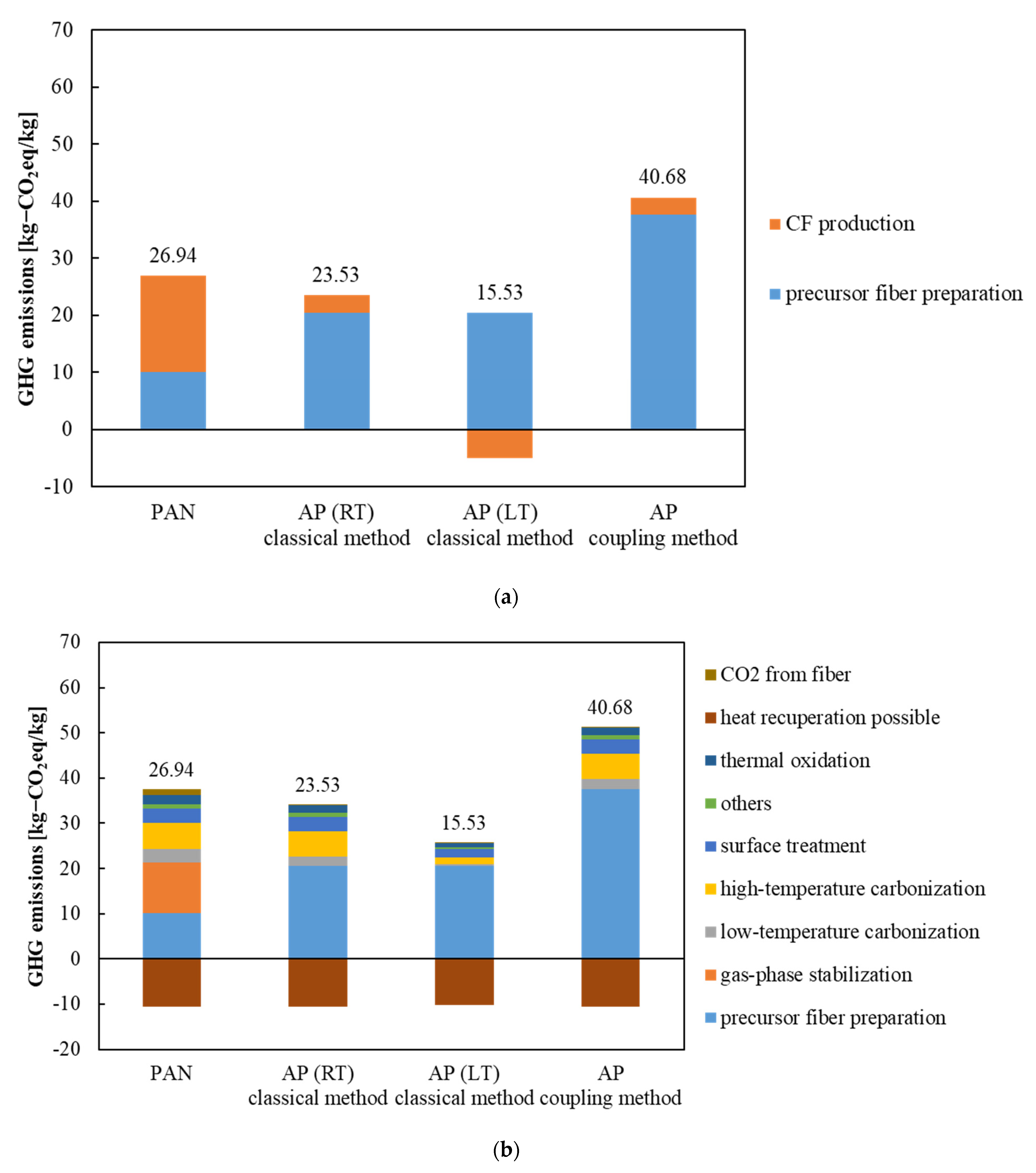
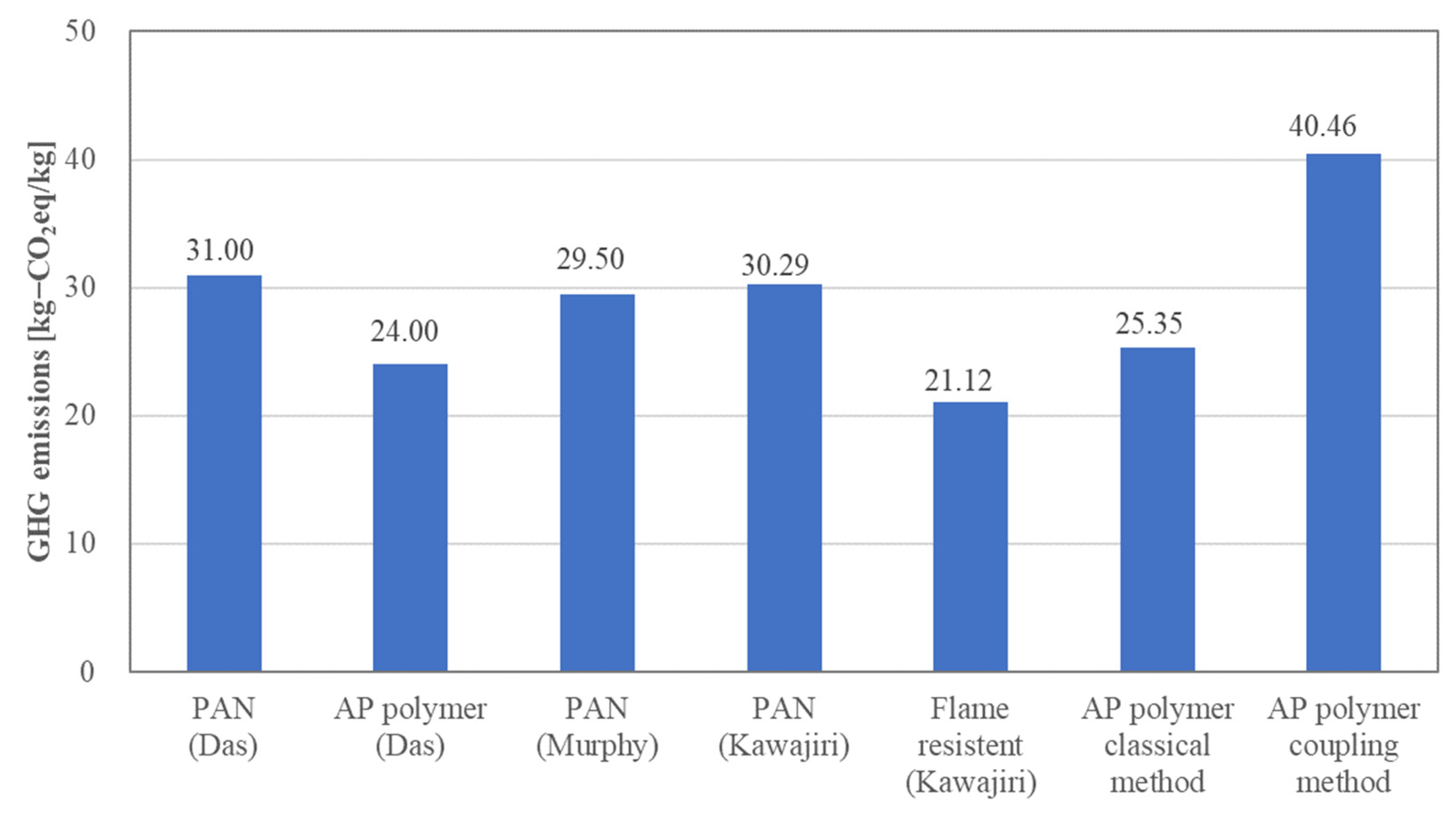
3.3. GHG Emissions from AP and PAN CFs
4. Discussion
5. Conclusions
- Production processes of AP CFs exhibit good potential in reducing GHG emissions compared with those from PAN CFs. Furthermore, these processes omit gas-phase stabilization, which is the bottleneck in the conventional production of CFs, thereby improving the performance of carbonization and reducing the GHG emissions from the fiber body by 11%.
- GHG emissions from APs were identified for the first time by the coupling method. The observed value of 40.46 kg-CO2 eq/kg was approximately 25% higher than that of PAN-based CFs and approximately 40% higher than that of CFs derived from APs using the classical method. Although CFs derived from APs from the coupling method are free from carcinogenic risk, this analysis raises a question regarding the utilization of the coupling method as far as GHG emissions are concerned. The use of APs in the coupling method requires a reduction in GHG emissions during precursor fabrication.
- The trade-off between GHG emissions and carcinogenic risk was confirmed by comparing the classical and coupling methods in TAB production. One solution is to use the benzidine process under control without leakage of hazardous substances.
- Our analysis results were consistent with the previous studies of GHG emissions for AP and PAN CFs. GHG emissions from AP CFs were approximately 20% lower than those from PAN CFs. This supports our analysis results. Since there are no data for GHG emissions from the coupling method, we need to wait for a new analysis or until the GHG coefficients of TAB and NTCA are defined in the IDEA_v2.1.3 database.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. The Intergovernmental Panel on Climate Change, Transport. 2018. Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/ipcc_wg3_ar5_chapter8.pdf (accessed on 8 September 2021).
