Abstract
When drought occurs during the maize-filling period, the probability of yield decline increases. Abscisic acid (ABA) plays a regulatory role in physiological and metabolic activities during plant development. However, its effect on the antioxidant system of maize leaves during the grain-filling stage is unclear. Maize plants (Zhengdan958) were used as an experimental material, and ABA was sprayed on the leaves during the grain-filling stage. The plants were placed under drought conditions to analyze the relationship between the ascorbate-glutathione (AsA-GSH) cycle and hydrogen peroxide (H2O2) removal. Exogenous ABA significantly reduced the malondialdehyde content, relative electrolyte leakage, and H2O2 under drought stress. This is similar to the exogenous ABA effect on the AsA-GSH cycle. Exogenous ABA upregulated the transcription of related genes and alleviated the inhibition of drought stress on the monodehydroascorbate reductase and dehydroascorbate reductase activities, thereby further increasing the ascorbate peroxidase and glutathione reductase activities. It contributed to an increase in the AsA and GSH levels and inhibited the decrease in the AsA/dehydroascorbic acid and GSH/oxidized glutathione ratios. Therefore, exogenous ABA plays an important role in improving the antioxidant capacity and drought resistance physiology of maize by enhancing antioxidant enzyme activity and stabilizing the AsA and GSH redox state.
1. Introduction
Current estimates indicate that the world population will reach 9.6 billion by 2050 [1,2]. It is expected that grain production must increase by 50% in the next 40 years to meet the needs of the population [3]. Global warming leads to an increase in drought stress frequency. Water is a key factor in plant growth and water deficit leads to growth retardation, metabolism obstruction, and productivity decline. The sources of energy and protein in human nutrition makes maize (Zea mays L.) the third-largest crop globally [4]. Maize is sensitive to water deficits during the key growth and development period, such as the grain-filling stage. Therefore, it is imperative to improve the drought resistance of maize during the grain-filling period to ensure global food security and establish sustainable agriculture.
The imbalance between the production and clearance of reactive oxygen species (ROS) in plants inhibits its growth and photosynthetic capacity [5]. This includes superoxide anions (O2−), hydrogen peroxide (H2O2), singlet oxygen (1O2), and hydroxyl radicals (OH). Some studies have suggested that the damage to chloroplast development under water stress is caused by the metabolic imbalance of oxygen free radicals [6]. Although H2O2 is not a free radical, it is the source of many oxygen-containing free radicals and the conversion of other ROS. H2O2 is an important signaling molecule in plant cells, capable of mediating the response of plants to stress [7]. H2O2 may mediate ABA-induced stomatal closure by controlling potassium channels on the cytoplasmic membrane and activating calcium channels [8]. H2O2 affects gene expression and metabolism and induces programmed death of plant cells [9,10,11]. The physiological regulation of H2O2 is crucial for metabolic activities, as abnormal accumulation causes damage to the membrane system of tissues and organs. Under water-deficit conditions, the massive ROS accumulation will destroy the stability of protein, sugar, and nucleic acid molecules [12]. This, in turn, results in the peroxidation of unsaturated fatty acids in the biofilm, that is, membrane peroxidation, resulting in a decrease in membrane fluidity, structural damage, and an increase in permeability [13,14]. The degree of membrane lipid peroxidation is reflected by the malondialdehyde (MDA) content. MDA poisons plant cells by causing intramolecular or intermolecular cross-linking of proteins [15].
Aerobic organisms continuously produce active oxygen, which is more serious under abiotic stress. The ROS level must be regulated. After long-term adaptation to their environment, plants have formed a relatively perfect active oxygen scavenging system. This system has evolved to regulate cellular redox status and coordinate gene expression to resist damage to the cell structure and function caused by ROS accumulation with strong oxidation ability and promote plant resistance [16,17,18,19]. The ascorbate-glutathione (AsA-GSH) cycle has proven to be the main metabolic pathway for scavenging H2O2 [20]. This cycle can remove reactive oxygen free radicals and protect the structure of the cell membrane and maintain its function. Therefore, it is reasonable to believe that the strength of intracellular antioxidant systems is the basis for determining the stress resistance of plants [21].
The AsA-GSH reduction system includes AsA, GSH, ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) [22]. They cooperate to complete the removal of H2O2 under drought stress [23]. The AsA-GSH cycle varied slightly in different tests, but the basic framework was similar, including some related metabolic reactions (Figure 1) [24]. APX is the first catalytic enzyme for H2O2 removal in the AsA-GSH cycle. It can catalyze AsA to reduce H2O2 to H2O and form monodehydroascorbate (MDHA). However, the half-life of MDHA is not long and unstable. One part can be reduced to AsA by MDHAR, while the other can spontaneously and disproportionately produce dehydroascorbic acid (DHA). Under the catalysis of DHAR, DHA can use GSH as a reducing agent to form AsA and oxidized glutathione (GSSG) to complete the regeneration of AsA. GSSG can be catalytically reduced to GSH by GR, which is the key enzyme that maintains the stability of GSH levels in cells [25]. The AsA-GSH circulatory system exists in the chloroplasts, cytoplasm, mitochondria, and peroxisomes of plants. Moreover, several genes encoding AsA-GSH circulating enzymes have been identified, including two GR genes (GR1 and GR2) and three DHAR genes (DHAR1, DHAR2, and DHAR3) [26].
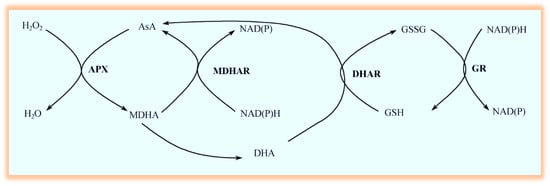
Figure 1.
Illustration of the ascorbate-glutathione (AsA-GSH) cycle.
