Abstract
The glaciers in China have an important role as one of the most climate-sensitive constituents of the Tibetan Plateau which is known as the Asian Water Tower. Although the cryosphere is one of the most extreme environments for organisms, the soils of the glacier foreland harbor surprisingly rich microbiomes. A large amount of accelerated glacier retreat accompanied by global warming will not only raise the sea level, but it will also lead to the massive release of a considerable amount of carbon stored in these glaciers. The responses of glacier microbiomes could alter the biogeochemical cycle of carbon and have a complex impact on climate change. Thus, understanding present-day and future glacier microbiome changes is crucial to assess the feedback on climate change and the impacts on ecosystems. To this end, we discuss here the diversity and biogeochemical functions of the microbiomes in Chinese mountain glacier ecosystems.
1. Introduction
Glaciers are a major component of the cryosphere, which is one of the five major spheres of the climate system [1]. Glaciers can be divided into ice caps and mountain glaciers based on their sizes and geographical characteristics [2]. Although the glacier environment is cold, nutrient-poor, and receives a large amount of UV radiation, it still harbors diverse and active microorganisms [3,4,5]. The microorganisms in this important ecosystem are involved in the biogeochemical cycles of various elements, including carbon, nitrogen, and sulfur [6,7,8]. The community structure and functions of these microorganisms are affected by the physicochemical properties of the glaciers, which are closely related to climate and environmental changes [9,10,11,12,13].
Current global warming is an undoubtedly trend characterized by increasingly serious temperature increase. According to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC), the global surface temperature in 2011–2020 increased by 1.09 °C compared to 1850–1900 [14,15]. Under the impact of human activities and the self-reinforcing feedbacks of the Earth, this temperature increase will continue [16]. The cryosphere, which includes ice sheets, glaciers, and permafrost, is the most sensitive to these temperature changes and will be the most strongly affected. Arctic summer sea ice, the Greenland Ice Sheet, Alpine glaciers, and the west Antarctic Ice Sheet will face the threat of disappearance when the global surface temperature increases by 1–3 °C [17]. Several studies have concluded that mountain glaciers will be more easily affected by climate change, and their retreat rate is higher than those of the Arctic and Antarctic Ice Sheets [17,18]. Based on the observed global mean sea level change in 1901–2018, the retreat of mountain glaciers contributed 67.2 mm to the sea level rise [15,19].
China has the largest and most numerous glaciers in the middle- and low-latitude regions of the world [20,21]. These mountain glaciers tend to melt because their temperatures are usually equal to or exceed the melting point of ice. When glacier ice melts, newly exposed habitats are formed, with diverse microbial communities. These diverse microbial communities conduct important biogeochemical processes (e.g., the microbially mediated carbon cycle), which are important aspects of glacier ecology [22,23]. Although it is difficult to culture the special glacier microorganisms using traditional cultivation methodologies, a novel technique based on uncultured methods (e.g., next-generation sequencing) provides opportunities for improving geomicrobiological studies of mountain glaciers [23,24].
Here, we review the existing knowledge of microbial diversity and functions, as well as the biogeochemical interactions, in Chinese mountain glacier ecosystems. Cyanobacteria and algae, the important components of the cryosphere microbiome, are not discussed in detail due to the few related reports in Chinese glaciers. Global warming is closely related with carbon cycle, which would be greatly affected by the microorganisms. We also discuss the interactions between the microbial community of mountain glaciers and the changing climate from the involvement of microbes in carbon biogeochemical cycle, highlighting the importance of understanding the ecological implications of climate change and glacier melting.
2. Status of Glacier Distribution in China
According to the Second Chinese Glacier Inventory (SCGI), 48,571 glaciers were identified in Tibetan, Qinghai, Gansu, Sichuan, and Yunnan provinces, with a total area of 51,766.08 km2 and an ice volume of 4494.00 ± 175.93 km3 [2]. They are mainly small glaciers with areas of less than 1.0 km2, accounting for 80% of all glaciers in China [2]. Based on the development and geomorphological conditions as well as movement properties, Chinese glaciers are classified as mountain glaciers, located in the western mountains, including the Hengduan Mountains, Himalayan Mountains, Nyainqentanglha Mountains, Karakoram Mountains, Kunlun Mountains, Qilian Mountains, Tianshan Mountains, and Altay Mountains from south to north (Figure 1).
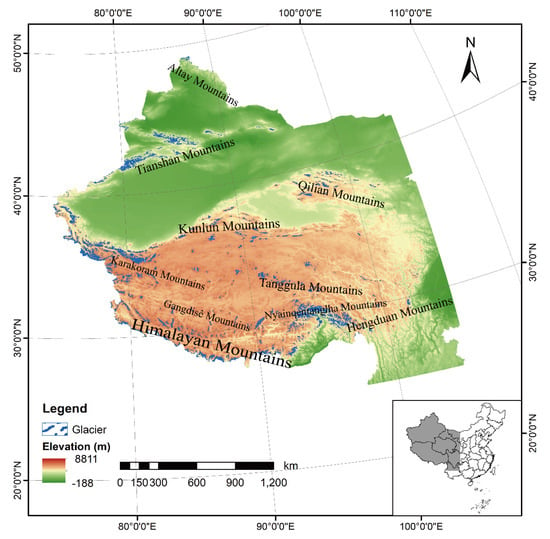
Figure 1.
The distribution map of glaciers in western China based on the Second Chinese Glacier Inventory.
According to the development and physical properties of glaciers, Chinese glaciers can be divided into continental glaciers, sub-continental glaciers, and temperate glaciers [25,26,27,28]. Covering an area of more than 13,200 km2, with abundant summer monsoon precipitation and an annual retreat rate of 4–66 m/year [29,30], the temperate glaciers are distributed in the eastern Himalayan Mountains, the central-eastern Nyainqentanglha Mountains, and the Hengduan Mountains [28]. This type of glacier is more sensitive to air temperature than precipitation changes, and the equilibrium altitude line would rise by 153 m if the temperature increased by 1 °C [31]. Sub-continental glaciers account for 46% (about 27,200 km2) of the all glaciers in China and are mainly distributed in the Qilian Mountains, the eastern Kunlun Mountains, the eastern Tanggula Mountains, the western Nyainqentanglha Mountains, part of the Gangdise Mountains, the north slopes of the central-western Himalayas, and the north slopes of the Karakoram Mountains [28]. With an annual precipitation of 500–1000 m, they have lower retreat rates; for example, Tianshan Glacier No. 1 has retreated by about 5 m every year [32]. In comparison, continental glaciers account for one-third of the total glacier area, covering 19,000 km2, and are characterized by the lowest annual average temperature (below 0 °C). Their dry climate, with an annual precipitation of 200–500 mm, causes the ice to flow slowly. Such glaciers are located in the western Qilian Mountains, western Gangdise Mountains, western Tanggula Mountains, eastern Pamir Plateau, the central and western Kunlun Mountains, and the Qiangtang Plateau [28,31].
As a result of continued global warming, glaciers are generally exhibiting a retreating trend. Nearly all of the Chinese glaciers have retreated significantly and at an accelerated rate (Table 1). From 1960 to the beginning of the 21st century, the annual average rate of change of the glacier area in China was 0.3%, and their total area shrank by 10.1% [33]. The area of glaciers decreased by 1393.97 km2 in only 10 years (2008–2018), which accounted for 3.74% of the total area of glaciers in China [34]. Based on remote sensing monitoring, 82.2% of these glaciers were found to be shrinking. The retreat size and proportion varied for each glacier, with significant regional differences [35]. In particular, the smaller glaciers were found to be more sensitive to global warming [36,37,38].

Table 1.
Retreating rates of glacier areas from western China.
3. Diversity and Community Structure of Glacial Microbiomes in China
Despite the harsh environment with low temperatures and limited nutrients, microorganisms play important roles in the glacier forelands and have a high level of diversity, including abundant bacteria, archaea, fungi, and viruses [68]. In the early stage, microbial research mainly depended on traditional microbial isolation and culture methods, which hindered our acknowledgement of cryospheric microbes. Since the 1990s, the emergence of non-culturally microbial research methods, including cloning libraries and high-throughput sequencing, has made the study of cryospheric microbiomes more comprehensive and accurate as well as accelerating the cryospheric microbial research [69]. The glaciers in the Tibetan Plateau cover an area of 47,000 km2, accounting for 80% of the total glacier area in China [70], making this main region suitable for glacial microbial research in China. The Pamir Plateau and the Tianshan Mountains are other active regions for glacial microbial research (Table 2).

Table 2.
Summary of the microbial diversity data for glaciers in China.
