Museal Indoor Air Quality and Public Health: An Integrated Approach for Exhibits Preservation and Ensuring Human Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Indoor Microclimate (Temperature, Relative Humidity and Carbon Dioxide)
2.2. Determination of Fungal Load in the Air and on the Exhibits
2.3. Non-Invasive Solution for Exhibits Cleaning
3. Results
3.1. Determination of Indoor Microclimate (Temperature, Relative Humidity and Carbon Dioxide)
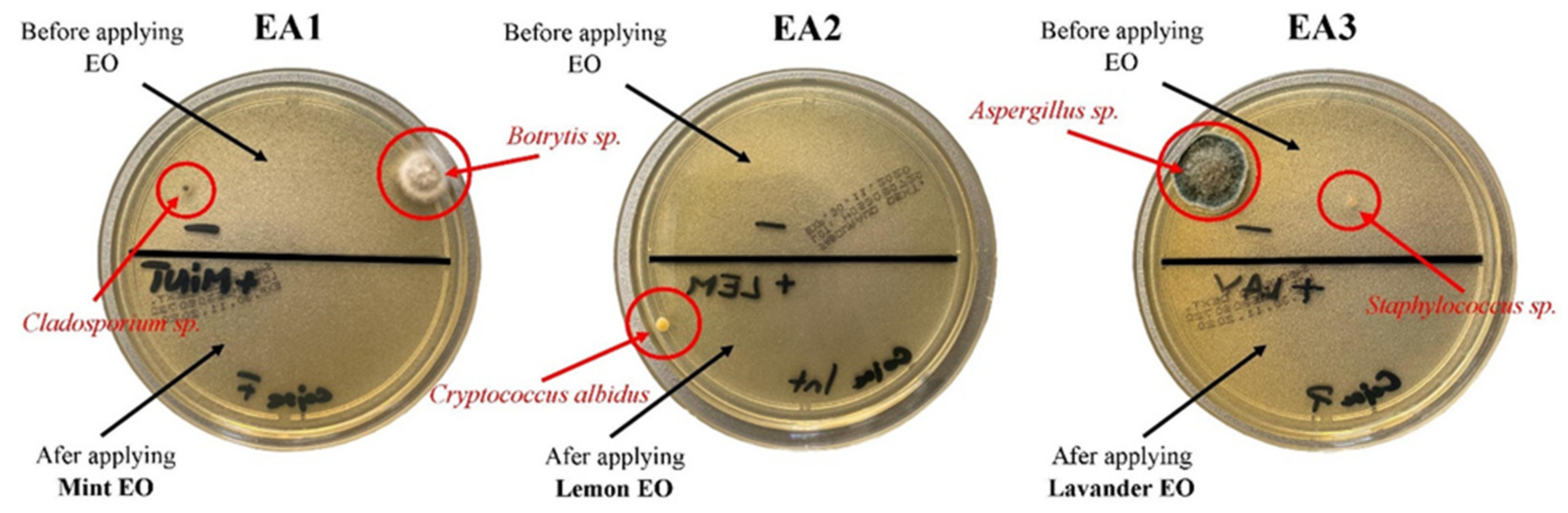
3.2. Determination of Fungal Load in the Air and on the Exhibits
3.3. Non-Invasive Solution for Exhibits Cleaning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deac, L.A.; Gozner, M.; Sambou, A. Ethnographic museums in the rural areas of Crisana region, Romania–keepers of local heritage, tradition and lifestyle. GeoJournal Tour. Geosites 2019, 27, 1251–1260. [Google Scholar] [CrossRef]
- Ilies, D.C.; Caciora, T.; Herman, G.V.; Ilies, A.; Ropa, M.; Baias, S. Geohazards affecting cultural heritage monuments. A complex case study from Romania. GeoJournal Tour. Geosites 2020, 31, 1103–1112. [Google Scholar] [CrossRef]
- Litti, G.; Audenaert, A. An integrated approach for indoor microclimate diagnosis of heritage and museum buildings: The main exhibition hall of Vleeshuis museum in Antwerp. Energy Build. 2018, 162, 91–108. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Lazaridis, M.; Katsivela, E.; Kopanakis, I.; Raisi, L.; Panagiaris, G. Indoor/outdoor particulate matter concentrations and microbial load in cultural heritage collections. Heritage Sci. 2015, 3, 34. [Google Scholar] [CrossRef]
- Gorny, A.R.; Harkawy, A.; Lawniczek-Walczyk, A.; Karbowska-Berent, J.; Wlazlo, A.; Nielsler, A.; Golofit-Szymczak, M.; Cyprowski, M. Exposure to culturable and total microbiota in cultural heritage conservation laboratories. Int. J. Occup. Med. Environ. Health 2016, 29, 255–275. [Google Scholar] [CrossRef]
- Karbowska-Berent, J.; Górny, R.L.; Strzelczyk, A.B.; Wlazło, A. Airborne and dust borne microorganisms in selected Polish libraries and archives. Build. Environ. 2011, 46, 1872–1879. [Google Scholar] [CrossRef]
- Camuffo, D. Microclimate for Cultural Heritage—Conservation, Restoration and Maintenance of Indoor and Outdoor Monuments, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Awad, A.H.A.; Saeed, Y.; Shakour, A.A.; Abdellatif, N.M.; Ibrahim, Y.H.; Elghanam, M.; Elwakeel, F. Indoor air fungal pollution of a historical museum, Egypt: A case study. Aerobiologia 2020, 36, 197–209. [Google Scholar] [CrossRef]
- Kumar, D.; Shah, N.R. Biodeterioration in Textiles: A Review. Int. J. Humanit. Soc. Sci. 2018, 3, 167–175. [Google Scholar]
- Marcu, F.; Ilies, D.C.; Wendt, J.; Indrie, L.; Ilies, A.; Burta, L.; Caciora, T.; Herman, G.V.; Todoran, A.; Baias, S.; et al. Investigations Regarding the Biodegradation of Cultural Heritage. Case Study of Traditional Embroidered Peasent Shirt (Maramures, Romania). Rom. Biotechnol. Lett. 2020, 25, 1362–1368. [Google Scholar] [CrossRef]