In different plant growth stages, the regulatory effect of plant hormones on stress responses has proven to be valuable. Abscisic acid (ABA) is a stress hormone that affects the growth and development of plants under abiotic stress (drought, heavy metals, heat, high salinity, low temperature, and radiation), especially water-related stress [27]. This allows the effective transport of ABA between cells because of its metabolism, perception, transport, and systemic effects [28]. Studies have shown that ABA, as a signal molecule, actively regulates fast and slow ABA communication pathways, endowing cells with the ability to resist dehydration, improve osmotic stress tolerance, and maintain the balance between plant internal growth and the environment. Under drought stress, the overexpression of 9-cis-epoxy carotenoid dioxygenase in tomatoes increased ABA accumulation and drought resistance and reduced transpiration levels [29]. It has been reported that ABA may be related to oxidative stress in maize cells [30]. Exogenous ABA is known to have a positive effect on plant stress tolerance. Some studies have found that ABA improves the activity of antioxidant enzymes in drought-resistant crops [31] and up regulates the expression of a variety of water stress-related genes. Environmental and human regulatory factors can affect the metabolic pattern and intensity of the AsA-GSH cycle [32]. It has been proven that it can induce antioxidant defenses by increasing the activities of APX, MDHAR, DHAR, and GR to provide plants with oxidative stress tolerance. Therefore, external regulation is an important method to improve plant antioxidant capacity.
Many photosynthetic products must be transported to the grain during the filling stage of maize. To improve the stress resistance of leaves, it is necessary to understand the regulatory mechanisms of exogenous hormones. In this study, the maize cultivar, Zhengdan958 (ZD958), was pretreated with exogenous ABA and subjected to water stress during grain filling. The effects of ABA on AsA-GSH in maize under water stress conditions were studied. This study provides a theoretical basis and technical support for exogenous ABA to improve antioxidant mechanisms and reduce the damage caused by drought stress.
2. Materials and Methods
2.1. Plant Material, Growth Conditions, Experimental Design, and Sampling
The inbred maize line, ZD958, was used as the test material (Henan Agricultural Materials Company, Zhengzhou, China). The experimental site was an artificial dry shed of the Northeast Agricultural University in Harbin. The Meidilianhua chemical company (Beijing, China) provided the chemical reagent ABA.
The treatment during the test was performed in the dry shed. The experimental plastic buckets measured 27.5 cm (top diameter) × 20 cm (bottom diameter) × 25 cm (height) with drainage holes at the bottom. Put 12.5 kg (pH 6.85) of black soil into each bucket. The main nutrients of black soil are analyzed as follows: total nitrogen (1.43 g·kg−1), available potassium (254.55 mg·kg−1), available phosphorus (33.93 mg·kg−1), organic matter (4.86 g·kg−1), and alkali hydrolysable nitrogen (186.17 mg·kg−1). All the potted plants were placed on the same plot, and soil indicators were measured according to unified standards, applying fertilizer on time to ensure that they are not disturbed by climate and nutrient factors, and standardizing the system of pest control and weed removal.
After pollination, maize plants were treated with one of the following three treatments: (1) CK, untreated; (2) drought stress (for 4, 7, or 10 days); (3) drought stress + ABA, i.e., foliar sprayed with ABA (0.5 mmol L−1) under drought stress. This ABA dose was selected based on previous experiments (data not shown). This experiment was repeated five times.
An SWP-100 portable soil water potential meter (Nanjing Institute of soil research, Chinese Academy of Sciences) was used to test soil water potential for 24 h. Three fixed measuring points were set for each treatment (the buried depth of the probe is about 15 cm). The amount of irrigation water was determined by a weighing method. The irrigation water for normal water supply and rewatering treatment was 80 mm (soil relative water content = 75–80%). When the soil water potential is lower than the set value, supplementary irrigation should be used to restore the preset water potential, and the irrigation water volume should be recorded. After treatment, samples (leaves) were collected every three days. To determine the antioxidant index, the middle of the maize ear leaf was sampled, either fresh or frozen in liquid nitrogen (−80 °C).
2.2. Measurement of H2O2 Content
Fresh corn leaves (2 g) were added into a 4 mL buffer solution (PBS, 50 mM, pH 6.8), ground in an ice bath, and transferred to a centrifugal tube for centrifugation (10,000× g, 4 °C, 15 min). The supernatant was then added to a mixture of titanium disulfate and 20% sulfuric acid (v/v). Measure the absorbance value at 415 nm and calculate the H2O2 content according to the standard curve [33].
2.3. Measurement of MDA Content
Weigh 1g of corn leaves, grind with 5 mL buffer (TCA, 10%, w/v) and centrifuge (10,000× g, 20 min 4 °C); take the supernatant as the test sample. Take 2 mL of centrifuged sample, mix 2 mL of thiobarbituric acid (TBA, 0.6%, w/v), cool quickly after reaction (100 °C, 15 min), and centrifuge (10,000× g, 10 min, 4 °C) [34]. The absorbance values of the supernatant were measured at 450 nm, 532 nm, and 600 nm.
2.4. Determination of Electrolyte Leakage
The leaves were washed with deionized water, and the surface water was absorbed using filter paper. Deionized water (10 mL) was added to the weighing bottle containing the leaves and placed in a vacuum drying oven, and the air was pumped out with a vacuum pump for 10 min. The weighing bottles were placed on the oscillator and oscillated for 1 h. Measure the initial conductivity (S1) with a conductivity meter. After measurement, each weighing bottle was placed in a boiling water bath for 10 min to kill the plant tissue. After removing the test tube, it was cooled with tap water, shaken evenly. The final conductivity value (S2) was measured according to the formula for electrolyte leakage (EL) = S1/S2 × 100.