3.1. Bacteria and Archaea
At the end of the 19th century, Christner [72] analyzed the Guliya ice cores from the Tibetan Plateau glaciers and found that the number of bacteria in them reached 180 cells/mL. Microbial diversity research was also subsequently conducted on Malan Glacier, Urumqi Glacier No. 1 in Tianshan Mountains and Dongkemadi Glacier on the Tibetan Plateau [72,75,84]. The number and major groups of bacteria in the ice cores varied in the different glaciers. The microorganisms in the Malan ice core were mainly Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, and Fibrobacterium/Flavobacterium/Proteobacteria (CFB), with a total amount of 50–410 cells/mL, and the culturable microorganisms were 0–85 CFU/mL [73]. Tianshan Glacier No. 1 had a higher total number of microorganisms (103–105 cells/mL) compared to Malan Glacier [76]. The dominant taxa were found to be Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, CFB, high G+C% bacteria (HGC), and low G+C Gram-positive bacteria (LGC) [76,88]. The culturable microorganisms in the Muztagata ice cores from the Pamir Plateau ranged from 9 to 96 CFU/mL with main taxa of Alphaproteobacteria, Betaproteobacteria, Cryobacteria, CFB, and HGC [75]. It can be seen that although there are differences in the microorganisms in the different glacial ice cores, there are also commonalities, which may be because microorganisms in ice cores mainly originate from atmospheric deposition. The atmospheric deposition in different periods may drive the different bacterial abundances and community structures at different ice core depths. For example, the composition of the bacterial taxa varied at four depths in the Malan ice core: Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria (35 m); Betaproteobacteria (64 m); Gammaproteobacteria, CFB, Marinobacter, and agricultural soil bacterium (70 m); and Betaproteobacteria (82 m) [74]. The number of culturable bacteria in the Muztagata ice cores also varied with depth in the cores, and the variations were few and small at depths of 0–2.7, 3.0–5.5, 12.5–14.7, and 16.0–17.7 m, while the number of culturable bacteria (2 to 56 CFU/mL) was greater at depths of 8–9.2 m [75].
With the continuous development of biomolecular information technology, microbial studies of glacier surface ice and snow have gradually developed, and the bacterial taxa present have been identified, including Alphaproteobacteria, Betaproteobacteria, Actinobacteria, Bacteroidetes, Firmicutes, Flavobacterium, and Cyanobacteria [81,85]. As the important autotrophic microbes, Oscillatoriales and Chroococcales were the main orders of cyanobacteria in the glacial surface, which could be found in the cryoconites of Urumqi Glacier No.1 and Yushugou Glacier [77,78]. The CFB and Proteobacteria taxa were dominant in the snow pits of the Miaoergou glacier in the Tianshan Mountains, while Actinobacteria was the dominant bacterial group in the snow samples from ZhuoAoyou on the Tibetan Plateau [88,89]. Alphaproteobacteria was the dominant culturable bacterial group, accounting for 52%, isolated from the snow pits on Yulong Snow Mountain [87]. However, the dominant taxon in the surface snow and meltwater from Qiyi Glacier in the Qilian Mountains was Bacteroidetes [88]. This shows that the geographic location of the glacier has an impact on the bacterial community structure because of the distinctly different climatic and nutritional conditions [94,95]. In addition, the seasons also led to changes in the number of bacteria and the community’s structure; e.g., the number of bacteria in snow deposited in summer (2.3 × 104 cells/mL) was much higher than that in the snow deposited in winter (6.9 × 103 cells/mL) in Rongbuk Glacier on Mt. Qomolangma [86]. Archaea have a lower diversity in glacier forelands and have received more research attention because of their important role in biogeochemical cycles, especially that of methane [96]. Euryarchaeota, Crenarchaeota, and Thaumarchaeota are the three dominant archaea phyla in glacier forelands, and they can withstand the low temperatures and remain active, but reports of archaea in glacial ice and snow environments in China are still rare [97,98,99].
Microbial activity and diversity are closely related to climatic factors such as temperature. Environmental changes induced by glacial retreat due to global warming are the main cause of the varied distribution of microbial communities in glacial retreat areas [100]. The microbial community in the glacier retreat area is dominated by bacterial communities, and archaea only account for a very small proportion [101]. Yue et al. reported that there was no correlation between the culturable bacteria and retreat time in the Zadang glacier foreland in the Nianqing Tanggula Mountains [102], while the number of culturable bacteria in the Tanggula Dongkemadi Glacier and Tianshan Glacier No. 1 increased with increasing glacier retreat time [103,104]. Focusing on Tianshan Glacier No. 1, we found that the copy number and microbial α-diversity of the soil bacteria and archaea increased with increasing retreat age, and the relative abundance of the Bacillus phylum was found to increase, as well [105]. The abundances and community structure of the microorganisms shifted due to climate warming, and they generally exhibited positive correlations with the retreat time. However, because of the climate and environmental heterogeneity, there are some exceptions [106]. Further study is needed to clarify the underlying reasons for the specific successional changes in glacier microbiomes.
3.2. Fungi
Compared with bacteria and archaea, fungi are rare in the glacier surface and can be more easily found in the glacier forelands [107]. The dominant fungal phyla in the retreat area are Ascomycota and Basidiomycota [90,108,109]. There are also geographical differences in the community structure of glacial fungi; for example, the fungi in Himalayan glaciers include the genus Lemonniera and species Tetracladium nainitalense (Sati and P. Arya) and Thelebolus microsporus (Berk. and Broome) [69,108]; those in the Tibetan Plateau glaciers include Phoma sclerotioides (Preuss ex Sacc.), Pseudogymnoascus pannorum (Minnis and D.L. Lindner), Psychrophila antarctica (M.M. Wang and Xing Z. Liu), P. lutea (M.M. Wang and Xing Z. Liu), P. olivacea (M.M. Wang and Xing Z. Liu), Tetracladium ellipsoideum (M.M. Wang and Xing Z. Liu), T. globosum (M.M. Wang and Xing Z. Liu), and T. psychrophilum (M.M. Wang and Xing Z. Liu) [69,109]. In addition, differences in the structure of the fungal communities have been observed in different locations in the same glacier. The dominance of Basidiomycota and Ascomycota in Tianshan Glacier No. 1 is consistent with the dominant fungal taxa in the Hailuogou Glacier [91]. At the genus level, Rhodotorula and Leucosporidium dominate the glacier surface, but the unique genus Cladosporium also exists here. The genus Aureobasidium is unique to the sediment layer at the bottom of the glacier, and the dominant genera are Aspergillus and Simplicillium [110].
3.3. Viruses
Viruses are not only important drivers of microbial evolution due to their unique mode of survival and reproduction, but they also play important roles in the cycling of nutrients and organic matter in ecosystems. Because of the fragile, low biodiversity, and short biological chains in glacial ecosystems, viruses in glaciers have a more significant impact on their material cycles and microbial community survival [111]. Viruses have been found in relatively high abundances and diversity in the South and Arctic glaciers and the Greenland glaciers, and a high virus-to-bacteria ratio has been reported [112]. Analysis of their genetic information has revealed that there were 33 different viral species, including four known genera and 28 unknown genera, in the ice cores from Guria Glacier on the Tibetan Plateau. In addition, genetic prediction analysis revealed that 18 viral species were closely related to the number of multiple bacteria in the ice cores [113]. Viruses in the cryosphere may be released due to climate change, permafrost thawing, and glacier retreat. Based on an average glacier virus-to-bacteria ratio of 30 to 1, the amount of viruses released from Arctic glaciers into the downstream environment could be up to approximately 1023, which has a significant impact on downstream ecosystems [114,115]. The study of cryosphere viruses in China and around the world is still in its infancy. The impacts of these released viruses and how to cope with the possible threat under climate warming are important research directions for the future.
4. Involvement of Glacier Microbes in Carbon Cycle
According to statistics, approximately 6 Pg of organic carbon is stored in glaciers worldwide, which could be released after glacier retreat due to the increased temperature and increased microbial activity [116]. The impacts on the ecosystems and the feedback on climate change due to the increased carbon release from the glacier ecosystem must be assessed. It is estimated that 0.58 ± 0.07 Tg of dissolved organic carbon would be released from meltwater of mountain glaciers, which would account for about 56% of dissolved organic carbon loss from all glaciers in the world [116,117,118]. In addition, as glaciers melt, subglacial sediments are gradually exposed and large amounts of carbon are released in different forms, further influencing the balance of the original global carbon cycle [119].
Glacier forelands provide an ideal study area and are a spatial representative of the temporal changes in important biogeochemical processes such as the accumulation of soil organic carbon (SOC) [120,121]. Expect for external input such as supraglacial input and aerial deposition, biological activity is the major source of the nutrients in glacier forelands [120,122]. Thus, there are three distinct carbon sources in glacier forelands: the ancient organic pools originating from under the glacier, exogenous deposition such as soot particles, and the primary production of vegetation and autotrophic microorganisms [122]. Soil development requires the accumulation of SOC, but the speed of soil development is significantly affected by the climate conditions and regional geographic environment. The microbial biogenic carbon content varies significantly with successional age, ranging from 4.78 to 145.53 mg/kg in the soils exposed by the retreat of Hailuogou Glacier, which is a typical temperate glacier in China [123]. Shrubs appeared in less than 30 years in the foreland of Hailuogou Glacier [124]. Meadow vegetation began to colonize the area after the soil had been exposed for about 20 years in the forelands of Tianshan Glacier No. 1, and in more than 10 years for the Laohugou Glacier [125].