- Cui, X.; Zhang, H.; Wang, S.; Zhang, L.; Ko, J. Design of lightweight multi-material automotive bodies using new material performance indices of thin-walled beams for the material selection with crashworthiness consideration. Mater. Des. 2011, 32, 815–821. [Google Scholar] [CrossRef]
- Landi, D.; Vita, A.; Germani, M. Interactive optimization of the resin transfer molding using a general-purpose tool: A case study. Int. J. Interact. Des. Manuf. 2019, 14, 295–308. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Zhang, L.; Bao, H. Lifecycle assessment of automotive hoods made of aluminum alloy and glass mat reinforced thermoplastic. J. Hefei Univ. Technol. 2012, 35, 433–438. [Google Scholar]
- Dubreuil, A.; Bushi, L.; Das, S.; Tharumarajah, A.; Gong, X. A Comparative Life Cycle Assessment of Magnesium Front End Autoparts; Society of American Engineers World Congress: Detroit, MI, USA, 2010. [Google Scholar]
- Meng, F.; Mckechnie, J. Towards a circular economy for end-of-life carbon fiber composite materials via fluidised bed process. In Proceedings of the 21st International Conference on Composite Materials, Xi’an, China, 20–25 August 2017. [Google Scholar]
- IDEA_v2.1.3 Database. Advanced Industry Science and Technology. 2017. Available online: https://en.aist-riss.jp/ (accessed on 5 August 2020).
- Kawajiri, K.; Kobayashi, M.; Sakamoto, K. Lightweight materials equal lightweight greenhouse gas emissions?: A historical analysis of greenhouse gases of vehicle material substitution. J. Clean. Prod. 2019, 253, 119805. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Libre, L. Lignin Based Carbon Fibres for Composites. 2016. Available online: libre2020.eu (accessed on 20 November 2021).
- GreenLight. GreenLight-Cost Effective Lignin-Based Carbon Fibres for Innovative Light-Weight Applications. 2016. Available online: https://greenlight-project.eu (accessed on 25 November 2021).
- Giuntoli, J.; Commission, E.; Bulgheroni, C.; Commission, E.; Marelli, L.; Commission, E.; Sala, S.; Commission, E. Brief on the Use of Life Cycle Assessment (LCA) to Evaluate Environmental Impacts of the Bioeconomy. Technical Report. 2019. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC109817 (accessed on 1 January 2021).
- ISO. Environmental management—Life cycle assessment—Requirements and guidelines. Int. Stand. Org. ISO 2006, 14044. Available online: https://scholar.google.com.hk/scholar?hl=en&as_sdt=0%2C5&q=Environmental+management%E2%80%94Life+cycle+assessment%E2%80%94Requirements+and+guidelines.+&btnG= (accessed on 1 January 2022).
- ISO. Environmental Management—Life Cycle Assessment—Principles and Framework. In Proceedings of the Technical Committee ISO/TC, Geneva, Switzerland, 14–15 March 2006; Volume 207. [Google Scholar]
- Hermansson, F.; Janssen, M.; Svanström, M. Allocation in life cycle assessment of lignin. Int. J. Life Cycle Assess. 2020, 25, 1620–1632. [Google Scholar] [CrossRef]
- Janssen, M.; Xiros, C.; Tillman, A.M. Life cycle impacts of ethanol production from spruce wood chips under high gravity conditions. Biotechnol. Biofuels 2016, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aryapratama, R.; Janssen, M. Prospective life cycle assessment of bio-based adipic acid production from forest residues. J. Clean. Prod. 2017, 164, 434–443. [Google Scholar] [CrossRef] [Green Version]
- Hermansson, F.; Janssen, M.; Gellerstedt, F. Environmental evaluation of Durapulp biocomposite using LCA: Comparison of two different applications. J. For. 2016, 5, 68–76. [Google Scholar]
- Janssen, M.; Gustafsson, E.; Echardt, L.; Wallinder, J.; Wolf, J. Life cycle assessment of lignin-based carbon fibers. In Proceedings of the 14th Conference on Sustainable Development of Energy, Water and Environment Systems (SDEWES), Dubrovnik, Croatia, 1–6 October 2019. [Google Scholar]
- Maghe, M.; Creighton, C.; Henderson, L.C.; Huson, M.G.; Nunna, S.; Atkiss, S.; Byrne, N.; Fox, B.L. Using ionic liquids to reduce energy consumption for carbon fibre production. J. Mater. Chem. A 2016, 4, 16619–16626. [Google Scholar] [CrossRef]
- Irisawa, T.; Hatori, H.; Soneda, Y.; Kodama, M. Polybenzimidazole Carbon Fiber and Method for Manufacturing Same. U.S. Patent US 20,170,152,612 A1, 1 June 2017. [Google Scholar]
- Karp, E.M.; Eaton, T.R.; Nogué, V.S.; Vorotnikov, V.; Biddy, M.J.; Tan, E.C.D.; Brandner, D.G.; Cywar, R.M.; Liu, R.; Manker, L.P.; et al. Renewable acrylonitrile production. Science 2017, 358, 1307–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawajiri, K.; Sakamoto, K. Environmental impact of carbon fibers fabricated by an innovative manufacturing process on life cycle greenhouse gas emissions. Sustainability 2021. under Review. [Google Scholar] [CrossRef]
- Moretti, C.; Corona, B.; Hoefnagels, R.; Vural-Gürsel, I.; Gosselink, R.; Junginger, M. Review of life cycle assessments of lignin and derived products: Lessons learned. Sci. Total Environ. 2021, 770, 144656. [Google Scholar] [CrossRef] [PubMed]
- Das, S. Life cycle assessment of carbon fiber-reinforced polymer composites. Int. J. Life Cycle Assess. 2011, 16, 268–282. [Google Scholar] [CrossRef]
- Yasuda, H.; Domitrovichi, O.V. Method of Manufacturing 3,3′4,4′-Tetraaminobiphenyl. U.S. Patent US 7,601,873 B2, 13 October 2009. [Google Scholar]
- Clyde, O.H.; Wilmington, D.; Benner, R.G. Process for the Manufacture of Benzidine. U.S. Patent US 2,194,938 A, 23 April 1938. [Google Scholar]
- United States Environmental Protection Agency. Integrated risk Information System (IRIS). 1987. Available online: https://iris.epa.gov/static/pdfs/0135_summary.pdf (accessed on 1 January 2022).