2.5. Measurement of Antioxidant Enzyme Activities
To determine APX enzyme activity, we followed the methods described by Zhu [35]. The reaction system consisted of 850 µL phosphate-buffered saline (PBS, pH 7.0, containing 0.1 mM EDTA), 50 µL AsA (5 mM), and 50 µL H2O2 (20 mM). The AsA oxidation was initiated by monitoring H2O2 levels (extinction coefficient ε = 2.8 mM cm−1). The enzyme activity was calculated by the decrease in absorbance at 290 nm.
The decrease in absorbance value per minute at a wavelength of 349 nm (by the oxidation of NADH, ε = 6.2 mM cm−1) was used to measure the activity of MDHAR. The reaction system included Tris-HCl buffer (50 mM, pH 7.5), NADH (0.2 mM), AsA (2.5 mM), AsA oxidase (0.15 U), and 50 µL enzyme solution [36].
DHAR activity was determined according to the method described by Doulis [37]. The reaction system included 50 µL enzyme activity, 850 µL PBS (pH 7.0, containing 0.1 mM EDTA), 50 µL GSH (2.5 mM), and 50 µL DHA (0.1 mM). Record the change in absorbance per minute at 265 nm (by forming ASA, ε = 14 mm cm−1) to calculate DHAR activity.
GR activity was calculated by recording the change in absorbance per minute at 340 nm (through the oxidation of NADPH, ε = 6.2 mM cm−1). The reaction system consisted of 1.2 mL Tris-HCl buffer (100 mM, pH 8.0), 0.4 mL GSSH (1 mM), 0.3 mL NADPH (0.2 mM), and 0.1 mL enzyme solution [38].
The standard curve of bovine serum protein was used to calculate the protein concentration [39].
2.6. Measurements of AsA/DHA and GSH/GSSG in Leaves
Weigh 0.5 g of corn leaves, grind with 5 mL phosphoric acid (5%, v/v) buffer, and centrifuge (20 min, 10,000× g, 4 °C). Take the supernatant after centrifugation as the determination sample. The contents of AsA, DHA, and total AsA were determined according to Hodges’ method [40]: DHA = total AsA-AsA. The contents of GSH, GSSG, and total GSH were determined according to Griffith’s method [41]: GSH = total GSH-GSSG.
2.7. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction
First, total RNA was isolated from ear position leaves of maize. Trizol reagent (Invitrogen, Carlsbad, CA, USA) was used. Specific primers for each gene were designed from the 3′ ends of the gene sequences using Primer Premier 5.0 (Table 1). The primer melting temperature (Tm) value was between 55–60 °C. The cDNA sample was diluted five times for use as a template for computer detection. The reagent used for real-time quantitative PCR was 2× SG Fast qPCR Master Mix (B639271, BBI), and PCR was performed on a LightCycler 480 II (Roche Molecular Systems Inc., Pleasanton, CA, USA) [26]. The 2−ΔΔCt method was used to calculate the relative transcript levels.

Table 1.
Primer sequences for real-time RT-PCR.
2.8. Statistical Analysis
Experimental data are represented as the mean ± standard deviation (SD). Microsoft Excel 2007 and SPSS 17 software were used for statistical analysis. Fisher’s least significant difference test was used to compare the treatment methods, and the significance level was p < 0.05.
3. Results
3.1. Effects of Drought Stress and/or ABA Application on the Relative Water Content, Relative Electrolyte Leakage, MDA Content, and H2O2 Content of Leaves
Drought stress significantly reduced the relative water content of maize leaves, and exogenous ABA alleviated the downward trend (Figure 2a). Drought stress significantly increased the relative EL of maize plants (Figure 2b). With the extension of drought stress time, the relative EL of the plants increased gradually. The application of exogenous ABA inhibited the increase in the relative EL under drought stress conditions. Compared to drought treatment alone, ABA treatment decreased the relative EL by 20.86%, 19.3%, and 27.36% on the 4th, 7th, and 10th day of drought treatment, respectively.
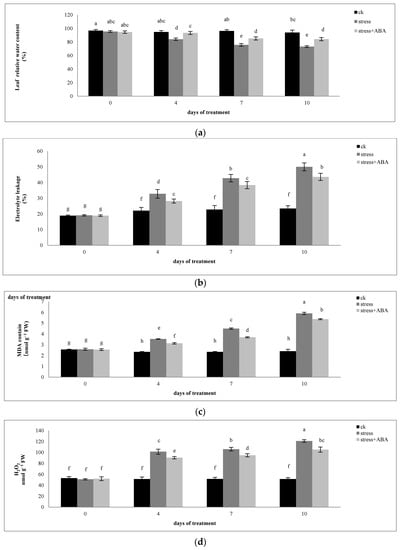
Figure 2.
Effects of exogenous ABA application on (a) Relative water content, (b) EL, (c) MDA content, and (d) H2O2 content in leaves of maize exposed to drought stress for 10 days. The data represents the means of independent measurements for five replicates. Standard deviation (SD) is indicated by vertical error bars. Values with the same lowercase letters on the bars are not significantly different (p < 0.05).
As shown in Figure 2, the MDA content in functional maize leaves continued to increase with the extension of drought stress time. The MDA content reached a maximum on the 10th day of drought stress treatment, which increased by 145.31% compared to the control treatment. On the 4th, 7th, and 10th days of drought stress, after exogenous ABA treatment, the content of MDA in the functional leaves of ZD958 decreased by 17.35%, 34.50%, and 22.24%, respectively, compared with drought treatment alone. The most obvious effect of ABA was on the seventh day. This shows that ABA can effectively inhibit membrane lipid peroxidation damage to the cell membrane under drought stress and protect the integrity of the cell membrane.