As the dominant undertaker of life activities in the fragile glacier ecosystem, microorganisms are the main contributors to the carbon cycles, and they have various metabolism and fixation capacities, which determine the dynamic storage of the carbon pool before the emergence of vegetation [8,126]. Traditionally, detection of enzyme activity and microbial culture methods have been used to study the biogeochemical processes mediated by microbial communities [127,128,129]. With the development of high-throughput sequencing and the microarray-based metabolic Geochip, a better understanding of the relationships between element biogeochemical cycles and microorganisms can be obtained from the microbial community’s composition, as well as the diversity and abundance of the functional genes [130].
Under the low temperature, high UV exposure, and unstable water conditions of the glacier ecosystem, microorganisms, including fungi, bacteria, and archaea, in glacier forelands are characterized by strong carbon metabolism abilities [131]. Zhu et al. [92] determined that the microbial community in the Laohugou Glacier can use amino acids, carbohydrates, carboxylic acids, and amines as metabolic substrates using the BiologEco microporous plate method. By detecting the soil enzymes, such as urease, sucrase, protease, polyphenol oxidase, catalase, and dehydrogenase, Wu et al. [127] found that the enzyme activity increased as the exposure time increased in the foreland of Tianshan Glacier No. 1, indicating active microbial metabolism. This is consistent with the increased microbial activity during the soil chronosequence of the foreland of Tianshan Glacier No. 1 [132]. Sanyal et al. [133] found that the microbial communities in the Himalayan region are able to utilize more diverse types of carbonaceous substrates compared to those in the Antarctic region. The effects of the heterogeneity of mountain glaciers on the responses and feedback of microbial communities must be studied further.
Nearly all microorganisms need to decompose carbon nutrients to survive; only photoautotrophs such as Cyanobacteria and chemoautotrophs and some members of Alphaproteobacteria can synthesize carbon compounds independently through utilization of inorganic carbon such as CO2 and bicarbonate [121,134,135]. Cyanobacteria are important contributors to the carbon in glacier forelands and cryconite [76,133,136]. For example, Cyanobium (Synechococcales), Trichormus, and Nostoc (Nostocales) can fix CO2 into organic carbon compounds using light energy via the Calvin cycle (CBB cycle). This typical carbon fixation pathway utilizes one molecule of CO2 and ribulose 1, 5-disphoshate to synthesize third molecules of triose phosphate [137,138]. Some members of Cyanobacteria can upregulate the carbon concentration to improve the carbon fixation ability [134]. Chemoautotrophs such as sulfate-reducing bacteria and Chlorobiales have been found in Antarctic soils [135], and they can utilize organic carbon through the reductive tricarboxylic acid cycle (rTCA cycle). This pathway converts four molecules of CO2 into one molecule of oxalacetate [137]. The reductive acetyl-CoA pathway (WL cycle) and the hydroxypropionate bicycle (3HP cycle) convert two molecules of CO2 into acetic acid or glyoxylate [136], and they have been found to occur in Antarctica based on detection of Chloroflexi, Armatimonadetes, and Nitrospirae [119,139]. Generally, the Calvin cycle is the main pathway in the surface environments of mountain glaciers, such as in the glacial runoff of Qiangyong and Satrundi glaciers on the Tibetan Plateau [140,141]. Based on bioinformation analysis, Actinobacteria, Cyanobacteria, and Proteobacteria, which are also the main microbial groups found in Chinese glaciers, are the dominant carbon sequestration microbial groups [140,142,143,144]. The mean relative abundance of Actinobacteria, Cyanobacteria, and Proteobacteria was found to be higher in Tibetan Plateau glaciers than in the South and Arctic glaciers, which implies a higher carbon fixation ability in this area [119]. The assessment of the rate of carbon sequestration is key to understanding the carbon cycle; however, few relevant studies have been conducted on the Chinese glacier ecosystem.
5. Concluding Remarks
At present, climate change has accelerated glacier ablation, accompanied by a large amount of glacier foreland exposure. With the abundant carbon storage and more intense microbial activities, not only have microbial succession and soil development received wide attention, but changes in the carbon cycle in glacier forelands have also been intensively studied, such as carbon sources [8,122], the carbon release rate [145], and the dynamic processes involving carbon [146,147].
Climate warming will have direct and indirect effects on microbial communities, including changes in the microbial community structures, the accelerated release of CO2, and the indirect impact of changes in the soil properties [148]. Based on field experiments and indoor simulation experiments, the carbon loss would be intensified as the temperature increases due to the more actively microbial activities [149,150,151]. Higher carbon fluxes have been detected in short-term warming experiments compared with long-term warming experiments [152], which is related to the lack of labile carbon [153], the low carbon utilization rate [154], and changes in the thermal adaptability of microorganisms [155,156].
Furthermore, the biogeochemical cycle in the glacier ecosystem is mediated by microorganisms, which are affected by the environmental conditions, such as nutrient limitations [157]. Jiang et al. [158] investigated the microbial processes and nutrient limitations and also found that carbon and nitrogen are the main limiting nutrients in the early succession stage in Hailuogou Glacier, followed by a shift to phosphorus in the later stages. Climate warming and the retreat time affect the soil properties and microbial community, which can result in positive or negative feedback.
As one of the indicators of climate change, glacier microbiomes and their response to climate warming can provide a better understanding of glacier ecosystems and the effects of climate warming. At present, although a series of results have been achieved regarding microbial diversity and community structure, research on Chinese glacial microbiomes still needs to be strengthened. Under the context of climate change, future sustained research, including the following topics, is required.
- 1.
- Response of glacial microbes to climate change
Microbial responses to climate change are complex. The microbial response to climate warming varies in the different types of glaciers. In particular, mountain glaciers have a higher environmental heterogeneity, so enhancing the research on these glaciers may improve our understanding of global glacier microbes. In addition, ancient viruses that lie dormant in glaciers may be gradually released with the melting of glaciers. Whether they are still infectious and pose a great threat to glacial ecosystems locally or globally is an issue that needs to be explored.
- 2.
- Evolutionary and biogeographical studies of glacial microbiomes
Many studies have found that glacial microorganisms mostly originate from atmospheric deposition, and they subsequently undergo gradual adaptation over time to become glacier-specific, but there are different opinions on this subject. Therefore, where and how glacial microbes originate, how they colonize, and their environmental and evolutionary drivers need to be studied further. The microbial diversity in the glaciers in China has both uniqueness and similarities compared to glaciers elsewhere in the world. Whether there is a certain pattern in the distribution of glacial microbes across the country or even globally must be determined. All of these issues are related to the origin and evolution of glacial microorganisms and biogeography.
- 3.
- Glacial microbes and geochemical cycles
At present, research on the mediation of the geochemical cycles by glacial microbiomes in China is in the preliminary stage. More attention is devoted to the carbon and nitrogen cycles, while other elements, such as sulfur, phosphorus, and metals, have been studied less. With the retreat of glaciers, various elements contained in the glaciers are gradually being released, and their effects on glacial microorganisms and the underlying mechanisms of these effects need to be studied further.
- 4.
- Glacial microbial diversity and microbial resources
Glacier microorganisms often have their own unique metabolic characteristics and products, such as protease producing low-temperature resistant bacteria, low-temperature amylase-producing strains, and purple pigment synthesized strains [159,160,161]. The diversity of glacier microorganisms is rapidly decreasing as a result of climate warming, which suggests that the biodiversity of the environment-specific microorganisms in glaciers will also decrease. We recognize the effects of climate change on glacier microbiomes and their feedback; however, we know far less about this issue. It is urgent to strengthen research on glacier microbiomes to conserve the biodiversity and these unique microbial resources.