- Ilies, D.C.; Herman, G.V.; Caciora, T.; Ilies, A.; Indrie, L.; Wendt, J.A.; Axinte, A.; Diombera, M.; Lite, C.; Berdenov, Z.; et al. Considerations Regarding the Research for the Conservation of Heritage Textiles in Romania. In Waste in Textile and Leather Sectors; Körlü, A., Ed.; IntechOpen: Rijeka, Croatia, 2020; Chapter 10; pp. 88–93. [Google Scholar]
- Borrego, S.; Guiamet, P.; de Saravia, S.G.; Batistini, P.; Garcia, M.; Lavin, P.; Perdomo, I. The quality of air at archives and the biodeterioration of photographs. Int. Biodeterior. Biodegrad. 2010, 64, 139–145. [Google Scholar] [CrossRef]
- Caneva, G.; Nugari, M.P.; Salvadori, O. Biology in the Conservation of Works of Art; Sintesi Grafica, s.r.l.: Roma, Italy, 1991. [Google Scholar]
- Kakakhel, M.A.; Wu, F.; Gu, J.-D.; Feng, H.; Shah, K.; Wang, W. Controlling biodeterioration of cultural heritage objects with biocides: A review. Int. Biodeterior. Biodegrad. 2019, 143, 104721. [Google Scholar] [CrossRef]
- Ilies, D.C.; Onet, A.; Herman, G.V.; Indrie, L.; Ilies, A.; Burtă, L.; Gaceu, O.; Marcu, F.; Baias, S.; Caciora, T.; et al. Exploring the indoor environment of heritage buildings and its role in the conservation of valuable objects. Environ. Eng. Manag. J. 2019, 18, 2579–2586. [Google Scholar] [CrossRef]
- Di Carlo, E.; Barresi, G.; Palla, F. Biodeterioration. In Biotechnology and Conservation of Cultural Heritage; Palla, F., Barresi, G., Eds.; Springer: New York, NY, USA, 2017; pp. 1–32. [Google Scholar]
- Lucchi, E. Environmental Risk Management for Museums in Historic Buildings through an Innovative Approach: A Case Study of the Pinacoteca di Brera in Milan (Italy). Sustainability 2020, 12, 5155. [Google Scholar] [CrossRef]
- Ilieș, D.C.; Marcu, F.; Caciora, T.; Indrie, L.; Ilieș, A.; Albu, A.; Costea, M.; Burtă, L.; Baias, Ș.; Ilieș, M.; et al. Investigations of Museum Indoor Microclimate and Air Quality. Case Study from Romania. Atmosphere 2021, 12, 286. [Google Scholar] [CrossRef]
- Saini, J.; Dutta, M.; Marques, G. Indoor Air Quality Monitoring Systems Based on Internet of Things: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4942. [Google Scholar] [CrossRef]
- Gaceu, O.; Ilieș, D.C.; Baias, Ș.; Georgiță, M.; Ilieș, A.; Caciora, T.; Indrie, L.; Albu, A.; Herman, G.V.; Baidog, A.; et al. Microclimatic characteristics and air quality inside the National Archives of Bihor County, Romania. Environ. Eng. Manag. J. 2021, 20, 459–466. [Google Scholar]
- Buiuc, D.; Negut, M. Tratat de Microbiologie Clinică, 3rd ed.; Editura Medicală: Bucuresti, Romania, 2009. [Google Scholar]
- Bitnun, A.; Nosal, R.M. Stachybotrys chartarum (atra) contamination of the indoor environment: Health implications. Paediatr. Child Health 1999, 4, 125–129. [Google Scholar] [CrossRef]