Under drought stress, the H2O2 content in maize plants increased gradually with increasing stress time. On the 4th, 7th, and 10th days of drought stress, the H2O2 content increased by 97.40%, 105.90%, and 135.56%, respectively, compared to the control; the application of ABA decreased by 21.46%, 21.79%, and 30.91%, respectively. The effect of exogenous ABA was almost the same on the 4th and 7th days of drought stress and reached a maximum on the 10th day.
3.2. Effects of Drought Stress and/or ABA Application on APX, MDHAR, DHAR, and GR Activity
As shown in Figure 3a,b, under drought stress, the activities of APX and GR in maize leaves were significantly higher than those in the control. Exogenous application of ABA further increased the activities of the two enzymes. On the 4th, 7th, and 10th days of drought stress, the activities of APX and GR in maize leaves increased by 96.89%, 115.48%, and 124.05%, and 48.71%, 30.32%, and 27.65%, respectively. After exogenous ABA treatment, the activities of APX and GR increased by 156.01%, 152.53%, and 157.81%, and 143.94%, 154.14%, and 166.25%, respectively. The degree of stress influenced the changes in APX and GR activities. There was no significant difference between the activities of APX and GR in maize plants on the 10th day of drought stress and those on the seventh day of drought stress. However, they were significantly higher than those on the fourth day of drought stress. The effect of exogenous ABA on APX activity did not change with the stress duration. However, the effect of ABA on GR activity under drought stress escalated with increasing stress days.
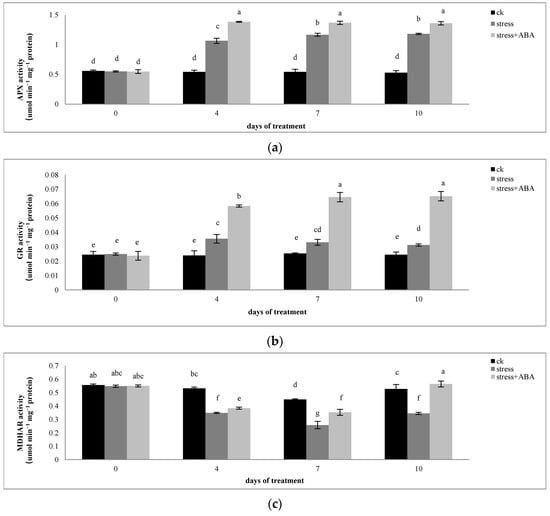
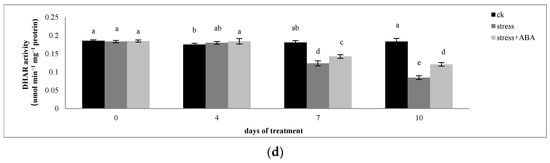
Figure 3.
Effects of exogenous ABA application on the activities of (a) APX, (b) GR, (c) MDHAR, and (d) DHAR in leaves of maize exposed to drought stress for 10 days. The data represent the means of independent measurements for five replicates. SD is indicated by vertical error bars. Values with the same lowercase letters on the bars are not significantly different (p < 0.05).
Drought stress reduced the activities of MDHAR and DHAR in maize leaves, and the application of ABA inhibited the decline in enzyme activity. On the 4th, 7th, and 10th days of drought stress, the activity of MDHAR in maize leaves decreased by 34.43%, 42.54%, and 34.56%, respectively. After exogenous ABA treatment, MDHAR activity increased by 6.53%, 21.2%, and 41.77%, respectively. On the 7th day of drought stress, the DHAR activity of maize leaves decreased by 31.48% compared to that of the control and then continued to decline. On the 7th and 10th days of drought stress, there was no difference in the effect of exogenous ABA on DHAR activity in maize leaves under drought stress conditions.
3.3. Effects of Drought Stress and/or ABA Application on Non-Enzymatic Antioxidants
As shown in Figure 4, under drought conditions, the AsA content and AsA/DHA ratio in maize leaves were significantly lower than those in the control treatment, but the DHA content increased significantly. Exogenous application of ABA significantly increased the AsA content and the AsA/DHA ratio in maize leaves and inhibited the increase in DHA content. The decrease in AsA content in the leaves of ZD958 escalated with increasing stress days. On the 10th day of stress, the DHA content in leaves of ZD958 was the highest, increasing 103.26% higher than the control.
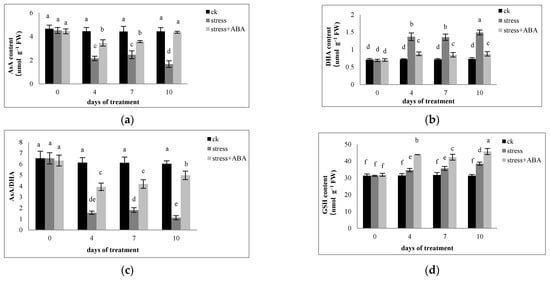
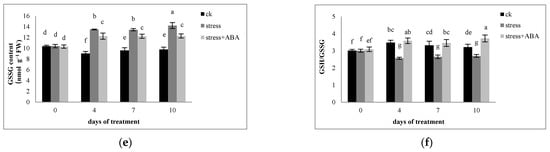
Figure 4.
Effects of exogenous ABA application on the contents of (a) AsA and (b) DHA, (c) the AsA/DHA ratio, (d) reduced GSH, (e) oxidized GSSG, and (f) the GSH/GSSG ratio in leaves of maize exposed to drought stress for 10 days. The data represents the means of independent measurements for five replicates. SD is indicated by vertical error bars. Values with the same lowercase letters on the bars are not significantly different (p < 0.05).
The contents of GSH and GSSG in the functional leaves of maize increased significantly under drought stress. The application of exogenous ABA further increased the GSH content and inhibited the increase in GSSG under drought stress conditions. Exogenous ABA increased the GSH content by 21.01–29.26% and reduced the GSSG content by 12.68–19.87% compared to the drought stress alone. The GSH/GSSG ratio in maize leaves reduced significantly under drought stress, and the ABA application could increase it by 24.28–31.76%.