Author Contributions
Conceptualization, A.M., J.Z. and G.Z.; original draft preparation, A.M. and J.Z.; review and editing, G.L. and X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Second Tibetan Plateau Scientific Expedition and Research Program (2019QZKK0402, 2019QZKK0307) and the National Key Research and Development Program of China (2018YFA0901200).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qin, D.; Yao, T.; Ding, Y.; Ren, J. Establishment and Significance of the Scientific System of Cryospheric Science. Bull. Chin. Acad. Sci. 2020, 35, 394–406. [Google Scholar] [CrossRef]
- Liu, S.; Yao, X.; Guo, W.; Xu, J.; Shangguan, D.; Wei, J.; Bao, W.; Wu, L. The Contemporary Glaciers in China Based on the Second Chinese Glacier Inventory. Acta Geogr. Sin. 2015, 70, 3–16. [Google Scholar] [CrossRef]
- Boetius, A.; Anesio, A.M.; Deming, J.W.; Mikucki, J.A.; Rapp, J.Z. Microbial Ecology of the Cryosphere: Sea Ice and Glacial Habitats. Nat. Rev. Microbiol. 2015, 13, 677–690. [Google Scholar] [CrossRef]
- Eva, G.; Cristna, C. Glaciers and Ice Sheets as Analog Environments of Potentially Habitable Icy Worlds. Front. Microbiol. 2017, 8, 1407. [Google Scholar] [CrossRef]
- Makowska, N.; Zawierucha, K.; Mokracka, J.; Koczura, R. First Report of Microorganisms of Caucasus Glaciers (Georgia). Biologia 2016, 71, 620–625. [Google Scholar] [CrossRef]
- Martinez-alonso, E.; Pena-perez, S.; Serrano, S.; Garcia-lopez, E. Taxonomic and Functional Characterization of a Microbial Community from a Volcanic Englacial Ecosystem in Deception. Sci. Rep. 2019, 9, 12158. [Google Scholar] [CrossRef]
- Margesin, R.; Collins, T. Microbial Ecology of the Cryosphere (Glacial and Permafrost Habitats): Current Knowledge. Appl. Microbiol. Biotechnol. 2019, 103, 2537–2549. [Google Scholar] [CrossRef]
- Stibal, M.; Šabacká, M.; Žárský, J. Biological Processes on Glacier and Ice Sheet Surfaces. Nat. Geosci. 2012, 5, 771–774. [Google Scholar] [CrossRef]
- Xu, H.; Wang, F.; Li, T.; Wu, X. A Review of Freezing-Thawing Cycle Effects on Key Processes of Soil Nitrogen Cycling and the Underlying Mechanisms. Acta Ecol. Sin. 2020, 40, 3168–3182. [Google Scholar] [CrossRef]
- Zarsky, J.D.; Stibal, M.; Hodson, A.; Sattler, B. Large Cryoconite Aggregates on a Svalbard Glacier Support a Diverse Microbial Community Including Ammonia-Oxidizing Archaea. Environ. Res. Lett. 2013, 8, 035044. [Google Scholar] [CrossRef]
- Ambrosini, R.; Musitelli, F.; Navarra, F.; Tagliaferri, I.; Gandolfi, I.; Bestetti, G.; Mayer, C.; Minora, U.; Azzoni, R.S.; Diolaiuti, G.; et al. Diversity and Assembling Processes of Bacterial Communities in Cryoconite Holes of a Karakoram Glacier. Environ. Microbiol. 2017, 73, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Vick-majors, T.J.; Priscu, J.C.; Yao, T.; Kang, S.; Liu, K.; Cong, Z.; Xiong, J. Biogeography of Cryoconite Bacterial Communities on Glaciers of the Tibetan Plateau. FEMS Microbiol. Ecol. 2017, 93, 10. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.; Mccutcheon, J.; Mcquaid, J.B.; Benning, L.G. The Diversity of Ice Algal Communities on the Greenland Ice Sheet as Revealed by Oligotyping. Microb. Genomics 2018, 4, e000159. [Google Scholar] [CrossRef] [PubMed]
- Kadow, C.; Hall, D.M.; Ulbrich, U. Artificial Intelligence Reconstructs Missing Climate Information. Nat. Geosci. 2020, 13, 408–413. [Google Scholar] [CrossRef]
- Arias, P.A.; Bellouin, N.; Coppola, E.; Jones, R.G.; Krinner, G.; Marotzke, J.; Naik, V.; Palmer, M.D.; Plattner, G.-K.; Rogelj, J.; et al. Technical Summary. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 42–44. [Google Scholar]
- Steffen, W.; Rockström, J.; Richardson, K.; Lenton, T.M.; Folke, C.; Liverman, D.; Summerhayes, C.P.; Barnosky, A.D.; Cornell, S.E.; Crucifix, M.; et al. Trajectories of the Earth System in the Anthropocene. Proc. Natl. Acad. Sci. USA 2018, 115, 8252–8259. [Google Scholar] [CrossRef] [PubMed]
- Veettil, B.K.; Kamp, U. Global Disappearance of Tropical Mountain Glaciers: Observations, Causes, and Challenges. Geosciences 2019, 9, 196. [Google Scholar] [CrossRef]
- Yao, T.; Xue, Y.; Chen, D.; Chen, F.; Thompson, L.; Cui, P.; Koike, T.; Lau, W.K.-M.; Lettenmaier, D.; Mosbrugger, V.; et al. Recent Third Pole’s Rapid Warming Accompanies Cryospheric Melt and Water Cycle Intensification and Interactions between Monsoon and Environment: Multidisciplinary Approach with Observation, Modeling and Analysis. Bull. Am. Meteorol. Soc. 2019, 100, 423–444. [Google Scholar] [CrossRef]
- Zemp, M.; Huss, M.; Thibert, E.; Eckert, N.; McNabb, R.; Huber, J.; Barandun, M.; Machguth, H.; Nussbaumer, S.U.; Gärtner-Roer, I.; et al. Global Glacier Mass Changes and Their Contributions to Sea-Level Rise from 1961 to 2016. Nature 2019, 568, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, S.; Xu, J.; Wu, L.; Shangguan, D.; Yao, X.; Wei, J.; Bao, W.; Yu, P.; Liu, Q.; et al. The Second Chinese Glacier Inventory: Data, Methods and Results. J. Glaciol. 2015, 61, 357–372. [Google Scholar] [CrossRef]
- Wang, Z.; Su, H. Glaciers in the World and China: Distribution and Their Significance as Water Resources. J. Glaciol. Geocryol. 2003, 25, 498–503. [Google Scholar] [CrossRef]
- Rime, T.; Hartmann, M.; Brunner, I.; Widmer, F.; Zeyer, J.; Frey, B. Vertical Distribution of the Soil Microbiota along a Successional Gradient in a Glacier Forefield. Mol. Ecol. 2015, 24, 1091–1108. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Rime, T.; Phillips, M.; Stierli, B.; Hajdas, I.; Widmer, F.; Hartmann, M. Microbial Diversity in European Alpine Permafrost and Active Layers. FEMS Microbiol. Ecol. 2016, 92, fiw018. [Google Scholar] [CrossRef] [PubMed]
- Botnen, S.; Mundra, S.; Kauserud, H.; Eidesen, P. Glacier Retreat in the High Arctic: Opportunity or Threat for Ectomycorrhizal Diversity? FEMS Microbiol. Ecol. 2020, 96, fiaa171. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zheng, B.; Yao, T. Glaciers and Environments during the Last Glacial Maximum (LGM) on the Tibetan Plateau. J. Glaciol. Geocryol. 1997, 19, 97–113. [Google Scholar]
- Shi, Y.; Ren, B.; Xie, Z. Progress of Glaciology Research in China in the Past 30 Years. J. Glaciol. Geocryol. 1979, 2, 1–6. [Google Scholar]
- Huang, M.; Shi, Y. Progress in the Study on Basic Features of Glaciers in China in the Last Thirty Years. J. Glaciol. Geocryol. 1988, 10, 228–237. [Google Scholar]
- Shen, Y.; Chang, X.; Li, Y.; Wang, C. The Special of Chinese Glaciers. For. Humankind 2018, 12, 192–207. [Google Scholar]
- Zemp, M.; Haeberli, W.; Hoelzle, M.; Paul, F. Alpine Glaciers to Disappear within Decades? Geophys. Res. Lett. 2006, 33, L13504. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Pu, T.; Jia, W.; He, X.; Pang, H.; Zhang, N.; Liu, Q.; Qiao, L.; Wang, S.; et al. Changes of Climate, Glaciers and Runoff in China’s Monsoonal Temperate Glacier Region during the Last Several Decades. Quat. Int. 2010, 218, 13–28. [Google Scholar] [CrossRef]
- Xu, X.; Pan, B.; Hu, E.; Li, Y.; Liang, Y. Responses of Two Branches of Glacier No. 1 to Climate Change from 1993 to 2005, Tianshan, China. Quat. Int. 2011, 236, 143–150. [Google Scholar] [CrossRef]
- Sun, M.; Li, Z.; Yao, X.; Jin, S. Rapid Shrinkage and Hydrological Response of a Typical Continental Glacier in the Arid Region of Northwest China—Taking Urumqi Glacier No. 1 as an Example. Ecohydrology 2013, 6, 909–916. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Li, Z.; Wang, F. Variation of Glacier Area in China against the Warming in the Past 50 Years. Acta Geogr. Sin. 2011, 66, 1155–1165. [Google Scholar]