- Chouaki, T.; Lavarde, V.; Lachaud, L.; Raccurt, C.P.; Hennequin, C. Invasive infections due to Trichoderma species: Report of 2 cases, findings of in vitro susceptibility testing, and review of the literature. Clin. Infect. Dis. 2002, 35, 1360–1367. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.A.; Salem, N.; Ali, S. Evaluation of indoor air quality in a museum (Bait Al Zubair) and residential homes. Indoor Built Environ. 2015, 24, 244–255. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Blades, N.; Camuffo, D.; Sturaro, G.; Valentino, A.; Gysels, K.; Van Grieken, R.; Busse, H.J.; Kim, O.; Ultych, U.; et al. The indoor environment of a modern museum building, the Sainsbury Centre for Visual Arts, Norwich, UK. Indoor Air 1999, 9, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Camuffo, D.; Pegan, E.; Rissanen, S.; Bratasz, L.; Kozlowski, R.; Camuffo, M.; Della Valle, A. An advanced church heating system favorable to artworks: A contribution to European standardization. J. Cult. Herit. 2010, 11, 205–219. [Google Scholar] [CrossRef]
- Camuffo, D.; Van Grieken, R.; Busse, H.J.; Sturaro, G.; Valentino, A.; Bernardi, A.; Blades, N.; Shooter, D.; Gysels, K.; Deutsch, F.; et al. Environmental monitoring in four European museums. Atmos. Environ. 2001, 35, 127–140. [Google Scholar] [CrossRef]
- Godoi, R.H.M.; Carneiro, B.H.B.; Paralovo, S.L.; Campos, V.P.; Tavares, T.M.; Evangelista, H.; Van Grieken, R.; Godoi, A.F.L. Indoor air quality of a museum in a subtropical climate: The Oscar Niemeyer museum in Curitiba, Brazil. Sci. Total Environ. 2013, 452–453, 314–320. [Google Scholar]
- Lech, T.; Ziembinska-Buczynska, A.; Krupa, N. Analysis of microflora present on historical textiles with the use of molecular techniques. Int. J. Conserv. Sci. 2015, 6, 137–144. [Google Scholar]
- Iwata, T.; Masahiro, H.; Hiroyasu, T. Field investigations on IAQ in museums and examination of methods for monitoring: Pilot Study for Air Quality Guidelines in Museums Part 1. J. Archit. Plan. 2001, 66, 55–62. [Google Scholar] [CrossRef][Green Version]
- Varas-Muriel, M.J.; Fort, R.; Martínez-Garrido, M.I.; Zornoza-Indart, A.; López-Arce, P. Fluctuations in the indoor environment in Spanish rural churches and their effects on heritage conservation: Hygro-thermal and CO2 conditions monitoring. Build. Environ. 2014, 82, 97–109. [Google Scholar] [CrossRef]
- Dăneasă, C.M. The study of conservation state of a wooden church from Boz Village, Hunedoara County, Romania. Int. J. Conserv. Sci. 2013, 4, 53–64. [Google Scholar]
- Herbel, R.G. Wooden church from Cetăţeaua Village, Mitrofani Commune, Vâlcea County. Rev. Transilv. 2015, 3–4, 89–95. [Google Scholar]
- Grøntoft, T.; Marincas, O. Indoor Air Pollution Impact on Cultural Heritage in an Urban and a Rural Location in Romania: The National Military Museum in Bucharest and the Tismana Monastery in Gorj County. Herit. Sci. 2018, 6, 73. [Google Scholar] [CrossRef]
- Auner, N.; Bucşa, C.; Bucşa, L.; Ciocşan, O. Technology of Consolidation, Restoration and Protection Against Biodegradation of Wood Structures from Historical Monuments; Alma Mater Publishing House: Sibiu, Romania, 2005. [Google Scholar]