3.4. Effects of Drought Stress and/or ABA Application on the Expression of Genes Encoding AsA-GSH Cycle Enzymes
Drought-induced changes in GR activity (Figure 3b) were also evident at the mRNA levels (Figure 5a,b). The relative levels of GR1 and GR2 mRNAs increased under drought stress conditions (Figure 5a,b), and the highest levels were both observed on the fourth day. GR1 mRNA levels under drought stress conditions + ABA were higher than only drought stress conditions, but GR2 mRNA levels showed the opposite result.
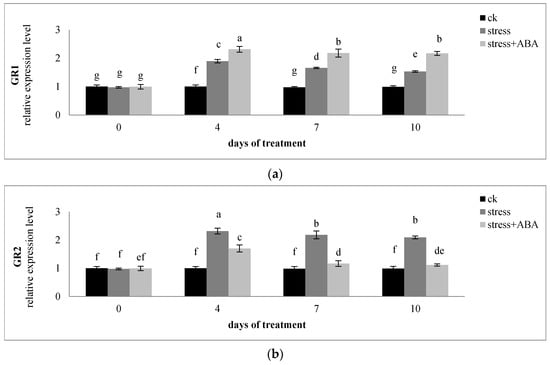
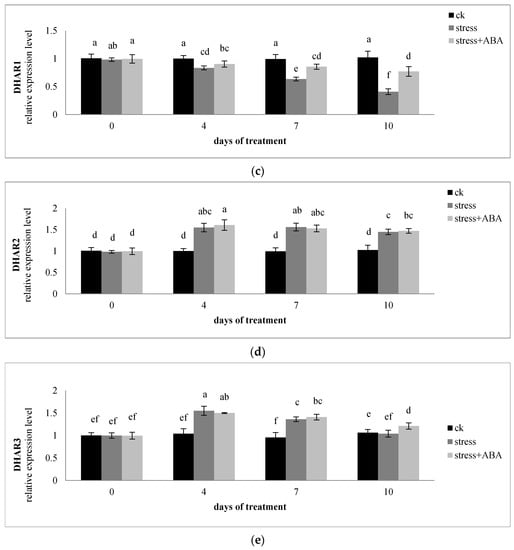
Figure 5.
Effects of exogenous ABA application on the relative expression level of (a) GR1, (b) GR2, (c) DHAR1, (d) DHAR2, and (e) DHAR3 in leaves of maize exposed to drought stress for 10 days. The data represent the means of independent measurements for five replicates. SD is indicated by vertical error bars. Values with the same lowercase letters on the bars are not significantly different (p < 0.05).
On the fourth and seventh days, the relative levels of DHAR2 and DHAR3 under drought stress were higher than those in well-watered controls. Under drought stress, the transcription level of DHAR1 decreased, and the decline in the transcription level was inhibited by exogenous ABA, having a significant effect on the 7th and 10th days. Furthermore, on the fourth and seventh days, the transcription level of DHAR3 was upregulated to a greater extent than that of DHAR2 under water-deficit conditions. Lastly, the effect of ABA treatment on the levels of DHAR2 and DHAR3 mRNAs did not differ significantly from that of drought stress.
4. Discussion
The antioxidant defense system of leaves has attracted much attention. One of its principles is that leaves are the main sensor of drought stress. Moreover, some research results show that the drought tolerance of maize varieties is closely related to the ability of antioxidant systems [42]. In this experiment, the H2O2 content in maize plants increased in water deficit conditions, which explains the role of H2O2 as a stress signal molecule [43]. With the aggravation of water stress, H2O2 attacks the cell membrane as a free radical [44]. When treated with exogenous ABA, the ability to scavenge H2O2 was enhanced in maize plants. Between the fourth and seventh days, the increase in H2O2 content in ZD958 was not different, which proved the effectiveness of oxidative stress control. On the 10th day of drought stress, the H2O2 content of maize plants treated with exogenous ABA did not decrease significantly. One possible explanation is that exogenous ABA treatment increases the internal ABA content, resulting in H2O2 accumulation (a signal molecule that activates antioxidant enzymes) [45]. This theory has been confirmed in rice [46] and maize [30]. The accumulated H2O2 maximally activated the enzyme activity participating in the clearance reaction.
Malondialdehyde (MDA), the end-product of membrane damage peroxidation caused by the accumulation of reactive oxygen species, can react with a variety of components in cells and damage a variety of enzymes and membrane systems. This is a sign of plant leaf senescence. Drought conditions exhibited greater accumulation in MDA contents, a product of lipid peroxidation in maize plants, fulvic acid-treated plants considerably lowered MDA contents which reported that lower MDA contents could be correlated with stress tolerance [47]. Under water-deficit conditions, the average content of MDA in maize leaves was higher, and the application of ABA significantly reduced membrane damage. Other studies observed that ABA treatment of maize significantly alleviated membrane damage (decreased MDA levels) [31]. Some studies found that, in the later stage of stress, the MDA and H2O2 contents in resistant crop varieties decreased, which may be because the antioxidant enzyme activity was activated to a maximum extent, thus reducing oxidative damage [48]. However, in this study, the MDA and H2O2 contents in maize leaves did not decrease under water stress conditions with an increase in stress days. This may be because the imbalance between the accumulation and clearance of reactive oxygen species (ROS) leads to the accumulation of MDA, which, in turn, partially inhibits the activity of antioxidant enzymes.