- Zhao, H.; Wang, X.; Zhao, X.; Guo, W.; Liu, S.; Wei, J.; Zhang, Y. Analysis of Glacier Changes in China from 2008 to 2018. J. Glaciol. Geocryol. 2021, 43, 976–986. [Google Scholar] [CrossRef]
- Duan, J.; Wang, L.; Ren, J.; Li, L. Progress in Glacier Variations in China and Its Sensitivity to Climatic Change during the Past Century. Prog. Geogr. 2009, 28, 231–237. [Google Scholar] [CrossRef]
- Feng, T.; Liu, S.; Xu, J.; Guo, W.; Wei, J.; Zhang, Z. Glacier Change of the Yarkant River Basin from 1968 to 2009 Derived from the First and Second Glacier Inventories of China. J. Glaciol. Geocryol. 2015, 37, 1–13. [Google Scholar] [CrossRef]
- Xie, Z.C.; Wang, X.; Feng, Q.H.; Kang, E.; Liu, C.H.; Li, Q.Y. Modeling the Response of Glacier Systems to Climate Warming_Taking Glaciers in China as an Example. Res. Soil Water Conserv. 2005, 12, 313–316. [Google Scholar] [CrossRef][Green Version]
- Su, Z.; Shi, Y. Response of Monsoonal Temperate Glaciers in China to Global Warming since the Little Ice Age. J. Glaciol. Geocryol. 2000, 22, 223–229. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Luo, S.; Bai, J.; Huai, B.; Wang, F.; Li, H.; Wang, W.; Wang, L. Five Decades of Changes in the Glaciers on the Friendship Peak in the Altai Mountains, China: Changes in Area and Ice Surface Elevation. Cold Reg. Sci. Technol. 2015, 116, 24–31. [Google Scholar] [CrossRef]
- Huai, B.; Li, Z.; Wang, F.; Wang, P. Variation of Glaciers in the Sawuer Mountain within Chinese Territory during 1959–2013. J Glaciol. Geocryol. 2015, 37, 1141–1149. [Google Scholar] [CrossRef]
- Zhang, C.; Yao, X.; Liu, S.; Zhang, D.; Xu, J. Variation of Glacier Length in the Altun Mountains during 1970—2016. J Glaciol. Geocryol. 2021, 43, 49–60. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, J.; Wang, J.; Yang, L.; Zhang, S. Remote Sensing Analysis of Glacier Dynamic Changes in Parlung Zangbo River. Sci. Technol. Eng. 2019, 19, 56–62. [Google Scholar] [CrossRef]
- Liu, J.; Yao, X.; Liu, S.; Guo, W.; Xu, J. Glacier Changes in the Gangdisê Mountains from 1970 to 2016. Acta Geogr. Sin. 2019, 74, 1333–1344. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, N.; Li, X.; Liu, K. Glacier Changes and Its Response to Climate Change in the Gilgit River Basin, Western Karakorum Mountains over the Past 20 Years. Mt. Res. 2019, 37, 347–358. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Liu, S.; Guo, W.; Wei, J.; Feng, T. Glacier Changes since the Early 1960s, Eastern Pamir, China. J. Mt. Sci. 2016, 13, 276–291. [Google Scholar] [CrossRef]
- Jia, B.; Hou, S.; Wang, Y. Variation of Glaciers at Zangser Kangri on the Qiangtang Plateau during 1971–2015. J. Glaciol. Geocryol. 2020, 42, 307–317. [Google Scholar] [CrossRef]
- Sun, M.; Liu, S.; Yao, X.; Guo, W.; Xu, J. Glacier Changes in the Qilian Mountains in the Past Half Century: Based on the Revised First and Second Chinese Glacier Inventory. Acta Geogr. Sin. 2015, 70, 1402–1414. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wu, L.; Guo, L.; Li, J. Using Remote Sensing Images to Monitor the Glacier Changes in Qilian Mountains during 1987–2018 and Analyzing the Impact Factors. J. Glaciol. Geocryol. 2020, 42, 344–356. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Gao, W. Rapid Shrinking of Glaciers in the Middle Qilian Mountain Region of Northwest China during the Last ~50 Years. J. Earth Sci. 2011, 22, 539–548. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Yu, G.; Li, H.; Wang, W.; Huai, B.; Zhou, P.; Jin, S.; Wang, L.; Zhang, H. Glacier Shrinkage in the Daxue and Danghenan Ranges of the Western Qilian Mountains, China, from 1957 to 2010. Environ. Earth Sci. 2016, 75, 127. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, W.; Teng, F.; Gu, S. Effects of Changing Climate on Glacier Shrinka Age and River Flow in the Upper Heihe River Basin, China. J. Coast. Res. 2014, 68, 121–128. [Google Scholar] [CrossRef]
- Chen, H.; Li, Z.Q.; Wang, P.Y.; Lai, Z.P.; Chen, R.S.; Huai, B.J. Five Decades of Glacier Changes in the Hulugou Basin of Central Qilian Mountains, Northwest China. J. Arid Land 2015, 7, 159–165. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, X.; Chen, J.; Li, Z.; Wang, J.; Du, W.; Guo, W. Variations of Laohugou Glacier No. 12 in the Western Qilian Mountains, China, from 1957 to 2015. J. Mt. Sci. 2018, 15, 25–32. [Google Scholar] [CrossRef]
- Ou, J.; Xu, L.; Pu, T. Glacier Change and Its Response to Climate Change in the Que’er Mountains, 1987—2016. J. Glaciol. Geocryol. 2021, 43, 36–48. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Li, Z.; Zhang, M. Glacier Changes in the Sawuer Mountain during 1977-2017 and Their Response to Climate Change. J. Nat. Resour. 2019, 34, 802–814. [Google Scholar] [CrossRef]
- Xing, W.; Li, Z.; Zhang, H.; Zhang, M.; Liang, P.; Mu, J. Spatial-Temporal Variation of Glacier Resources in Chinese Tianshan Mountains since 1959. Acta Geogr. Sin. 2017, 72, 1594–1605. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Z.; Xu, C.; Huai, B. Glacier Change of Western China since the Little Ice Age: A Case of the Urumqi River Watershed. Arid L Geogr. 2016, 39, 486–494. [Google Scholar] [CrossRef]
- Wang, W.; Li, K.; Gao, J. Monitoring Glacial Shrinkage Using Remote Sensing and Site-Observation Method on Southern Slope of Kalik Mountain, Eastern Tian Shan, China. J. Earth Sci. 2011, 22, 503–514. [Google Scholar] [CrossRef]
- Huang, X.; Bao, A.; Guo, H.; Meng, F.; Zhang, P. Change of Typical Glaciers and Its Response to Meteorological Factors in the Eastern Tianshan Mountains in China in Recent 20 Years. Arid Zone Res. 2017, 34, 870–880. [Google Scholar] [CrossRef]
- Zhao, J.; Mansur, S.; Mailikai, A.; Nijiate, Y. Changing Rates of Glacier in Tomur National Nature Reserve from 1992 to 2017 ZHAO. Arid Zone Res. 2020, 37, 1079–1086. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Li, Z.; Wang, P.; Xu, C.; Liu, S.; Jin, S.; Zhang, Z.; Xu, L. Research on the Changes of the Urumqi Glacier No. 1, Tianshan Mountains Based on Multi-Source Remote Sensing Data. J. Glaciol. Geocryol. 2021, 43, 1018–1026. [Google Scholar] [CrossRef]
- Ji, Q.; Liu, R.; Yang, T. Glacier Variations in the Himalayas during 1990–2015 JI. Geogr. Res. 2020, 39, 2403–2414. [Google Scholar] [CrossRef]
- Wu, K.; Liu, S.; Guo, W. Glacier Variation and Its Response to Climate Change in the Mount Namjagbarwa from 1980 to 2015. J. Glaciol. Geocryol. 2020, 42, 1115–1125. [Google Scholar] [CrossRef]
- Wang, J.; Yang, T.; Ji, Q.; Hu, F. Change of the Modern Glaciers in the Eastern Himalaya near China and Bhutan Border Area from 1990 to 2015. Arid L Geogr. 2019, 42, 542–550. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, N.; Chen, A.; Liu, K. Monitoring Variation of Glaciers Based on Remote Sensing Images in the Chenab Basin, Western-Himalaya, 1993~2016. Remote Sens. Technol. Appl. 2020, 35, 712–722. [Google Scholar] [CrossRef]
- Lhakpa, D.; Yu, X. Study on the Variation of Chema Yongdrung Glacier in Tibet Using Remote Sensing during 1976–2019. Plateau Sci. Res. 2020, 4, 17–29, 54. [Google Scholar] [CrossRef]
- Li, C.; Jing, Z.; He, X. Remote Sensing Monitoring of Glacier Variation in Geladandong, Source Regions of the Yangtze River from 1986 to 2015. J. Glaciol. Geocryol. 2021, 43, 405–416. [Google Scholar] [CrossRef]