- Sato, T.; Ito, I.; Takeda, K. Production Process of 1,4,5,8-Naphthalene Tetracarboxylc Acid. U.S. Patent US 5,354,899 A, 11 October 1994. [Google Scholar]
- Bennett, C.W.; Wilson, B.R.; Charles, B.; Hilton, A.J.E.; Kenneth, G. Davenport Method for the Production of 3,3′4,4′-Tetraaminobiphenyl. U.S. Patent US 5,041,666 A, 20 April 1991. [Google Scholar]
- Geisler, G.; Hofstetter, T.B.; Hungerbuhler, K. Production of fine and specialty chemicals: Procedure for the estimation of LCIs. Int. J. LCA 2004, 9, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Harper International. Processing advancements within reach for achieving significant reductions in carbon fiber cost of manufacturing. In Proceedings of the JEC Europe 2013, ICS Carbon Conference, Paris, France, 12–14 March 2013. [Google Scholar]
- Kawajiri, K.; Goto, T.; Sakurai, S.; Hata, K.; Tahara, K. Development of life cycle assessment of an emerging technology at research and development stage: A case study on single-wall carbon nanotube produced by super growth method. J. Clean. Prod. 2020, 255, 120015. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Preventing Health Hazards from Exposure to Benzidine Congener Dyes. 1983. Available online: https://www.cdc.gov/niosh/docs/83-105/default.html (accessed on 8 August 2021).
- Sigma-Aldrich. Safety Data Sheet Version 4.6. Safety Data Sheet (t3db.ca). 2014. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjClr-dosz2AhVSsVYBHZiVB3wQFnoECAUQAQ&url=http%3A%2F%2Fwww.t3db.ca%2Fsystem%2Fmsds%2Fattachments%2F000%2F001%2F371%2Foriginal%2FT3D0026.pdf%3F1413587672&usg=AOvVaw1WnNYXE6J_tCSZcJMnLYCA (accessed on 1 January 2022).
- Murphy, T. The New Face of CAFÉ. Ward’s Autoworld 2008, 34, 36–40. Available online: https://trid.trb.org/view/858441 (accessed on 1 January 2022).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, K.; Kawajiri, K.; Hatori, H.; Tahara, K. Impact of the Manufacturing Processes of Aromatic-Polymer-Based Carbon Fiber on Life Cycle Greenhouse Gas Emissions. Sustainability 2022, 14, 3541. https://doi.org/10.3390/su14063541
Sakamoto K, Kawajiri K, Hatori H, Tahara K. Impact of the Manufacturing Processes of Aromatic-Polymer-Based Carbon Fiber on Life Cycle Greenhouse Gas Emissions. Sustainability. 2022; 14(6):3541. https://doi.org/10.3390/su14063541
Chicago/Turabian StyleSakamoto, Kaito, Kotaro Kawajiri, Hiroaki Hatori, and Kiyotaka Tahara. 2022. "Impact of the Manufacturing Processes of Aromatic-Polymer-Based Carbon Fiber on Life Cycle Greenhouse Gas Emissions" Sustainability 14, no. 6: 3541. https://doi.org/10.3390/su14063541
APA StyleSakamoto, K., Kawajiri, K., Hatori, H., & Tahara, K. (2022). Impact of the Manufacturing Processes of Aromatic-Polymer-Based Carbon Fiber on Life Cycle Greenhouse Gas Emissions. Sustainability, 14(6), 3541. https://doi.org/10.3390/su14063541