AsA and GSH are non-enzymatic substances with low moleculars, which widely exist in cells and participate in antioxidant metabolic reactions. They participate in the scavenging of reactive oxygen species through the AsA-GSH cycle. The AsA oxidation and reduction system consist of AsA, MDHA, and DHA. AsA is oxidized to MDHA by APX and further converted to DHA during H2O2 removal. MDHAR can reduce MDHA to AsA, and DHA can regenerate AsA through DHAR. Therefore, AsA/DHA can represent oxidation and reduction states in cells. The concentrations of AsA and GSH vary with the external environment, especially abiotic stress [49]. Under drought stress, the AsA content in ZD958 decreased continuously. This is consistent with the relevant test conclusions for maize [50,51] and Brassica [20]. This may be because the water deficit improved the activity of APX and promoted the H2O2 scavenging reaction of AsA in this experiment. At the same time, it reduces the activities of MDHAR and DHAR and hinders the regeneration of AsA.
APX is the core enzyme in the AsA-GSH cycle and plays a crucial role in H2O2 metabolism [52]. In a certain range, high temperatures [53], water shortages [54], and high salt contents [55] can lead to an increase in APX activity. This is consistent with the results of the present study. The APX activity of ZD958 significantly increased under drought stress, and the application of exogenous ABA further improved its activity. The high activity of APX and DHAR can improve the salt and alkali tolerance of cabbage [56]. Conversely, decreased APX, GR, and MDHAR activities at high temperatures resulted in more severe membrane lipid peroxidation [57]. It can be inferred that exogenous ABA enhanced the activity of APX, which can increase the drought resistance of maize and reduce the content of MDA.
The AsA/DHA ratio measures the level of available AsA [22]. Exogenous application of ABA inhibited the decrease in the AsA/DHA ratio in maize plants under water stress conditions. This can be explained by the significant increase in MDHAR and DHAR activities in ZD958 treated with exogenous ABA. Therefore, exogenous ABA can stabilize the redox state of maize cells by improving the activity of antioxidant enzymes. However, according to the conclusion of Li [51], it is predicted that if the stress time is prolonged in this experiment, ABA may not continue to improve the antioxidant capacity of maize. When the mediated ability of AsA-GSH reaches its maximum, its mediated ability decreases with the extension of stress time, resulting in plant damage.
As an important antioxidant related to AsA regeneration in the AsA-GSH cycle, GSH also regulates the metabolism of H2O2 [14]. As a regulator of enzyme activity, GSH activates various defense mechanisms by participating in redox signals, such as cold injury [58], heat injury [59], salt injury [5], and water stress [19], thus protecting cells from adverse stress. In this signaling pathway, GSH interacts with the plant hormone abscisic acid (ABA) [60]. In this study, drought stress increased the GSH content in maize plants. This is consistent with the results for tomatoes [53] under mildly high-temperature stress. However, a water deficit reduced the ratio of GSH/GSSG in ZD958. Because GSH is oxidized in the process of scavenging accumulated H2O2, this is consistent with the results of previous studies. However, Selote [12] believed that the changes in GSH content and the GSH/GSSG ratio are not synchronous under different drought intensities. The redox state of GSH affects the adaptation of crops to the stress environment, and the amount of GSH involved in the response largely determines the efficacy of AsA. Exogenous ABA restored the high GSH/GSSG ratio in maize and could effectively protect plants from reactive oxygen species induced by abiotic stress. A high ratio of GSH/GSSG is a sign of plant tolerance. The transformation of GSSG to GSH can enhance the tolerance of plants to environmental stress [61]. The mechanism may operate through increasing GR activity and GR1 expression, increasing GSH synthesis, and reducing GSH degradation [60].
Under abiotic stress, GR, an antioxidant enzyme, plays an important regulatory role in the glutathione cycle. The increase in GR activity under stress promoted the effective removal of H2O2 by GSH and maintained a high GSH/GSSG ratio [60]. In soybean [43], H2O2 and GSH have interacted with stress signals. This can explain why, in this present experiment, the application of exogenous ABA inhibited the accumulation of H2O2 by increasing GR activity and GR1 levels to increase GSH content. Analysis of enzyme gene expression revealed that the levels of DHAR1 and GR1 mRNAs were highly consistent with the physiological activity trends of DHAR and GR, respectively. Therefore, it is concluded that DHAR1 and GR1 regulate the two enzymes more closely in this study.
5. Conclusions
Through the water stress test of ZD958 maize at the pustulation period, it was found that ABA enhanced the scavenging ability of H2O2 and MDA by regulating the physiological process of the AsA-GSH cycle. In addition, under water deficit conditions, ABA-induced the upregulation of GR activity and transcription maintained the stability of the GSH/GSSG ratio and helped to maintain the intracellular redox state. The GR1 and DHAR1 expression level changes were consistent with those in GR and DHAR enzyme activity levels. ABA promotes the regeneration of AsA by stabilizing the activity and transcriptional expression of MDHAR and DHAR. Therefore, the ABA-mediated AsA-GSH cycle improves the antioxidant capacity, alleviates the damage ofROS, reduces growth inhibition, and enhances the tolerance of plants to drought stress.