- Anesio, A.M.; Lutz, S.; Chrismas, N.A.M.; Benning, L.G. The Microbiome of Glaciers and Ice Sheets. NPJ Biofilms Microbiomes 2017, 3, 10. [Google Scholar] [CrossRef]
- Li, S.; Chen, T.; Zhang, W.; Liu, G. Cryomicrobiology: Retrospect and Prospect. J. Glaciol. Geocryol. 2019, 41, 1221–1234. [Google Scholar] [CrossRef]
- Yao, T.; Qin, D.; Shen, Y.; Zhao, L.; Wang, N.; Lu, A. Cryospheric Changes and Their Impacts on Regional Water Cycle and Ecological Conditions in the Qinghai-Tibetan Plateau. Chin. J. Nat. 2013, 35, 179–186. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.K.; Si, J.; Wu, G.J.; De Tian, L.; Xiang, S.R. Changes of the Bacterial Abundance and Communities in Shallow Ice Cores from Dunde and Muztagata Glaciers, Western China. Front. Microbiol. 2016, 7, 1716. [Google Scholar] [CrossRef]
- Christner, B.C.; Mosley-thompson, E.; Thompson, L.G.; Zagorodnov, V.; Sandman, K.; Reeve, J.N. Recovery and Identification of Viable Bacteria Immured in Glacial Ice. Icarus 2000, 144, 479–485. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, T.; Ma, X.; Wang, N. Malan Glacier: Analysis of Microbial Signatures in a Deep Ice Core. Sci. China (Ser. D) 2001, 31, 295–299. [Google Scholar]
- Xiang, S.; Yao, T.; An, L.; Li, Z.; Wu, G.; Wang, Y.; Xu, B.; Wang, J. The Relationship between the Structural Changes of Malan Ice Core Bacteria and Climatic Environment. Chin. Sci. Bull. 2004, 49, 1762–1769. [Google Scholar] [CrossRef]
- Xiang, S.; Yao, T.; An, L.; Wu, G.; Xu, B.; Ma, X.; Li, Z.; Wang, J.; Yu, W. The Quantity Distribution of the Culturable Bacteria and the Change of the Main Bacterial Community Structure with Depth in The Muztagata Ice Core. Sci. China (Ser. D) 2005, 35, 252–262. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, X.; An, L.; Yao, T.; Li, Z.; Wang, F.; Xu, S. Study on the Diversity of Ice Core Bacteria in Tianshan Mountain. J. Glaciol. Geocryol. 2008, 3, 1033–1040. [Google Scholar]
- Ni, X.; Qi, X.; Gu, Y.; Zheng, X.; Dong, J.; Ni, Y.; Cheng, G. Community Structure and Phylogenetic Analysis of Cyanobacteria in Cryoconite from Surface of the Glacier No.1 in the Tianshan Mountains. Acta Microbiol. Sin. 2014, 54, 1256–1266. [Google Scholar]
- Xu, H.; Li, Z.; Takeuchi, N.; Zhang, X.; Luo, S. Characteristics and Formation Analysis of Cryoconite Granules of Yushugou Glacier. Arid L Geogr. 2014, 37, 429–438. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, T.; Jiao, N.; Kang, S.; Huang, S.; Li, Q.; Wang, K.; Liu, X. Culturable Bacteria in Glacial Meltwater at 6350 m on the East Rongbuk Glacier, Mount Everest. Extremophiles 2009, 13, 89–99. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, Y.; Hu, Y.; Cai, J.; Liu, X.; Bai, C.; Tang, X.; Zhang, Y.; Jang, K.S.; Spencer, R.G.M.; et al. Microbial Production and Consumption of Dissolved Organic Matter in Glacial Ecosystems on the Tibetan Plateau. Water Res. 2019, 160, 18–28. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Sawut, G. Culturable Bacterial Diversity in Snow, Ice and Meltwater of the Yangbark Glacier, Muztag Ata. J. Glaciol. Geocryol. 2015, 37, 1634–1641. [Google Scholar] [CrossRef]
- Segawa, T.; Takeuchi, N. Cyanobacterial Communities on Qiyi Glacier, Qilian Shan, China. Ann. Glaciol 2010, 51, 135–144. [Google Scholar] [CrossRef]
- Xie, J.; Wang, N.; Chen, L.; Li, Q.; He, J.; Jiang, X.; Wu, X. Bacterial Diversity Recovered from Qiyi Glacier and Runoff, Qilian Mts. Chin. J. Environ. Sci. 2009, 30, 2735–2740. [Google Scholar]
- Liu, Y.; Yao, T.; Jiao, N.; Tian, L.; Hu, A.; Yu, W.; Li, S. Microbial Diversity in the Snow, a Moraine Lake and a Stream in Himalayan Glacier. Extremophiles 2011, 15, 411–421. [Google Scholar] [CrossRef]
- Xie, J.; Wang, N.; Pu, J.; Chen, L. Study of the Bacterial Diversity Recovered from Glacial Snow of the Northern Tibetan Plateau. J. Glaciol. Geocryol. 2009, 31, 342–349. [Google Scholar]
- Liu, Y.; Yao, T.; Kang, S.; Jiao, N.; Zeng, Y.; Shi, Y.; Luo, T.; Jing, Z.; Huang, S. Microbial Community of Ice and Snow and Its Seasonal Variation in DongRongbu Glacier in Qomolangma Region. Chin. Sci. Bull. 2006, 51, 1287–1296. [Google Scholar] [CrossRef]
- Ma, X.; Liu, W.; Hou, S.; Chen, T.; Qin, D. Bacterial Diversity and Community at Yulong Mountains and Their Relationship to Climatic and Environmental Changes. J. Lanzhou Univ. Nat. Sci. 2009, 45, 94–100. [Google Scholar] [CrossRef]
- Liu, W.; Ma, X.; Hou, S.; Chen, T.; Qin, D. Study on Microbial Diversity and Community in Miaoergou Snow of East Tianshan Mountains and Their Relation to Climatic and Environmental Changes. Acta Microbiol. Sin. 2007, 47, 1019–1026. [Google Scholar] [CrossRef]
- Tong, X.; Chen, F.; Yu, J.; Hua, S.; Ciren, S.; Jiangbai, L.; Wang, W.; Liang, Y.; Zheng, X.; Wang, J. Analysis of Bacterial Flora Structure and Diversity in ZhuoAoyou Peak (8201 m) Snow Cover. Chin. Sci. Bull. 2008, 53, 2216–2222. [Google Scholar] [CrossRef]
- Yang, G.L.; Hou, S.G.; Le Baoge, R.; Li, Z.G.; Xu, H.; Liu, Y.P.; Du, W.T.; Liu, Y.Q. Differences in Bacterial Diversity and Communities between Glacial Snow and Glacial Soil on the Chongce Ice Cap, West Kunlun Mountains. Sci. Rep. 2016, 6, 36548. [Google Scholar] [CrossRef]
- Sun, H.; Wu, Y.; Zhou, J.; Bing, H. Variations of Bacterial and d Fungal Communities along a Primary Successional Chronosequence in the Hailuogou Glacier Retreat Area (Gongga Mountain, SW China). J. Mt. Sci. 2016, 13, 1621–1631. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Chen, H.; Wang, Y.; Cao, F.; Sun, W.; Qi, X.; Zhao, Y. Soil Properties and Microbial Diversity at the Frontier of Laohugou Glacier Retreat in Qilian Mountains. Curr. Microbiol. 2020, 77, 425–433. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Sun, W.; Ji, X.; Wei, Y. Diversity of Culturable Low-Temperature Bacteria in Vertical Climate Zones of Mingyong Glacier. Genomics Appl. Biol. 2019, 38, 2070–2077. [Google Scholar] [CrossRef]
- Donhauser, J.; Frey, B. Alpine Soil Microbial Ecology in a Changing World. FEMS Microbiol. Ecol. 2018, 94, 1–31. [Google Scholar] [CrossRef]
- Hultman, J.; Waldrop, M.P.; Mackelprang, R.; David, M.M.; McFarland, J.; Blazewicz, S.J.; Harden, J.; Turetsky, M.R.; McGuire, A.D.; Shah, M.B.; et al. Multi-Omics of Permafrost, Active Layer and Thermokarst Bog Soil Microbiomes. Nature 2015, 521, 208–212. [Google Scholar] [CrossRef]
- Nauer, P.A.; Dam, B.; Liesack, W.; Zeyer, J.; Schroth, M.H. Activity and Diversity of Methane-Oxidizing Bacteria in Glacier Forefields on Siliceous and Calcareous Bedrock. Biogeosciences 2012, 9, 2259–2274. [Google Scholar] [CrossRef]
- Boyd, E.S.; Skidmore, M.; Mitchell, A.C.; Bakermans, C.; Peters, J.W. Methanogenesis in Subglacial Sediments. Environ. Microbiol. Rep. 2010, 2, 685–692. [Google Scholar] [CrossRef]
- Lopatina, A.; Medvedeva, S.; Shmakov, S.; Logacheva, M.D.; Krylenkov, V.; Severinov, K. Metagenomic Analysis of Bacterial Communities of Antarctic Surface Snow. Front. Microbiol. 2016, 7, 398. [Google Scholar] [CrossRef]
- Christner, B.C.; Priscu, J.C.; Achberger, A.M.; Barbante, C.; Carter, S.P.; Christianson, K.; Michaud, A.B.; Mikucki, J.A.; Mitchell, A.C.; Skidmore, M.L.; et al. A Microbial Ecosystem beneath the West Antarctic Ice Sheet. Nature 2014, 512, 310–313. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; Liu, G.; An, L.; Chen, T.; Li, Z. Distribution of Aerobic Heterotrophic Bacteria Managed by Environmental Factors in Glacier Foreland. J. Glaciol. Geocryol. 2012, 34, 965–971. [Google Scholar]