- Bucsa, L. Biological expertise in the restoration of historical monuments. Transsylvania Nostra 2004, 2, 81–92. [Google Scholar]
- Bucsa, L.; Bucsa, C. Biodegradation Agents at Historical Monuments in Romania Prevention and Fighting; Alma Mater Publishing House: Sibiu, Romania, 2006. [Google Scholar]
- Bucsa, L.; Bucsa, C. Biological degradation of wood structures to historical monuments and open-air museums. Transsylvania Nostra 2009, 2, 22–30. [Google Scholar]
- Bucsa, L.; Halasz, A.M. Wooden church in Bulgari, Salaj County, construction and biodegradation problems. Restor. Books 2013, 1, 178–188. [Google Scholar]
- Budu, A.-M.; Sandu, I. Monitoring of Pollutants in Museum Environment. Present Environ. Sustain. Dev. 2015, 9, 173–180. [Google Scholar]
- Iorga, G.; Raicu, C.B.; Stefan, S. Annual air pollution level of major primary pollutants in Greater Area of Bucharest. Atmos. Pollut. Res. 2015, 6, 824–834. [Google Scholar] [CrossRef]
- Sandu, I.C.A.; Luca, C.; Sandu, I.; Pohontu, M. Research regarding the soft wood support degradation evaluation in old paintings, using preparation layers. II. IR and FTIR Spectroscopy. Rev. Chim. 2001, 52, 409–419. [Google Scholar]
- Sciurpi, F.; Carletti, C.; Cellai, G.; Pierangioli, L. Environmental monitoring and microclimatic control strategies in ‘La Specola’ museum of Florence. Energy Build. 2015, 95, 190–201. [Google Scholar] [CrossRef]
- Lucchi, E. Review of preventive conservation in museum buildings. J. Cult. Herit. 2018, 29, 180–193. [Google Scholar] [CrossRef]
- Indrie, L.; Oana, D.; Ilies, M.; Ilies, D.C.; Lincu, A.; Ilies, A.; Baias, S.; Herman, G.; Onet, A.; Costea, M.; et al. Indoor air quality of museums and conservation of textiles art works. Case study: Salacea Museum House, Romania. Ind. Text. 2019, 70, 88–93. [Google Scholar]
- Stupar, M.; Grbić, M.L.; Simić, G.S.; Jelikić, A.; Vukojević, J.; Sabovljević, M. A sub-aerial biofilms investigation and new approach in biocide application in cultural heritage conservation: Holy Virgin Church (Gradac Monastery, Serbia). Indoor Built Environ. 2012, 23, 584–593. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential Oils as Natural Biocides in Conservation of Cultural Heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef] [PubMed]
- Savković, Ž.D.; Stupar, M.Č.; Grbić, M.V.L.; Vukojević, J.B. Comparison of anti-Aspergillus activity of Origanum vulgare L. essential oil and commercial biocide based on silver ions and hydrogen peroxide. Acta Bot. Croat. 2016, 75, 121–128. [Google Scholar] [CrossRef]
- Ilieș, D.C.; Hodor, N.; Indrie, L.; Dejeu, P.; Ilieș, A.; Albu, A.; Caciora, T.; Ilieș, M.; Barbu-Tudoran, L.; Grama, V. Investigations of the Surface of Heritage Objects and Green Bioremediation: Case Study of Artefacts from Maramureş, Romania. Appl. Sci. 2021, 11, 6643. [Google Scholar] [CrossRef]
- Muzeul Municipal. Available online: https://primariabeius.ro/pagina/muzeul-municipal (accessed on 30 August 2021).