Author Contributions
Conceptualization: M.L.; data curation, Z.J. and H.Z. (Hengguang Zhu); Formal analysis: Z.J.; funding acquisition: M.L.; investigation: J.L.; methodology: C.L. and M.L.; project administration: M.L.; resources, M.L.; supervision: C.L. and M.L.; validation, F.Y. and J.L.; writing—original draft, Z.J. and Y.T.; writing—review and editing, Z.J., H.Z. (Hanyu Zhu) and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful for the support from the grants from China Postdoctoral Science Foundation Project (2018M631905) and the Innovation project of ministry of science and technology of China (2017YFD0300506-2).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful for the high efficiency and valuable comments by the editor and reviewers who contributed significantly to improving our manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, R. The Outlook for Population Growth. Science 2011, 333, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiermeier, Q. Water risk as world warms. Nature 2014, 505, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Dalal, V.K.; Tripathy, B.C. Water-stress induced downsizing of light-harvesting antenna complex protects developing rice seedlings from photo-oxidative damage. Sci. Rep. 2018, 8, 5955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, G.D.S.; Lopes, L.D.S.; Paula-Marinho, S.D.O.; Mesquita, R.O.; Gomes-Filho, E. H2O2 priming induces proteomic responses to defense against salt stress in maize. Plant Mol. Biol. 2021, 106, 33–48. [Google Scholar] [CrossRef]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.; Silva, P.; Matos, R.M.D.; Matos, R.; Neto, J.; Melo, Y. Induction of salt stress tolerance in chives by priming with H2O2 in hydroponic cultivation. Chil. J. Agric. Res. 2021, 81, 317–325. [Google Scholar] [CrossRef]
- Carvalho, F.; Silveira, J. H2O2-retrograde signaling as a pivotal mechanism to understand priming and cross stress tolerance in plants. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Academic Press: Cambridge, MA, USA, 2020; pp. 57–78. [Google Scholar]
- Cho, B.O.; Ryu, H.W.; Lee, C.W.; Lee, C.W.; Jin, C.H.; Seo, W.D.; Ryu, J.; Jeong, I.Y. Protective effects of new blackberry cultivar MNU-32 extracts against H2O2-induced oxidative stress in HepG2 cells. Food Sci. Biotechnol. 2015, 24, 643–650. [Google Scholar] [CrossRef]
- Selote, D.S.; Khanna, C.R. Drought acclimation confers oxidative stress tolerance by inducing coordinated antioxidant defense at cellular and subcellular level in leaves of wheat seedlings. Physiol. Plant. 2006, 127, 494–506. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Yaghubi, K.; Ghaderi, N.; Vafaee, Y.; Javadi, T. Potassium silicate alleviates deleterious effects of salinity on two strawberry cultivars grown under soilless pot culture. Sci. Hortic. 2016, 213, 87–95. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Drought Induces Oxidative Stress and Enhances the Activities of Antioxidant Enzymes in Growing Rice Seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Goher, M.; Iobal, N. Drought induced programmed cell death and associated changes in antioxidants, proteases, and lipid peroxidation in wheat leaves. Biol. Plant. 2013, 57, 370–374. [Google Scholar] [CrossRef]
- Hojati, M.; Modarres-Sanavy, S.A.M.; Karimi, M.; Ghanati, F. Responses of growth and antioxidant systems in Carthamus tinctorius L. under water deficit stress. Acta Physiol. Plant 2011, 33, 105–112. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Prasad, S.M. Sulphur and calcium attenuate arsenic toxicity in Brassica by adjusting ascorbate–glutathione cycle and sulphur metabolism. Plant Growth Regul. 2020, 91, 221–235. [Google Scholar] [CrossRef]
- Zhou, W. Deteriorative effects of cadmium stress on antioxidant system and cellular structure in germinating seeds of Brassica napus L. J. Agric. Sci. Technol. 2018, 17, 63–74. [Google Scholar]
- Ma, J.; Zheng, G.; Pei, C.M.; Zhang, Z.Y. The function of ascorbate-glutathione cycle in salt tolerance of alfalfa mutant. Plant Physiol. 2015, 79, 287–297. [Google Scholar]
- Guo, P.; Baum, M.; Grando, S.; Ceccarelli, S.; Bai, G.; Li, R.; Korff, M.; Varshney, R.K.; Graner, A.; Valkoun, J. Differentially expressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. Exp. Bot. 2009, 60, 3531–3544. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Zhang, J. Advances in the research on the AsA-GSH cycle in horticultural crops. Front. Agric. Chin. 2010, 4, 84–90. [Google Scholar] [CrossRef]
- Moothoo-Padayachie, A.; Varghese, B.; Pammenter, N.W.; Govender, P.; Naidoo, S. Germination associated ROS production and glutathione redox capacity in two recalcitrant-seeded species differing in seed longevity. Botany 2016, 94, 1103–1114. [Google Scholar] [CrossRef]
- Liu, Y.J.; Yuan, Y.; Liu, Y.Y.; Liu, Y.; Fu, J.J.; Zheng, J.; Wang, G.Y. Gene families of maize glutathione–ascorbate redox cycle respond differently to abiotic stresses. J. Plant Physiol. 2012, 169, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, V.; Neha, U.; Nitin, K.; Gaurav, Y.; Jaspreet, S.; Mishra, R.K.; Vivek, K.; Rishi, V.; Upadhyay, R.G.; Mayank, P. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Andrews, J.; Mulholland, B.J.; McKee, J.M.T.; Hilton, H.W.; Horridge, J.S.; Farquhar, G.D.; Smeeton, R.C.; Smilie, I.R.A.; Black, C.R.; et al. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol. 2007, 143, 1905–1917. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.L.; Jiang, M.Y.; Zhang, A.Y.; Lu, J. Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves. Planta 2005, 223, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Kellos, T.; Tímár, I.; Szilágyi, V.; Szalai, G.; Galiba, G.; Kocsy, G. Stress hormones and abiotic stress have different effects on antioxidants in maize lines with different sensitivity. Plant Biol. 2008, 10, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Song, X.S.; Tiao, C.L.; Shi, K.; Mao, W.H.; Ogweno, J.O.; Zhou, Y.H.; Yu, J.Q. The response of antioxidant enzymes in cellular (Cucumissativus L.) leaves to methyl viologen-induced photo-oxidative stress. Plant Growth Regul. 2006, 49, 85–93. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T.; Ibrahim, A.S. Alleviation of drought-induced oxidative stress in maize (Zeamays L.) plants by dual application of 24-epibrassinolide and spermine. Environ. Exp. Bot. 2015, 113, 47–58. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.Q.; Yu, J.Q. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.; Okuma, E.; Amako, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline and glycinebetaine increase NaCl-induced ascorbate-glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2suspension-cultured cells. J. Plant Physiol. 2007, 164, 1457–1468. [Google Scholar] [CrossRef]