- Rime, T.; Hartmann, M.; Frey, B. Potential Sources of Microbial Colonizers in an Initial Soil Ecosystem after Retreat of an Alpine Glacier. ISME J. 2016, 10, 1625–1641. [Google Scholar] [CrossRef]
- Yue, J.; Liu, G.; Zhang, G.; Zhang, W.; Xu, S. Changes in Soil Properties and Culturable Bacteria Diversity in Zhadang Glacier Foreland. J. Glaciol. Geocryol. 2010, 32, 1180–1185. [Google Scholar]
- Liu, G.; Hu, P.; Zhang, W.; Wu, X. Variations in Soil Culturable Bacteria Communities and Biochemical Characteristics in the Dongkemadi Glacier Forefield along a Chronosequence. Folia Microbiol. (Praha) 2012, 57, 485–494. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Liu, G.; Yang, X.; Hu, P.; Chen, T.; Zhang, G.; Li, Z. Bacterial Diversity in the Foreland of the Tianshan No. 1 Glacier, China. Environ. Res. Lett. 2012, 7, 014038. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, A.; Zhong, G.; Xie, F.; Zhou, H.; Liu, G.; Zhuang, G. Effect of Simulated Warming on Microbial Community in Glacier Forefield. Environ. Sci. 2020, 41, 2918–2923. [Google Scholar] [CrossRef]
- Wu, X.; Mao, W.; Tai, X.; Zhang, W.; Liu, G.; Chen, T.; Long, H.; Zhang, B.; Chen, N. Progress in Studies of Microbial Ecology in Glacier Foreland. J. Glaciol. Geocryol. 2013, 35, 217–223. [Google Scholar] [CrossRef]
- Tian, J.; Qiao, Y.; Wu, B.; Chen, H.; Li, W.; Jiang, N.; Zhang, X.; Liu, X. Ecological Succession Pattern of Fungal Community in Soil along a Retreating Glacier. Front. Microbiol. 2017, 8, 1028. [Google Scholar] [CrossRef]
- Hassan, N.; Rafiq, M.; Hayat, M.; Aamer, A.; Fariha, H. Psychrophilic and Psychrotrophic Fungi: A Comprehensive Review. Rev. Environ. Sci. Biotechnol. 2016, 15, 147–172. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, X.; Wu, W.; Hao, Y.; Cai, L.; Xiang, M.; Liu, X. Psychrophilic Fungi from the World’s Roof. Persoonia Mol. Phylogeny Evol. Fungi 2015, 34, 100–102. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Y.; Ni, X.; Guan, B.; Ni, Y. Composition and Phylogeny of Fungal Community in Supraglacial Cryoconite and Subglacial Sediments of the Glacier No.1 at Headwaters of the Urumqi River in Tianshan Mountains. J. Glaciol. Geocryol. 2017, 39, 781–791. [Google Scholar] [CrossRef]
- Zang, L.; Liu, Y.; Liu, X. Advance in Researches of Virus in Cryoconite on Glacier Surface. J. Glaciol. Geocryol. 2019, 41, 1–9. [Google Scholar]
- Chen, T.; Zhang, W.; Liu, G.; Li, S. Microbes in Cryosphere: Opportunities and Challenges. Bull. Chin. Acad. Sci. 2020, 35, 434–442. [Google Scholar] [CrossRef]
- Zhong, Z.-P.; Solonenko, N.E.; Li, Y.-F.; Gazitúa, M.C.; Roux, S.; Davis, M.E.; Van Etten, J.L.; Mosley-Thompson, E.; Rich, V.I.; Sullivan, M.B.; et al. Glacier Ice Archives Fifteen-Thousand-Year-Old Viruses. bioRxiv 2020. [Google Scholar] [CrossRef]
- Anesio, A.; Mindl, B.; Laybourn-Parry, J.; Hodson, A.; Sattler, B. Viral Dynamics in Cryoconite Holes on a High Arctic Glacier (Svalbard). J. Geophys. Res. Biogeosci. 2007, 112, G04S31. [Google Scholar] [CrossRef]
- Irvine-Fynn, T.D.L.; Edwards, A. A Frozen Asset: The Potential of Flow Cytometry in Constraining the Glacial Biome. Cytom. Part A J. Int. Soc. Anal. Cytol. 2014, 85, 3–7. [Google Scholar] [CrossRef]
- Hood, E.; Battin, T.J.; Fellman, J.; O’neel, S.; Spencer, R.G.M. Storage and Release of Organic Carbon from Glaciers and Ice Sheets. Nat. Geosci. 2015, 8, 91–96. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, A.; Liu, G.; Ma, J.; Wei, J.; Zhou, H.; Brandt, K.K.; Zhuang, G. Potential Feedback Mediated by Soil Microbiome Response to Warming in a Glacier Forefield. Glob. Chang. Biol. 2020, 26, 697–708. [Google Scholar] [CrossRef]
- Treat, C.C.; Natali, S.M.; Ernakovich, J.; Iversen, C.M.; Lupascu, M.; Mcguire, A.D.; Norby, R.J.; Roy Chowdhury, T.; Richter, A.; Šantrůčková, H.; et al. A Pan-Arctic Synthesis of CH4 and CO2 Production from Anoxic Soil Incubations. Glob. Chang. Biol. 2015, 21, 2787–2803. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Liu, Y. Research Progress of Carbon Fixation Microorganisms in Glaciers. Acta Microbiol. Sin. 2020, 60, 2012–2029. [Google Scholar] [CrossRef]
- Bradley, J.A.; Singarayer, J.S.; Anesio, A.M. Microbial Community Dynamics in the Forefield of Glaciers. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140882. [Google Scholar] [CrossRef]
- Wietrzyk-Pełka, P.; Rola, K.; Szymański, W.; Węgrzyn, M.H. Organic Carbon Accumulation in the Glacier Forelands with Regard to Variability of Environmental Conditions in Different Ecogenesis Stages of High Arctic Ecosystems. Sci. Total Environ. 2020, 717, 135151. [Google Scholar] [CrossRef]
- Schulz, S.; Brankatschk, R.; Dümig, A.; Kögel-Knabner, I.; Schloter, M.; Zeyer, J. The Role of Microorganisms at Different Stages of Ecosystem Development for Soil Formation. Biogeosciences 2013, 10, 3983–3996. [Google Scholar] [CrossRef]
- Li, W.; Yang, G.; Hu, J.; Zhu, Q.; Chen, H.; Peng, C.; Zhu, D.; Gao, Y. Soil Microbial Biomass Carbon and Nitrogen along the Glacial Retreat Sites of the Hailuogou Glacier. Chin. J. Appl. Environ. Biol. 2015, 21, 512–516. [Google Scholar] [CrossRef]
- Lei, Y.; Zhou, J.; Xiao, H.; Duan, B.; Wu, Y.; Korpelainen, H.; Li, C. Soil Nematode Assemblages as Bioindicators of Primary Succession along a 120-Year-Old Chronosequence on the Hailuogou Glacier Forefield, SW China. Soil Biol. Biochem. 2015, 88, 362–371. [Google Scholar] [CrossRef]
- Liu, G.; Li, S.; We, X.; Zhang, B.; Zhang, B.; Long, H.; Tai, X.; Li, Z. Studies on Rule and Mechanism of the Succession of Plant Community in the Retreat Forefield of the Tianshan Mountain Glacier No.1 at the Headwater of Urumqi River. J. Glaciol. Geocryol. 2012, 34, 1134–1141. [Google Scholar]
- Bardgett, R.D.; Richter, A.; Bol, R.; Garnett, M.H.; Bäumler, R.; Xu, X.; Lopez-capel, E.; Manning, D.A.C.; Hobbs, P.J.; Hartley, I.R.; et al. Heterotrophic Microbial Communities Use Ancient Carbon Following Glacial Retreat Heterotrophic Microbial Communities Use Ancient Carbon Following Glacial Retreat. Biol. Lett. 2007, 3, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, G.; Zhang, W.; Liu, G.; Chen, T. Variations in Culturable Bacterial Communities and Biochemical Properties in the Foreland of the Retreating Tianshan No. 1 Glacier. Braz. J. Microbiol. 2018, 49, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, A.; Bernasconi, S.M.; Zeyer, J.; Frey, B. Microbial Community and Activity Shifts after Soil Transplantation in a Glacier Forefield. Appl. Geochem. 2011, 26, S326–S329. [Google Scholar] [CrossRef]
- Hahn, A.S.; Quideau, S.A. Shifts in Soil Microbial Community Biomass and Resource Utilization along a Canadian Glacier Chronosequence. Can. J. Soil Sci. 2013, 93, 305–318. [Google Scholar] [CrossRef]
- Zheng, B.; Zhu, Y.; Sardans, J.; Peñuelas, J.; Su, J. QMEC:A Tool for High-Throughput Quantitative Assessment of Microbial Functional Potential in C, N, P and S Biogeochemical Cycling. Sci. China Life Sci. 2018, 61, 1451–1462. [Google Scholar] [CrossRef]
- Esperschütz, J.; Pérez-De-Mora, A.; Schreiner, K.; Welzl, G.; Buegger, F.; Zeyer, J.; Hagedorn, F.; Munch, J.C.; Schloter, M. Microbial Food Web Dynamics along a Soil Chronosequence of a Glacier Forefield. Biogeosciences 2011, 8, 3283–3294. [Google Scholar] [CrossRef]
- Sigler, W.V.; Zeyer, J. Microbial Diversity and Activity along the Forefields of Two Receding Glaciers. Microb Ecol. 2002, 43, 397–407. [Google Scholar] [CrossRef]