- Caciora, T.; Herman, G.V.; Baias, S. Computer analysis of a heritage item—The traditional sheepskin waistcoat in Beius area. Rev. Etnogr. Folc. J. Ethnogr. Folk. 2021, 1–2, 195–209. [Google Scholar]
- Corgnati, S.P.; Fabi, V.; Filippi, M. A methodology for microclimatic quality evaluation in museums: Application to a temporary exhibit. Build. Environ. 2009, 44, 1253–1260. [Google Scholar] [CrossRef]
- Nelson, R.J. Seasonal immune function and sickness responses. Trends Immunol. 2004, 25, 187–192. [Google Scholar] [CrossRef]
- Commission of European Communities: Biological Particles in Indoor Environments, European Collaborative Action-Indoor Air Quality and its Impact on Man; Report No. 12; CEC: Luxembourg, 1993; Available online: http://www.inive.org/medias/ECA/ECA_Report12.pdf (accessed on 12 January 2022).
- ANSI/ASHRAE Standard 621-2010, Ventilation for Acceptable Indoor Air Quality; American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 2010; Available online: https://www.ashrae.org/file%20library/doclib/public/200418145036_347.pdf (accessed on 30 August 2021).
- Chapter 23: Museums, Galleries, Archives and Libraries. In ASHRAE Handbook–HVAC Applications, SI ed.; ASHRAE Research: Atalanta, GA, USA, 2011; pp. 1–23.
- World Heritage Encyclopedia. Oil painting. Available online: http://www.worldheritage.org/articles/Oil_painting (accessed on 30 August 2021).
- Puchianu, G.; Necula, V.; Enache, D.V. Research on active and passive monitoring aeromicroflora in the milk units processing. Annals of the Academy of Romanian Scientists Series on Agriculture. Silvic. Vet. Med. Sci. 2016, 5, 91–103. [Google Scholar]
- Cernei, E.R.; Maxim, D.C.; Mavru, R.; Indrei, L.L. Bacteriological analysis of air (aeromicroflora) from the level of dental offices in IAŞI County. Rom. J. Oral Rehabil. 2013, 5, 53–68. [Google Scholar]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, 1231–1249. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Cotoras, M.; Castro, P.; Vivanco, H.; Melo, R.; Mendoza, L. Farnesol induces apoptosis-like phenotype in the phytopathogenic fungus Botrytis cinerea. Mycologia 2013, 105, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Huang, B.; Wang, Y. Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int. J. Food Microbiol. 2011, 145, 464–470. [Google Scholar] [CrossRef] [PubMed]
- EPA Standard. The National Ambient Air Quality Standards for Particulate Matter-Epa Retains Air Quality Standards for Particle Pollution (Particulate Matter): Fact Sheet. 2020. Available online: https://www.epa.gov/sites/production/files/2020-04/documents/fact_sheet_pm_naaqs_proposal.pdf (accessed on 5 January 2022).
- Andualem, Z.; Ayenew, Y.; Ababu, T.; Hailu, B. Assessment of airborne culturable fungal load in an indoor environment of dormitory rooms: The case of University of Gondar student’s dormitory rooms, northwest Ethiopia. Air Soil Water Res. 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Wanner, H.; Verhoeff, A.; Colombi, A.; Flannigan, B.; Gravesen, S.; Mouilleseaux, A.; Nevalainen, A.; Papadakis, J.; Seidel, K. Indoor Air Quality and Its Impact on Man: Report no. 12: Biological Particles in Indoor Environments; Brussels-Luxembourg, ECSC-EEC-EAEC: Brussels, Belgium, 1993. [Google Scholar]
- Satish, S.; Mohana, D.C.; Ranhavendra, M.P.; Raveesha, K.A. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. J. Agric. Technol. 2007, 3, 109–119. [Google Scholar]
- Stupar, M.; Grbić, M.L.J.; Džamić, A.; Unkovia, N.; Ristić, M.; Jelikić, A.; Vukojević, J. Antifungal activity of selected essential oils and biocide benzalkonium chloride against the fungi isolated from cultural heritage objects. S. Afr. J. Bot. 2014, 93, 118–124. [Google Scholar] [CrossRef]