- Doulis, A.G.; Debian, N.; Kingston Smith, A.H.; Foyer, C.H. Differential localization of antioxidants in maize leaves. Plant Physiol. 1997, 114, 1031–1037. [Google Scholar] [CrossRef] [Green Version]
- Ramiro, D.A.; Guerreiro-Filho, O.; Mazzafera, P. Phenol contents, oxidase activities, and the resistance of coffee to the leaf miner Leucoptera coffeella. J. Chem. Ecol. 2006, 32, 1977–1988. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. J. Exp. Bot. 1997, 48, 1105–1113. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinyl pyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Chugh, V.; Kaur, N.; Gupta, A.K. Evaluation of oxidative stress tolerance in maize (Zea mays L.) seedlings in response to drought. Indian J. Biochem. Biophys. 2011, 48, 47–53. [Google Scholar] [PubMed]
- Xing, X.; Xu, Z.; Tong, F.; Qi, Y.J.; Wang, X. Involvement of triadimefon induced early ABA-dependent H2O2 accumulation in soybean against water stress. Oil Crop Sci. 2020, 5, 41–47. [Google Scholar] [CrossRef]
- Queval, G.; Hager, J.; Gakiere, B.; Noctor, G. Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in the quantitative assay of leaf extracts. J. Exp. Bot. 2008, 59, 135–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, T.C.; Magalhães, P.C.; de Castro, E.M.; Carneiro, N.P.; Padilha, F.A.; JHYPER, C.C.G. ABA application to maize hybrids contrasting for drought tolerance: Changes in water parameters and in antioxidant enzyme activity. Plant Growth Regul. 2014, 73, 205–217. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Ye, Y.; Yang, J.C.; Zhang, J.H.; Ye, Y.X.; Wang, Z.Q.; Zhu, Q.S.; Liu, L.J. Involvement of abscisic acid and ethylene in the responses of rice grains to water stress during filling. Plant Cell Environ. 2004, 27, 1055–1064. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.; Farooq, M.; Xue, L.; Ali, S. Fulvic acid application improves the maize performance under well-watered and drought conditions. J. Agron. Crop Sci. 2011, 197, 409–417. [Google Scholar] [CrossRef]
- Moussa, H.R.; Abdel-Aziz, S.M. Comparative response of drought tolerant and sensitive maize genotypes to water stress. Aust. J. Crop Sci. 2008, 1, 31–36. [Google Scholar]
- Shan, C.; Wang, B.; Sun, H.; Gao, S.; Li, H. H2S induces NO in the regulation of AsA-GSH cycle in wheat seedlings by water stress. Protoplasma 2020, 257, 1487–1493. [Google Scholar] [CrossRef]
- Li, L.; Gu, W.; Li, C.; Li, W.; Li, C.; Li, J.; Wei, S. Exogenous spermidine improves drought tolerance in maize by enhancing the antioxidant defence system and regulating endogenous polyamine metabolism. Crop Pasture Sci. 2018, 69, 1076–1091. [Google Scholar] [CrossRef]
- Xie, T.; Gu, W.; Zhang, L.; Li, L.; Qu, D.; Li, C.; Meng, Y.; Li, J.; Wei, S.; Li, W. Modulating the antioxidant system by exogenous 2-(3, 4-dichlorophenoxy) triethylamine in maize seedlings exposed to polyethylene glycol-simulated drought stress. PLoS ONE 2018, 13, e0203626. [Google Scholar] [CrossRef]
- Ishikawa, T.; Shigeoka, S. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci. Biotechnol. Biochem. 2008, 72, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.Q.; Shu, S.; Shan, X.; Guo, S.R.; Sun, J. Effects of exogenous spermidine on antioxidant system of tomato seedlings exposed to high temperature stress. Russ. J. Plant Physiol. 2016, 63, 645–655. [Google Scholar] [CrossRef]
- Bai, J.; Tai, K.; Wu, H.; Lu, B.; Gong, C. Relative contribution of photorespiration and antioxidative mechanisms in Caragana korshinskii under drought conditions across the Loess Plateau. Funct. Plant Biol. 2017, 44, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Gicek, N.; Gakirlar, H. Changes in some antioxidant enzyme activities in response to long-term salinity at two different temperatures. Gen. Appl. Plant Physiol. 2008, 34, 267–280. [Google Scholar]
- Li, N.; Cao, B.; Chen, Z.; Xu, K. Root morphology ion absorption and antioxidative defense system of two Chinese cabbage cultivars (Brassica rapa L.) reveal the different adaptation mechanisms to salt and alkali stress. Protoplasma 2021, 259, 385–398. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Lu, Z.H.; Kang, C.M. Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int. J. Biol. Sci. 2008, 4, 8–14. [Google Scholar] [CrossRef]
- Guo, Z.; Ou, W.; Lu, S.; Zhong, Q. Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol. Biochem. 2006, 44, 828–836. [Google Scholar] [CrossRef]
- Dash, S.; Mohanty, N. Response of seedlings to heat-stress in cultivars of wheat: Growth temperature-dependent differential modulation of photosystem 1 and 2 activity, and foliar antioxidant defense capacity. J. Plant Physiol. 2002, 159, 49–59. [Google Scholar] [CrossRef]
- Szalai, G.; Hellos, T.; Galiba, G.; Kocsy, G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Verma, G.; Mishra, S.; Sangwan, N.; Sharma, S. Reactive oxygen species mediate axis-cotyledon signaling to induce reserve mobilization during germination and seedling establishment in Vigna radiata. J. Plant Physiol. 2015, 184, 79–88. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).