- Sanyal, A.; Antony, R.; Samui, G.; Thamban, M. Microbial Communities and Their Potential for Degradation of Dissolved Organic Carbon in Cryoconite Hole Environments of Himalaya and Antarctica. Microbiol. Res. 2018, 208, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Price, G.D. Inorganic Carbon Transporters of the Cyanobacterial CO2 Concentrating Mechanism. Photosynth. Res. 2011, 109, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R. Microbial Ecology of Antarctic Aquatic Systems. Nat. Rev. Microbiol. 2015, 13, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Menendez, C.; Bauer, Z.; Huber, H.; GAD’On, N.; Stetter, K.-O.; Fuchs, G. Presence of Acetyl Coenzyme A (CoA) Carboxylase and Propionyl-CoA Carboxylase in Autotrophic Crenarchaeota and Indication for Operation of a 3-Hydroxypropionate Cycle in Autotrophic Carbon Fixation. J. Bacteriol. 1999, 181, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.C.W.; Buchanan, B.B.; Arnon, D.I. A New Ferredoxin-Dependent Carbon Reduction Cycle in a Photosynthetic Bacterium. Proc. Natl. Acad. Sci. USA 1966, 55, 928–934. [Google Scholar] [CrossRef]
- Li, Y.; Cha, Q.; Dang, Y.; Chen, X.; Wang, M.; Mcminn, A.; Espina, G.; Zhang, Y.; Blamey, J.M.; Qin, Q. Reconstruction of the Functional Ecosystem in the High Light, Low Temperature Union Glacier Region, Antarctica. Front. Microbiol. 2019, 10, 2408. [Google Scholar] [CrossRef]
- Angeles Lezcano, M.; Moreno-Paz, M.; Carrizo, D.; Prieto-Ballesteros, O.; Angel Fernandez-Martinez, M.; Sanchez-Garcia, L.; Blanco, Y.; Puente-Sanchez, F.; de Diego-Castilla, G.; Garcia-Villadangos, M.; et al. Biomarker Profiling of Microbial Mats in the Geothermal Band of Cerro Caliente, Deception Island (Antarctica): Life at the Edge of Heat and Cold. Astrobiology 2019, 19, 1490–1504. [Google Scholar] [CrossRef]
- Kong, W.; Liu, J.; Ji, M.; Yue, L.; Kang, S.; Morgan-kiss, R.M. Autotrophic Microbial Community Succession from Glacier Terminus to Downstream Waters on the Tibetan Plateau. FEMS Microbiol. Ecol. 2019, 95, fiz074. [Google Scholar] [CrossRef]
- Kumar, V.; Thakur, V.; Ambika; Kumar, V.; Kumar, R.; Singh, D. Genomic Insights Revealed Physiological Diversity and Industrial Potential for Glaciimonas Sp. PCH181 Isolated from Satrundi Glacier in Pangi-Chamba Himalaya. Genomics 2020, 112, 637–646. [Google Scholar] [CrossRef]
- Cameron, K.; Hodson, A.; Osborn, A. Carbon and Nitrogen Biogeochemical Cycling Potentials of Supraglacial Cryoconite Communities. Polar Biol. 2012, 35, 1375–1393. [Google Scholar] [CrossRef]
- Franzetti, A.; Tagliaferri, I.; Gandolfi, I.; Bestetti, G.; Minora, U.; Mayer, C.; Azzoni, R.S.; Diolaiuti, G.; Smiraglia, C.; Ambrosini, R. Light-Dependent Microbial Metabolisms Drive Carbon Fluxes on Glacier Surfaces. ISME J. 2016, 10, 2984–2988. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.S.; Hamilton, T.L.; Havig, J.R.; Mark, L.; Shock, E.L.; Boyd, E.S.; Hamilton, T.L.; Havig, J.R.; Skidmore, M.L.; Shock, L. Chemolithotrophic Primary Production in a Subglacial Ecosystem. Appl. Environ. Microbiol. 2014, 80, 6146. [Google Scholar] [CrossRef] [PubMed]
- Guelland, K.; Hagedorn, F.; Smittenberg, R.H.; Göransson, H.; Bernasconi, S.M.; Hajdas, I.; Kretzschmar, R. Evolution of Carbon Fluxes during Initial Soil Formation along the Forefield of Damma Glacier, Switzerland. Biogeochemistry 2013, 113, 545–561. [Google Scholar] [CrossRef]
- Rime, T.; Hartmann, M.; Stierli, B.; Anesio, A.M.; Frey, B. Assimilation of Microbial and Plant Carbon by Active Prokaryotic and Fungal Populations in Glacial Forefields. Soil Biol. Biochem. 2016, 98, 30–41. [Google Scholar] [CrossRef]
- Guelland, K.; Esperschütz, J.; Bornhauser, D.; Bernasconi, S.M.; Kretzschmar, R.; Hagedorn, F. Mineralisation and Leaching of C from 13C Labelled Plant Litter along an Initial Soil Chronosequence of a Glacier Forefield. Soil Biol. Biochem. 2013, 57, 237–247. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Freeman, C.; Ostle, N.J. Microbial Contributions to Climate Change through Carbon Cycle Feedbacks. ISME J. 2008, 2, 805–814. [Google Scholar] [CrossRef]
- Crowther, T.W.; Todd-Brown, K.E.O.; Rowe, C.W.; Wieder, W.R.; Carey, J.C.; MacHmuller, M.B.; Snoek, B.L.; Fang, S.; Zhou, G.; Allison, S.D.; et al. Quantifying Global Soil Carbon Losses in Response to Warming. Nature 2016, 540, 104–108. [Google Scholar] [CrossRef]
- Treat, C.C.; Wollheim, W.M.; Varner, R.K.; Grandy, A.S.; Talbot, J.; Frolking, S. Temperature and Peat Type Control CO2 and CH4 Production in Alaskan Permafrost Peats. Glob. Chang. Biol. 2014, 20, 2674–2686. [Google Scholar] [CrossRef]
- Hagedorn, F.; Martin, M.; Rixen, C.; Rusch, S.; Bebi, P.; Zürcher, A.; Siegwolf, R.T.W.; Wipf, S.; Escape, C.; Roy, J.; et al. Short-Term Responses of Ecosystem Carbon Fluxes to Experimental Soil Warming at the Swiss Alpine Treeline. Biogeochemistry 2010, 97, 7–19. [Google Scholar] [CrossRef]
- Melillo, J.M.; Steudler, P.A.; Aber, J.D.; Newkirk, K.; Lux, H.; Bowles, F.P.; Catricala, C.; Magill, A.; Ahrens, T.; Morrisseau, S. Soil Warming and Carbon-Cycle Feedbacks to the Climate System. Science 2002, 298, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, C.; Beer, C.; Sosnin, A.; Wagner, D.; Pfeiffer, E.M. Predicting Long-Term Carbon Mineralization and Trace Gas Production from Thawing Permafrost of Northeast Siberia. Glob. Chang. Biol. 2013, 19, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.; Mayes, M.A.; Allison, S.D.; Frey, S.D.; Shi, Z.; Hu, X.M.; Luo, Y.; Melillo, J.M. Reduced Carbon Use Efficiency and Increased Microbial Turnover with Soil Warming. Glob. Chang. Biol. 2019, 25, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Monteux, S.; Weedon, J.T.; Blume-Werry, G.; Gavazov, K.; Jassey, V.E.J.; Johansson, M.; Keuper, F.; Olid, C.; Dorrepaal, E. Long-Term in Situ Permafrost Thaw Effects on Bacterial Communities and Potential Aerobic Respiration. ISME J. 2018, 12, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- MacKelprang, R.; Waldrop, M.P.; Deangelis, K.M.; David, M.M.; Chavarria, K.L.; Blazewicz, S.J.; Rubin, E.M.; Jansson, J.K. Metagenomic Analysis of a Permafrost Microbial Community Reveals a Rapid Response to Thaw. Nature 2011, 480, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Miteva, V. Diversity and Ecology of Psychrophilic Microorganisms. Res. Microbiol. 2011, 162, 346–361. [Google Scholar] [CrossRef]
- Jiang, Y.; Lei, Y.; Qin, W.; Korpelainen, H.; Li, C. Revealing Microbial Processes and Nutrient Limitation in Soil through Ecoenzymatic Stoichiometry and Glomalin-Related Soil Proteins in a Retreating Glacier Fore Fi Eld. Geoderma 2019, 338, 313–324. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, J. Screening and Phylogenetic Analysis of Lipase-Producing Yeast from Tianshan No.1 Glacier. Light Ind. Sci. Technol. 2016, 32, 1–3, 6. [Google Scholar]
- Wu, Y.; Kumula; Zhang, X.; Wang, W.; Gulinazi; Wanglu; Chen, W.; Miaosen; Gao, W. Molecular Biologic Identification of Some Bacteria Strains Producing Low Temperature Amylase Isolated and Enzymatic Characterization from Frozen Soil in Xinjiang Glacier Ice Margin. Xinjiang Agric. Sci. 2013, 50, 1288–1296. [Google Scholar]
- Ni, Y.; Gu, Y.; Shi, X.; Zheng, X.; Han, L.; Zhou, H.; Cheng, G. Phylogenetic and Physiological Diversity of Cold-Adapted Bacteria Producing Protease from Sediiments of the Bottom Layer of the Glacier No. 1 in the Tianshan Mountains. Acta Microbiol. Sin. 2013, 53, 164–172. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).