- De Nuntiis, P.; Palla, F. Bioaerosols. In Biotechnology and Conservation of Cultural Heritage; Palla, F., Barresi, G., Eds.; Springer International Publishing: Cham, Germany, 2017; pp. 31–48. [Google Scholar]
- Pasanen, A.-L.; Kalliokoski, P.; Pasanen, P.; Jantunen, M.J.; Nevalainen, A. Laboratory studies on the relationship between fungal growth and atmospheric temperature and humidity. Environ. Int. 1991, 17, 225–228. [Google Scholar] [CrossRef]
- Sindt, C.; Besancenot, J.P.; Thibaudon, M. Airborne Cladosporium fungal spores and climate change in France. Aerobiologia 2016, 32, 53–68. [Google Scholar] [CrossRef]
- Ritschkoff, A.C.; Viitanen, H.; Koskela, K. The response of building materials to the mould exposure at different humidity and temperature conditions. In Healthy Buildings, 1st ed.; Seppänen, O., Säteri, J., Eds.; Finnish Society of Indoor Air Quality and Climate: Espoo, Finland, 2000; pp. 317–322. [Google Scholar]
- Anaf, W.; Horemans, B.; Madeira, T.I.; Carvalho, M.L.; De Wael, K.; Van Grieken, R. Effects of a constructional intervention on airborne and deposited particulate matter in the Portuguese National Tile Museum, Lisbon. Environ. Sci. Pollut. Res. Int. 2013, 20, 1849–1857. [Google Scholar] [CrossRef]
- Dalal, L.; Bhowal, M.; Kolbeude, S. Incidence of deteriorating fungi in the air inside the college libraries of Wardha city. Arch. Appl. Sci. Res. 2011, 3, 479–485. [Google Scholar]
- Krakoval, L.; Chovanova, K.; Selim, S.A.; Simonovicova, A.; Puskarova, A.; Makova, A.; Pangallo, D. A multiphasic approach for investigation of microbial diversity and its biodegradative abilities in historical paper and parchment documents. Int. Biodeterior. Biodegrad. 2012, 70, 117–125. [Google Scholar] [CrossRef]
- May, E.; Lewis, F.J.; Pereira, S.; Tayler, S.; Seaward, M.R.D.; Allsopp, D. Microbial deterioration of building stone—A review. Biodeterior. Abstr. 1993, 7, 109–123. [Google Scholar]
- Sakamoto-Momiyama, M. Seasonality in Human Mortality; University of Tokyo Press: Tokyo, Japan, 1977. [Google Scholar]
- Fich, F.; Abarzúa-Araya, A.; Pérez, M.; Nauhm, Y.; León, E. Candida parapsilosis and Candida guillermondii: Emerging pathogens in nail candidiasis. Indian J. Dermatol. 2014, 59, 24–29. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Mendez, M.; Kibbler, C.; Erzsebet, P.; Chang, S.C.; Newell, V.A. Candida guilliermondii, an opportunistic fungal pathogen with decreased susceptibility to fluconazole: Geographic and temporal trends from the ARTEMIS DISK antifungal surveillance program. J. Clin. Microbiol. 2006, 44, 3551–3556. [Google Scholar] [CrossRef]
- Silva, F.C.; Viana, V.O.; Araújo, B.P.D.; Campos, L.A.N.L.; Rosa, L.P. Prevalence of Candida yeasts in oral samples from children with AIDS and children exposed and not exposed to HIV served by SUS in the state of Bahia, Brazil. Rev. Gaúcha Odontol. 2015, 63, 7–12. [Google Scholar] [CrossRef][Green Version]
- Choe, Y.J.; Blatt, D.B.; Yalcindag, A.; Geffert, S.F.; Bobenchik, A.M.; Michelow, I.C. Cryptococcus albidus Fungemia in an Immunosuppressed Child: Case Report and Systematic Literature Review. J. Pediatric Infect. Dis. Soc. 2020, 9, 100–105. [Google Scholar] [CrossRef]
- Ragupathi, L.; Reyna, M. Case report of Cryptococcus albidus peritonitis in a peritoneal dialysis patient and a review of the literature. Perit. Dial. Int. 2015, 35, 421–427. [Google Scholar] [CrossRef]
- Cheng, M.F.; Chiou, C.C.; Liu, Y.C.; Wang, H.Z.; Hsieh, K.S. Cryptococcus laurentii fungemia in a premature neonate. J. Clin. Microbiol. 2001, 39, 1608–1611. [Google Scholar] [CrossRef]
- Gullo, F.P.; Rossi, S.A.; de Sardi, J.; Teodoro, V.L.I.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Cryptococcosis: Epidemiology, fungal resistance, and new alternatives for treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1377–1391. [Google Scholar] [CrossRef]
- Hurtado, J.C.; Castillo, P.; Fernandes, F.; Navarro, M.; Lovane, L.; Casas, I.; Quinto, L.; Marco, F.; Jordao, D.; Ismail, M.R.; et al. Mortality due to Cryptococcus neoformans and Cryptococcus gattii in low-income settings: An autopsy study. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Damji, R.; Mukherji, A.; Mussani, F. Sporobolomyces salmonicolor: A case report of a rare cutaneous fungal infection. SAGE Open Med. Case Rep. 2019, 7, 1–2. [Google Scholar] [CrossRef]
- Wong, S.Y.; Wong, K.F. Penicillium marneffei infection in AIDS. Patholog. Res. Int. 2011, 11, 1–10. [Google Scholar] [CrossRef]
- Yuen, K.Y.; Pascal, G.; Wong, S.S.; Glaser, P.; Woo, P.C.; Kunst, F.; Cai, J.J.; Cheung, E.Y.; Medigue, C.; Danchin, A. Exploring the Penicillium marneffei genome. Arch. Microbiol. 2003, 179, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Paulussen, C.; Hallsworth, J.E.; Álvarez-Pérez, S.; Nierman, W.C.; Hamill, P.G.; Blain, D.; Rediers, H.; Lievens, B. Ecology of aspergillosis: Insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb. Biotechnol. 2017, 10, 296–322. [Google Scholar] [CrossRef] [PubMed]
- Huerta, A.; Soler, N.; Esperatti, M.; Guerrero, M.; Menendez, R.; Gimeno, A.; Zalacain, R.; Mir, N.; Aguado, J.M.; Torres, A. Importance of Aspergillus spp. isolation in Acute exacerbations of severe COPD: Prevalence, factors and follow-up: The FUNGI-COPD study. Respir. Res. 2014, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, R.; Andersson, M.A.; Kredics, L.; Grigoriev, P.A.; Sundell, N.; Salkinoja-Salonen, M.S. 20-Residue and 11 residue peptaibols from the fungus T richoderma longibrachiatum are synergistic in forming N a+/K+-permeable channels and adverse action towards mammalian cells. FEBS J. 2012, 279, 4172–4190. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Sutton, D.A.; Martin-Vicente, A.; Cano-Lira, J.F.; Wiederhold, N.; Guarro, J.; Genéa, J. Cladosporium Species Recovered from Clinical Samples in the United States. J. Clin. Microbiol. 2015, 53, 2990–3000. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Gené, J.; Sutton, D.A.; Wiederhold, N.P.; Cano-Lira, J.F.; Guarro, J. New species of Cladosporium associated with human and animal infections. Pers. Mol. Phylogeny Evol. Fungi 2016, 36, 281–298. [Google Scholar] [CrossRef]
- Jürgensen, C.W.; Madsen, A.M. Exposure to the airborne mould Botrytis and its health effects. Ann. Agric. Environ. Med. 2009, 16, 183–196. [Google Scholar]
- Mansour, M.M.A.; EL-Hefny, M.; Salem, M.Z.M.; Ali, H.M. The Biofungicide Activity of Some Plant Essential Oils for the Cleaner Production of Model Linen Fibers Similar to Those Used in Ancient Egyptian Mummification. Processes 2020, 8, 79. [Google Scholar] [CrossRef]
- Cappitelli, F.; Cattò, C.; Villa, F. The Control of Cultural Heritage Microbial Deterioration. Microorganisms 2020, 8, 1542. [Google Scholar] [CrossRef] [PubMed]
- Bou-Belda, E.; Indrie, L.; Ilieș, D.C.; Hodor, N.; Berdenov, Z.; Herman, G.V.; Caciora, T. Chitosan—A non-invasive approach for the preservation of historical textiles. Ind. Text. 2020, 71, 576–579. [Google Scholar] [CrossRef]
- Indrie, L.; Bonet-Aracil, M.; Ilies, D.C.; Albu, A.V.; Ilies, G.; Herman, G.V.; Baias, S.; Costea, M. Heritage ethnographic objects-antimicrobial effects of chitosan treatment. Ind. Text. 2021, 72, 284–288. [Google Scholar] [CrossRef]
- Spada, M.; Cuzman, O.A.; Tosini, I.; Galeotti, M.; Sorella, F. Essential oils mixtures as an eco-friendly biocidal solution for a marble statue restoration. Int. Biodeterior. Biodegrad. 2021, 163, 105280. [Google Scholar] [CrossRef]
- Gatti, L.; Troiano, F.; Vacchini, V.; Cappitelli, F.; Balloi, A. An In Vitro Evaluation of the Biocidal Effect of Oregano and Cloves’ Volatile Compounds against Microorganisms Colonizing an Oil Painting—A Pioneer Study. Appl. Sci. 2021, 11, 78. [Google Scholar] [CrossRef]












| Name of Genera/Species | Identification | Pathogenicity | Potential Disorders | |||
|---|---|---|---|---|---|---|
| Air | Exhibites | Pathogenic | Rare Pathogenic | |||
| Yeast | Candida guilliermondii | ● | ● | Onychomycosis; Rarely, invasive candidiasis [80,81]. | ||
| Candida sphaerica | ● | ● | Opportunist infections—especially in children [82]. | |||
| Cryptococcus albidus | ● | ● | ● | Pulmonary, Cutaneous or Disseminated Cryptococcosis—especially in immunocompromised patients [83,84]. | ||
| Cryptococcus laurentii | ● | ● | Cutaneous Cryptococcosis, Keratitis, Endophthalmitis, Pulmonary abscess, Peritonitis, Meningitis, Fungemia [85,86]. | |||
| Cryptococcus neoformans | ● | ● | Pneumonia, Meningoencephalitis—in patients with immunocompromising disorders [87]. | |||
| Sporobolomyces salmonicolor | ● | ● | Dermatitis, Endophthalmitis—in patients with immunocompromising disorders [88]. | |||
| Moulds | Penicillium sp. | ● | ● | ● | Sindrom respirator allergic, Keratitis, Endophthalmitis, Otomycosis, Pneumonia, Endocarditis, Peritonitis, Urinary tract infections [89,90]. | |
| Aspergillus sp. | ● | ● | ● | Obstructive bronchial aspergillosis, Pulmonary aspergillosis and aspergilloma, Acute/chronic allergic sinusitis, Allergic bronchopulmonary aspergillosis, Endocarditis, Osteomyelitis, Endophthalmitis [91,92] | ||
| Trichoderma sp. | ● | ● | ● | Sneezing, asthma attacks, prolonged coughing and lung infections—in patients with immunocompromising disorders [93]. | ||
| Cladosporium sp. | ● | ● | ● | Allergic respiratory syndrome, Allergic sinusitis, Dermatitis, Opportunist infections—in patients with immunocompromising disorders [94,95]. | ||
| Stachybothris sp. | ● | ● | ● | Respiratory, cutaneous, ophthalmic allergic syndrome—especially in children [23]. | ||
| Botrytis sp. | ● | ● | Various allergies and respiratory problems—in patients with immunocompromising disorders [96]. | |||
| Bacteria | Staphylococcus sp. | ● | ● | Bacteremia, Pneumonia, Endocarditis, Osteomyelitis [69]. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilieș, D.C.; Safarov, B.; Caciora, T.; Ilieș, A.; Grama, V.; Ilies, G.; Huniadi, A.; Zharas, B.; Hodor, N.; Sandor, M.; et al. Museal Indoor Air Quality and Public Health: An Integrated Approach for Exhibits Preservation and Ensuring Human Health. Sustainability 2022, 14, 2462. https://doi.org/10.3390/su14042462
Ilieș DC, Safarov B, Caciora T, Ilieș A, Grama V, Ilies G, Huniadi A, Zharas B, Hodor N, Sandor M, et al. Museal Indoor Air Quality and Public Health: An Integrated Approach for Exhibits Preservation and Ensuring Human Health. Sustainability. 2022; 14(4):2462. https://doi.org/10.3390/su14042462
Chicago/Turabian StyleIlieș, Dorina Camelia, Bahodirhon Safarov, Tudor Caciora, Alexandru Ilieș, Vasile Grama, Gabriela Ilies, Anca Huniadi, Berdenov Zharas, Nicolaie Hodor, Mircea Sandor, and et al. 2022. "Museal Indoor Air Quality and Public Health: An Integrated Approach for Exhibits Preservation and Ensuring Human Health" Sustainability 14, no. 4: 2462. https://doi.org/10.3390/su14042462
APA StyleIlieș, D. C., Safarov, B., Caciora, T., Ilieș, A., Grama, V., Ilies, G., Huniadi, A., Zharas, B., Hodor, N., Sandor, M., Zsarnóczky, M. B., Pantea, E., Herman, G. V., Dejeu, P., Szabo-Alexi, M., & Denes David, L. (2022). Museal Indoor Air Quality and Public Health: An Integrated Approach for Exhibits Preservation and Ensuring Human Health. Sustainability, 14(4), 2462. https://doi.org/10.3390/su14042462









